Abstract
Maternal smoking during pregnancy (SDP) is associated with lower academic achievement in offspring. The current study, which was based on all births in Sweden from 1983 through 1991, explored the possible causal processes underlying the association between SDP and offspring school grades and a standardized assessment of mathematic proficiency at age 15. The analyses compared relatives who varied in their exposure to SDP and who varied in their genetic relatedness. Although SDP was statistically associated with academic achievement when comparing unrelated individuals, the results suggest that SDP does not cause poorer academic performance, as full siblings differentially exposed to SDP did not differ in their academic scores. The pattern of results suggests that genetic factors shared by parents and their offspring explain significant variance in why offspring opposed to SDP have lower levels of academic achievement. Nevertheless, SDP impacts pregnancy-related outcomes. Reducing SDP, therefore, remains a major public health issue.
Maternal smoking during pregnancy (SDP) has been consistently linked with numerous birth complications and problems in the offspring, including unsuccessful pregnancy outcomes, lower intellectual abilities, lower academic achievement (AA), more inattention/hyperactivity problems, and increased antisocial behavior (reviews in Cnattingius, 2004; Huizink & Mulder, 2006; Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002). Many researchers have noted that the current state of research suggests the associations are consistent with a causal connection as the findings are frequently consistent across studies, there is some specificity in the outcomes, the associations follow a dose-dependent relationship, and basic research (e.g., animal studies) has identified plausible biological mechanisms through which SDP may interfere with development. The interest in exploring the effects of SDP stems from a growing appreciation of the role of prenatal risk factors for later development (e.g., Coe & Lubach, 2008) and the fact that SDP may be one of the most preventable risk factors for poor pregnancy outcomes (Cnattingius, 2004) and later antisocial behavior (Wakschlag et al., 2002).
It is important to note, however, that SDP is correlated with many risk factors that also predict poor outcomes in children. SDP is correlated with psychosocial risks, such as early age at childbearing; parental history of psychopathology and antisocial behavior, low socioeconomic status, poor parenting, and ongoing exposure to parental substance use (reviews in Huizink & Mulder, 2006; Maughan, Taylor, Caspi, & Moffitt, 2004). These correlated risks could confound the association between SDP and offspring adjustment. A number of researchers have also argued that genetic confounds may explain the associations between SDP and offspring adjustment (D’Onofrio et al., 2003; D’Onofrio et al., 2008; Fergusson, 1999; Knopik et al., 2005; Silberg et al., 2003). Behavior genetic studies have documented that genetic factors influence maternal SDP (Agrawal et al., 2008; D’Onofrio et al., 2003). Genetic factors that influence SDP, such as risk for substance abuse and dependence (Agrawal et al., 2008), could be passed from parents to their offspring and influence the behavior of their offspring. Thus, SDP may not be causally related to offspring behavior but rather the genetic factors shared by both generations could explain the association.
Approaches to Control for Selection Factors
Most studies of SDP have tried to control for selection factors that could lead to both SDP and offspring adjustment by statistically controlling for characteristics of mothers that covary with SDP (e.g., maternal education, SES, and history of delinquency). To date, reviews suggest that the associations between SDP and birth outcomes (Cnattingius, 2004), and antisocial behavior (Wakschlag et al., 2002) remain largely robust to the inclusion of statistical covariates. With respect to the association between SDP and low AA/intellectual abilities, the literature is less conclusive. Numerous studies have found an independent association between SDP and offspring AA when controlling for various familial characteristics (e.g., Butler & Goldstein, 1973; Cornelius, Ryan, Day, Godschmidt, & Willford, 2001; Fogelman & Manor, 1988; Rantakallio, 1983). In contrast, large national cohort (Fergusson & Lloyd, 1991) and nationally representative studies (Batty, Der, & Deary, 2006) suggest the association between SDP and academic problems is completely confounded by covariates, such as detailed measures of maternal intellectual abilities. Ultimately, the use of measured covariates is limited; finding an independent association while controlling for possible confounds can help support a possible causal inference, but it is impossible to know whether every salient confound was included and measured accurately in each study.
Because SDP co-occurs with numerous psychosocial and genetic risks for offspring the effects of SDP are difficult to isolate using standard family research designs. Quasi-experimental studies, approaches that disentangle co-occurring genetic and environmental risks, are therefore required to elucidate the processes through which SDP is associated with poor adjustment (Moffitt, 2005; Rutter, 2007). Because of the inherent limitations and strengths of each approach, converging evidence from multiple designs is needed to infer a causal association (Rutter, Pickles, Murray, & Eaves, 2001).
Researchers must use numerous approaches, including quasi-experimental designs, to study the putative effects of SDP. Recently, a number of genetically informed approaches have been used as quasi-experimental approaches. Two studies of SDP have utilized a Children of Twins design (D’Onofrio et al., 2003; Heath, Kendler, Eaves, & Markell, 1985; Silberg & Eaves, 2004), an approach that helps control for environmental factors that are not measured by comparing cousins differentially exposed to a putative risk factor. In this design environmental factors that influence all cousins (e.g., SES, religious affiliation) are controlled. The approach also accounts for genetic risk passed down from parents to their offspring because the offspring of an identical twin shares 25% of his/her genetic makeup with the offspring of his/her parent's cotwin (socially they are cousins, but genetically they are half siblings), whereas the offspring of a fraternal twin shares 12.5% of his/her genetic makeup with his/her cousin. D’Onofrio et al. (2003) found that the association between SDP and offspring birth weight was not due to environmental or genetic confounds. Rather, the association appeared to be mediated via environmental risks specifically associated with SDP, consistent with a causal inference. Knopik et al. (2005), however, found that the association between SDP and offspring ADHD was largely accounted for by genetic and environmental correlates of SDP, not the teratogenic effects of prenatal nicotine exposure.
A number of studies have recently utilized a sibling comparison approach, a design that compares siblings who are differentially exposed to SDP (the mother varied her smoking among her pregnancies). The comparison of siblings within families rules out all unmeasured environmental risks that effect siblings similarly (Rodgers, Cleveland, van den Oord, & Rowe, 2000). The comparison of siblings also controls for genetic factors transmitted from the mother that influence both SDP and AA in offspring (D’Onofrio et al., 2007; Harden et al., 2007; Lahey, D’Onofrio, & Waldman, in press). This is because each child receives a random draw of their mother’s genes through the process of meiosis, regardless of the mother’s SDP during each pregnancy. It is also important to note that child effects, including the possibility of active or evocative gene-environment correlation, cannot confound the findings, as it is unlikely that traits in the offspring influence their mother’s decision to smoke while pregnant (Rutter, 2007).
Recent use of the approach suggests that the direct effects of SDP on certain outcomes are minimal. D’Onofrio et al. (2008) found no association between SDP and offspring conduct and behavioral problems when comparing differentially exposed offspring. The association between SDP and attention/impulsivity problems was still statistically significant, but the magnitude of the association was greatly reduced when compared to the association among unrelated individuals. Of particular relevance to the current manuscript, Lambe et al. (2006) found that among mothers who varied in their SDP across pregnancies, the risk for poor AA at age 15 was increased in all offspring regardless of whether they were specifically exposed to SDP. The study was a population-based study in Sweden, created by merging national registries. The results suggested that the association between SDP and school performance at age 15 was due to unmeasured familial traits that differ between offspring exposed and unexposed to SDP.
The sibling comparison design is limited, however, in its ability to identify the source of the confounds when the risk factors are not associated with the outcome among siblings (D’Onofrio et al., 2008). Comparing siblings differentially exposed to SDP can disconfirm a causal hypothesis (e.g., Lambe et al., 2006), but the design cannot determine why there is a statistical relation between SDP and AA in the population if differentially exposed siblings do not also differ in AA. Researchers must, therefore, use additional, genetically informed approaches to explore whether factors that confound the association between SDP and offspring AA are genetic or environmental in origin. Numerous genetically informed approaches are available (Rutter et al., 2001).
One approach to disentangling the source of the confounds is to compare the magnitude of the association between SDP and offspring AA in groups of relatives who differ in their exposure to SDP and who differ in their genetic relatedness (D’Onofrio et al., 2008). When multiple types of relatives are included in a study (e.g., cousins, full-siblings, and half-siblings) researchers can examine whether the degree to which the groups of relatives share genetic risk moderates the association between SDP and AA (i.e. the degree to which relatives share genetic background influences how strongly SDP and AA are associated). If the association between SDP and AA is smaller when comparing groups of relatives who share more genetic background, then genetic factors would be implicated. Stated differently, if the magnitude of the association between SDP and AA is smaller when using comparison groups that better “control” for genetic risk (comparing groups of relatives who share more genetic background), the results would suggest that genetic factors help account for the statistical relation between SDP and AA that is found in the population.
When large family pedigrees are available, researchers can compare two types of cousins who are differentially exposed to SDP. Like children of fraternal twins, cousins whose mothers are full siblings share 12.5% of their genetic makeup (referred to as full cousins), whereas cousins of mothers who are half siblings provide a comparison group that shares only 6.25% of their genes (half cousins). Using full and half-cousins, therefore, provides two groups of relatives who differ in the degree to which they share genetic risk; the comparison of full cousins would “control” for (or “account” for) more genetic risk than the comparison of half cousins.
Using different types of siblings can also help differentiate the possible underlying mechanisms. The comparison of differentially exposed half siblings from the same mother (siblings who share 25% of their genetic makeup) only controls for genetic factors that are passed down from mothers. In contrast, the comparison of full sibling (offspring who share the same mothers and fathers) differentially exposed to SDP rules out genetic factors passed down from both mothers and fathers (Harden et al., 2007; Lahey et al., in press). If genetic (or environmental) factors from fathers were important in explaining the association between SDP and AA, the association between SDP and AA would be smaller in full siblings than in half siblings (because the comparison of full siblings controls for more genetic risk). A difference in the associations between SDP and AA when comparing full siblings and half siblings would suggest that paternal effects due to assortative mating (the nonrandom mating of individuals based on similar backgrounds) are important in understanding the effects of SDP.
If the magnitude of the SDP and AA association decreases according to increasing genetic similarity in the groups of relatives (unrelated offspring > cousins sharing 6.25% of their genes > cousins sharing 12.5% of their genes > siblings who share 25% of their genes > siblings sharing 50% of their genes), genetic factors would be implicated. The findings would not prove that genetic factors account for the association because the various groups of relatives can also differ on environmental risk associated with SDP (see discussion). In contrast, the results would suggest the importance of environmental confounds (factors that influence both SDP and AA) if the association was of the same magnitude in all the relative groups. Environmental confounds would be implicated because the factors accounting for the association between SDP and AA would not depend on genetic risk associated with SDP. In sum, large family studies including relative groups who vary both in their exposure to SDP and in their genetic relatedness can help to differentiate whether genetic and/or environmental factors account for the statistical relation between SDP and AA.
The Current Study
The current study used a number of statistical and quasi-experimental approaches to explore the underlying causal mechanisms responsible for the association between maternal SDP and offspring AA. The current study expands on the study by Lambe et al. (2006), which compared siblings differentially exposed to SDP on school grades using offspring born in Sweden between 1983 and 1987. We use a larger sample to explore the association between SDP and grade average (using data on every individual born in Sweden between 1983 and 1991), also include scores from a standardized assessment of mathematic proficiency given to all students in grade 9 throughout the country, explore the possible confounding role of measured maternal and paternal characteristics, and utilize multiple quasi-experimental designs to help specify the causal mechanisms through which SDP is associated with lower AA.
There were four specific aims of the analyses: (1) identify the magnitude of the associations between SDP and two measures of AA, school grades and mathematics proficiency at age 15, (2) examine whether the relations were robust to the inclusion of statistical covariates for individual, maternal, and paternal characteristics, (3) explore the strength of the associations when comparing cousins and siblings differentially exposed to SDP, and (4) test whether genetic and/or environmental confounds help explain the statistical association between SDP and AA. The last aim was based on our ability to explore whether the strength of the statistical association between SDP and AA was dependent on the degree to which the exposed and unexposed individuals shared genetic risk. The analyses explicitly tested whether the association between SDP and AA was stronger when comparing cousins who share 6.25% of their genes (cousins of mothers who were half siblings) than when comparing cousins who share 12.5% of their genes (cousins of mothers who were full siblings). Likewise, the analyses explored whether SDP was more strongly associated with AA in half-siblings (who share 25% of their genes) than in full siblings (who share 50%) who were differentially exposed to SDP.
METHODS
Sample
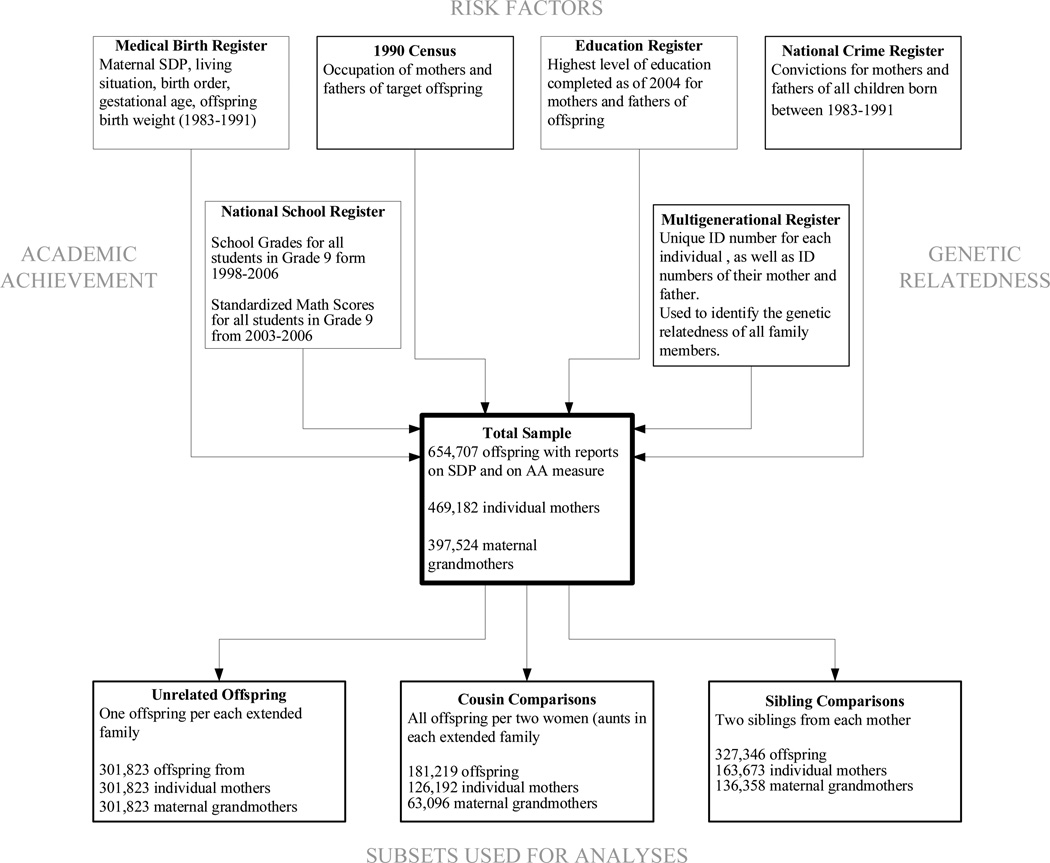
The current study was based on merging numerous Swedish population registries. Various governmental agencies and research institutes in Sweden maintain the registries. Each register includes a unique identifier for each individual in the country, which enables Statistics Sweden to link the various datasets. The registries and the agencies that maintain them are described in detail below (see Figure 2 in the appendix for a diagram of the registries included).
The Medical Birth Register
The Swedish Medical Birth Register (MBR) is administered by the Swedish Centre for Epidemiology at the National Board of Health and Welfare. The MBR includes over 99% of all births in Sweden since 1973 (Swedish_Centre_for_Epidemiology, 2001). Information is gathered throughout the pregnancy and delivery using standardized records. Since 1982, the register has also included information from the first visit to antenatal care. Over 95% of pregnant women in Sweden receive such care by the 15th gestational week (Lindmark & Cnattingius, 1991). The register includes detailed demographic and pregnancy information, including maternal age at birth, maternal living situation, maternal SDP, birth order of the child, infant physical attributes at birth, pregnancy complications, and delivery complications. No offspring who died at childbirth, had serious malformations at birth, and/or were missing the identification number of their mother were included. Because the current study explores prenatal risk factors, all multiple births (e.g., twins and triplets) were entirely excluded from the analyses. Children born in Sweden from 1983 to 1991 were included (n = 918,028).
The National School Register
The School Register, a collaboration between the Swedish School Authority and Statistics Sweden, includes educational achievement (scores in multiple subjects and summary grades) for all students at the end of grade nine (Swedish_National_Agency_for_Education). The register began in 1983. The analyses in the current manuscript were conducted on information from students in all public and private schools throughout the country between 1998 and 2006. If students repeated the grade, the student’s scores for his/her first year were used. The total School Register included grades for 972,979 individuals, including some individuals not included in the current analyses (e.g., those from multiple births and children who immigrated to Sweden).
Starting in 2003 all students in grade nine throughout Sweden completed a compulsory comprehensive test that provides standardized assessments of students’ proficiency in Swedish, English, and Mathematics. Thus, the standardized testing permits the comparison of students throughout Sweden on the same test. Scores were available for 456,007 students (from 2003–2006).
The National Crime Register
The National Crime Register is maintained by the Swedish National Council for Crime Prevention and includes all registered convictions in the country from 1973–2004. The register includes detailed information about the nature of every conviction, number of offenses, time of the criminal activity, and sentence type and length. Merging the dataset in the study provided criminal background information from age 15 (age of criminal responsibility) for all of the mothers and fathers of the offspring born in Sweden from 1983 to 1991.
The Education Register
The Education Register is maintained by Statistics Sweden and includes information about the highest obtained level of education for each individual each year since 1990. We used the highest level of education in 2004 for the mothers and fathers of the target children in the study.
The 1990 Census
The Swedish Population and Housing Census was undertaken every ten years until 1990. Its completion was enforced by law, and included measures of individuals in each household, such as assessments of employment, occupation, and income. Occupational status information was obtained for individuals from the 1990 Census.
Multi-Generation Register
The Multi-Generation Register, maintained by Statistics Sweden, contains information on the identity of the parents of each child born in Sweden since 1932, or who immigrated and became Swedish citizens before age 18 (Statistics_Sweden, 2003). The register is part of the Total Population Register, which collects information from the national registration records maintained by the National Tax Board. For each target individual, the register includes information on the identification number of biological parents and adoptive parents, if appropriate. The information allows researchers to link all children to their parents and identify all types of relationships for each index person (e.g., full siblings, half siblings, grandparents, cousins, etc.). Because some of our analyses comprise comparisons among cousins, offspring who had missing information about the identification of their maternal grandmother (e.g., their mother immigrated to Sweden from another country) were not included in the analyses.
Total Sample and Subsets Included in Analyses
The total sample used for analyses included 654,707 offspring with information on maternal SDP and at least one of the educational outcomes (either school grades from 1998–2006 or standardized testing from 2003–2006). The population included 469,182 individual mothers from 397,524 extended families (based on maternal lineage). See Figure 2 in the appendix for more details.
The first subset of the data included unrelated offspring so that the between families effect of SDP on AA could be estimated. One grandchild from each extended family, indexed by the maternal grandmother, was initially selected. Offspring who shared any first-degree relative (e.g., their father) or second-degree relatives (e.g., their maternal grandfather, paternal grandmother, or grandfather) were excluded from the analyses. The highest genetic relatedness of any two offspring in the sample, therefore, would be minimal (second cousins, rg = 0.55 = 0.03125). The unrelated subset included 301,823 offspring from the same number of mothers and maternal grandmothers.
The second subset of data was selected to compare cousins, especially cousins differentially exposed to mothers with a history of any SDP. Because the current study explores the putative effects of a maternal behavior, SDP, the identification of cousins was based on offspring of female sisters (i.e. each offspring in an extended family shared the same maternal grandmother). All offspring from extended families with two adult sisters were included in the analyses. Offspring of women with no adult sisters were excluded from the analyses because their offspring had no cousins who were related through maternal aunts. For extended families that contained more than two adult sisters, two adult women and their offspring were selected. If all the women in the extended family had similar histories of SDP (either never smoked during any pregnancy or smoked during every one) then the first two adult sisters and their families were included in the analyses. If there was any variability in SDP histories among the sisters (one woman had a history of ever SDP and one of her adult sisters did not) one adult sister with a history of SDP and one adult sister without and their offspring were included. The subset maximized, where possible, the number of cousins differentially exposed to mothers with histories of SDP. The subset, referred to as the cousin subset, included 181,219 offspring from 126,192 mothers and 63,096 maternal grandmothers.
The third subset of the data sought to compare siblings differentially exposed to SDP. The identification of siblings was based on sharing the same biological mother. All nuclear families that included only one offspring were excluded. All nuclear families with two children were included. If a woman had more than two children, two children were selected for inclusion in the subset. For mothers with no variation in their SDP across their pregnancies, her first two children were selected. For mothers who varied their SDP across their pregnancies, siblings were selected to include one offspring unexposed and one offspring exposed. The subset, referred to as the sibling subset, included 327,346 offspring from 163,673 mothers and 136,358 maternal grandmothers.
Variables
Offspring Outcomes
School performance was based on a summary grade-point score of 16 different subjects from the National School Register. Students were given separate grades (10 = pass, 15 = pass with distinction, and 20 = pass with honors) for each subject. The summary grade-point score summed the subject scores for each student. As was found in previous studies (Lambe et al., 2006), the average grade-point score in the population (m = 204.5, sd = 64.3, n = 761,970) was skewed toward higher values. Therefore, the variable was normalized using a Blom transformation, a procedure that has been shown to help when analyzing skewed data using genetically-informed designs (van den Oord et al., 2000). The score was then converted to a z-score for ease of interpretation.
The compulsory standardized testing of students in grade nine provided assessment of student proficiency in mathematics (n = 331,093). Students could receive four possible scores: goals not achieved (10.6%), pass with lowest pass grade (52.1%), pass with distinction or intermediate pass (26.4%), and pass with special distinction or highest pass grade (11.0%). The variable was transformed using a Blom transformation and standardized as a z-score.
One birth characteristic, birth weight (BW) as measured in grams, was also included in the current study as a sensitivity analysis because previous findings strongly suggest SDP has a direct effect on offspring BW (e.g., Cnattingius, 2004). Offspring BW, excluding multiple births and children with significant physical malformations, was approximately normally distributed (m = 3,522.5 grams, sd = 539.7, n = 759,627). Because birth weight was measured on an interpretable scale, no transformations were completed.
Risk Factors
Maternal SDP was measured on a three-point scale: non-smokers, 1–9 cigarettes per day (moderate smokers), 10+ cigarettes per day (heavy smokers). Given the complexity of the analyses, the analyses treated SDP as dichotomous (no smoking versus smoking). As has been reported previously (Cnattingius, 2004) the prevalence of SDP declined during the years of 1983–1992; women reported smoking during 31.3% of pregnancies in 1983 and 24.3% in 1992.
Risk factors specific to each pregnancy were also included. The MBR assessed maternal age at the time of birth, birth order, gestational age (in days), year of birth, and whether the mother was living with a partner at the time of birth, coded as not cohabiting versus cohabiting. Paternal age at the birth of the child was calculated based on his and the child’s dates of birth.
Characteristics of the mothers and fathers were also included in the analyses. Socioeconomic status for both parents was measured by a frequently used, standard socioeconomic index variable, based on occupational group (Statistics_Sweden, 1982). The groupings include (a) unskilled blue collar worker, (b) skilled blue collar worker, (c) low-level white collar worker, (d) intermediate-level white collar worker, (e) high-level white collar worker, (f) self-employed, (g) employed but uncategorized, and (h) not working/missing. The final category included individuals with no information about occupational status. It includes individuals too young to be assessed (below the age of 15) and individuals that were not working at the time of the census (because they were students or unemployed). Mothers and fathers SES was based on the occupation of each individual separately. Previous research has shown that the groupings cannot be ordered (Lichtenstein, Pedersen, & McClearn, 1992). Therefore, dummy codes were created to compare each occupational group to the unskilled blue collar worker category.
Maternal and paternal highest level of education in 2004 was based on seven categories: (a) less than 9 years of primary and lower secondary education, (b) 9 years of primary and lower secondary education, (c) 1–2 years of upper secondary education, (d) 3 years of upper secondary education, (e) less than 3 years of post-secondary education, (f) more than 3 years of post-secondary education, and (g) postgraduate education. Because the assessment of educational level was not on an interval scale, dummy codes were used to compare higher levels of education to having completed less than 9 years of primary education. History of criminal behavior for mothers and fathers was based on the presence or absence of a conviction for any criminal offense in the National Crime Register through 2004.
Estimates of Genetic Relatedness
The biological mother of each offspring was determined by the MBR. The Multi-Generation register provided information about the biological father of each child. From the information, two offspring from the same mother could be designated as full siblings (sharing the same biological father) or half-siblings (having different biological fathers).
The determination of genetic relatedness of cousins was based on information from the Multi-Generation register. The genetic relatedness of the cousins is based on the genetic correlations of adult sisters within an extended family (the mothers of the offspring in the current study). Genetic relatedness of the adult sisters in the study was determined contingent on their mothers, the maternal grandmothers of the offspring in the current study. Adult sisters who shared the same fathers (the maternal grandfather of the offspring in the study) were determined to be full siblings (sharing 50% of their genetic makeup), whereas adult sisters with different fathers were designated as half-siblings (sharing 25% of their genetic makeup). Thus, offspring of full siblings, referred to as full cousins, shared 12.5% of their genes with their cousins; whereas offspring of half siblings, referred to as half cousins, shared 6.25% of their genes with their cousins. The process of determining the relationship of adult sisters contingent on the maternal grandmother does not include aunts who are half siblings because they had the same father but were born to different mothers. We restricted the sample to only include aunts who shared the same mother because the analyses explored the risk of a maternal characteristic (SDP) and we wanted to control for environmental factors that may be shared by women growing up in the same household; most children historically have lived with their mothers after separations or divorces.
Analyses
Association between Smoking During Pregnancy and Risk Factors
Linear and logistic regression models estimated the association between SDP and the risk factors at the individual, maternal, and paternal levels. Clustering for extended family members was taken into account using a sandwich estimator and Full Information Maximum Likelihood (review in Schafer & Graham, 2002) was utilized to account for missing data using the software program Mplus (Muthén & Muthén, 1998–2007).
Comparison of Offspring Exposed and Unexposed to SDP Contingent on Biological Relatedness
To describe the association between SDP and the AA variables and the underlying processes, the mean AA scores are presented for offspring differentially exposed using the three subsets of the data: unrelated offspring, cousins, and siblings. The difference between the AA means suggest the magnitude of the association between SDP and each academic measure. Formal significance testing of the differences was completed using regression analyses (see next section). The key to understanding the underlying causal mechanisms associated with SDP stems from the difference in exposure, not the overall mean levels, which are heavily influenced by family type. The means for AA among offspring differentially exposed (e.g., cousins and siblings who varied in their exposure) are tabled.
The first comparison involved unrelated offspring who were exposed and unexposed to SDP. The difference between those exposed and unexposed represents the association between SDP and each AA variable in standard deviation units (the AA variables were converted to Z-scores). The comparison of cousins differentially exposed to mothers with a history of SDP, regardless of genetic relatedness, was then completed. The comparison controlled for factors influencing all offspring within an extended family. Next, the cousin comparison was estimated separately for full cousins (those sharing 12.5% of their genes) and half cousins (sharing 6.25% of their genes) to examine whether the genetic relatedness of the cousins moderated the association between SDP and AA.
Mean comparisons were then estimated for differentially exposed siblings, regardless of genetic relatedness. Because the identification of siblings was based on sharing the same mother in the current sample, the comparison controls for genetic factors passed from the mother to her offspring, as well as environmental factors shared by siblings. Next, the sibling comparison was calculated separately for full siblings (those who share 50% of their genes) and half siblings (those only sharing 25% of their genetic makeup) to examine the possible influence of paternal factors.
Regression Analyses of the Relations between SDP and Offspring Academic Achievement
A series of regression analyses were performed to formally test the associations between SDP and AA across the groups of relatives and include the statistical covariates. Five regression models were fit for school grades and math proficiency. Descriptions of the models, including the main parameters of interest, an explanation of the models, and the samples utilized, are found in Table 3. All models accounted for the clustering of the data and used Full Information Maximum Likelihood to handle missing values using Mplus (Muthén & Muthén, 1998–2007). Model 1, an epidemiological model, examined the magnitude of the association between SDP and school grades in the entire sample, controlling for offspring sex. Model 2, the standard approach for controlling for selection factors, estimated the association of SDP and grades in the full sample while statistically controlling for the measured covariates.
Table 3.
Description of Analytical Models
| Description | Parameters of Interest | Explanation | Sample Used and Controls |
|
|---|---|---|---|---|
| 1 | Epidemiological model | SDP parameter | Estimates the difference in AA between unrelated offspring exposed and unexposed to SDP. | Includes all offspring in entire dataset. |
| 2 | Association controlling for statistical covariates | Compare magnitude of SDP parameter to Model 1 | Estimates the independent association between SDP and AA while statistically controlling for covariates. | Includes all offspring in the entire dataset. |
| 3 | Cousin and sibling comparison | SDP: Cousin comparison | Estimates whether offspring whose mother had more SDP across her pregnancies have lower AA than their cousins whose mothers had less SDP on average. | Includes all offspring in entire dataset, while controlling offspring birth order. |
| SDP: Sibling comparison | Estimates whether siblings exposed to SDP had lower AA than their siblings who were not exposed. | |||
| 4 | Comparison of full and half cousins | SDP: Half cousins | Estimates the association between SDP and AA when comparing half cousins (share 6.25% of genetic makeup) who were differentially exposed. | Includes all offspring from two mothers per extended family, while controlling for the main effects of cousin relatedness and offspring birth order. |
| SDP: Half cousin – Full cousin | Estimates the difference in the cousin SDP-AA association between half and full cousins, which was based on the interaction between the cousin parameter and cousin genetic relatedness, coded 0 = half cousin and 1 = full cousin. To calculate the magnitude of the SDP-AA association in full cousins requires adding the two cousin parameters in the model. | |||
| 5 | Comparison of full and half siblings | SDP: Half siblings | Estimates the association between SDP and AA when comparing half siblings (share 25% of genetic makeup) who were differentially exposed. | Includes two offspring from all mothers with two or more offspring, while controlling for main effects of sibling relatedness and offspring birth order. |
| SDP: Half sibling – Full sibling | Estimates the difference in the cousin SDP-AA association between half and full siblings, which was based on the interaction between the sibling parameter and sibling genetic relatedness, coded 0 = half sibling and 1= full sibling. To calculate the magnitude of the SDP-AA association in full siblings requires adding the two sibling parameters in the model. | |||
Note. All models accounted for the clustering in the data using a sandwich estimator in Mplus. Each model also included the main effect of offspring sex.
Model 3 examined the association when comparing cousins and siblings differentially exposed in the full sample while controlling for birth order effects. The models used a series of contrast codes that have been shown to accurately estimate within and between family effects at multiple levels (Neuhaus & McCulloch, 2006). The statistical approach results in parameters equivalent to that of a fixed effects model (Greene, 2003). For further details concerning the analytical approach, including explanations of the coding, see D’Onofrio et al. (2008) and Harden et al. (2007). Sibling comparisons were based on contrast codes created by subtracting the mother’s average SDP across all of her pregnancies from the child’s specific exposure. For example, if a mother had two children and only smoked during one pregnancy, the exposed sibling would receive a score = 0.50 and the unexposed sibling would receive a contrast code of −0.50. The contrast codes, therefore, compare exposure to SDP relative to their sibling’s exposure. A similar approach was used to compare cousins. Contrast codes were calculated by subtracting the average level of SDP across all women in an extended family (the adult sisters) from each mother’s average SDP across all of her pregnancies. The contrast codes compared siblings from a nuclear family who were more exposed to SDP on average to their cousins who were less exposed to SDP on average. The mean SDP across all cousins was also included in the model because the parameter associated with the variable provides a comparison of unrelated individuals (i.e. the parameter compares cousins who are all exposed to more SDP on average to unrelated sets of cousins who were exposed to less SDP on average). Model 3, therefore, provides estimates of the association between SDP and AA when comparing unrelated individuals, cousins, and siblings.
The next two models were conducted to further explore the cousin comparisons (Model 4) and the sibling comparisons (Model 5). Model 4 specifically examined whether the magnitude of the cousin comparison depended on whether the cousins were full or half cousins. The model was fit to a subset of the data that only included two adult sisters and their offspring from each extended family so that only one cousin comparison could be made per extended family (a conservative approach to handling extended families with more than two adult sisters). The model utilized the contrast coding scheme for cousin comparisons explained above (recalculated for the subset) and the interaction of the contrast coding with a variable that identified whether the cousins were half- (coded = 0) or full cousins (coded = 1). The interaction term, therefore, estimated the difference in the magnitudes of the associations between SDP and school grades in the two types of cousin pairs. The parameter associated with the interaction specifically estimated whether cousin type (full vs. half) moderates the association between SDP and AA, providing a statistical test of whether the SDP-AA association was stronger when comparing half cousins than when comparing full cousins (e.g., D’Onofrio et al., 2005). The model also included the main effect of cousin relatedness.
Model 5 explored whether the strength of the association between SDP and school grades varied among full and half siblings. The model was fit to the subset of data that only included two offspring per nuclear family, which permitted one sibling comparison per mother. The sibling comparison contrast code approach described above was used (recalculated on the subset). Plus, the interaction of the contrast code with a variable noting the genetic relatedness of the siblings (0 = half siblings, 1 = full siblings) was included in the model (as well as the main effect of sibling type). The parameter associated with the interaction term assessed whether the SDP-AA association differed between full and half siblings.
Comparison of Fathers of Half Siblings Differentially Exposed to Smoking During Pregnancy
Studying nuclear families that include half siblings (the siblings had different fathers) who were differentially exposed to SDP provides a unique opportunity to examine risk factors associated with SDP. By comparing the characteristics of the two fathers and the pregnancies that are associated with the mothers smoking during pregnancy allows us to examine whether any pregnancy-specific or paternal risk factors were specifically associated with the pregnancy during which the mother smoked. Therefore, regression models predicting prenatal smoking exposure were fit to a sample of offspring (n = 2,773) from families that included siblings with different biological fathers and differential exposure to SDP.
Sensitivity Analyses
A series of analyses examined the robustness of the findings presented in the manuscript and are briefly summarized in the text (full results available upon request).
RESULTS
Association between Smoking During Pregnancy and Risk Factors
Table 1 presents the relation between maternal SDP and each risk factor. The associations indicate that maternal SDP is associated with the covariates at all levels. When exploring each pregnancy, SDP was associated with earlier age at childbearing for parents, shorter gestational age, and a higher likelihood that the mother was not living with a partner at the time of birth.
Table 1.
Association between Maternal Smoking During Pregnancy and Child-Specific, Maternal, Paternal, and Familial Risks
| Risk Factor | b/OR | Confidence Interval |
n |
|---|---|---|---|
| Child-Specific Risksa | |||
| Maternal age at childbirth (years) | b = −1.17* | −1.20 to −1.14 | 654,707 |
| Paternal age at childbirth (years) | b = −0.95* | −0.99 to −0.92 | 654,707 |
| Birth order | b = 0.014* | 0.008 to 0.019 | 654,707 |
| Gestational age (days) | b = −1.15* | −1.22 to −1.08 | 654,707 |
| Year of birth (years) | b = −0.25* | −.026 to −0.24 | 654,707 |
| Not cohabiting | OR = 3.58* | 3.46 to 3.71 | 654,707 |
| Maternal Risksb | |||
| Occupation | |||
| Unskilled blue collar | ref | ref | 181,772 |
| Skilled blue collar | 1.00 | 0.98 to 1.01 | 76,421 |
| Low-level white collar | OR = 0.89* | 0.88 to 0.91 | 94,642 |
| Intermediate-level white collar | OR = 0.39* | 0.38 to 0.40 | 114,688 |
| High-level white collar | OR = 0.36* | 0.34 to 0.37 | 40,467 |
| Self-employed | OR = 0.78* | 0.75 to 0.81 | 16,563 |
| Employed, uncategorized | OR = 1.46* | 1.44 to 1.48 | 43,791 |
| Not working / Missing | OR = 1.39* | 1.36 to 1.41 | 85,262 |
| Educational level | |||
| Less than 9 years of education | ref | ref | 7,751 |
| 9 years of education | OR = 2.69* | 2.64 to 2.75 | 68,264 |
| 1–2 years upper secondary education | OR = 1.67* | 1.65 to 1.69 | 250,267 |
| 3 years upper secondary education | OR = 0.88* | 0.86 to 0.89 | 89,323 |
| Less than 3 years post-secondary education | OR = 0.48* | 0.47 to 0.49 | 112,728 |
| 3+ years post-secondary education | OR = 0.37* | 0.36 to 0.38 | 115,699 |
| Postgraduate education | OR = 0.21* | 0.18 to 0.24 | 3,091 |
| History of criminal conviction | OR = 2.58* | 2.54 to 2.63 | 469,182 |
| Paternal Risksb | |||
| Occupation | |||
| Unskilled blue collar | ref | ref | 135,529 |
| Skilled blue collar | OR = 1.34* | 1.32 to 1.36 | 147,089 |
| Low-level white collar | OR = 0.81* | 0.79 to 0.83 | 55,462 |
| Intermediate-level white collar | OR = 0.56* | 0.55 to 0.58 | 103,552 |
| High-level white collar | OR = 0.38* | 0.37 to 0.39 | 87,224 |
| Self-employed | OR = 0.91* | 0.89 to 0.93 | 46,690 |
| Employed, uncategorized | OR = 1.80* | 1.77 to 1.83 | 30,128 |
| Not working / Missing | OR = 1.82* | 1.78 to 1.86 | 42,507 |
| Educational level | |||
| Less than 9 years of education | ref | ref | 28,812 |
| 9 years of education | OR = 1.77* | 1.74 to 1.80 | 101,413 |
| 1′2 years upper secondary education | OR = 1.52* | 1.50 to 1.54 | 240,474 |
| 3 years upper secondary education | OR = 0.81* | 0.80 to 0.83 | 77,191 |
| Less than 3 years post-secondary education | OR = 0.55* | 0.54 to 0.56 | 83,455 |
| 3+ years post-secondary education | OR = 0.36* | 0.35 to 0.36 | 89,417 |
| Postgraduate education | OR = 0.24* | 0.22 to 0.26 | 8,946 |
| History of criminal conviction | OR = 2.22* | 2.20 to 2.25 | 469,182 |
Note. All estimates accounted for clustering using a sandwich estimator and were based on full maximum likelihood approaches to account for missing data.
Estimates for the child-specific risks are based on maternal smoking during each specific pregnancy for all births.
Estimates are unique for each individual mother and are based on maternal history of ever smoking during any pregnancy. The associations with paternal characteristics are based on the father of the first child per mother.
p < .001.
The association between SDP and maternal, paternal, and familial risks were based on a woman’s history of ever smoking across her pregnancies. SDP was associated with maternal occupational status; compared to women in unskilled blue collar jobs, SDP was associated with lower likelihood of working in white collar jobs. SDP was also associated with greater likelihood of being in jobs that were difficult to categorize or being unemployed. SDP was also associated with maternal highest level of educational achievement; women with a history of SDP were less likely to receive secondary and higher levels of education. Being convicted of a criminal offense was also associated with history of SDP. The same general pattern held for paternal risks. SDP was associated with occupational status, highest educational level achieved, and history of criminal offenses in fathers.
Comparison of Offspring Exposed and Unexposed to SDP Contingent on Biological Relatedness
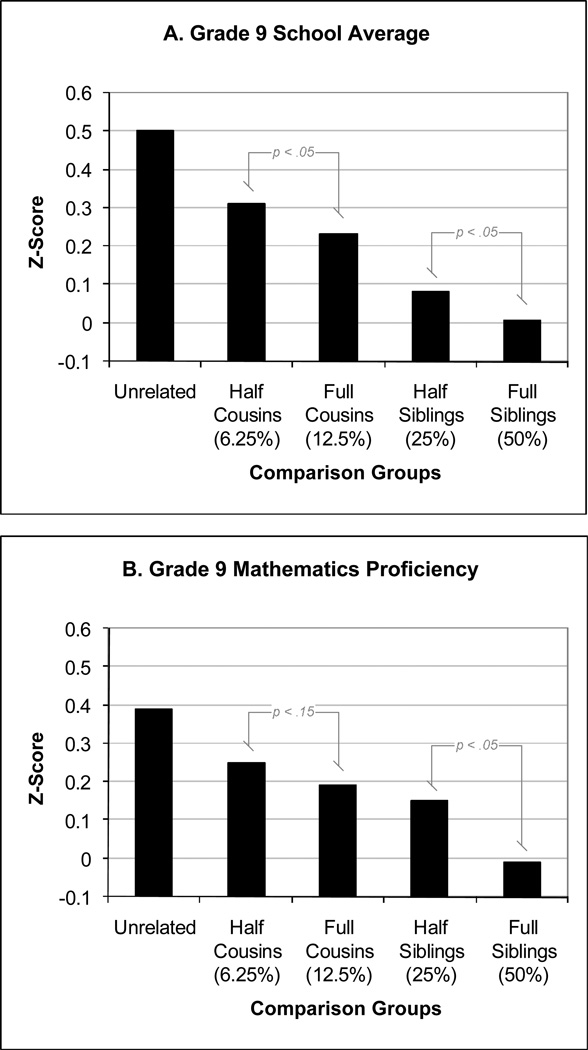
Table 2 presents the mean comparisons of offspring differentially exposed, based on family type. The mean AA scores for offspring from extended and nuclear families with no variation in SDP are presented in Table 6 (in the appendix). The comparison of unrelated offspring suggests a 0.49 standard deviation difference in grades associated with exposure to SDP. The comparison of all cousins differentially exposed to mothers with a history of SDP, regardless of genetic relatedness, suggests the association is attenuated (compared to the study of unrelated individuals), as the difference between the exposed and unexposed was approximately 0.25 standard deviations. The comparison of full cousins was comparable (0.24 standard deviation difference), but the comparison of half cousins yielded a larger difference (0.32 standard deviations), suggesting that the degree of genetic relatedness moderates the association.
Table 2.
Mean offspring outcomes based on exposure to maternal smoking during pregnancy and genetic relatedness to comparison group
| Grades | Math Scores | |||
|---|---|---|---|---|
| SDP | m | n | m | n |
| Unrelated Offspringa | ||||
| Unexposed | 0.19 | 210,858 | 0.14 | 97,286 |
| Exposed | −0.30 | 79,954 | −0.24 | 31,417 |
| Difference (SD units) | 0.49 | 0.38 | ||
| Cousin Comparison (All Cousins)b | ||||
| No history SDP | −0.07 | 30,656 | −0.04 | 13,618 |
| History of SDP | −0.32 | 31,273 | −0.24 | 12,440 |
| Difference (SD units) | 0.25 | 0.20 | ||
| Cousin Comparison (Aunts were Full Siblings, rg = 0.125)b | ||||
| No history SDP | −0.04 | 26,524 | −0.02 | 11,839 |
| History of SDP | −0.28 | 26,794 | −0.22 | 10,667 |
| Difference (SD units) | 0.24 | 0.20 | ||
| Cousin Comparison (Aunts were Half Siblings, rg = 0.0625)b | ||||
| No history SDP | −0.23 | 3,313 | −0.14 | 1,428 |
| History of SDP | −0.55 | 3,351 | −0.41 | 1,322 |
| Difference (SD units) | 0.32 | 0.27 | ||
| Sibling Comparison (All Siblings)c | ||||
| Unexposed | −0.19 | 16,467 | −0.16 | 8,117 |
| Exposed | −0.16 | 16,465 | −0.18 | 5,423 |
| Difference (SD units) | 0.03 | 0.02 | ||
| Sibling Comparison (Full Siblings, rg = 0.50)c | ||||
| Unexposed | −0.16 | 14,675 | −0.15 | 7,356 |
| Exposed | −0.13 | 14,579 | −0.14 | 4,801 |
| Difference (SD units) | 0.03 | 0.02 | ||
| Sibling Comparison (Half Siblings, rg = 0.25)c | ||||
| Unexposed | −0.54 | 1,377 | −0.34 | 583 |
| Exposed | −0.63 | 1,396 | −0.51 | 525 |
| Difference (SD units) | 0.09 | 0.17 | ||
Note.
Based on one grandchild per maternal grandmother. No offspring shared any other first or second degree relatives in the subset.
Based on cousins from two aunts from each extended family.
The comparison of differentially exposed siblings suggests SDP is not associated with lower grades. When all siblings, regardless of genetic relatedness, are compared there was no difference (exposure is associated with a 0.03 standard deviation increase in grades). The comparison of full siblings revealed a similar difference (0.03 standard deviation increase). In contrast, the comparison of half-siblings (−0.09 standard deviations) suggests that SDP is associated with offspring grades when comparing siblings with different fathers.
The same pattern of results was true when comparing the math proficiency scores. The comparison of unrelated individuals revealed a large difference (0.38 standard deviations). The comparison of cousins revealed an attenuated difference that was partially dependent on genetic relatedness (a 0.20 difference in full cousins but a 0.27 difference in half cousins). There was no difference in math proficiency when all siblings were compared (0.02 difference). Similarly, no difference emerged when full siblings were compared (0.02 increase), but the exposure to SDP was associated with poorer math proficiency (0.17 standard deviation difference) in half siblings.
The mean comparisons suggested two main findings. First, for both school grades and math proficiency, the comparison of full siblings differentially exposed to SDP, a comparison that controls for genetic and environmental confounds shared by siblings, revealed no differences. This finding strongly suggests that SDP does not cause lower AA; rather, differences between families associated with SDP appear to be responsible. Second, the association between SDP and AA appears to covary with degree of genetic relatedness. Table 2 illustrates how the difference between the exposed and unexposed offspring depended on genetic relatedness. For both measures of AA, the magnitude of the association between SDP and AA declined as genetic relatedness increased, suggesting that genetic factors help to explain the statistical relation between SDP and AA.
Regression Analyses of the Relations between SDP and Offspring Academic Achievement
School Grades
The results of the regression models for school grades are presented in Table 4. Model 1, an epidemiological model, indicated SDP is associated with a 0.50 standard deviation decrease in school grades (given the large sample size, significance values will only be noted when they are not p < 0.05). Model 2, which statistically controlled for the covariates, indicated that SDP was associated with a 0.21 standard deviation decrease, independent of the effects of the other covariates in the model (Table 7 in the appendix includes the parameter estimates for all of the covariates). The results from Model 3, a model that compared all cousins and siblings differentially exposed, show that SDP is associated with lower school grades when comparing unrelated offspring (b = −0.58). The association is attenuated when comparing cousins (b = −0.26) but is nonexistent when comparing siblings (b = 0.00, p = 0.82).
Table 4.
Regression analyses of the association between Maternal Smoking During Pregnancy and Offspring Grade Average
| Model 1 | Model 2a | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor | b | SE | b | SE | b | SE | b | SE | b | SE |
| Smoking During Pregnancy | ||||||||||
| Unrelated comparison | −0.50 | 0.00 | −0.21 | 0.00 | −0.58 | .00 | −0.67 | 0.01 | −0.56 | 0.00 |
| Cousin comparison | ||||||||||
| All cousins | −0.26 | .01 | ||||||||
| Half cousins | −0.31 | 0.02 | ||||||||
| Full cousin – Half cousin | 0.08 | 0.02 | ||||||||
| Sibling comparison | ||||||||||
| All siblings | 0.00+ | 0.01 | ||||||||
| Half siblings | −0.08 | 0.03 | ||||||||
| Full sibling – Half sibling | 0.08 | 0.03 | ||||||||
| Offspring sex | 0.39 | 0.00 | 0.39 | 0.00 | 0.39 | 0.00 | 0.39 | 0.00 | 0.39 | 0.00 |
| Birth order | −0.14 | 0.00 | −0.12 | 0.00 | −0.10 | 0.00 | −0.10 | 0.00 | ||
| Aunts are full siblings | 0.24 | 0.01 | ||||||||
| Siblings are full fiblings | 0.45 | 0.01 | ||||||||
| Number of offspring | 654,707 | 654,707 | 654,707 | 181,099 | 327,161 | |||||
The results of the Model 4, a model that assessed whether cousin type moderated the association between SDP and AA, suggest that SDP exposure is more strongly associated with school grades when comparing half cousins (b = −0.31) than when comparing full cousins (b = −0.23 = −0.31 + 0.08). It is important to note that the difference (b = 0.08) was statistically significant, indicating that cousin type moderates the association between SDP and school grades.
Model 5 examined whether the association between SDP and AA was stronger in half siblings than in full siblings. The model indicated that the half siblings who were differentially exposed to SDP differed in their school grades (b = −0.08), whereas full siblings differentially exposed did not differ (b = 0.00 = −0.08 + 0.08). The difference was statistically significant.
Figure 1 (Panel A) presents the estimates of the association between SDP and school grades separately for the different comparison groups (unrelated offspring, half cousins, full cousins, half siblings, and full siblings). The figure illustrates how the association between SDP and school grades is dependent on the genetic relatedness of the relatives used in the comparisons.
Figure 1.
Associations between Smoking During Pregnancy and Offspring Academic Achievement Based on Degree of Genetic Relatedness
Note. Statistical tests of the differences among the cousin and sibling groups are based on Models 4 and 5, respectively.
Mathematics Proficiency
Model 1 indicated that offspring exposed to SDP performed less well on a standardized mathematics test (b = −0.39 standard deviations). The association (b = −0.15) was attenuated when statistically controlling for measured risk factors in Model 2 (See Table 8 in the appendix). Model 3 indicated that the association between SDP and math scores was smaller when comparing cousins (b = −0.20) than unrelated individuals, and there was no association when siblings were compared (b = 0.00, p = 0.94). Model 4, which explored whether the magnitude of the cousin comparison was dependent on the type of cousins suggested that the association between SDP and mathematics proficiency was stronger when comparing half cousins (b = −0.25) than full cousins (b = −0.19 = −0.25 + 0.06), although the difference between the two cousin types could not be estimated precisely (p = 0.14). Model 5, however, indicated that the association between SDP and math scores is stronger (and statistically different) in half siblings (b = −0.15) than full siblings (b = 0.01 = −0.15 + 0.16). Figure 1 (Panel B) shows how the association between SDP and math scores varies across the types of relatives.
Comparison of Fathers of Half Siblings Differentially Exposed to Smoking During Pregnancy
The model fitting described above suggest that SDP is associated with slightly poorer grades and math scores when comparing siblings who have different fathers. Since half- and full siblings are equally genetically related to their mothers, the differences in association between SDP and AA for half- and full siblings may be due to a genetic correlation between fathers and mothers. A series of regression analyses were conducted to examine which, if any, child-specific and paternal risks factors were associated with the pregnancy during which the mother smoked when the siblings were (a) differentially exposed to SDP and (b) had different fathers (n = 2,773 births). Birth order, maternal and parental age at childbearing, paternal education, and paternal occupational status were not associated with the pregnancy during which the mother smoked. However, the father of the child who was exposed to SDP was more likely to have a history of criminality (OR = 1.40, CI = 1.19 to 1.62, p < 0.05) and the mother was more likely not to be living with any partner at the time of the birth (OR = 2.05, CI = 1.60 to 2.63, p < 0.05).
Sensitivity Analyses
A number of additional analyses were performed to test the validity of the findings. Analyses of birth weight were completed to replicate previous research findings. When comparing unrelated individuals, SDP was associated with a 205 gram decrease in birth weight. The comparison of siblings differentially exposed indicated SDP was associated with a 101 gram decrease. The difference in magnitude of the effect when comparing half and full siblings (20 grams) was not statistically significant (p = 0.32). Since no moderation by genetic relatedness was found, the results strongly suggest that SDP has a specific effect on offspring birth weight.
Results of regression models predicting dichotomous measure AA, a measure of school grades (below passing) using the same cutoff as previous studies (Lambe et al., 2006) and predicting failing mathematics (goals not achieved), were comparable to those reported in the current manuscript (data not shown). Results of analyses using dummy codes to compare moderate smoking and heavy smoking to non-smokers revealed comparable findings to the results of considering SDP as present versus absent.
DISCUSSION
The current study combined two general quasi-experimental approaches to study the mechanisms through which SDP influences offspring AA. The study relied on the historical decline in SDP, due in part to public health campaigns to deter pregnant women and women of childbearing ages from smoking (Cnattingius, 2004), to explore whether variations in SDP by the same mother were associated with differences in her offspring’s AA. The study also utilized a large family study, including a mixture of sibling and cousin types, to compare relatives who differ in their exposure to SDP and who vary in the degree to which they share genetic risk associated with SDP. By combining these two approaches, the current study sought to pull apart the co-occurring genetic and environmental risks associated with SDP.
The results provide support for two main conclusions. First and foremost, the results strongly suggest that SDP does not cause offspring to have lower AA. We compared full siblings who were differentially exposed to SDP, an approach that accounts for the genetic factors and environmental factors that siblings share (Lahey et al., in press; Rutter, 2007). If SDP causes offspring to have lower AA, perhaps through the neurotoxic effects of exposure to prenatal nicotine, then a child exposed to SDP would be expected to have poorer AA than his/her sibling who was not exposed. For both school grades and mathematics proficiency, however, full siblings differentially exposed to SDP did not differ in their levels of AA. The results strongly suggest that familial risks correlated with SDP, and not the specific effects of SDP, are responsible for the lower AA found in offspring whose mothers smoked during pregnancy. The findings are consistent with previous research on the role of SDP and AA in Sweden (Lambe et al., 2006), as well as a sibling comparison study of conduct problems in the U.S. (D’Onofrio et al., 2008) and a children of twins study of ADHD in Australia (Knopik et al., 2005). The results are also consistent with traditional family studies that used extensive covariates (Batty et al., 2006; Fergusson & Lloyd, 1991). These designs, however, do not explain why SDP is associated with lower levels of AA in the population.
The second conclusion involves the underlying processes by which SDP is associated with AA. The results suggest that genetic factors passed from parents to their offspring (passive gene-environment correlation) account for at least part of the statistical association. Genetic factors are implicated because the degree to which relatives were genetically correlated moderated the association between SDP and AA when comparing types of cousins and types of siblings. Models 4 and 5, in fact, provided statistical tests of these differences. When relatives were more genetically similar (full cousins versus half cousins and full siblings versus half siblings), the association between SDP and AA was weaker. Stated differently, when genetic factors were more controlled (by comparing relatives who shared more genetic factors), the association between SDP and AA went down. Figure 1 graphically illustrates this finding. It is important to note that the findings were consistent across levels of analysis (i.e. the comparisons of half versus full cousins were similar to those comparing full and half siblings) and measures of AA, which provides converging evidence for the confounding role of genetic factors.
We stress that the results do not prove that the underlying causal mechanisms are genetic—the findings only suggest that genetic factors confound the association (i.e., the results are consistent with the role of genetic confounds). Half cousins and full cousins differ in their genetic relatedness, but they also may differ on environmental risks. Full cousins may see each other (and their aunts) more often than half cousins, which could make full cousins more similar if the level of contact influenced AA—the equal environments assumption one generation removed (D’Onofrio et al., 2003). Offspring of half siblings may also be exposed to more environmental risk than offspring of full siblings, which could influence the variability in AA. Similarly, there may be differences in the variability of AA between half and full siblings. These limitations, which apply to all step-family behavioral genetic designs (e.g., Reiss, Neiderhiser, Hetherington, & Plomin, 2000), need to be tested in future studies. Using the Children of Twins Design would help test these assumptions. Studies of adult twins frequently include measures of contact between the twins (and their families), which could help account for differences in levels of family contact. Differences in genetic risk among cousins also would not be based on comparing offspring from intact families to those from divorced/separated parents; rather, differences in genetic relatedness among cousins in the Children of Twins design are due to the zygosity type of the adult twins.
The comparison of fathers of half siblings discordant for exposure to prenatal smoking indicated that the father of the child exposed to SDP more often had a history of criminal convictions. Exposure to prenatal smoking, thus, covaries with paternal risk of criminality, even within-families. For the paternal phenotype to influence a genetic association between mother and offspring, the paternal phenotype has to be genetically correlated with the maternal phenotype. It is well-known that there is substantial assortative mating for antisocial behavior (e.g., Krueger, Moffitt, Caspi, Bleske, & Silva, 1998), and in the current sample women with a history of a criminal conviction were much more likely to have children with men with convictions (OR = 3.38, CI = 3.32 – 3.46). Thus, the pattern of associations between SDP and AA for half- and full siblings is congruent with genetic confounding, even though environmental explanations cannot be excluded. Additional research is required to further delineate the underlying causal mechanisms.
The fact that SDP is associated with lower AA in half siblings provides compelling evidence that researchers must explore the role of fathers when studying the putative effects of SDP. Although most research on SDP has not included measured characteristics of the fathers (review in Maughan et al., 2004), the current results indicate that characteristics of fathers, whether due to genetic risk passed down to the children or psychosocial risk factors, help explain some of the association between SDP and offspring AA.
In addition to the ability to use quasi-experimental approaches to study the risks associated with SDP, the current study also benefits from a number of key strengths. The study was conducted on a large, national sample of offspring. The analyses also included two measures of AA, school grades and mathematics proficiency. The inclusion of the mathematics scores greatly helps the interpretation of the data because the assessment was standardized across the entire study. School grades could vary substantially by schools or communities. The fact that both measures of AA provide similar results provides converging evidence for the conclusions drawn. The large sample size also permits the comparison of unique types of relatives (e.g., half siblings differentially exposed to SDP) that would be hard to find using traditional samples. SDP was also assessed during pregnancy rather than relying on maternal retrospective reports, a key limitation of some of the previous sibling comparison tests of SDP (Rutter, 2007). The analyses were also able to include measured covariates of both mothers and fathers. The exclusion of paternal information is a key limitation of previous research on SDP (Maughan et al., 2004).
There are also a number of limitations of the current study. The measure of SDP was based on maternal report, usually during the beginning of her pregnancy. Exposure to smoking later in life was also not available. Therefore, the study cannot explore whether the timing of the exposure was critical for later AA. The study could also not explore the importance of paternal smoking during pregnancy. The analyses also did not study whether individual or familial factors moderate the association between SDP and offspring AA. Research has suggested that factors, such as offspring sex and birth complications (Brennan, Grekin, & Mednick, 1999), interact with SDP to substantially increase risk for poor offspring adjustment. The comparison of siblings differentially exposed relies on assumptions that mothers who vary their smoking across pregnancies are comparable to those who smoked during every pregnancy (D’Onofrio et al., 2008). Furthermore, the analyses did not estimate the degree to which environmental and genetic confounds explain the association between SDP and AA. The results imply that genetic factors (and environmental confounds) are important. We are currently working on the analytical models required to specifically quantify the role of genetic and environmental factors when studying exposure to SDP with the various types of relative pairs available. More research, therefore, is needed to understand the causal process responsible for the association between SDP and poor offspring AA.
Overall, the study illustrates the importance of quasi-experimental approaches to studying putative environmental risk factors (Moffitt, 2005; Rutter, 2007). Similar to the two previous sibling comparison studies of SDP (D’Onofrio et al., 2008; Lambe et al., 2006), we would have drawn the wrong conclusion about the role of SDP if we only relied on the statistical covariates to account for confounds. Even though the regression analyses on the epidemiological sample controlled for measures of maternal and paternal traits, we would have wrongly concluded that SDP had an independent association with AA after controlling for maternal and paternal characteristics, consistent with a causal inference.
Finally, it is important to emphasize that the current results do not suggest that SDP has no impact on offspring adjustment. The results are limited to measures of AA at age 15 only. The findings for birth weight suggest, as have previous genetically informed (D’Onofrio et al., 2003) and other quasi-experimental studies (Cnattingius, 2004), that SDP has a specific effect on offspring birth weight that is environmentally mediated. SDP appears to impact particular outcomes more strongly than others, particularly poor pregnancy outcomes (Cnattingius, 2004). Reducing SDP, therefore, remains a major public health issue.
Table 5.
Regression analyses of the association between Maternal Smoking During Pregnancy and Offspring Grade 9 Mathematics Proficiency
| Model 1 | Model 2a | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor | b | SE | b | SE | b | SE | b | SE | b | SE |
| Smoking During Pregnancy | ||||||||||
| Unrelated comparison | −0.39 | 0.01 | −0.15 | 0.01 | −0.45 | 0.00 | −0.52 | 0.00 | −0.44 | 0.01 |
| Cousin comparison | ||||||||||
| All cousins | −0.20 | 0.01 | ||||||||
| Half cousins | −0.25 | 0.04 | ||||||||
| Full cousin – Half cousin | 0.06+ | 0.03 | ||||||||
| Sibling comparison | ||||||||||
| All siblings | 0.00+ | 0.02 | ||||||||
| Half siblings | −0.15 | 0.06 | ||||||||
| Full sibling – Half sibling | 0.16 | 0.06 | ||||||||
| Offspring sex | 0.02 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 |
| Birth order | −0.11 | 0.00 | −0.08 | 0.00 | −0.08 | 0.00 | −0.10 | 0.00 | ||
| Aunts are full siblings | 0.18 | 0.01 | ||||||||
| Siblings are full fiblings | 0.30 | 0.01 | ||||||||
| Number of offspring | 283,938 | 283,938 | 283,938 | 77,622 | 142,559 | |||||
Acknowledgement
Funding for the study was provided by grants from the Indiana University Faculty Research Support Program, NARSAD, and the Swedish Research Council.
Appendix
Figure 2.
Explanation of Linkage of National Registries and Creation of Subsets for Analyses
Table 6.
Mean offspring outcomes based on exposure to maternal smoking during pregnancy and genetic relatedness to comparison group (including offspring of concordant aunts and siblings concordant for exposure to SDP)
| Grades | Math Scores | |||
|---|---|---|---|---|
| SDP | m | n | m | n |
| Unrelated Offspringa | ||||
| Unexposed | 0.19 | 210,858 | 0.14 | 97,286 |
| Exposed | −0.30 | 79,954 | −0.24 | 31,417 |
| Cousin Comparison (All Cousins)b | ||||
| Concordant – No history SDP | 0.20 | 90,167 | 0.17 | 40,357 |
| Discordant - No history SDP | −0.07 | 30,656 | −0.04 | 13,618 |
| Discordant - History of SDP | −0.32 | 31,273 | −0.24 | 12,440 |
| Concordant – History of SDP | −0.48 | 29,003 | −0.36 | 11,207 |
| Cousin Comparison (Aunts were Full Siblings, rg = 0.125) b | ||||
| Concordant – No history SDP | 0.22 | 85.985 | 0.18 | 38,550 |
| Discordant - No history SDP | −0.04 | 26,524 | −0.02 | 11,839 |
| Discordant - History of SDP | −0.28 | 26,794 | −0.22 | 10,667 |
| Concordant – History of SDP | −0.45 | 25,280 | −0.34 | 9,771 |
| Cousin Comparison (Aunts were Half Siblings, rg = 0.0625) b | ||||
| Concordant – No history SDP | −0.10 | 4,182 | −0.08 | 1,807 |
| Discordant - No history SDP | −0.23 | 3,313 | −0.14 | 1,428 |
| Discordant - History of SDP | −0.55 | 3,351 | −0.41 | 1,322 |
| Concordant – History of SDP | −0.64 | 3,723 | −0.47 | 1,436 |
| Sibling Comparison (All Siblings)c | ||||
| Concordant - Unexposed | 0.19 | 225,645 | 0.14 | 101881 |
| Discordant - Unexposed | −0.19 | 16,467 | −0.16 | 8,117 |
| Discordant - Exposed | −0.16 | 16,465 | −0.18 | 5,423 |
| Concordant - Exposed | 0.41 | 68,584 | −0.33 | 27,138 |
| Sibling Comparison (Full Siblings, rg = 0.50)c | ||||
| Concordant - Unexposed | −0.20 | 219,716 | 0.15 | 99,354 |
| Discordant - Unexposed | −0.16 | 14,675 | −0.15 | 7,356 |
| Discordant - Exposed | −0.12 | 14,579 | −0.14 | 4,801 |
| Concordant - Exposed | −0.37 | 61,041 | −0.30 | 24,262 |
| Sibling Comparison (Half Siblings, rg = 0.25) c | ||||
| Concordant - Unexposed | −0.36 | 5,929 | −0.26 | 2,527 |
| Discordant - Unexposed | −0.54 | 1,377 | −0.34 | 583 |
| Discordant - Exposed | −0.63 | 1,396 | −0.51 | 525 |
| Concordant - Exposed | −0.76 | 7,543 | −0.56 | 2,876 |
Table 7.
Full Results for Model 2 for Average Grades
| Risk Factor | b | se | p |
|---|---|---|---|
| SDP | −0.201 | 0.004 | 0.000 |
| Maternal age at childbirth (years) | 0.007 | 0.000 | 0.000 |
| Paternal age at childbirth (years) | 0.000 | 0.000 | 0.693 |
| Gestational age (days) | 0.000 | 0.000 | 0.010 |
| Year of birth (years) | 0.021 | 0.001 | 0.000 |
| Not cohabiting | 0.158 | 0.012 | 0.000 |
| Offspring Sex | 0.393 | 0.003 | 0.000 |
| Maternal less than 9 years of education | - | - | - |
| 9 years of education | 0.048 | 0.015 | 0.002 |
| 1–2 years upper secondary education | 0.206 | 0.015 | 0.000 |
| 3 years upper secondary education | 0.345 | 0.015 | 0.000 |
| Less than 3 years post-secondary education | 0.440 | 0.015 | 0.000 |
| 3+ years post-secondary education | 0.529 | 0.015 | 0.000 |
| Postgraduate education | 0.663 | 0.027 | 0.000 |
| Maternal unskilled blue collar | - | - | - |
| Skilled blue collar | 0.109 | 0.005 | 0.000 |
| Low-level white collar | 0.197 | 0.005 | 0.000 |
| Intermediate-level white collar | 0.200 | 0.006 | 0.000 |
| High-level white collar | 0.289 | 0.008 | 0.000 |
| Self-employed | 0.188 | 0.011 | 0.000 |
| Employed, uncategorized | 0.047 | 0.007 | 0.000 |
| Not working / Missing | 0.031 | 0.008 | 0.000 |
| Maternal History of criminal conviction | −0.176 | 0.005 | 0.000 |
| Paternal less than 9 years of education | |||
| 9 years of education | 0.026 | 0.009 | 0.003 |
| 1–2 years upper secondary education | 0.100 | 0.008 | 0.000 |
| 3 years upper secondary education | 0.266 | 0.009 | 0.000 |
| Less than 3 years post-secondary education | 0.325 | 0.009 | 0.000 |
| 3+ years post-secondary education | 0.440 | 0.010 | 0.000 |
| Postgraduate education | 0.593 | 0.016 | 0.000 |
| Paternal unskilled blue collar | |||
| Skilled blue collar | 0.044 | 0.005 | 0.000 |
| Low-level white collar | 0.116 | 0.006 | 0.000 |
| Intermediate-level white collar | 0.146 | 0.006 | 0.000 |
| High-level white collar | 0.194 | 0.007 | 0.000 |
| Self-employed | 0.137 | 0.007 | 0.000 |
| Employed, uncategorized | 0.011 | 0.008 | 0.191 |
| Not working / Missing | 0.046 | 0.010 | 0.000 |
| Paternal History of criminal conviction | −0.164 | 0.003 | 0.000 |
Table 8.
Full Results for Model 2 for Mathematics Proficiency
| Risk Factor | b | se | p |
|---|---|---|---|
| SDP | −0.148 | 0.006 | 0.000 |
| Maternal age at childbirth (years) | 0.012 | 0.001 | 0.000 |
| Paternal age at childbirth (years) | 0.001 | 0.001 | 0.200 |
| Gestational age (days) | 0.001 | 0.000 | 0.000 |
| Year of birth (years) | −0.035 | 0.002 | 0.000 |
| Not cohabiting | 0.100 | 0.018 | 0.000 |
| Offspring Sex | 0.034 | 0.005 | 0.000 |
| Maternal less than 9 years of education | - | - | - |
| 9 years of education | −0.019 | 0.031 | 0.536 |
| 1–2 years upper secondary education | 0.080 | 0.030 | 0.007 |
| 3 years upper secondary education | 0.192 | 0.030 | 0.000 |
| Less than 3 years post-secondary education | 0.296 | 0.030 | 0.000 |
| 3+ years post-secondary education | 0.395 | 0.031 | 0.000 |
| Postgraduate education | 0.498 | 0.045 | 0.000 |
| Maternal unskilled blue collar | - | - | - |
| Skilled blue collar | 0.061 | 0.008 | 0.000 |
| Low-level white collar | 0.127 | 0.008 | 0.000 |
| Intermediate-level white collar | 0.135 | 0.008 | 0.000 |
| High-level white collar | 0.226 | 0.012 | 0.000 |
| Self-employed | 0.159 | 0.017 | 0.000 |
| Employed, uncategorized | 0.046 | 0.010 | 0.000 |
| Not working / Missing | 0.005 | 0.011 | 0.611 |
| Maternal History of criminal conviction | −0.106 | 0.008 | 0.000 |
| Paternal less than 9 years of education | |||
| 9 years of education | 0.038 | 0.015 | 0.011 |
| 1–2 years upper secondary education | 0.095 | 0.014 | 0.000 |
| 3 years upper secondary education | 0.248 | 0.015 | 0.000 |
| Less than 3 years post-secondary education | 0.323 | 0.016 | 0.000 |
| 3+ years post-secondary education | 0.435 | 0.016 | 0.000 |
| Postgraduate education | 0.597 | 0.026 | 0.000 |
| Paternal unskilled blue collar | - | - | - |
| Skilled blue collar | 0.027 | 0.007 | 0.000 |
| Low-level white collar | 0.086 | 0.009 | 0.000 |
| Intermediate-level white collar | 0.131 | 0.008 | 0.000 |
| High-level white collar | 0.183 | 0.010 | 0.000 |
| Self-employed | 0.129 | 0.011 | 0.000 |
| Employed, uncategorized | 0.038 | 0.012 | 0.001 |
| Not working / Missing | −0.010 | 0.014 | 0.505 |
| Paternal History of criminal conviction | −0.096 | 0.005 | 0.000 |
Footnotes
Preliminary analyses were presented at the Behavior Genetics Association Conference (June 2007, Amsterdam).
References
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, et al. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine and Tobacco Research. 2008;10:567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring's cognitive ability: Empirical evidence for complete confounding in the US National Longitudinal Survey of Youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mednick SA. Maternal smoking during pregnancy and adult male criminal outcomes. Archives of General Psychiatry. 1999;56:215–219. doi: 10.1001/archpsyc.56.3.215. [DOI] [PubMed] [Google Scholar]
- Butler N, Goldstein H. Smoking in pregnancy an subsequent child development. British Medical Journal. 1973;4:573–575. doi: 10.1136/bmj.4.5892.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Fetal programming: Prenatal origins of health and illness. Current Directions in Psychological Science. 2008;17:36–41. [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Godschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental and Behavioral Pediatrics. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Eaves LJ, Corey LA, Berg K, Solaas MH, et al. The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer EN, Emery RE, Slutske W, Heath A, Madden PAF, et al. A genetically informed study of marital instability and its association with offspring psychopathology. Journal of Abnormal Psychology. 2005;114:570–586. doi: 10.1037/0021-843X.114.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, et al. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- Fergusson DM. Prenatal smoking and antisocial behavior. Archives of General Psychiatry. 1999;56:223–224. doi: 10.1001/archpsyc.56.3.223. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lloyd M. Smoking during pregnancy and its effects on child cognitive ability from teh ages of 8 to 12 years. Paediatric and Perinatal Epidemiology. 1991;5:189–200. doi: 10.1111/j.1365-3016.1991.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Fogelman K, Manor O. Smoking in pregnancy and subsequent development into early adulthood. British Medical Journal. 1988;297:1233–1236. doi: 10.1136/bmj.297.6658.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WH. Econometric Analysis. 5th ed. Upper Saddle River, NJ: Prentice-Hall; 2003. [Google Scholar]
- Harden KP, Lynch SK, Turkheimer E, Emery RE, D’Onofrio BM, Slutske WS, et al. A behavior genetic investigation of adolescent motherhood and offspring mental health problems. Journal of Abnormal Psychology. 2007;116:667–683. doi: 10.1037/0021-843X.116.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: Informativeness of different relationships. Behavior Genetics. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PAF, Bucholz KK, Hudziak JJ, Reich W, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychological Medicine. 2005;35:625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Moffitt TE, Caspi A, Bleske A, Silva PA. Assortative mating for antisocial behavior: Developmental and methodological implications. Behavior Genetics. 1998;28:173–186. doi: 10.1023/a:1021419013124. [DOI] [PubMed] [Google Scholar]
- Lahey BB, D’Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2008.01980.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Pedersen NL, McClearn GE. The origins of individiual differences in occupational status and educational level. Acta Sociologica. 1992;35:13–31. [Google Scholar]
- Lindmark G, Cnattingius S. The scientific basis of antenatal care. Report from a state-of-the-art conference. Acta Obstetricia et Gynecologica Scandinavica. 1991;70:105–109. doi: 10.3109/00016349109006190. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: Gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s Guide. Fifth Edition. Los Angeles, CA: Muthén and Muthén; 1998–2007. [Google Scholar]
- Neuhaus JM, McCulloch CE. Separating between- and within-cluster covariate effects by using conditional and partitioning methods. Journal of the Royal Statistical Society. 2006;68:859–872. [Google Scholar]
- Rantakallio P. A follow-up study up to the age of 14 of children whose mothers smoked during pregnancy. Acta Paediatrica Scandinavica. 1983;72:747–753. doi: 10.1111/j.1651-2227.1983.tb09805.x. [DOI] [PubMed] [Google Scholar]
- Reiss D, Neiderhiser JM, Hetherington EM, Plomin R. The relationship code: Deciphering genetic and social patterns in adolescent development. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Rodgers JL, Cleveland H, van den Oord E, Rowe D. Resolving the debate over birth order, family size, and intelligence. American Psychologist. 2000;55:599–612. doi: 10.1037//0003-066x.55.6.599. [DOI] [PubMed] [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Silberg JL, Eaves LJ. Analyzing the contribution of genes and parent-child interaction to childhood behavioral and emotional problems: A model for the children of twins. Psychological Medicine. 2004;34:347–356. doi: 10.1017/s0033291703008948. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys' conduct disturbance: An examination of the causal hypothesis. Biological Psychiatry. 2003;53:130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Statistics_Sweden. Swedish socioeconomic classification: Reports on statistical coordination. Sweden: Orebro; 1982. [Google Scholar]
- Statistics_Sweden. Multi-Generation Register 2002, A description of contents and quality. Sweden: Örebro; 2003. [Google Scholar]
- Swedish_Centre_for_Epidemiology. The Swedish Medical Birth Registry: A summary of content and quality. 2001. Retrieved. from http://www.sos.se/fulltext/112/2003-112-3/summary.htm. [Google Scholar]
- Swedish_National_Agency_for_Education. from http://www.skolverket.se/ [Google Scholar]
- van den Oord EJ, Simonoff E, Eaves LJ, Pickles A, Silberg J, Maes H. An evaluation of different approaches for behavior genetic analyses with psychiatric symptom scores. Behavior Genetics. 2000;30:1–18. doi: 10.1023/a:1002095608946. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. American Journal of Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]