Abstract
Summary
In older men, severe abdominal aortic calcification and vertebral fracture (both assessed using dual-energy X-ray absorptiometry) were positively associated after adjustment for confounders including bone mineral density.
Introduction
Abdominal aortic calcification (AAC) is associated with higher fracture risk, independently of low bone mineral density (BMD). Dual-energy X-ray absorptiometry (DXA) can be used to assess both vertebral fracture and AAC and requires less time, cost, and radiation exposure.
Methods
We conducted a cross-sectional study of the association between AAC and prevalent vertebral fractures in 901 men ≥50 years old. We used DXA (vertebral fracture assessment) to evaluate BMD, vertebral fracture, and AAC.
Results
Prevalence of vertebral fracture was 11 %. Median AAC score was 1 and 12 % of men had AAC score >6. After adjustment for age, weight, femoral neck BMD, smoking, ischemic heart disease, diabetes, and hypertension, AAC score >6 (vs ≤6) was associated with 2.5 (95 % CI, 1.4–4.5) higher odds of vertebral fracture. Odds of vertebral fracture for AAC score >6 increased with vertebral fracture severity (grade 1, OR=1.8; grade 2, OR=2.4; grade 3, OR=4.4; trend p<0.01) and with the number of vertebral fractures (1 fracture, OR=2.0, >1 fracture, OR=3.5). Prevalence of vertebral fracture was twice as high in men having both a T-score<−2.0 and an AAC score>6 compared with men having only one of these characteristics.
Conclusions
Men with greater severity AAC had greater severity and greater number of vertebral fractures, independently of BMD and co-morbidities. DXA can be used to assess vertebral fracture and AAC. It can provide a rapid, safe, and less expensive alternative to radiography. DXA may be an important clinical tool to identify men at high risk of adverse outcomes from osteoporosis and cardiovascular disease.
Keywords: Aortic calcification, DXA, Men, Vertebral fracture, Vertebral fracture assessment
Introduction
Previous studies suggest that arterial calcification is associated with decreased bone mineral density (BMD) [1–8] and increased risk of fracture [1–6, 9, 10], although findings can vary according to population, skeletal site, and method used to measure arterial calcification. Calcification in the aortic media is a highly regulated process involving hormones, cytokines, transdifferentiation of vascular cells into bone cells, mineral deposition, and other factors [11].
An association between arterial calcification and skeletal fragility has significant clinical implications. A single assessment of bone and vascular calcification could identify individuals for treatment and prevention of future cardiovascular events and fracture, which are major causes of morbidity and mortality [12, 13]. Dual-energy X-ray absorptiometry (DXA) can be used to assess both vertebral fracture and abdominal aortic calcification (AAC) [14–17]. Compared with radiography, DXA evaluation of vertebral fracture is more rapid, less costly, and requires less exposure to radiation. In addition, DXA assessment of vertebral fracture predicts subsequent fragility fracture [18], and DXA evaluation of AAC predicts incidence of cardiovascular events [17]. Identifying individuals at risk for both cardiovascular events and osteoporotic fracture may help reduce morbidity and mortality associated with these highly common conditions.
Therefore, we conducted a cross-sectional study to determine the association between prevalent vertebral fracture and AAC, each assessed by DXA, in older men from the Structure of the Aging Men’s Bone (STRAMBO) study.
Subjects and methods
Participants
Participants in this study are members of the ongoing STRAMBO study, a single-center, prospective, cohort study of the determinants of skeletal fragility in men [19]. The study is a collaboration between INSERM (National Institute of Health and Medical Research) and private health insurance company MTRL (Mutuelle des Travailleurs de la Région Lyonnaise). The study obtained authorization from the local ethics committee and is performed according to the Helsinki Declaration of 1975 and 1983.
A randomly selected sample of men aged 20–87 years were recruited by letters of invitation in 2006–2008 from the MTRL health insurance rolls in Greater Lyon. The STRAMBO study enrolled 1,169 men aged 20–87. During the baseline visit in 2006–2008, all men completed a questionnaire and had clinical examination and DXA measurements. No exclusion criteria were used. This study was carried out in 920 men age 50 years and greater. DXA scans for 19 men could not be evaluated for assessment of vertebral fracture and/or AAC and were excluded from this study. Thus, the current analysis was performed in 901 men.
Dual-energy X-ray absorptiometry
BMD was measured at the lumbar spine, femoral neck, total hip, whole body, and distal non-dominant forearm by dual-energy X-ray absorptiometry using a Hologic Discovery, a device equipped with rotatory C-arm (HOLOGIC Inc., Bedford, MA). T-scores were calculated on the basis of the BMD values obtained in 116 healthy men aged 20 to 35 from the STRAMBO cohort. We used BMD and T-scores for femoral neck in the current study. Long-term stability of the device was assessed by daily measurements of the Hologic spine phantom (CV=0.35 %). Lateral single-energy scans of the thoracic and lumbar (T4–L4) spine were obtained in the dorsal decubitus (supine) position using Vertebral Fracture Assessment software (VFA) [16].
Abdominal aortic calcification
Aortic calcification was assessed from VFA scans using a semiquantitative method [20]. Calcific deposits in the anterior and posterior walls of the abdominal aorta adjacent to the first four lumbar vertebrae were assessed using the midpoint of the intervertebral space above and below the vertebrae as boundaries. Severity scores for the eight segments of the posterior and anterior walls (0–3) were added to yield an AAC score ranging from 0 to 24.
Intra- and inter-rater reproducibility was assessed by two readers (PS, DPK) for 76 randomly selected scans. Intra-and inter-rater agreement (intraclass correlation coefficient) for AAC score (continuous scale) was 0.95 (95 % confidence interval (CI) 0.91, 0.99) and 0.90 (95 % CI 0.86, 0.94), respectively. Intra- and inter-rater agreement (kappa) for AAC score >6 vs ≤6 (a cut-point used in the current study) was =0.93 (95 % CI 0.89, 1.00) and =0.83 (95 % CI 0.69, 0.98), respectively.
Vertebral fracture
Prevalent vertebral fractures from T4 to L4 were assessed by one reader (PS) using Genant’s semiquantitative score, modified for use in men [21, 22]. Vertebral fractures due to major trauma and non-fracture vertebral deformities (e.g., arthritis and Scheuerman’s disease) were classified as non-fractured [23]. Vertebrae that could not be assessed due to poor visibility of vertebrae (23 % at T4, 11 % at T5 and T6) were considered as non-fractured. We considered two definitions of vertebral fracture. First, fracture was defined as a vertebral body graded at least mildly deformed (grade ≥1 vs grade 0). Second, we restricted the definition to include moderate to severe fracture (grade ≥2 vs grade ≤1). We determined inter- and intra-rater agreement for vertebral fracture assessment for two readers (PS and ESR) assessed using a sample of scans for 45 participants, enriched to include 32 individuals (71 %) with fracture to obtain meaningful results. Each reader assessed the scans for each individual on two separate occasions, 3 weeks apart, blinded to each other’s readings and to his/her first reading. Intra-rater agreement (κ) for vertebral fracture (grade ≥1 vs 0) ranged from 0.89 to 0.93, and inter-rater agreement (κ) was 0.84–0.91. Intra-rater agreement for grade ≥2 vs grade ≤1 ranged from κ=0.85 to 0.87, and inter-rater agreement ranged from 0.77 to 0.79. Intra-rater agreement (κ) for severity of vertebral fracture, classified as grade 0–3 (four classes) ranged from 0.86 to 0.88, and inter-rater agreement ranged from 0.76 to 0.81.
Covariates
Information on covariates was obtained from a standardized questionnaire administered by an interviewer. Men self-reported age, history of smoking, and chronic disease (ischemic heart disease, diabetes mellitus, hypertension). Weight (kilograms) and height (centimeter) were measured in light clothes without shoes using standard clinical equipment.
Statistical analyses
Men with more than one vertebral fracture were classified according to the fracture with greatest severity. Bivariate comparisons between AAC score and class variables (smoking status and comorbidities) were performed by Kruskal–Wallis tests. We categorized AAC score into four groups: 0, 1–2, 3–6, and >6 or dichotomously as >6 versus ≤6. These thresholds were used in a similar study of men [5] and also provided sufficient numbers in each category for analysis.
We performed Chi2 test and Fisher’s exact test to evaluate prevalence of co-morbidities and of smoking habits as well as differences in vertebral fracture prevalence, severity, and number by category of AAC score (0, 1–2, 3–6, and >6). BMD across the four groups of the AAC score was assessed using analysis of covariance adjusted for age, weight, smoking, hypertension, ischemic heart disease, and diabetes mellitus. We used logistic regression to calculate age- and multivariable-adjusted odds ratios (OR) of vertebral fracture and 95 % CI associated with AAC score >6 (vs ≤6). The multivariable model also included age, weight, femoral neck BMD, smoking, hypertension, ischemic heart disease, and diabetes. We selected covariates based on the model that provided the highest area under the curve for presence of vertebral fracture (c statistic). For definitions of vertebral fractures (dependent variable) including more than two fracture severity levels (grade 1, grade 2, and grade 3 vs grade 0; one fracture and >1 fracture vs no fracture), we used multivariate polytomous logistic regression. We calculated ORs of vertebral fracture according to a four-level, independent, class variable created to represent men according to both femoral neck BMD T-score and AAC score as follows: [1] T-score≥ −2 and AAC score ≤6 (reference), [2] T-score≥ −2 and AAC score >6, [3] T-score<−2 and AAC score ≤6, and [4] T-score<−2 and AAC score >6. Since there were few men with T-score of −2.5, we used T-score of −2 in order to have sufficient numbers of men in each category for analysis. Analysis was performed using SAS 9.1 software (SAS Institute Inc., Cary, NC).
Results
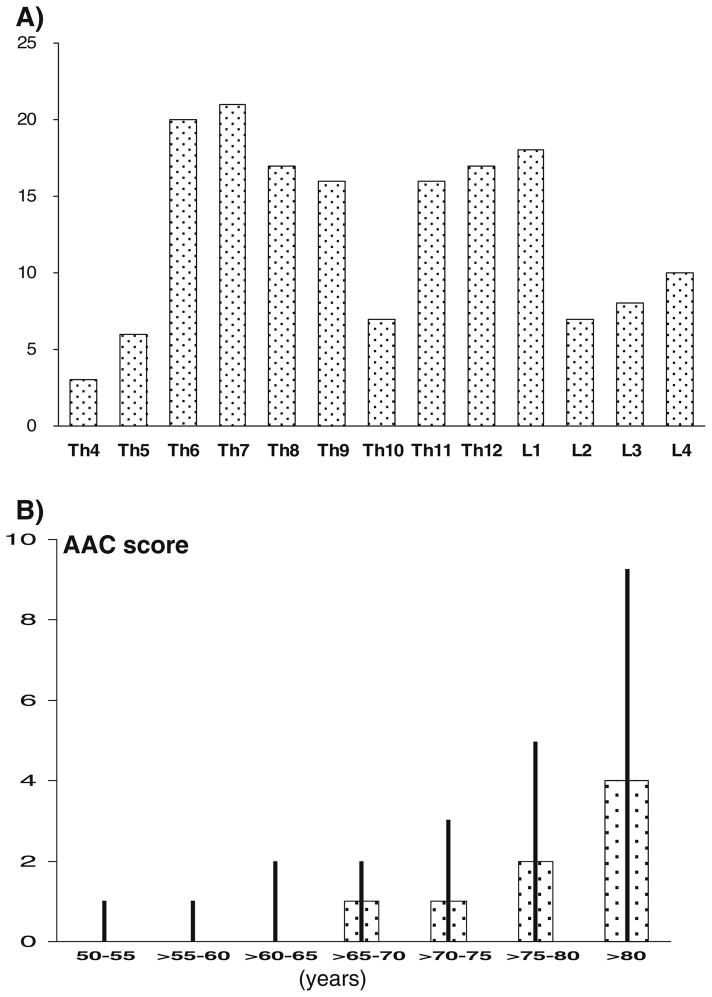
Average age of the 901 men was 70 years and mean BMI was 27.7 kg/m2 (Table 1). Seven percent were current smokers, 38 % reported hypertension, 11 % diabetes, and 14 % ischemic heart disease. Prevalence of vertebral fracture (grade ≥1) was 11 %, and prevalence of moderate and severe fractures (grade ≥2) was 9 %. Moderate grade 2 fracture was most common with 7 % prevalence whereas prevalence was as low as 2 % for mild (grade 1) fracture and 2 % for severe (grade 3) fracture. Sixty-one (7 %) men had one fracture and 4 % had more than one fracture. Sixty-four men had only thoracic fractures, 16 men had only lumbar fractures and 18 men had both thoracic and lumbar fractures. The distribution of vertebral fractures across the spine followed the expected bimodal distribution centered around T7/T8 and T12/L1 (Fig. 1a).
Table 1.
Characteristics of 901 men aged 50 years and older from the STRAMBO cohort
| Characteristic | Mean±SD or n (%) |
|---|---|
| Age (years) | 70±9 |
| Body weight (kg) | 79±11 |
| Body height (cm) | 169±7 |
| Smokers | |
| Current | 61 (7) |
| Former | 551 (60) |
| Never | 301 (33) |
| Hypertension : yes | 342 (37) |
| Ischemic heart disease: yes | 127 (14) |
| Diabetes mellitus: yes | 104 (11) |
| Abdominal aortic calcification score | |
| 0 | 397 (44) |
| 1 to 2 | 242 (27) |
| 3 to 6 | 151 (17) |
| >6 | 111 (12) |
| Fracture severitya (n, %) | |
| Normal (grade 0) | 803 (89) |
| Mild (grade 1) | 18 (2) |
| Moderate (grade 2) | 60 (7) |
| Severe (grade 3) | 20 (2) |
| Fracture definitiona | |
| Mild–severe (grade ≥1) | 98 (11) |
| Moderate–severe (grade ≥2) | 80 (9) |
| Fracture number | |
| 1 | 61 (7) |
| >1 | 37 (4) |
Men classified according to fracture of greatest severity
Fig. 1.
a Distribution (number) of vertebral fractures according to vertebral level in 901 men aged 50 years and older from the STRAMBO cohort. b Distribution of abdominal aortic calcification (AAC) score by age in 901 men from the STRAMBO cohort. Data are presented as median and interquartile range per 5-year age groups
Median AAC score was 1 (range, 0–22; interquartile range, 1–3). AAC score was 0 (no aortic calcification) for 44 % of men, 27 % had AAC=1–2, 17 % had AAC=3–6, and 12 % had AAC>6. AAC score increased with age (Fig. 1b). Smoking habits and prevalence of co-morbidities (ischemic heart disease, hypertension, diabetes) were similar in the three lower classes of AAC score but higher in men with AAC score >6 (Table 2). After adjustment for confounders, BMD at all the skeletal sites was not associated with AAC score (p>0.10). There was no trend for any site (p>0.25).
Table 2.
Co-morbidities, bone mineral density as well as severity and number of prevalent vertebral fractures in the groups of men categorized according to the severity of abdominal aortic calcification score (AAC)
| AAC=0 (n=397) | AAC=1 to 2 (n=242) | AAC=3 to 6 (n=151) | AAC>6 (n=111) | p | |
|---|---|---|---|---|---|
| Ever-smoker (vs never-smoker) | 248 (63 %) | 166 (68 %) | 102 (68 %) | 91 (80 %) | <0.01a |
| Ischemic heart disease | 36 (9 %) | 28 (11 %) | 28 (18 %) | 41 (35 %) | <0.001a |
| Hypertension | 125 (31 %) | 91 (36 %) | 63 (41 %) | 71 (61 %) | <0.001a |
| Diabetes mellitus | 41 (10 %) | 31 (12 %) | 13 (8 %) | 31 (27 %) | <0.001a |
| Bone mineral density (g/cm2) | |||||
| Lumbar spine | 1.032±0.180 | 1.038±0.188 | 1.030±0.182 | 1.051±0.196 | 0.80b |
| Femoral neck | 0.776±0.127 | 0.791±0.136 | 0.780±0.119 | 0.774±0.114 | 0.45b |
| Total hip | 0.955±0.136 | 0.969±0.143 | 0.952±0.132 | 0.939±0.130 | 0.20b |
| Trochanter | 0.741±0.121 | 0.760±0.129 | 0.743±0.120 | 0.735±0.119 | 0.13b |
| One third distal radius | 0.763±0.070 | 0.772±0.076 | 0.760±0.080 | 0.765±0.085 | 0.33b |
| Ultra-distal radius | 0.457±0.071 | 0.465±0.075 | 0.460±0.076 | 0.463±0.079 | 0.51b |
| Whole body | 1.120±0.106 | 1.128±0.117 | 1.136±0.115 | 1.132±0.115 | 0.44b |
| Vertebral fractures | |||||
| Fracture severity | |||||
| Mild (grade 1) | 6 (2 %) | 6 (2 %) | 2 (1 %) | 4 (4 %) | 0.44c |
| Moderate (grade 2) | 26 (6 %) | 10 (4 %) | 8 (5 %) | 16 (14 %) | <0.005a |
| Severe (grade 3) | 7 (2 %) | 4 (2 %) | 3 (2 %) | 6 (5 %) | <0.005a |
| Grade ≥1 (mild–moderate–severe) | 39 (10 %) | 20 (8 %) | 13 (9 %) | 26 (23 %) | <0.001a |
| Grade ≥2 (moderate–severe) vs Grade ≤1 | 33 (8 %) | 14 (6 %) | 11 (7 %) | 22 (20 %) | <0.001a |
| Number of fractures | |||||
| 1 fracture | 23 (6 %) | 14 (6 %) | 9 (6 %) | 15 (14 %) | <0.05a |
| >1 fracture | 16 (4 %) | 6 (3 %) | 4 (3 %) | 11 (10 %) | <0.05c |
Chi2 test
Covariance analysis adjusted for age, weight, smoking habits, hypertension, ischemic heart disease, and diabetes mellitus
Fisher’s exact test—comparisons with men without vertebral fracture
Abdominal aortic calcification and vertebral fracture
Prevalence, severity, and number of vertebral fracture were similar in the three categories with lower AAC score (0, 1–2, and 3–6) and significantly higher in men with the AAC score>6; however, the trend was not statistically significant for frequency of mild (grade 1) fracture.
Multivariable-adjusted odds of vertebral fracture were 2.5 times higher in men with AAC score >6 vs ≤6 (Table 3). Multivariable-adjusted odds of fracture associated with AAC score >6 vs ≤6 increased with increasing severity of fracture and with increasing number of fractures. Men with AAC score >6 had 1.8 increased odds of grade 1 fracture, 2.4 increased odds of grade 2 fracture and 4.4 increased odds of grade 3 fracture (trend: p<0.01). Men with AAC score >6 had 2.0 increased odds of one fracture and 3.5 increased odds of multiple vertebral fractures.
Table 3.
Odds of vertebral fracture associatied with abdominal aortic calcification (AAC score >6 versus≤6) in 901 men aged 50 and older from the STRAMBO cohort assessed using age-adjusted and multivariable-adjusted logistic regression
| Age-adjusted OR (95 % CI) | Multivariable-adjusteda OR (95 % CI) | |
|---|---|---|
| Fracture prevalence | ||
| Grade ≥1 (n=98) vs grade 0 | 2.1 (1.2–3.6) | 2.5 (1.4–4.5) |
| Grade ≥2 (n=80) vs grade ≤1 | 2.1 (1.2–3.9) | 2.6 (1.4–5.1) |
| Fracture severity b | ||
| Grade 1 (n=18) | 1.6 (0.4–6.1) | 1.8 (0.4–7.3) |
| Grade 2 (n=60) | 2.0 (1.0–3.9) | 2.4 (1.2–5.1) |
| Grade 3 (n=20) | 2.8 (0.99–8.1) | 4.4 (1.3–15.1) |
| Number of fractures b | ||
| 1 fracture | 1.7 (0.9–3.4) | 2.0 (0.98–4.2) |
| >1 fracture | 2.7 (1.2–6.0) | 3.5 (1.4–8.6) |
Data are presented as odds ratios (OR) and 95 % confidence interval (95%CI)
Adjusted for age (years), weight (kilogram), femoral neck BMD (grams per square centimeter), smoking (ever/never), ischemic heart disease (yes/no), diabetes (yes/no), and hypertension (yes/no)
Assessed by polytomous logistic regression using men without vertebral fracture (grade 0) as the reference group
Prevalence of vertebral fracture (grade ≥1) was twice as high in men having both a low T-score (T<–2.0) and AAC score >6 compared with men having only one of these characteristics (Table 4). This pattern also held for grade >1 vertebral fracture (moderate and severe vs no or mild). Multivariable-adjusted odds of vertebral fracture was eight times as high in men having both a low T-score (T<−2.0) and AAC score >6 compared with the reference group. Furthermore, odds of grade>1 vertebral fracture was two times as high for both a low T-score (T<−2.0) and≥grade 2 fracture compared with the reference group.
Table 4.
Odds of vertebral fractures associated with low femoral neck bone mineral density (T-score<−2.0) and severe abdominal aortic calcification score (AAC score>6) in older men from the STRAMBO cohort
| Skeletal site | AAC score | Number (%) | Multivariable-adjusteda OR (95 % CI) |
|---|---|---|---|
| Femoral neck bone mineral density | |||
| Grade ≥1 fracture (mild–moderate–severe) | |||
| T-score ≥ −2.0 | 0 to 6 | 42 (7 %) | 1.00 |
| >6 | 14 (18 %) | 2.7 (1.3–5.7) | |
| T-score < −2.0 | 0 to 6 | 30 (19 %) | 3.3 (1.9–5.7) |
| >6 | 12 (39 %) | 8.1 (3.3–19.7) | |
| Grade ≥2 fracture (moderate–severe) vs grade ≤1 | |||
| T-score ≥ −2.0 | 0 to 6 | 30 (5 %) | 1.00 |
| >6 | 10 (13 %) | 2.8 (1.2–6.4) | |
| T-score < −2.0 | 0 to 6 | 28 (18 %) | 4.4 (2.4–8.1) |
| >6 | 12 (39 %) | 12.2 (4.8–31.2) | |
Data are presented as odds ratios (OR) and 95 % confidence interval (95 %CI) Femoral neck BMD T-score was calculated in 116 men aged 20–35 from the STRAMBO cohort (T-score=−2 corresponds to 0.675 g/cm2 )
Adjusted for age (years), weight (kilogram), femoral neck BMD (grams per square centimeter), smoking (ever/never), ischemic heart disease (yes/no), diabetes (yes/no), and hypertension (yes/no)
We found similar results when we changed the AAC score threshold (AAC score >5 or >7) and when we used BMD of another skeletal site (distal radius, total hip, and whole body).
Discussion
In this cohort of older men, severity of aortic calcification, assessed on lateral DXA scan (obtained using the VFA software), was associated with a higher prevalence number and severity of vertebral fracture independent of age, BMD, smoking habits, and co-morbidities.
Our finding that higher AAC was associated with higher vertebral fracture prevalence in the general population is supported by some [1, 3–6, 10], but not all [2, 24–26] studies. Higher risk of vertebral fracture was also found in hemodialysis patients with severe AAC and in postmenopausal women with echogenic carotid artery plaque [9, 27, 28]. Moreover, older men with arterial disease had higher risk of non-spine fracture [29]. Interestingly, vertebral fracture severity was associated with higher cardiovascular risk in postmenopausal women [30]. These studies (including this one) show that the link between fragility fracture and cardiovascular disease is driven by more severe fractures (hip fracture, grade ≥2 vertebral fracture) and by severe cardiovascular disease (severe arterial calcification, renal failure, major cardiovascular event). In addition, sufficient number of events and temporal proximity are necessary to detect the association. The association between fragility fracture and cardiovascular disease has not been detected in small groups, especially composed of younger individuals without severe cardiovascular pathology, or in the long-term follow-up with a large time elapse between the cardiovascular event (or the assessment of cardiovascular status) and fracture event [31, 32].
In our study, severe AAC was associated with vertebral fracture but not with lower BMD, and the link between AAC and vertebral fracture was significant after adjustment for BMD. Thus, similar to previous studies, this association is independent of BMD. It is possible that poor health status (chronic diseases, side effects of drugs, bad lifestyle habits) might be an underlying explanation as to why the association is independent of BMD, although AAC was associated with vertebral fracture after adjustment for age and co-morbidities. Another possible explanation is that cardiovascular diseases or medications may increase the risk of falling and cause vertebral fractures [33, 34]. However, the link between AAC score and fracture risk persisted after adjustment for a history of falls [5]. Adjustment for co-morbidities had a small impact on the results in this study probably, because few vertebral fractures are related to falls.
Men with severe AAC may have some bone defect not detected by DXA. Vertebral fracture has been associated with poor cortical microarchitecture [23]. However, in men, AAC correlated with trabecular number only weakly [35]. Abdominal obesity has been associated with severe AAC and lower BMD, but not with higher fracture risk [36–38]. Higher homocysteine (Hcy) level was associated with severe aortic calcification in one study [39]; however, data on the link between higher Hcy and vertebral fracture are scanty [40]. Hcy inhibits lysyl oxidase and decreases formation of collagen crosslinks. Low deoxypyridinoline content was found in the aortic calcified lesions [41]. However, data on the link between bone crosslink content and bone strength are discordant [42, 43]. Accumulation of the glycation end-product, pentosidine, in bone may reduce bone strength by affecting the non-mineralized matrix of bone [44]. However, in a small group of samples, pentosidine content did not differ between the calcified and non-calcified aortic lesions [41]. In the femoral neck of hip fracture patients, osteopontin (OPN) content was low [45]. In low density lipoprotein receptor −/− mice, low OPN content correlated with higher aortic calcification [46]. However, one study had a modest sample size and the second study used an experimental model. Thus, the mechanism of the link between AAC and bone fragility remains elusive.
Reproducibility of the diagnosis of vertebral fractures and AAC assessed by intra- and inter-reader agreement was similar to the agreement found previously both for AAC score and for vertebral fracture assessed on radiographs [20, 21]. This indicates that DXA lateral scans provide image quality which may be comparable to radiographs for the diagnosis of AAC and vertebral fractures.
Our study has limitations. First, the cohort composed of volunteers may not be representative of the general population, mainly among the oldest men. Second, in a cross-sectional study such as this one, we do not know the temporal relation between vertebral fractures and AAC appearance. Third, although we excluded vertebral fractures due to self-reported severe trauma, we did not assess circumstances of all fractures. Fourth, not all DXA scans could be assessed such as those of poor quality or with scoliosis, and not all the thoracic vertebrae could be assessed for the presence of fracture. The advantage of lateral DXA scans compared with radiographs is the absence of parallax affecting the vertebral contours; however, the image resolution may be poorer. Finally, co-morbidities and smoking habits were self-reported and not further ascertained.
Most importantly, vertebral fractures and AAC were assessed by one reader and from the same scan but on two separate occasions. This may introduce a bias in the assessment. We believe this is not the case for four reasons. We had satisfactory intra- and inter-rater agreement of the diagnosis of vertebral fractures and of AAC. Higher prevalence of vertebral fractures was observed only in men with AAC >6, whereas the vertebral fracture prevalence did not vary in men with AAC ≤6. A biased (“wishful thinking”) reading may have produced more fractures in men with low AAC score. Although the difference between two estimations of AAC may vary within a couple of units, men with AAC>6 did have severe AAC. The association between AAC and vertebral fractures was driven by the association with the grades 2 and 3 fractures, whereas that with the grade 1 fracture was not significant. Biased reading may have been more likely to produce false-positive grade 1 fractures leading to associations driven by grade 1 fractures and not by the grade 2 and 3 fractures; however our findings were strongest for more severe grades of fractures. The association between AAC and vertebral fractures was driven by the multiple fractures, whereas that with a single fracture was weaker. Biased reading may have been more likely to produce false-positive single fractures, thus, the association would have been driven by the single and not by the multiple fractures. Overall, these observations argue against a systematic bias during the reading of scans.
A cross-sectional epidemiological study does not permit us to study mechanisms underlying the association between AAC and bone fragility. In addition, it is not possible to determine if vertebral fracture and AAC occur simultaneously, or if one precedes the other. Nevertheless, our results suggest that men with vertebral fracture, especially men with moderate and severe fracture or men with more than one fracture, are likely to concurrently have significant AAC. Our study demonstrates that DXA may be used for the assessment of vertebral fractures and AAC in research studies and, potentially, in clinical practice. Our results are in line with previous data obtained using radiography and showing that higher risk of fracture is observed mainly in subjects with severe AAC. In addition, we show that the reproducibility of the diagnosis of vertebral fracture and AAC on lateral DXA scans is comparable to that observed for radiographs. Thus, our data suggest that the assessment of AAC and vertebral fracture by DXA may be a safe, rapid, and inexpensive tool to study the link between cardiovascular diseases and osteoporosis. The association between severe AAC and vertebral fracture implies that DXA examinations may provide opportunity to identify men for prevention of future fracture and cardiovascular events.
Acknowledgments
Funding This study is supported by grants from the Roche pharmaceutical company, Baselle, Switzerland, from Agence Nationale de la Recherche and from Hospices Civils de Lyon. Dr. Kiel’s efforts are supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR/AG 41398) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH. Dr. Samelson’s efforts are supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K01AR053118).
Footnotes
Conflicts of interest The authors have no conflict of interest to disclose
Contributor Information
P. Szulc, Email: pawel.szulc@inserm.fr, INSERM UMR 1033, University of Lyon, Hospices Civils de Lyon, Lyon, France. INSERM UMR 1033, Hôpital Edouard Herriot, Place d’Arsonval, 69437 Lyon, France
E. J. Samelson, Institute for Aging Research, Hebrew SeniorLife, Harvard Medical School, Boston, MA, USA
E. Sornay-Rendu, INSERM UMR 1033, University of Lyon, Hospices Civils de Lyon, Lyon, France
R. Chapurlat, INSERM UMR 1033, University of Lyon, Hospices Civils de Lyon, Lyon, France
D. P. Kiel, Institute for Aging Research, Hebrew SeniorLife, Harvard Medical School, Boston, MA, USA
References
- 1.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang TK, Bolland MJ, Pelt NC, Horne AM, Mason BH, Ames RW, Grey AB, Ruygrok PN, Gamble GD, Reid IR. Relationships between vascular calcification, calcium metabolism, bone density, and fractures. J Bone Miner Res. 2010;25:2501–2509. doi: 10.1002/jbmr.183. [DOI] [PubMed] [Google Scholar]
- 3.Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 4.Rajzbaum G, Roger VL, Bézie Y, Chauffert M, Bréville P, Roux F, Safar ME, Blacher J. French women, fractures and aortic calcifications. J Intern Med. 2005;257:117–119. doi: 10.1111/j.1365-2796.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 5.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 6.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 7.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 8.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamal SA, Leiter RE, Bauer DC. Hyperhomocysteinaemia and aortic calcification are associated with fractures in patients on haemodialysis. QJM. 2005;98:575–579. doi: 10.1093/qjmed/hci092. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto J, Matsumoto H, Takeda T, Sato Y, Uzawa M. A radiographic study on the associations of age and prevalence of vertebral fractures with abdominal aortic calcification in Japanese postmenopausal women and men. J Osteoporos. 2010;2010:748380. doi: 10.4061/2010/748380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis—from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 13.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 14.Rea JA, Li J, Blake GM, Steiger P, Genant HK, Fogelman I. Visual assessment of vertebral deformity by X-ray absorptiometry: a highly predictive method to exclude vertebral deformity. Osteoporos Int. 2000;11:660–668. doi: 10.1007/s001980070063. [DOI] [PubMed] [Google Scholar]
- 15.Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–289. doi: 10.1007/s00198-005-2010-5. [DOI] [PubMed] [Google Scholar]
- 16.Schousboe JT, Wilson KE, Hangartner TN. Detection of aortic calcification during vertebral fracture assessment (VFA) compared to digital radiography. PLoS One. 2007;8:e715. doi: 10.1371/journal.pone.0000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schousboe JT, Taylor BC, Kiel DP, Ensrud KE, Wilson KE, McCloskey EV. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J Bone Miner Res. 2008;23:409–416. doi: 10.1359/jbmr.071024. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey EV, Vasireddy S, Threlkeld J, Eastaugh J, Parry A, Bonnet N, Beneton M, Kanis JA, Charlesworth D. Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res. 2008;23:1561–1568. doi: 10.1359/jbmr.080515. [DOI] [PubMed] [Google Scholar]
- 19.Chaitou A, Boutroy S, Vilayphiou N, Munoz F, Delmas PD, Chapurlat R, Szulc P. Association between bone turnover rate and bone microarchitecture in men—the STRAMBO study. J Bone Miner Res. 2010;25:2313–2323. doi: 10.1002/jbmr.124. [DOI] [PubMed] [Google Scholar]
- 20.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 21.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 22.Szulc P, Munoz F, Marchand F, Delmas PD. Semiquantitative evaluation of prevalent vertebral deformities in men and their relationship with osteoporosis: the MINOS study. Osteoporos Int. 2001;12:302–310. doi: 10.1007/s001980170120. [DOI] [PubMed] [Google Scholar]
- 23.Szulc P, Boutroy S, Vilayphiou N, Chaitou A, Delmas PD, Chapurlat R. Cross-sectional analysis of the association between fragility fractures and bone microarchitecture in older men: the STRAMBO study. J Bone Miner Res. 2011;26:1358–1367. doi: 10.1002/jbmr.319. [DOI] [PubMed] [Google Scholar]
- 24.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O’Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flipon E, Liabeuf S, Fardellone P, Mentaverri R, Ryckelynck T, Grados F, Kamel S, Massy ZA, Dargent-Molina P, Brazier M. Is vascular calcification associated with bone mineral density and osteoporotic fractures in ambulatory, elderly women? Osteoporos Int. 2012;23:1533–1539. doi: 10.1007/s00198-011-1762-3. [DOI] [PubMed] [Google Scholar]
- 26.Frye MA, Melton LJ, 3rd, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-García M, Gómez-Alonso C, Naves-Díaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andía JB. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24:239–246. doi: 10.1093/ndt/gfn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SH, Kim YM, Cho MA, Rhee Y, Hur KY, Kang ES, Cha BS, Lee EJ, Lee HC, Lim SK. Echogenic carotid artery plaques are associated with vertebral fractures in post-menopausal women with low bone mass. Calcif Tissue Int. 2008;82:411–417. doi: 10.1007/s00223-008-9141-6. [DOI] [PubMed] [Google Scholar]
- 29.Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 31.Vestergaard P, Rejnmark L, Mosekilde L. Hypertension is a risk factor for fractures. Calcif Tissue Int. 2009;84:103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- 32.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaëlsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–1362. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 33.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–717. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gribbin J, Hubbard R, Gladman JR, Smith C, Lewis S. Risk of falls associated with antihypertensive medication: population-based case–control study. Age Ageing. 2010;39:592–597. doi: 10.1093/ageing/afq092. [DOI] [PubMed] [Google Scholar]
- 35.Chow JT, Khosla S, Melton LJ, 3rd, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. J Bone Miner Res. 2008;23:1601–1612. doi: 10.1359/JBMR.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk–the MINOS study. J Bone Miner Res. 2010;25:1446–1454. doi: 10.1002/jbmr.13. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi T, Kanazawa I, Yamamoto M, Kurioka S, Yamauchi M, Yano S, Sugimoto T. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45:174–179. doi: 10.1016/j.bone.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454–5461. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 39.Van Campenhout A, Moran CS, Parr A, Clancy P, Rush C, Jakubowski H, Golledge J. Role of homocysteine in aortic calcification and osteogenic cell differentiation. Atherosclerosis. 2009;202:557–566. doi: 10.1016/j.atherosclerosis.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiraki M, Kuroda T, Shiraki Y, Tanaka S, Higuchi T, Saito M. Urinary pentosidine and plasma homocysteine levels at baseline predict future fractures in osteoporosis patients under bisphosphonate treatment. J Bone Miner Metab. 2011;29:62–70. doi: 10.1007/s00774-010-0191-2. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino H, Takahashi M, Kushida K, Ohishi T, Kawana K, Inoue T. Quantitation of the crosslinks, pyridinoline, deoxypyridinoline and pentosidine, in human aorta with dystrophic calcification. Atherosclerosis. 1995;112:39–46. doi: 10.1016/0021-9150(94)05395-y. [DOI] [PubMed] [Google Scholar]
- 42.Follet H, Viguet-Carrin S, Burt-Pichat B, Dépalle B, Bala Y, Gineyts E, Munoz F, Arlot M, Boivin G, Chapurlat RD, Delmas PD, Bouxsein ML. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011;29:481–488. doi: 10.1002/jor.21275. [DOI] [PubMed] [Google Scholar]
- 43.Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone. 2006;38:300–309. doi: 10.1016/j.bone.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Narusawa K, Onishi H, Miura M, Hijioka A, Kanazawa Y, Nishida S, Ikeda S, Nakamura T. Lower osteocalcin and osteopontin contents of the femoral head in hip fracture patients than osteoarthritis patients. Osteoporos Int. 2011;22:587–597. doi: 10.1007/s00198-010-1328-9. [DOI] [PubMed] [Google Scholar]
- 46.Shao JS, Sierra OL, Cohen R, Mecham RP, Kovacs A, Wang J, Distelhorst K, Behrmann A, Halstead LR, Towler DA. Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1821–1833. doi: 10.1161/ATVBAHA.111.230011. [DOI] [PMC free article] [PubMed] [Google Scholar]