Abstract
Objective:
To evaluate the incidence, characteristics, and clinical consequences of delayed intraventricular hemorrhage (dIVH).
Methods:
Patients with primary intracerebral hemorrhage (ICH) were enrolled into a prospective registry between December 2006 and February 2012. Patients were managed, and serial neuroimaging obtained, per a structured protocol. Initial and delayed IVH were identified on imaging, along with ICH volumes, with outcomes blinded. Multivariate models were developed to test whether the occurrence of dIVH was a predictor of functional outcomes independent of known predictors, including the ICH score elements and ICH growth.
Results:
A total of 216 patients were studied, and 104 (48%) had IVH on initial imaging. Of the 112 with no IVH, 23 (21%) subsequently developed IVH. Emergent surgical intervention, mostly ventriculostomy placement, was required after discovery of dIVH in 10 (43%) of these 23. In multivariate models adjusting for all elements of the ICH score and hematoma growth, dIVH was an independent predictor of death at 14 days (p = 0.015) and higher modified Rankin Scale scores at 3 months (all p = 0.037). The effect of dIVH remained significant in a secondary analysis that adjusted for all other variables significant in the univariate analysis.
Conclusions:
Similar to hematoma expansion dIVH is independently associated with death and poor outcomes. Because IVH is easily detected by serial neuroimaging and often requires emergent surgical intervention, monitoring for dIVH is recommended.
Significant attention has been given to hematoma expansion as a dynamic, measurable, and potentially modifiable phenomenon in patients with primary intracerebral hemorrhage (ICH). The effect of hematoma volume on outcomes is substantial, and initial ICH volume is included as a variable in 2 widely used scoring systems for ICH severity.1,2 Hematoma expansion has also been associated with worse outcomes, and prevention of hematoma expansion has become a therapeutic goal.3 Other variables in those validated scales are clearly nonmodifiable (e.g., site of ICH, age, and premorbid cognitive impairment) or indirectly reflect an aggregate of many physiologic variables (e.g., the Glasgow Coma Scale [GCS]). The final important prognostic variable in the ICH score is the presence of intraventricular hemorrhage (IVH) on initial assessment. Like ICH volume, IVH is clearly associated with worse outcomes, but unlike the phenomenon of interval ICH expansion after initial assessment, interval development of IVH has not been systematically studied.
In this study, we tested the hypothesis that delayed intraventricular hemorrhage (dIVH), analogous to hematoma expansion, is associated with worse outcomes in patients with primary ICH.
METHODS
Patients presenting to Northwestern Memorial Hospital with ICH between December 2006 and February 2012 were prospectively enrolled in an observational cohort study. All cases were diagnosed by a board-certified vascular neurologist or neurointensivist using CT, MRI, or both. Patients with ICH attributed to trauma, hemorrhagic conversion of ischemic stroke, structural lesions, or vascular malformations were excluded. Demographic information, clinical data, laboratory results, and follow-up assessments were prospectively recorded. The likely etiology of ICH was adjudicated by a board-certified vascular neurologist or neurointensivist who used all available clinical and imaging data. Previously validated criteria were used for the diagnosis of cerebral amyloid angiopathy.4 All patients were admitted to the neurointensive care unit with a standard order set in the electronic order entry system.
Basic data, including demographic information, medical history, medication history, standardized clinical instruments (GCS, NIH Stroke Scale, and premorbid modified Rankin Scale [mRS]), pretreatment vital signs, and imaging data, were prospectively recorded by independent study staff. Data on medical management, surgical interventions, and medical complications were recorded contemporaneously during hospitalization. Per protocol, patients underwent serial noncontrast CT imaging to monitor for expansion of the hematoma and cerebral edema at 6, 24, and 48 hours after the initial brain imaging, although earlier imaging was obtained in response to a change in clinical status or deferred in the case of medical instability or withdrawal of care. At the discretion of the clinical team, CT angiography was performed, usually as a distinct study within the first 6 hours. MRI was obtained whenever feasible in patients with salvageable conditions on Siemens 1.5-T MRI scanners (Siemens AG, Munich, Germany) with a protocol including B1000, diffusion-weighted images, apparent diffusion coefficient maps, fluid-attenuated inversion recovery, T2/turbo spin echo, and T1 and T2* gradient echo. A certified examiner recorded the NIH Stroke Scale and mRS at 14 days or discharge, whichever came first. The mRS was also recorded prospectively at 28 days and 3 months with a validated questionnaire.5,6
Each neuroimaging study obtained during the initial hospitalization was prospectively reviewed by a board-certified neuroradiologist and then retrospectively confirmed by a board-certified neurointensivist (M.B.M.) and board-certified neuroradiologist (A.J.N.) to identify the appearance of intraventricular blood. Interrater reliability was evaluated by κ coefficient. Hematoma volumes were measured from initial and final head CT scans on industry-standard Digital Imaging and Communications in Medicine images from both referring hospitals and ours by using Analyze software (Mayo Clinic, Rochester, MN) with a semiautomated process, a technique with high reliability that has been used as an endpoint in other ICH studies, as we have previously described.7 Both reviewers were blinded to patient outcomes. One-way analysis of variance (for >2 groups of normally distributed data), χ2 (for categorical variables), and independent-samples Kruskal-Wallis tests (for groups of non–normally distributed data) were used to compare characteristics of subjects with IVH on their initial brain imaging, subjects who developed IVH after their initial brain imaging, and subjects who never developed IVH. Regression models were developed to test whether the occurrence of dIVH contributed independent prognostic value to the validated and widely used ICH score variables: GCS score, age 80 years or older, infratentorial origin, ICH volume 30 mL or greater, and IVH, all assessed at first evaluation as originally defined.1 ICH growth was added to the ICH score variables to clarify whether dIVH is an epiphenomenon for ICH growth or a true, independent mediator of poor outcome. We defined ICH growth as 26% or greater growth in the volume of the hematoma between initial and final head CT imaging, consistent with a prior comprehensive study3 that evaluated multiple definitions for ICH growth and found this to be the most predictive cutoff by receiver operating curve analysis. A binary logistic regression model was used to predict death at 14 days (or discharge, if occurring before 14 days). An ordinal regression model was used to evaluate mRS at 3 months because such models are more informative than dichotomous outcomes.8 Validity of the ordinal logistic regression model was assessed with the test of parallel lines, and significance was confirmed by −2 log likelihood.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board. Written informed consent was obtained from patients or their legally authorized representatives. The institutional review board approved a waiver of consent for patients who died during initial hospitalization or who were incapacitated and for whom a legal representative could not be located.
RESULTS
There were 216 patients in the study cohort. Of those, 104 (48%) had IVH on their initial brain imaging, but more than 20% with no IVH on initial evaluation subsequently developed IVH, as confirmed on follow-up imaging. Interrater reliability for the identification of IVH was excellent (κ = 0.995). The median interval between symptom onset and discovery of dIVH was 8.9 hours (interquartile range, 6.8–28.0 hours), and the median interval between the first neuroimaging examination and the examination showing IVH was 6.7 hours (interquartile range, 4.7–23.8 hours). In 17 of the 23 patients with dIVH, the intraventricular extension was found on the second neuroimaging study (i.e., the next study after the baseline scan), with the latest diagnosis being on the sixth brain scan, performed at 72.4 hours from the time of symptom onset.
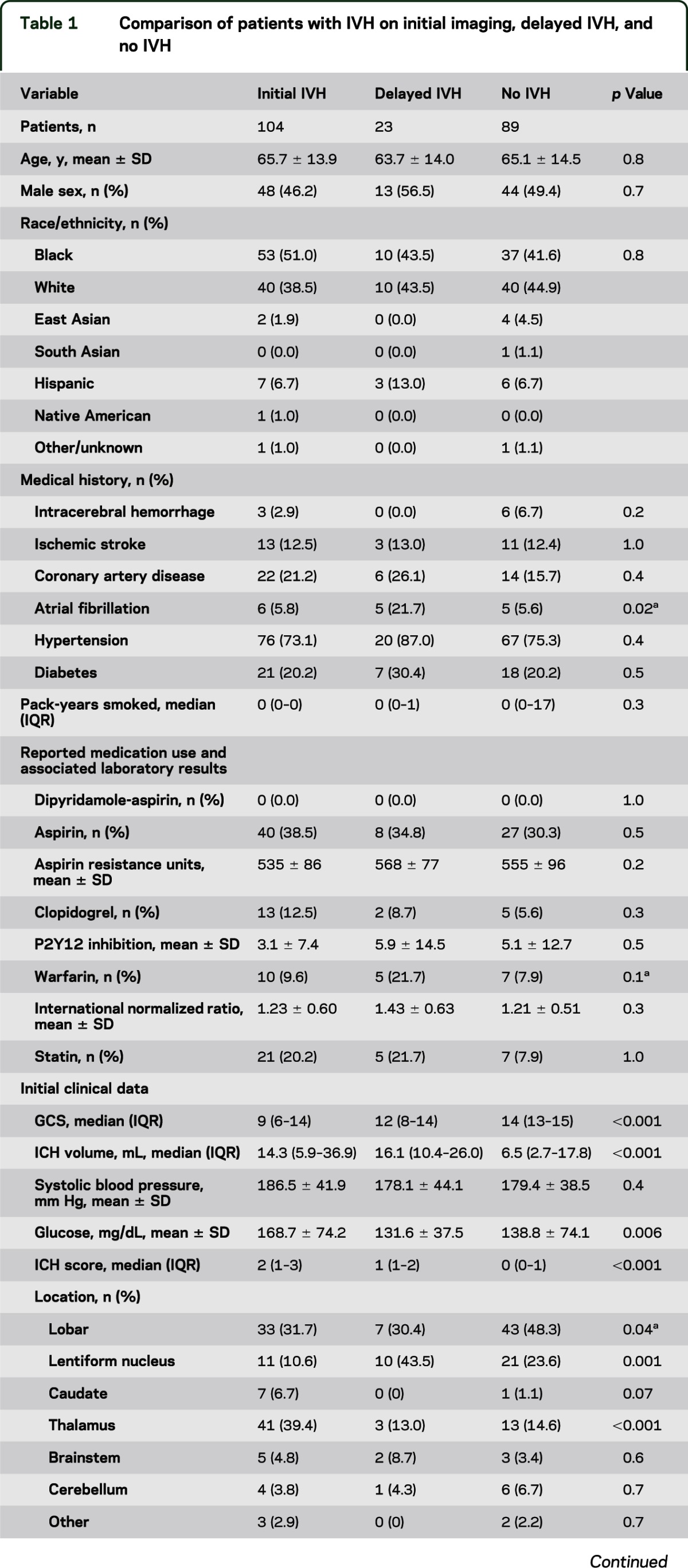
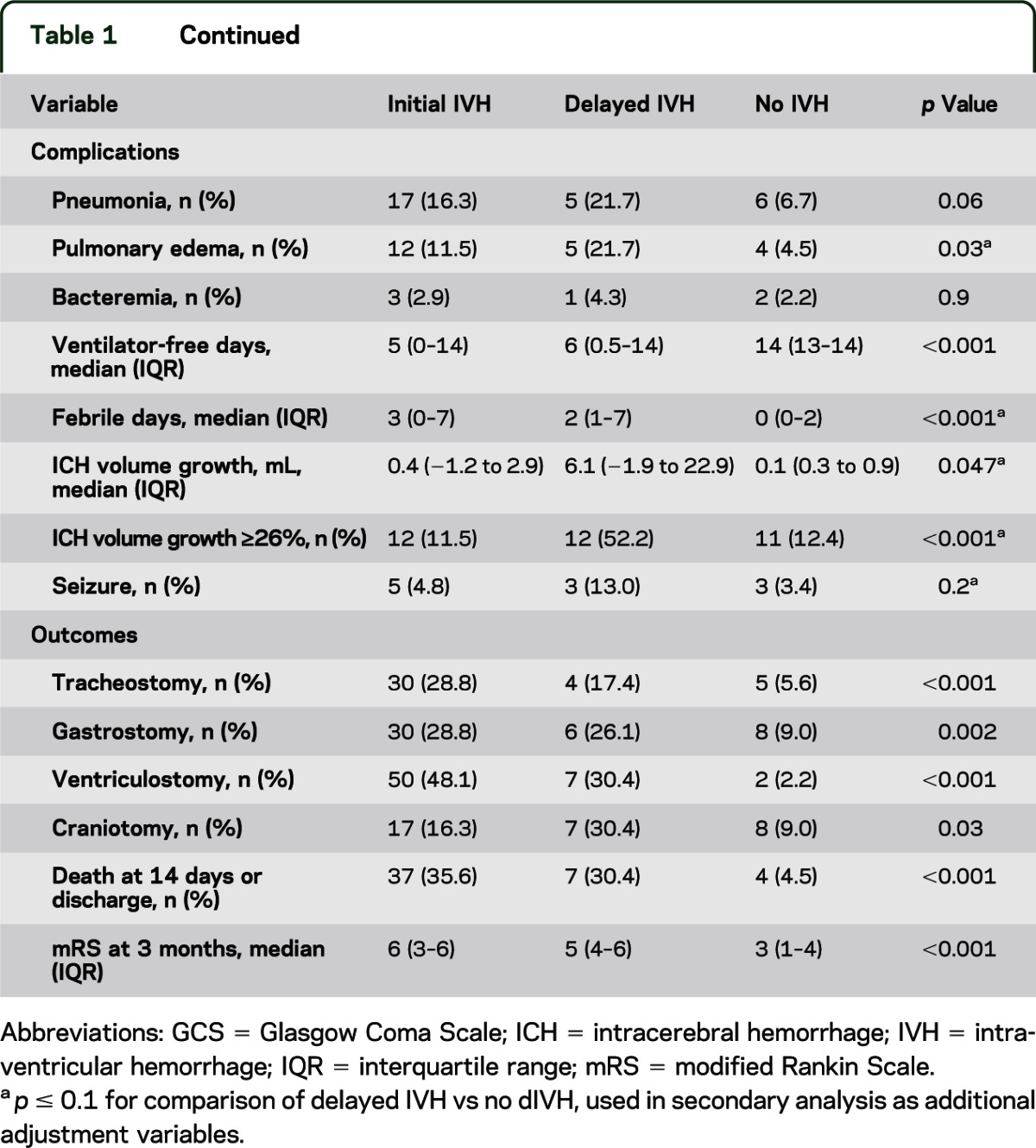
The characteristics of the 3 groups of patients (patients with IVH on first imaging, patients with IVH discovered on subsequent imaging, and those who never developed IVH) are summarized in table 1. Complication rates for dIVH patients were similar to, or higher than, those for patients with initial IVH. Most strikingly, hematoma expansion was overwhelmingly greater in patients with dIVH (median growth of 6.1 mL; interquartile range, −1.9 to 22.9 mL) than in either patients with initial (median growth of 0.4 mL; interquartile range, −1.2 to 2.9 mL) or no (median growth of 0.1 mL; interquartile range, −0.3 to 0.9 mL) IVH (p = 0.047), and craniotomy rates were higher as well (30% vs 16% and 9%, respectively; p = 0.03). As expected, those who never developed IVH were more likely to have lobar hemorrhage. With respect to hemorrhage location, patients with initial IVH were more likely to have hemorrhages in the thalamus (p < 0.001) and caudate (p = 0.07), whereas those with dIVH were more likely to have hemorrhages in the lentiform nucleus (p = 0.001). Of the 23 patients who developed dIVH, 10 (43%) required emergent surgical intervention, including emergent craniotomy in 3 and ventriculostomy with or without subsequent craniotomy in 7.
Table 1.
Comparison of patients with IVH on initial imaging, delayed IVH, and no IVH
When dIVH was tested in a regression model along with the other validated elements of the ICH score and ICH growth was defined at the optimized cutoff point, dIVH was associated with death at 14 days (or discharge) and worse functional outcome as measured by mRS at 3 months. The results of this analysis are summarized in table 2. The Hosmer and Lemeshow test indicated good fit for the death-at-14-days model (p = 0.64), as did the Pearsons goodness-of-fit test (p = 0.999) and −2 log-likelihood overall model test (p < 0.001) for the ordinal regression. All cases in this cohort had mRS prospectively collected at the 14-day/discharge timepoint, and 85.5% had mRS data at 3 months.
Table 2.
Predictive value of delayed IVH when added to ICH score variables
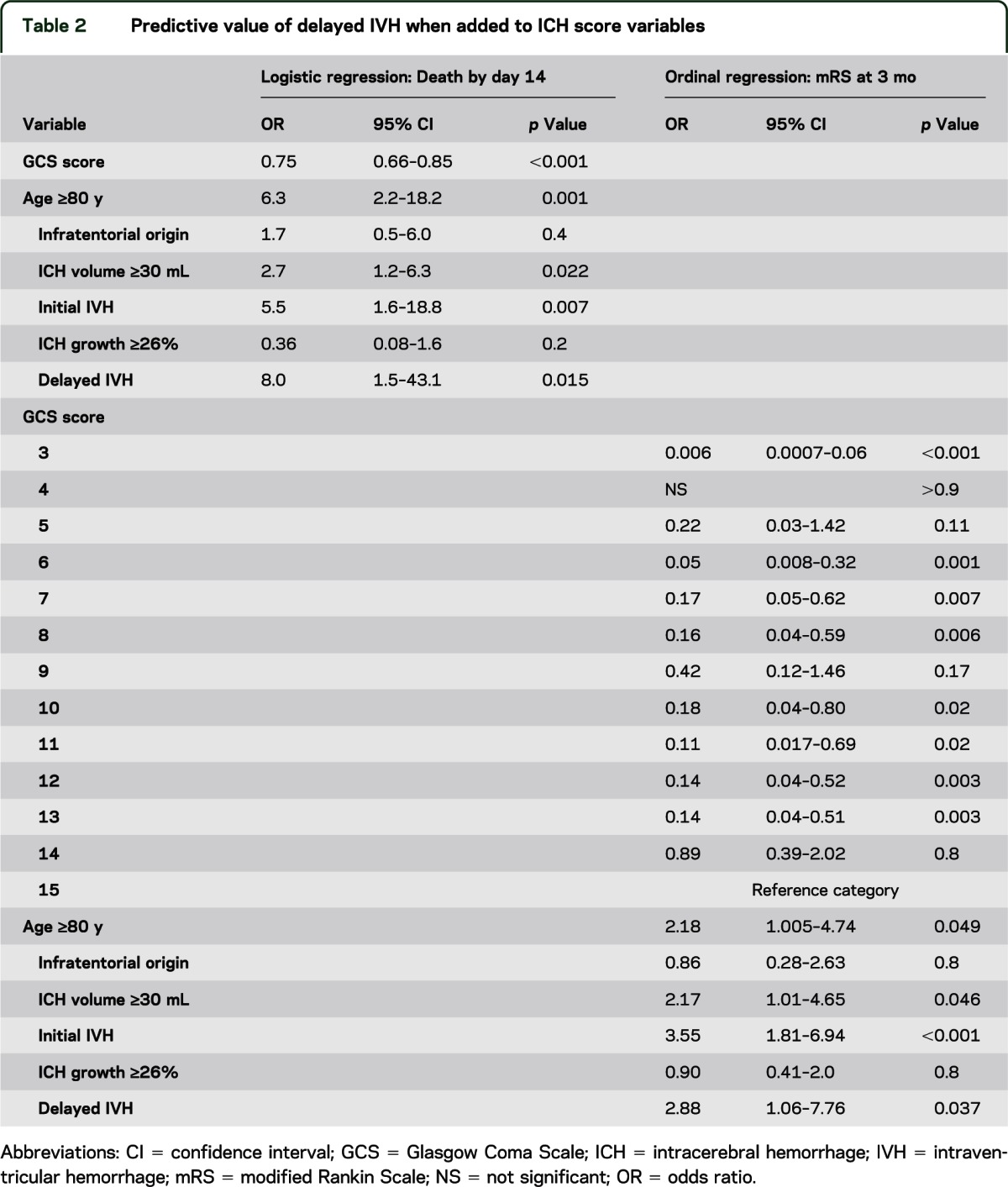
An association was seen between dIVH and several other variables that we had not prospectively chosen to adjust for but that, nevertheless, could be collinear predictors of outcome. To account for these possibilities, a final model was developed to show the independent effect of dIVH on death at 14 days by using all variables with imbalance among the dIVH, initial IVH, and no IVH groups at a significance level of p ≤ 0.1 (indicated by a in the p value column of table 1). Given the large number of variables, a backward stepwise method was used to remove variables of low significance (p > 0.1). dIVH remained a strong predictor (p = 0.024).
DISCUSSION
Our data demonstrate that dIVH is a significant, independent predictor of death and poor functional outcomes in addition to the 5 validated ICH score variables, as well as ICH growth as defined by optimized cutoff. This association remained significant, even with adjustment for a broader set of potential predictors. Several variables significant on univariate testing may represent possible risk factors for dIVH. For example, an association was seen between atrial fibrillation and dIVH, as were trends toward a higher rate of warfarin use and presenting INR. Patients with dIVH were overwhelmingly more likely to experience hematoma expansion as well. It is plausible that the underlying pathophysiology of dIVH and hematoma growth is similar, although our analysis demonstrated that the morbidity attributable to dIVH was independent of that phenomenon, atrial fibrillation, or warfarin use. We were interested to observe that ICH in the thalamus or caudate, structures abutting the ventricular system, were more associated with initial IVH, whereas hemorrhage in the lentiform nucleus, near but not directly adjacent to the ventricular system, was more frequent in dIVH. It is possible that the additional tissue separating the ICH from the ventricles slowed the dissection of blood and ventricular extension. Despite initially better clinical function compared with patients with initial IVH, as measured by the GCS, patients with dIVH fared nearly as poorly in hospital complications, need for emergent craniotomy, death, and functional outcomes.
Several strengths are worth noting about the data and study methods. All data were gathered prospectively, and the neuroimaging data were further subjected to confirmation by both a neurointensivist and neuroradiologist to optimize the reliability of the imaging interpretations. This cohort is relatively large—more than 40% larger than the one used to develop the ICH score—and all subjects underwent scheduled follow-up at prespecified timepoints to gather functional outcomes by mRS in addition to mortality information.1 Despite the methodologic rigor, generalization of these results is limited by their being derived from a single center. These results should be validated by other prospective and multicenter studies.
These findings raise several important issues related to patient management. First, the occurrence of dIVH is of similar importance to hematoma expansion. In essence, dIVH seems to be the ventricular equivalent of hematoma expansion in the parenchyma. Although neither of these morbidities was formulated into widely used ICH severity scales, they both clearly and independently affect patient outcomes and are thus valuable to monitor.1,2 Second, because both of these phenomena occur in the hospital and within the first 72 hours and are easily measureable, they may be suitable targets for hemostatic therapies and provide surrogate markers for outcomes.3
Finally, serial neuroimaging is a widely practiced component of the early management of these critically ill patients, yet limited objective evidence has been reported to support the practice. In fact, the most recent American Stroke Association guidelines9 for the early management of ICH recommend critical care monitoring for the first few days in a dedicated neuroscience intensive care unit, including vital sign monitoring, telemetry, pulse oximetry, and neurologic assessments, but do not mention or recommend repeat neuroimaging. Our data indicate that a sizeable proportion of subjects will develop a clinically meaningful condition—dIVH—discovered on serial imaging that will require emergent surgical intervention in nearly half of the cases. Further study of the utility of serial neuroimaging in the early management of primary ICH is warranted.
GLOSSARY
- dIVH
delayed intraventricular hemorrhage
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
AUTHOR CONTRIBUTIONS
Matthew B. Maas was responsible for study concept and design, data and imaging analysis and interpretation, and drafting of the manuscript. Alexander J. Nemeth was responsible for imaging analysis and critical revision of the manuscript for important intellectual content. Neil F. Rosenberg was responsible for critical revision of the manuscript for important intellectual content. Adam R. Kosteva was responsible for data acquisition and critical revision of the manuscript for important intellectual content. Shyam K. Prabhakaran was responsible for critical revision of the manuscript for important intellectual content. Andrew M. Naidech was responsible for critical revision of the manuscript for important intellectual content and for study supervision. The statistical analysis was performed by Dr. Maas and reviewed by Drs. Prabhakaran and Naidech.
STUDY FUNDING
Departmentally funded.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897 [DOI] [PubMed] [Google Scholar]
- 2.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309 [DOI] [PubMed] [Google Scholar]
- 3.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 2011;76:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539 [DOI] [PubMed] [Google Scholar]
- 5.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–1096 [DOI] [PubMed] [Google Scholar]
- 6.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002;33:2243–2246 [DOI] [PubMed] [Google Scholar]
- 7.Naidech AM, Jovanovic B, Liebling S, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke 2009;40:2398–2401 [DOI] [PubMed] [Google Scholar]
- 8.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke 2007;38:3055–3062 [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]


