Abstract
Context
Iron is essential for brain development and functioning. Emerging evidence suggests that iron deficiency in early life leads to long-lasting neural and behavioral deficits in infants and children. Adopting a life course perspective, we examined the effects of early iron deficiency on the risk of schizophrenia in adulthood.
Objective
To determine whether maternal iron deficiency, assessed by maternal hemoglobin concentration during pregnancy, increases the susceptibility to schizophrenia spectrum disorders (SSDs) among offspring.
Design
Data were drawn from a population-based cohort born from 1959 through 1967 and followed up for development of SSD from 1981 through 1997.
Participants
Of 6872 offspring for whom maternal hemoglobin concentration was available, 57 had SSDs (0.8%) and 6815 did not (99.2%).
Main Outcome Measure
Prospectively assayed, the mean value of maternal hemoglobin concentration was the primary exposure. Hemoglobin concentration was analyzed as a continuous and a categorical variable.
Results
A mean maternal hemoglobin concentration of 10.0 g/dL or less was associated with a nearly 4-fold statistically significant increased rate of SSDs (adjusted rate ratio, 3.73; 95% confidence interval, 1.41–9.81; P=.008) compared with a mean maternal hemoglobin concentration of 12.0 g/dL or higher, adjusting for maternal education and ethnicity. For every 1-g/dL increase in mean maternal hemoglobin concentration, a 27% decrease in the rate of SSDs was observed (95% confidence interval, 0.55–0.96; P=.02).
Conclusions
The findings suggest that maternal iron deficiency may be a risk factor for SSDs among offspring. Given that this hypothesis offers the potential for reducing the risk for SSDs, further investigation in independent samples is warranted.
Although the onset of overt schizophrenia typically manifests during adulthood, multidisciplinary evidence suggests that a considerable portion of its pathogenesis lies in early brain development, specifically the prenatal period. Pregnancy imposes substantial demands for oxygen and essential nutrients on the mother, the growing fetus, and the placenta. Failure of the maternal-placental unit to meet the demands of the fetus, particularly when occurring during critical periods of brain development,1 produces an adverse intrauterine milieu that can exert long-lasting, deleterious effects that extend into adulthood.
An extensive range of prenatal exposures has been linked to later development of schizophrenia among offspring. One potentially important exposure is maternal iron deficiency, which is a risk factor for several adverse birth outcomes2 and which can induce hypoxia during this period of high oxygen demand. Although iron deficiency during pregnancy has not been studied with regard to psychiatric outcomes among offspring, 2 of its correlates, fetal hypoxia3–8 and nutritional deprivation, 9,10 have been extensively examined in the schizophrenia literature and have been consistently implicated as risk factors for the disorder.
The primary direct consequence of maternal iron deficiency is anemia, indicated by low circulating hemoglobin levels, which impairs the oxygen-carrying capacity of the mother and reduces oxygen delivery to the developing fetus.11 Moreover, maternal iron deficiency might disrupt neurodevelopment through its effect on birth outcomes,2 several of which are putative risk factors for schizophrenia.12 Furthermore, iron is essential for a plethora of metabolic mechanisms associated with the development and maintenance of brain structures and functions relevant to schizophrenia,13 including myelination14,15 and dopaminergic neurotransmission. 16 Therefore, failure to meet iron demands of the developing brain may have long-term consequences.
The literature on childhood development provides support for a plausible role of iron deficiency in the pathogenesis of schizophrenia. Motor, cognitive, and behavioral deficits are often observed in children who later develop schizophrenia17–20 and comparable developmental abnormalities have been observed in iron-deficient infants and children,21,22 assessed via cord blood and maternal serum samples, suggesting that prenatal iron deficiency may set children on a similar developmental course, predisposing them to schizophrenia in adulthood.
Although these findings provide compelling grounds to hypothesize that maternal iron deficiency plays a role in the etiopathogenesis of schizophrenia, no study in the literature has explicitly tested this association. Iron is essential for synthesis of hemoglobin, the protein present within erythrocytes responsible for blood oxygenation. As an indicator of maternal iron status, prospectively acquired maternal hemoglobin concentration data were used to examine the hypothesis that maternal iron deficiency in pregnancy is a risk factor for schizophrenia among offspring. We also conducted exploratory analyses of the gestational timing of low maternal hemoglobin levels and the effect of sex of the offspring on schizophrenia risk. The data were drawn from the Prenatal Determinants of Schizophrenia (PDS) study,23 a prospective investigation of a large birth cohort that included direct bioassays of maternal hemoglobin levels and comprehensive measures of other prenatal and perinatal exposures.
METHODS
DESCRIPTION OF THE COHORT
As described in detail by Susser et al,23 the PDS study was derived from the birth cohort of the Child Health and Development Study (CHDS). Nearly all pregnant women receiving prenatal care from the Alameda County Kaiser Permanente Medical Care Plan (KPMCP) were recruited from 1959 through 1966.24,25 The 19 044 live-born offspring from this cohort were automatically enrolled in the KPMCP. The Kaiser membership was racially, educationally, and occupationally diverse and demographically similar to the population of the region at the time.
The PDS study cohort composed the subsample of 12 094 offspring born into the CHDS cohort and were members of the KPMCP at any time from January 1, 1981 (the year when medical records were computerized), through December 31, 1997, the last date for case ascertainment in the PDS study.23 Compared with the CHDS cohort, the PDS study sample had a slight underrepresentation of offspring of low-income and unmarried women and a modest overrepresentation of offspring of African American women but was otherwise comparable. Most individuals who left the KPMCP did so before age 10 years, before onset of the schizophrenic prodrome, thereby minimizing bias from loss to follow-up.
DIAGNOSES OF SSDs
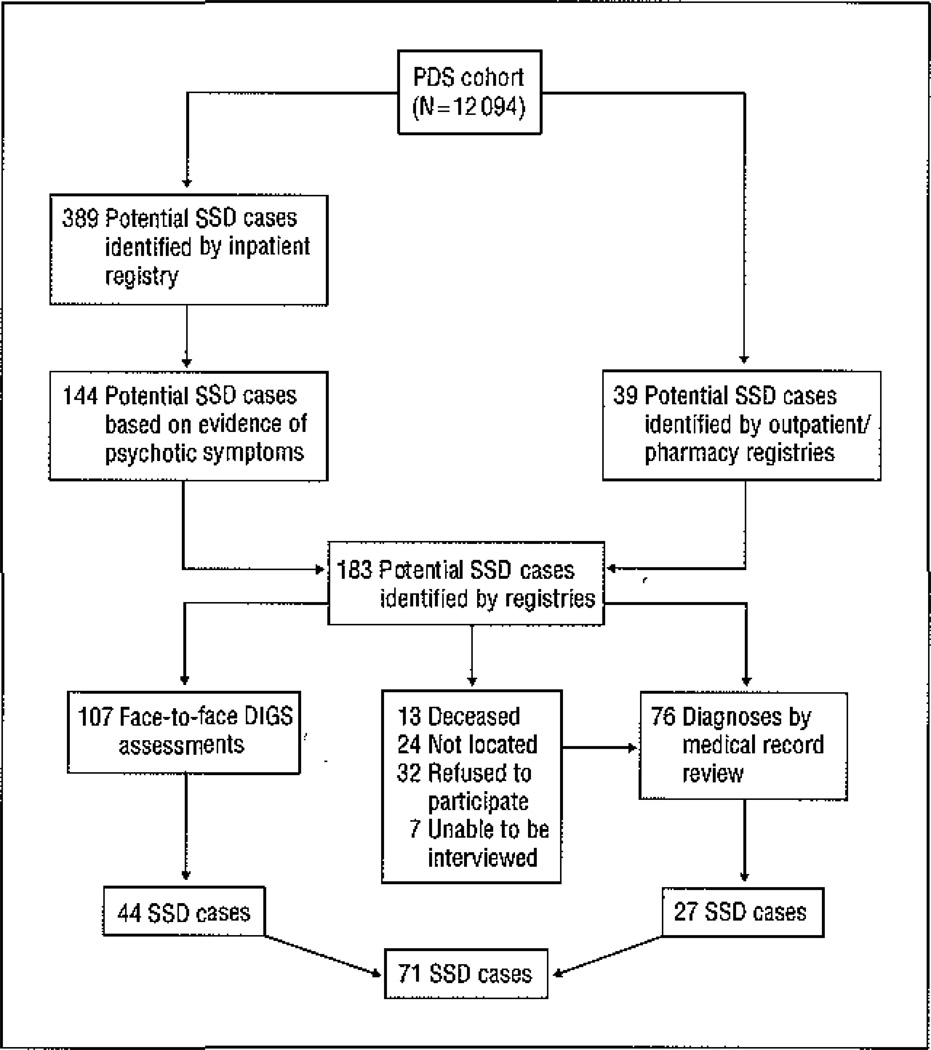
The outcome was schizophrenia and other schizophrenia spectrum disorders (SSDs), defined as schizophrenia, schizoaffective disorder, delusional disorder, psychotic disorder not otherwise specified, and schizotypal personality disorder, based on previous studies.26 Potential SSD cases were identified through computerized record linkages between CHDS and KPMCP identifiers (Figure). In the first stage of screening, the KPMCP inpatient registry identified subjects who were hospitalized with nonaffective or affective psychotiC disorder diagnostic codes 295 through 299 from the International Classification of Diseases, Ninth Revision. Following review of their psychiatric and medical records by an experienced, board-certified research psychiatrist (A.S.B. and E.S.S.) for evidence of psychotic symptoms, 144 participants with potential SSDs were targeted for a full diagnostic assessment. Participants identified from outpatient and pharmacy registries who may have been treated for psychosis without hospitalization were also targeted for a full diagnostic evaluation. Overall, inpatient and outpatient registry screening procedures identified 183 participants for diagnostic interviews. Among the 170 survivors, 146 were contacted, and 107 completed the Diagnostic Interview for Genetic Studies (DIGS),27 which was conducted by clinically experienced interviewers. The DSM-IV diagnoses were assigned by consensus of 3 experienced research psychiatrists, based on the written DIGS narrative, medical records, and discussions with the interviewer. For the 76 potential subjects who were not interviewed, DSM-IV diagnoses were assigned by medical record review and confirmed by a research psychiatrist (A.S.B. or E.S.S.). A total of 71 SSD cases were ascertained, 44 by DIGS and 27 by medical record review.
Figure 1.
Case ascertainment in the Prenatal Determinants of Schizophrenia (PDS) study cohort. DIGS indicates Diagnostic Interview for Genetic Studies; SSDs, schizophrenia spectrum disorders.
All participants in the PDS study provided written informed consent. The study protocol was approved by the institutional review boards of the New York State Psychiatric institute and the KPMCP.
ANALYTIC SAMPLE
The PDS analytic sample23 was defined to ensure independence of observations because the CHDS birth cohort included siblings, who represent nonindependent observations. This was achieved by randomly selecting only 1 sibling from each family. Because of the limited number of offspring diagnosed with SSD during follow-up, if a sibship included an affected sibling, only the affected sibling was chosen. If a sibship did not include an affected sibling, then 1 sibling was randomly chosen. The selection process produced a total of 7796 offspring, referred to as the PDS Cohort.23 In the present analysis, 5 offspring diagnosed as having SSDs were excluded (4 offspring diagnosed before the start of the PDS study and 1 who had an affected sibling in the PDS Cohort). Among the 66 participants with SSDs, maternal hemoglobin data were available for 57. The diagnoses of the 57 participants with SSDs were as follows: schizophrenia (n=34), schizoaffective disorder (n=13), delusional disorder (n=1), schizotypal personality disorder (n=4), and other schizophrenia spectrum psychosis (n=5).
MATERNAL IRON DEFICIENCY AND ANEMIA DATA
Hemoglobin concentration was chosen as a marker for iron deficiency because it is an excellent indicator of iron status and blood oxygenation.28,29 Extracted from well-documented prenatal medical records, maternal hemoglobin concentration data were available for 6872 of 7791 PDS Cohort offspring (88.2%). As the pregnancy of the mother advanced, the number of hemoglobin observations increased: 46% had hemoglobin assayed anytime during the first trimester, 78% during the second trimester, and 91% during the third trimester. Moreover, 59% of mothers of the offspring had 3 or more hemoglobin measurements, 31% had 2 measurements, and 10% had 1 measurement.
The mean value of maternal hemoglobin concentration during the entire pregnancy was chosen as the primary exposure because we did not have an a priori prediction that the effect of low maternal hemoglobin concentration on risk of SSDs was specific to any particular gestational period or trimester. This option maximized the number of participants in the analysis by enabling us to include all offspring with at least 1 maternal hemoglobin measurement. Nevertheless, because of the varying prenatal timing of the hemoglobin observations, we evaluated whether the temporal pattern might affect the results (see the “Data Analysis” subsection).
Hemoglobin concentration was first modeled as a continuous variable to assess SSD risk across the full spectrum of maternal hemoglobin values and to maximize statistical power. We also classified hemoglobin concentration into 3 main categories according to the World Health Organization’s criteria28; hemoglobin level of more than 12.0 g/dL (reference), more than 10.0 but less than 12.0 g/dL (moderate), and 10.0 g/dL or less (low). To convert hemoglobin to grams per liters multiply by #10.0. Analyzing hemoglobin concentration as a categorical variable enabled us to evaluate whether the rate of SSD linearly increased with decreasing hemoglobin concentration or whether the rate was constant for hemoglobin concentration below a threshold level. Analogously, we modeled mean trimester-specific hemoglobin level as a continuous and a categorical variable to explore whether a critical gestational period of susceptibility existed.
In a supplementary analysis, we used the Maternal Inter-current and Prenatal Conditions of the International Classification of Diseases, Ninth Revision, to identify women clinically diagnosed with anemic conditions during pregnancy: 2910 (iron deficiency), 2929 (other specified or pernicious), and 2930 (not otherwise specified). The 2 non-iron-deficient anemic categories were collapsed into a single category because of the limited number of women with these conditions and the lack of specific hypotheses concerning the “other specified” and “not otherwise specified” anemic conditions.
DATA ANALYSIS
Cox proportional hazards regression30 was used to account for varying durations of follow-up while simultaneously adjusting for multiple covariates. For offspring diagnosed with SSDs, the onset date was approximated by the date of the first hospital admission or outpatient visit, whichever came first. Therefore, the length of the follow-up for offspring diagnosed with SSDs was quantified in days elapsed from birth until the onset date of the disorder. For the unaffected offspring, the follow-up length was quantified as days since birth until the KPMCP membership termination date or until the end of the PDS study, whichever came first.
Initially, we investigated the temporal pattern of hemoglobin level observations across pregnancy to validate our use of mean hemoglobin level as the exposure statistic. Linear regression was used to approximate such changes for offspring with 2 or more hemoglobin observations; intercepts and slopes were calculated and then added as SSD predictors in the Cox regression model. We also added a variable reflecting the timing of the first maternal hemoglobin observation. None of the time-related variables were statistically significant (full model: P=.49 for intercept; P=.27 for slope; P=.23 for timing of first hemoglobin measure), indicating that adjustment for temporal variation of hemoglobin observations would not affect the results.
Variables selected as a priori potential confounders were maternal ethnicity,31 maternal age, maternal education, maternal prepregnancy body mass index (calculated as weight in kilograms divided by height in meters squared),32 parity, maternal smoking during pregnancy, and maternal mental conditions, defined as psychosis, anxiety reactions, and drug/alcohol addiction. Potential confounders were considered for inclusion in the Cox regression model if the variables were statistically associated with hemoglobin and were also related to SSD at P<.10, using the χ2 statistic for categorical variables and the t test for continuous variables, and were retained in the final model if their inclusion produced at least a 10% change in the regression coefficient for hemoglobin concentration.
We also examined whether observed consequence of maternal anemia,2,12 including low birth weight (<2500 g), prematurity (<37 gestational weeks), and small-for-gestational-age status (defined as birth weight in the lowest 10th percentile according to gestational week) were potential mediators. The presence of mediation effects was tested by determining whether the addition of the variable to the Cox model attenuated the regression coefficient for hemoglobin concentration. We also examined whether hypoxia (defined in accord with prior research7,33,34 as preeclampsia, gestational hypertension, hypotension, or diabetes mellitus) was an independent risk factor for SSDs.
The PDS Cohort included only 1 sibling per family. Excluding subjects has the potential to create bias. To address this concern, we also analyzed the full PDS sample, including all siblings, which contained hemoglobin data for 9971 offspring, including 58 participants with SSDs. We used a frailty model (Weibull regression with shared γ frailty, as implemented in Stata statistical software35) that adjusts for possible dependence between siblings; the full PDS sample had insufficient data to fit a Cox regression model with shared γ frailty. For ease of comparison with previous PDS study analyses, we used the PDS Cohort as the primary sample and the sample in the frailty model for confirmation of the results. Each of the approaches has advantages and disadvantages: although the frailty model adjusts for dependence between siblings, a disadvantage is that its baseline hazard assumes a parametric form, which is more restrictive than the Cox model, which allows a nonparametric baseline hazard.
RESULTS
SAMPLE CHARACTERISTICS
Maternal hemoglobin concentration ranged from 6.90 to 15.20 g/dL (mean [SD], 11.47 [0.98] g/dL). Women with low gestational hemoglobin concentrations (≤10 g/dL) were significantly younger, more likely to be a race/ethnicity other than white, of less educated, and had more prior live births and a higher prepregnancy body mass index compared with women who had adequate hemoglobin concentrations (Table 1). Maternal mental conditions were not significantly related to maternal hemoglobin concentration (P=.35); moreover, the prevalence of mental disorders was lower among mothers who had low hemoglobin concentrations compared with mothers who had adequate levels of hemoglobin. Although maternal ethnicity was the only variable to fulfill the confounder criteria, for comparability with studies that controlled for socioeconomic status and to limit finite sample bias,36 we also added maternal education to the final Cox regression model.
Table 1.
Maternal Characteristics and Prenatal and Perinatal Factors in Relation to Maternal Hemoglobin levels and SSDsa
| Mean Maternal Hemoglobin level, g/dL |
|||
|---|---|---|---|
| Characteristic | Reference >12.0 (n = 1967) |
Moderate >10.0 but ≤12.0 (n = 4447) |
Low ≤ 10.0 (n = 458) |
| Maternal | |||
| Age at delivery, yb | |||
| <20 | 54 (2.7) | 248 (5.6) | 55 (12.0) |
| 20–29 | 1146 (58.3) | 2591 (58.3) | 269 (58.7) |
| 30–39 | 683 (34.7) | 1386 (31.2) | 113 (24.7) |
| >40 | 84 (4.3) | 222 (5.0) | 21 (4.6) |
| Mean (SD) | 28.4 (5.9) | 27.9 (6.2) | 26.7 (6.5) |
| Race/ethnicityb,c | |||
| White | 1395 (70.9) | 2316 (52.1) | 105 (55.5) |
| Black | 300 (15.3) | 1435 (32.3) | 296 (29.6) |
| Other | 272 (13.8) | 696 (15.7) | 57 (14.9) |
| Education b | |||
| Did not finish high school | 268 (13.6) | 798 (17.9) | 138 (30.1) |
| High school graduate | 768 (39.0) | 1689 (38.0) | 174 (38.0) |
| Some college | 482 (24.5) | 1196 (26.9) | 111 (24.2) |
| College graduate/registered nurse | 449 (22.8) | 764 (17.2) | 35 (7.6) |
| No. of prior live birthsb | |||
| 0 | 512 (31.1) | 1353 (30.4) | 101 (30.1) |
| 1 | 540 (27.5) | 1166 (26.2) | 116 (26.5) |
| 2 | 381 (19.4) | 857 (19.3) | 77 (19.1) |
| ≥3 | 434 (22.1) | 1071 (24.1) | 164 (24.3) |
| Prepregnancy BMIb,c,d | |||
| <30 | 1366 (95.9) | 3791 (96.2) | 413 (93.2) |
| ≥30 | 59 (4.1) | 150 (3.8) | 30 (7.6) |
| Current smokere | |||
| No | 1276 (64.9) | 3023 (68.0) | 285 (62.2) |
| Yes | 691 (35.1) | 1424 (32.0) | 173 (37.8) |
| Chronic hypoxiab,f | |||
| No | 843 (42.9) | 2176 (48.9) | 239 (52.2) |
| Yes | 1124 (57.1) | 2271 (51.1) | 219 (47.8) |
| No. of prenatal visits, mean (SD) | 10.31 (2.90) | 10.06 (3.05) | 8.16 (3.36) |
| No. of hemoglobin assays, mean (SD) | 2.46 (0.72) | 2.57 (0.75) | 2.53 (1.11) |
| Mental conditionsg | |||
| No | 1775 (90.2) | 4041 (90.9) | 423 (92.4) |
| Yes | 192 (9.8) | 406 (9.1) | 35 (7.6) |
| Infant | |||
| Sexb,c | |||
| Male | 1073 (54.6) | 2212 (49.7) | 224 (48.9) |
| Female | 894 (45.4) | 2235 (50.3) | 234 (51.1) |
| Small for gestational age | |||
| No | 1770 (90.0) | 3990 (89.7) | 395 (86.2) |
| Yes | 197 (10.0) | 457 (10.3) | 63 (13.8) |
| Low birth weight, <2500g | |||
| No | 1864 (94.8) | 4202 (94.5) | 428 (93.4) |
| Yes | 103 (5.2) | 245 (5.5) | 30 (6.7) |
| Birth weight, mean (SD), oz | 117.1 (17.4) | 117.0 (18.2) | 114.8 (19.0) |
| Premature (<37 wk)e | |||
| No | 1846 (93.8) | 4124 (92.7) | 394 (86.0) |
| Yes | 121 (6.2) | 323 (7.3) | 64 (14.0) |
| Gestational age, mean (SD),d | 280.0 (13.3) | 278.7 (14.2) | 275.6 (17.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SSDs, schizophrenia spectrum disorders.
SI conversion factor: To convert hemoglobin to grams per liters multiply by 10.00
Data are presented as number (percentage) of participants unless otherwise indicated.
P < .001 for maternal hemoglobin level.
P < .05 for SSDs.
Maternal BMI data were not available for the entire sample.
P < .05 for maternal hemoglobin level.
Chronic hypoxia included preeclampsia or gestational hypertension, hypotension, or diabetes mellitus.
Maternal psychosis, anxiety reactions, and drug and alcohol addiction data were obtained from prenatal medical record reviews.
EFFECTS OF HEMOGLOBIN CONCENTRATION
We first examined hemoglobin concentration modeled as a continuous variable in relation to SSDs. The mean maternal hemoglobin concentration was significantly higher among the 6815 unaffected offspring (mean [SD], 11.47 [0.97] g/dL) compared with the 57 offspring diagnosed with SSDs (11.12 [1.09] g/dL) (P=.006; t test for equality of means). Increasing concentration of hemoglobin was associated with a lower rate of SSDs (rate ratio [RR], 0.66; 95% confidence interval [CI], 0.51–0.85; P=.001). Adjusting for maternal ethnicity and education, we observed that for every 1-g/dL increase in mean maternal hemoglobin level, the rate of SSDs decreased by 27% (adjusted RR, 0.73; 95% CI, 0.55–0.96; P=.02). The frailty model produced similar estimates (RR, 0.67; 95% CI, 0.51–0.87; P=.004) (adjusted RR, 0.75; 95% CI, 0.56–1.00; P=.049).
Using discrete categories of hemoglobin concentration (Table 2), we observed that as hemoglobin level decreased, the SSD rate increased. A maternal hemoglobin level of 10 g/dL or less was associated with a 5-fold increase in the SSD rate (RR, 5.17; 95% CI, 2.05–13.04; P<.001); the effect declined slightly following adjustment for maternal ethnicity and education (adjusted RR, 3.73; 95% CI, 1.41–9.81; P=.008). Moreover, an almost 8-fold increased rate for SSDs was observed for hemoglobin levels less than 9.0 g/dL, although its corresponding 95% CI was wide because only 2 offspring with SSDs had mothers with hemoglobin levels less than 9.0 g/dL (adjusted RR, 7.82; 95% CI, 1.64–37.27; P=.01). Similar estimates were produced by the frailty model; a hemoglobin level of less than 10 g/dL was associated with a greater than 5-fold increased rate of SSDs (RR, 5.37; 95% CI, 1.77–16.34; P=.003) (adjusted RR, 3.59; 95% CI, 1.20–10.78; P=.02). In summary, a significant dose-dependent effect of maternal hemoglobin concentration on the rate of SSDs was observed.
Table 2.
Mean Maternal Hemoglobin Level and SSDs
| Mean Maternal Hemoglobin Level, g/dL |
Study Sample (n = 6872) |
|||||
|---|---|---|---|---|---|---|
| No. of Offspring |
Unadjusted |
Adjusteda |
||||
| Without SSDs | With SSDs | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| >12.0 | 1958 | −9 | 1 [Reference] | NA | 1 [Reference] | NA |
| >10.0 to ≤ 12.0 | 4408 | 39 | 2.13 (1.03–4.40) | .04 | 1.91 (0.91–3.98) | .09 |
| ≤10.0 | 449 | 9 | 5.17 (2.05–13.04) | <.001 | 3.73 (1.41–9.81) | .008 |
Abbreviations: CI, confidence interval; NA. not applicable; RR, rate ratio; SSDs, schizophrenia spectrum disorders.
Adjusted for maternal ethnicity and level of education.
MATERNAL ANEMIA
To complement the hemoglobin findings, we examined the effect of clinically diagnosed maternal anemia during pregnancy on the SSD rate. Of 57 mothers with a clinical diagnosis of iron deficiency, 3 offspring were diagnosed with SSDs. In contrast, only 1 offspring among the 252 mothers identified with non-iron-deficient anemia developed SSDs. Adjusting for maternal ethnicity and education, iron-deficient anemia conferred an almost 5-fold increased rate of SSDs (adjusted RR, 4.78; 95% CI, 1.48–15.40; P= .009) compared with offspring whose mothers were not diagnosed with clinical anemia. No elevated rate of SSDs was observed for non-iron-deficient anemia (adjusted RR, 0.40; 95% CI, 0.06–2.89; P=.36).
To investigate the causal process by which maternal hemoglobin levels affected the SSD rate among offspring, we considered whether prematurity, low birth weight, and small-for-gestational-age status mediated the association between low maternal hemoglobin level and SSDs. Offspring whose mothers had low hemoglobin levels had significantly higher rates of prematurity (P<.001) and marginally higher rates of small-for-gestational-age status, but not of low birth weight compared with the offspring whose mothers had adequate hemoglobin levels. When each of these posited mediators was entered individually into the Cox model, there was no alteration in the hemoglobin coefficient, thereby providing no evidence of mediation. We also examined the effect of hypoxia, assessed as described in the “Data Analysis” subsection of the “Methods” section. We found that although the prevalence of hypoxia increased as hemoglobin decreased(P=.053), hypoxia was not associated with SSDs and did not alter the regression coefficient for hemoglobin concentration.
EXPLORATORY ANALYSES
Mean maternal hemoglobin concentration decreased from the first to the second trimester (Table 3). During the third trimester, the mean hemoglobin concentration of mothers of offspring without SSDs slightly increased, whereas the mean hemoglobin concentration of mothers of offspring with SSDs continued to decline. Using trimester-specific hemoglobin level classified as a continuous variable, we found an inverse association between increasing concentration of third-trimester hemoglobin and SSDs (adjusted RR, 0.74; 95% CI, 0.57–0.95; p=.02). No association was apparent between SSDs and either first-trimester (adjusted RR, 1.38; 95% CI, 0.89–2.1; p=.15) or second trimester hemoglobin concentration (0.88; 0.66–1.17; P=.31). When hemoglobin was analyzed as a categorical variable, a 3-fold, statistically significant increased rate of SSDs with a maternal hemoglobin concentration of 10.0 g/dL or less was observed during the second and third trimester, but we detected no evidence of a first-trimester effect.
Table 3.
Individual Trimester RRs of SSDs and Mean Maternal Hemoglobin Level as a Categorical Variable a
| Rate Ratio (95% Confidence Interval) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Offspring Without SSDs |
Offspring With SSDs |
Moderate Maternal Hemoglobin Level (>10.0 but ≤12.0 g/dL) |
Low Maternal Hemoglobin Level (≤10.0 g/dL) |
|||||
| Trimester | No. of Offspring |
Mean (Sd) Maternal Hemoglobin Level, g/dL |
No. of Offspring |
Mean (SD) Maternal Hemoglobin Level, g/dL |
Unadjusted | Adjustedb | Unadjusted | Adjustedb |
| First | 3120 | 11.94 (1.06) | 19 | 12.18 (1.20) | 0.87 (0.34–2.20) | 0.68 (0.26–1.76) | 1.84 (0.23–14.55) | 1.14 (0.14–9.35) |
| Second | 5300 | 11.25 (1.07) | 44 | 11.08 (1.04) | 1.89 (0.78–4.56) | 1.77 (0.73–4.29) | 3.69 (1.31–10.37) | 3.13 (1.08–9.09) |
| Third | 6191 | 11.34 (1.17) | 54 | 10.90 (1.28) | 1.87 (0.90– 3.91) | 1.68 (0.80–3.53) | 4.09 (1.69–9.87) | 3.09 (1.24–7.70) |
Abbreviation: SSDs, schizophrenia spectrum disorders.
SI conversion factor: To convert hemoglobin to grams per liters multiply by 10.00
Reference category is mean maternal trimester-specific hemoglobin level greater than 12.0 g/dL.
Adjusted for maternal ethnicity and education.
We stratified the data by sex of the offspring to explore the hypothesis that sex might modify the effect of low hemoglobin level on the rate of SSDs. Among women, 6 of 20 participants with SSDs had mothers with hemoglobin levels of 10.0 g/dL or less. The female adjusted RR for a hemoglobin level of 10.0 g/dL or less was 6.70 (95% CI, 1.54–29.03; P=.01). Among men, a weaker effect for maternal hemoglobin level of 10.0 g/dL or less was observed (adjusted RR, 2.06; 95% CI, 0.49–8.64; P=.32) reflecting the occurrence of maternal hemoglobin levels of 10.0 g/dL or less in only 3 of 37 men with SSDs.
COMMENT
Intrauterine exposure to low maternal hemoglobin levels was associated with a nearly 4-fold statistically significant increased rate of SSDs among offspring. A dose-response effect was observed; the SSD rate increased as maternal hemoglobin concentration decreased. Adjusting for potential confounders, including measures of parental socioeconomic status, did not appreciably alter the magnitude or significance of the hemoglobin effect. The validity of the finding is enhanced by 4 factors: (1) the offspring were drawn from a continuously monitored birth cohort; (2) maternal hemoglobin data were prospectively acquired; (3) a significant association between the clinical diagnoses of maternal iron deficiency anemia and SSDs was demonstrated, indicating internal consistency; and (4) two statistical approaches (frailty and Cox model) produced similar results.
This study is the first, to our knowledge, to specifically investigate the association between maternal hemoglobin levels and SSDs among offspring. A previous publication that examined many prenatal and obstetric events reported an odds ratio of 1.80 (95% CI, 0.8–3.8) for maternal anemia (defined as a hemoglobin level of <10.5 g/dL in the second trimester).37 Our results are compatible with past findings demonstrating that intrauterine exposure to famine in the Netherlands from 1944 through 1945 and in China from 1959 through 1961 were each associated with an increased risk of schizophrenia among offspring, although iron would have been only 1 of many nutrients that were deficient.9,10
We considered whether genetic loading for schizophrenia in mothers could nullify the association because women with schizophrenia often have suboptimal health behaviors, including nutritional intake.38 Conceivably, low hemoglobin levels resulting from poor iron intake might be a marker for mental illness. Therefore, the increased rate of SSDs among offspring might partly result from maternal genetic transmission of susceptibility for the disorder and not from low hemoglobin levels. Although we found that maternal mental conditions were marginally associated with SSDs (adjusted RR, 1.84; 95% CI, 0.90–3.74; P=.09), this effect was independent of low hemoglobin levels, as evidenced by no appreciable change in the hemoglobin coefficient following adjustment for this variable. We also explored whether low hemoglobin level was a surrogate for inadequate antenatal care. The addition of timing of initiation of prenatal care, the number and timing of the visits, and the number of hemoglobin assessments to the Cox model had no effect on the magnitude or the statistical significance of the hemoglobin estimate (results available from the corresponding author). Therefore, we found no evidence that either maternal mental illness or level of prenatal care confounded the association between low maternal hemoglobin level and SSDs.
BIOLOGICAL PLAUSIBILITY AND CAUSAL MECHANISMS
Because SSD is an etiologically heterogeneous syndrome, several mechanisms associated with maternal iron deficiency might be involved in its pathogenesis. We posit 2 main classes of causal mechanisms. The first class involves the direct effect of iron on brain development. Iron is necessary for a plethora of metabolic processes involved in the development of brain structures and functions associated with SSDS.39 Iron is a coenzyme of dopamine synthesis; its deficiency can alter dopamine receptor density and activity,l6 which has long been implicated in the pathophysiological mechanisms of schizophrenia. Iron is also an essential cofactor for lipid and cholesterol synthesis, integral to normal brain development; brains of persons with schizophrenia have profound myelin and oligodendrocyte abnormalities.15 Moreover, inadequate energy metabolism can ensue during times of iron deficiency, especially in rapidly developing brain structures.39 Support for these mechanisms is provided by preclinical studies, which found that myelin deficits persisted despite restoration of iron status at weaning40,41 and that perinatal iron deficiency altered the timing and the expression of critical genes relevant to hippocampal development.42 The second class comprises indirect pathways involving pregnancy and obstetric complications. Iron deficiency, the most common cause of maternal anemia, is a risk factor for poor pregnancy outcomes,2,12 including low birth weight, prematurity, and small-for-gestational-age status. However, our data provided no evidence of mediation by these 3 adverse outcomes of maternal anemia, thereby not substantiating the indirect mechanisms. In conclusion, although we cannot disregard the possibility that low maternal hemoglobin levels may merely be a marker for an independent schizophrenia risk factor, our results, in conjunction with strong biological plausibility for this exposure in the origins of neurodevelopmental perturbations, favor the contribution of low maternal hemoglobin level to the pathogenesis of schizophrenia.
EXPLORATORY ANALYSES
The effect of maternal hemoglobin level on SSDs varied by trimester: second- and third- trimester exposure to low hemoglobin levels, but not exposure during the first trimester, were each associated with an increased rate of SSDs. This may reflect physiological fluctuations of maternal and fetal iron demands throughout pregnancy; iron requirements in the first trimester are less than those before pregnancy and rapidly increase in the second and third trimesters to support the expansion of maternal and fetal blood supply and fetal development. The increased demand for iron as pregnancy advances suggests that the fetus may be more susceptible to the effects of maternal iron deficiency during later gestation rather than earlier. Although the Dutch famine study found that the increased schizophrenia risk from nutritional deficiency was limited to first-trimester exposure,9 these pregnant women experienced protein-calorie malnutrition, micronutrient deficiencies, and the psychological stress of war and/or famine, unlike the relatively healthy pregnant women in the CHDS. The trimester-specific results, however, must be viewed with caution, because statistical power for the first-trimester analysis was limited by a small sample size of 19 cases of SSDs.
Because sex differences in age of onset, symptom severity, and clinical course have been well documented in the schizophrenia literature,43 we explored whether the sex of the offspring modified the effect of low maternal hemoglobin levels on the rate of SSDs. We found some modest suggestion of a sex difference, such that a maternal hemoglobin level of 10.0 g/dL or less had a more deleterious effect on female than male offspring. Preclinical studies of iron deficiency provide support for this finding; brain iron and regional dopamine deficits persisted in female offspring but not in their male counterparts, despite restoration of iron status at weaning.40,41 Moreover, preclinical and human studies revealed sex differences in the turnover rates44 and gene expression of oligodendrocytes,44,45 the predominant iron-containing cells in the brain, as well as in erythropoietic activity.46 The role of chance cannot be excluded because the numbers of iron-deficient male and female offspring diagnosed with SSDs were relatively small.
LIMITATIONS
One limitation of this study is the modest number of offspring diagnosed with SSDs. This produced relatively broad CIs and limited our ability to comprehensively evaluate the effects of gestational timing and sex of the offspring on the hemoglobin-SSD association.
We also considered the potential bias introduced by the exclusion of 11.8% of the offspring whose mothers lacked hemoglobin data. Mothers with missing data were more likely to be white, to have more prior live births, to have experienced hypoxic complications, and to have smoked during pregnancy and were less likely to attend college; their offspring were less likely to have been premature and small for their gestational age. Thus, no clear pattern emerged, suggesting that the hemoglobin data were missing approximately at random. Most important, the SSD rate was not correlated with missing hemoglobin data (P=.65) and the insertion of a missing-value indicator into the Cox regression model did not alter the effect size and statistical significance for maternal hemoglobin level (adjusted RR, 1.40; 95% CI, 0.68–2.91; P=.36).
A further limitation concerns the use of maternal hemoglobin levels as a marker for fetal iron deficiency. Although cord blood would have provided a more direct indicator of iron deficiency and fetal hypoxia, we did not have cord serum available. However, previous studies have shown a strong association between maternal and cord serum iron levels at delivery,47,48 indicating that supply of this nutrient to the fetus is reduced in mothers with iron deficiency.49 Moreover, infants born to iron-deficient women were at risk for iron deficiency during the first year of life, although the infants exhibited normal iron endowment at birth.50,51
CONCLUSIONS
To our knowledge, this is the first study to report a significant association between low maternal hemoglobin and SSDs, among offspring. A postulated etiologic role of maternal iron deficiency is consistent with a large body of research, suggesting that perinatal iron deficiency has long-lasting neural and behavioral effects. A multitude of genetic and environmental factors govern brain development, many of which are involved in pathways that affect the delivery of oxygen and nutrients to the fetus. Maternal iron deficiency, assessed via maternal hemoglobin concentration, may disrupt these essential path-ways and other iron-dependent processes involving dopaminergic neurotransmission, myelination, and energy metabolism. Disturbances of these pathways during critical periods of fetal development might heighten the susceptibility to schizophrenia in adulthood. Therefore, further investigation in independent samples is warranted.
Acknowledgments
Funding/Support: This study was supported by grants 1R01MH 60249 and 1K02MH65422 (Dr Brown) from the National Institute of Mental Health, The Frontier Fund of Columbia University and grants N01HD13334 and N01HD63258 from The Eunice Kennedy Shriver Institute of Child Health and Human Development.
Footnotes
Author Contributions: Drs Insel, Susser, and Brown had full access to all the data in the study and Drs Schaefer and McKeague had partial access to the data in the study. All authors take full responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Previous Presentations: This study was presented in part at the Annual Meeting of the Society of Biological Psychiatry; May 17, 2007; San Diego, California.
Additional Contributions: Barbara A. Cohn, PhD, contributed to the study.
References
- 1.Susser E, Mane TD. Early antecedents of adult health. J Urban Health. 1998;75(2):236–241. doi: 10.1007/BF02345091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. J Nutr. 2000;130(2S suppl):443S–447S. doi: 10.1093/jn/130.2.443S. [DOI] [PubMed] [Google Scholar]
- 3.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 4.Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia, population-based case-control study. Br J Psychiatry. 2001;179:403–408. doi: 10.1192/bjp.179.5.403. [DOI] [PubMed] [Google Scholar]
- 5.Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other non-affective psychoses:a 19-year longitudinal study. Am J Psychiatry. 2000;157(2):196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
- 6.McNeil TF, Cantor-Graae E, Ismail B. Obstetric complications and congenital malformation in schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):166–178. doi: 10.1016/s0165-0173(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 7.Buka SL, Tsuang MT, Lipsin LP. Pregnancy/delivery complications and psychiatric diagnosis: a prospective study. Arch Gen Psychiatry. 1993;50(2):151–156. doi: 10.1001/archpsyc.1993.01820140077009. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophrenia Buff. 2000;26(2):351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 9.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovltz D, Gorman JM. Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry. 1996;53(1):25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 10.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294(5):557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 11.Viteri FE. The consequences of iron deficiency and anemia in pregnancy. Adv Exp Med Biol. 1994;352:127–139. doi: 10.1007/978-1-4899-2575-6_10. [DOI] [PubMed] [Google Scholar]
- 12.Kunugi H, Nanko S, Murray RM. Obstetric complications and schizophrenia, prenatal underdevelopment and subsequent neurodevelopmental impairment. Br J Psychiatry. 2001;40(Suppl.):s25–s29. doi: 10.1192/bjp.178.40.s25. [DOI] [PubMed] [Google Scholar]
- 13.Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129(1):199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- 14.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5, pt2):S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 16.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 17.Isohanni M, Murray GK, Jokelainen J, Croudace T, Jones PB. The persistence of developmental markers in childhood and adolescence and risk for schizophrenic psychoses in adult life: a 34-year follow-up of the Northern Finland 1966 birth cohort. Schizophr Res. 2004;71(2–3):213–225. doi: 10.1016/j.schres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97(1):65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Jones PB, Tarrant CJ. Specificity of developmental precursors to schizophrenia and affective disorders. Schizophr Res. 1999;39(2):121–125. doi: 10.1016/s0920-9964(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 20.Done OJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ. 1994;309(6956):699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140(2):165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 22.Perez EM, Hendricks MK, Beard JL, Murray-Kolb LE, Berg A, Tomlinson M, Irlam J, Isaacs W, Njengele T, Sive A, Vernon-Feagans L. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135(4):850–855. doi: 10.1093/jn/135.4.850. [DOI] [PubMed] [Google Scholar]
- 23.Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26(2):257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg BJ. The California Child Health and Development Studies: twenty years of research. World Health Stat Q. 1979;32(4):269–286. [PubMed] [Google Scholar]
- 25.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2(3):265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Neale MC, Walsh D. Evaluating the spectrum concept of schizophrenia in the Roscommon Family Study. Am J psychiatry. 1995;152(5):749–754. doi: 10.1176/ajp.152.5.749. [DOI] [PubMed] [Google Scholar]
- 27.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies: rationale, unique features, and training: NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 28.Dallman PR. Iron deficiency anemia: a synthesis of current scientific knowledge and U.S. recommendations for prevention and treatment. In: Earl R, Woteki CE, editors. Iron Deficiency Anemia, Recommended Guidelines for the Prevention, Detection, and Management Among U.S. Children and Women of Child bearing Age. Washington, DC: National Academies Press; 1993. pp. 41–98. [PubMed] [Google Scholar]
- 29.Tam KF, Lao TT. Hemoglobin and red cell indices correlated with serum ferritin concentration in late pregnancy. Obstet Gynecol. 1999;93(3):427–431. doi: 10.1016/s0029-7844(98)00422-0. [DOI] [PubMed] [Google Scholar]
- 30.Cox DR, Oakes D. Analysis of Survival Data. London, England: Chapman & Hall Ltd.; 1984. [Google Scholar]
- 31.Bresnahan M, Begg MD, Brown A, Schaefer C, Sohler N, Insel B, Vella L, Susser E. Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36(4):751–758. doi: 10.1093/ije/dym041. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer CA, Brown AS, Wyatt RJ, Kline J, Begg MD, Bresnahan MA, Susser ES. Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophr Bull. 2000;26(2):275–286. doi: 10.1093/oxfordjournals.schbul.a033452. [DOI] [PubMed] [Google Scholar]
- 33.Naeye RL, Peters EC. Antenatal hypoxia and low IQ values. Am J Dis Child. 1987;141(1):50–54. doi: 10.1001/archpedi.1987.04460010050022. [DOI] [PubMed] [Google Scholar]
- 34.Seidman LJ, Buka SL, Goldstein JM, Horton NJ, Rieder RO, Tsuang MT. The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal project. Schizophr Bull. 2000;26(2):309–321. doi: 10.1093/oxfordjournals.schbul.a033455. [DOI] [PubMed] [Google Scholar]
- 35.Cleves M, Gould WW, Gutierrez R. An Introduction to Survival Analysis Using Stata. Revised edition. College Station, Texas: Stata Press; 2004. Postestimation commands for parametric models; pp. 279–292. [Google Scholar]
- 36.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications, a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155(3):355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 38.McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, Paterson J. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ. 1998;317(7161):784–785. doi: 10.1136/bmj.317.7161.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff N MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121(3):475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 40.Kwik-Uribe CL, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice after brain iron concentrations and behavior despite postnatal iron supplementation. J Nutr. 2000;130(8):2040–2048. doi: 10.1093/jn/130.8.2040. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77(5):681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 42.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17(8):679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein JM. Sex differences in schizophrenia, epidemiology, genetics, and the brain. Int Rev Psychiatry. 1997;9(4):399–408. [Google Scholar]
- 44.Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26(5):1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byne W, Dracheva S, Chin B, Schmeidler JM, Davis KL, Haroutunian V. Schizophrenia and sex associated differences in the expression of neuronal and oligo-dendrocyte-specific genes in individual thalamic nuclei. Schizophr Res. 2008;98(1–):118–128. doi: 10.1016/j.schres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JW, Kim CS, Pai SH. Erythropoietic activity and soluble transferrin receptor level in neonates and maternal blood. Acta Paediatr. 2000;89(6):675–679. doi: 10.1080/080352500750043981. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Rai AK, Basu S, Dash D, Singh JS. Cord blood and breast milk iron status in maternal anemia. Pediatrics. 2008;121(3):e673–e677. doi: 10.1542/peds.2007-1986. [DOI] [PubMed] [Google Scholar]
- 48.Rusia U, Madan N, Agarwal N, Sikka M, Sood S. Effect of maternal iron deficiency anaemia on foetal outcome. Indian J Pathol Microbial. 1995;38(3):273–279. [PubMed] [Google Scholar]
- 49.Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, Donat J, Fernandez-Delgado R, Donat F, Alvarez-Dardet C. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;4(2):196–204. doi: 10.1111/j.1365-3016.1990.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 50.Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Int J Epidemiol. 1999;28(3):461–468. doi: 10.1093/ije/28.3.461. [DOI] [PubMed] [Google Scholar]
- 51.Strauss MB. Anemia of infancy from maternal iron deficiency in pregnancy. J Clin Invest. 1933;12(2):345–353. doi: 10.1172/JCI100507. [DOI] [PMC free article] [PubMed] [Google Scholar]