Abstract
Blood- and marrow-derived stem cells (BMDSCs) provide disease-ameliorating effects for cardiovascular and autoimmune diseases. Microchimerism from donor BMDSCs has been reported in several recipient tissues. We hypothesized that this finding suggests a potential use of BMDSCs in the treatment of salivary dysfunctions. We investigated the presence of Y chromosome-positive cells in salivary gland biopsies of 5 females who had received a marrow or blood stem cell transplant from male donors. One to 16 years after transplantation, all recipients exhibited scattered Y chromosome-positive cells in the acini, ducts, and stroma of their salivary glands (mean of 1.01%). Potentially, these cells can be markers of transplantation tolerance, contribute to neoplastic epithelial tissues, or engraft at sites of injury. In addition, transplantation of BMDSCs could be used for treatment of Sjögren’s syndrome and salivary glands damaged by therapeutic irradiation for cancers of the head and neck.
Keywords: Salivary glands, Microchimerism, Bone marrow stem cells, Blood stem cells, Transplantation
INTRODUCTION
Recent reviews suggest transplantation of blood- and marrow-derived stem cells (BMDSCs) provide disease-ameliorating effects for cardiovascular and autoimmune diseases [1,2]. Microchimerism arising from BMDSCs and organ transplantations has been reported in a variety of recipient tissues (heart, liver, kidney, gastrointestinal [GI] tract, lung, endometrium, buccal epithelium) [3–12]. We hypothesized that this phenomenon has implications for the potential use of BMDSCs in the treatment of salivary dysfunctions (eg, Sjögren’s syndrome and salivary glands damaged by irradiation) for which no suitable conventional treatments are currently available. Here we report, for the first time, evidence of microchimerism resulting from BMDSCs in salivary glands of recipients.
METHODS
This study was approved by the institutional review boards at McGill University, National Institutes of Health, and Veterans Affairs Medical Center. Labial salivary gland tissue was collected from 5 female subjects who had previously received either an allogeneic bone marrow or peripheral blood stem cell transplant from their brothers (Table 1).This gender-mismatched strategy allowed us to detect donor Y chromosomes in cells of the female-recipient salivary tissue. At the time of salivary gland biopsy, all patients were well, in hematologic remission, with full donor lymphohematopoietic engraftment. We chose to biopsy minor labial salivary glands because they share abundant similarities with the major salivary glands (ie, parotid, submandibular, and sublingual glands), they are routinely used to obtain information about all salivary tissue in patients with Sjögren’s syndrome and they can be obtained with little discomfort and morbidity compared to biopsies from the major glands [13]. We performed colocalization techniques to genetic and protein markers as we described previously [10]. Using fluorescence microscopy on 10-µm-thick frozen salivary tissue sections: (1) cells from the male donors were identified using fluorescence in situ hybridization (FISH) using a human Y chromosome probe conjugated with digoxigenin and visualized with Tyramide-FITC, and in the same sections, (2) salivary epithelial cells were identified by the presence of epithelial markers (cytokeratins; CK) using fluorescence immunohistochemistry (FIHC). The CK antibodies (BioGenex, San Ramon, CA) stain the salivary gland parenchymal cellswhile leaving nonepithelial cells (such as endothelial cells, stromal cells, and blood cells) unstained. We further determined whether male donor BMDSCs had fused with female recipient cells using FISH with X and Y chromosomal probes (Vysis, Downers Grove, IL). The sensitivity and specificity of the Y and X chromosomal probes used was between 97% and 100% [10]. As negative and positive controls, the DNA probes and antibodies were tested on labial salivary glands of normal healthy male and female volunteers.
Table 1.
Characteristics of the Female Transplant Recipients
| #1 | #2 | Recipient #3 | #4 | #5 | |
|---|---|---|---|---|---|
| Characteristic | |||||
| Age at transplant (years) | 31 | 36 | 37 | 52 | 28 |
| Reason for transplant | CML chronic phase | MDS (RAEB) | AML CR1 | Aplastic anemia | CML chronic phase |
| Type of transplant | BM | PBSC | PBSC | PBSC | BM |
| Conditioning | Cy 120 | Cy 120 | Cy 120 | Cy 120, Flu 125, | Cy 120, AraC 500 |
| TBI 13 Gy | TBI 12 Gy | TBI 12 Gy | ATG 120 | TBI 5.5 Gy | |
| Time from transplant to salivary gland biopsy | 57 months | 50 months | 40 months | 13 months | 201 months |
| History of pregnancy | Never | Never | 1 daughter 2 miscarriages |
1 daughter 1 son |
Never |
| History of blood transfusion | Never | Yes | Yes | Yes | Yes |
| GVHD | COP | None | Liver | Skin, oral | Skin, oral |
| Active at time of biopsy | no | — | no | yes | yes |
| % of positive male cells in salivary gland | 1.09% | 0.95% | 0.65% | 0.92% | 1.44% |
| % of positive male cells in buccal mucosa | 11.3% | 2.4% | 12.7% | n/a | n/a |
AML CR1 indicates acute myelogenous leukemia in first complete remission; AraC, Cytarabine 500 mg/m2; ATG, antithymocyte globulin 120 mg/kg; BM, bone marrow transplant; CML, chronic myelogenous leukemia; COP, cryptogenic organizing pneumonia; Cy, Cyclophosphamide 120 mg/kg; Flu, Fludarabine 125 mg/m2; MDS (RAEB), myelodysplastic syndrome with excess of blasts; PBSC, peripheral blood stem cell transplant; TBI, total-body irradiation; GVHD, graft-versus-host disease.
RESULTS AND DISCUSSION
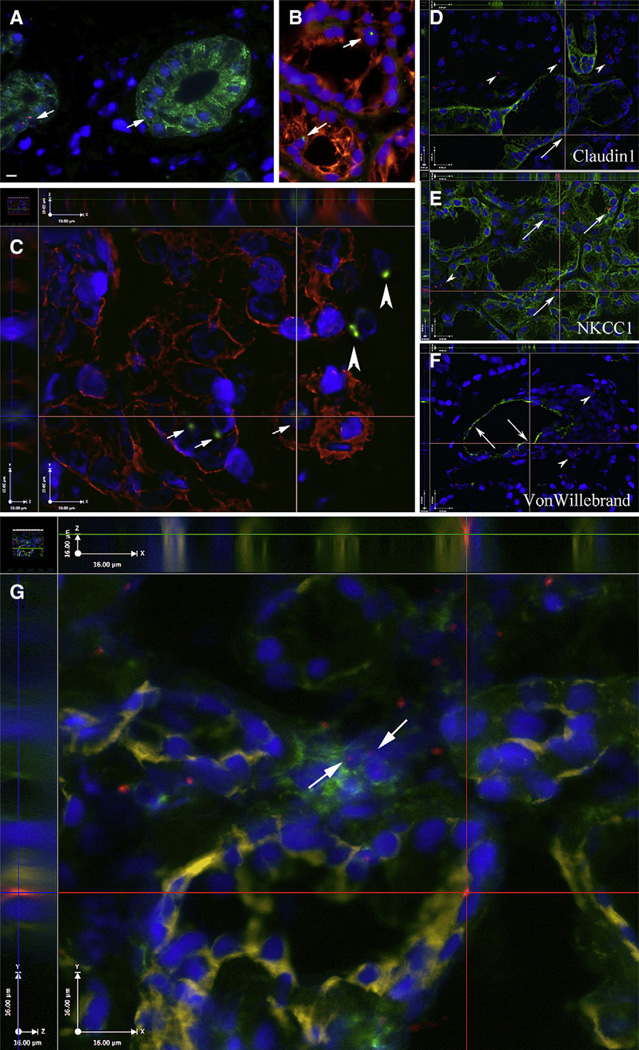
One to 16 years after male-to-female BMDSC transplantation, all 5 female recipients had Y chromosome-positive salivary cells (mean of 1.01%, range: 0.65%–1.44%) in the gland parenchymal tissue (Table 1). Our Y chromosome probe used in FISH has a false-positive rate between 0% to 0.2%, and a false-negative rate between 1.5% to 3.1% (ie, we were using a Y chromosome probe that underestimated the true frequency of Y chromosome-positive cells, but was unlikely to overestimate it). These Y chromosome-positive cells expressed CK-8, -9, -13, -18 (markers for salivary cells) (Figure 1A–C). Many of these cells were identified in each of the parenchymal components of the glands, that is, acini and intercalated, striated, and excretory ducts. We also used the following markers to further characterize the phenotypes of the Y chromosome-positive cells (Figure 1D–F): Na+-K+-2Cl− cotransporter (NKCC1, a marker for acini; donated by R.J. Turner), claudin-1 (a tight junction protein in salivary ducts; Zymed, San Francisco, CA), and von Willebrand factor IV (a marker for endothelial cells; Novocastra, Newcastle, UK). Our salivary gland specimens had few foci of lymphocytic infiltration. However, in situations where these lymphocytic infiltrations were detected by H&E staining in proximity to salivary cells, we immunostained an additional slide combining a hematopoietic lineage (CD45) marker, an epithelial marker (NKCC1) and the Y chromosome probe. This modification of the technique described earlier [14] using different fluorescent colors, showed scattered Y+/CD45+ cells in the stroma, but these were exceedingly rare in the epithelial structures (Figure 1G). Therefore, we conclude that the vast majority of the Y chromosome-positive cells in the intercalated ducts and acini of our specimens were of epithelial phenotype. Inmore than 1000 cells examined per patient, we detected no XXXY-positive cells (no cell fusion events).Wepreviously reported in our studies of buccal epithelial cells in a female recipient that DNA genotyping had excluded the possibility that all observed Y chromosome-positive cells were transferred in utero by her son during pregnancy (a process referred to as fetal microchimerism) [10].
Figure 1.
Labial salivary gland biopsies from female recipients of male BMDSCs. (A) Salivary cells are stained with the epithelial marker cytokeratin 13 (green). Y chromosome-positive cells (nuclei with red fluorescent dots, shown by the arrows) reside in an intercalated duct (left) and a striated duct (right). (B, C) Cytokeratins 8, 18, 19 are shown in red. Y-positive cells (green) reside in epithelial cells (arrows) and stromal cells (arrowheads). Panel C shows 3 planes restored from a Z-stack of sections taken at 0.5-µm intervals. The y and z dimensions are shown on the two sides of the x plane image and demonstrate the presence of the Y chromosome (green) in the same plane with the cytokeratins (red) and the nuclei (DAPI, blue). Further characterization of the phenotypes of Y chromosome-positive cells using additional cell markers (shown in green) such as: (D) claudin-1 (a tight junction protein in salivary ducts), (E) Na+-K+-2Cl− cotransporter (NKCC1; a marker for acini), and (F) von Willebrand factor IV (a marker for endothelial cells) indicate several epithelial and endothelial cells that are Y chromosome-positive (arrows); other Y-positive cells (arrowheads) in or near the wall of this blood vessel may be smooth muscle cells, fibroblasts, etc. (G) CD45 (a marker of the white blood cell lineage) in green (FITC); NKCC1 in yellow (CY5) and the Y chromosome in red (Alexa-594). All nuclei are blue (DAPI). The two arrows point to 2 CD45-positive cells in proximity to salivary cells. The intersection of the 2 red lines in this panel indicates a salivary epithelial cell (NKCC1, yellow) of male donor origin (Y chromosome, red dot). Panels D-G show 3 planes restored from a Z-stack of sections taken at 0.5-µm intervals. Scale bars equal 10 µm (A–C) and 16 µm in D–G.
Our results indicate that a small percentage (about 1%) of cells derived from marrow or granulocytecolony stimulating factor (G-CSF) mobilized blood cells can migrate into the salivary glands. Higher rates of microchimerism seem to occur in tissues with high cell turnover rates and/or that are sites of frequent injuries, for example, buccal (oral) epithelial cells (~10%) [10], endometrial glands [12,15], skin (~20%) [16], and liver (25%; an organ exposed to frequent chemical injuries) [7]. It is very interesting to compare our previous results on buccal cells to the results presented in this article in the partially overlapping patient population (3 out of the 5 patients were the same in both studies). The comparison clearly shows that tissues with high turnover rates (ie, the buccal epithelium) might need to replace stem cells more frequently during the life-span of the individual than tissues with slow turnover rates (ie, salivary glands). We found almost 10 times more differentiated epithelial cells of donor origin in the oral mucosa than in salivary glands of the same patients (Table 1).
The physiological significance of finding microchimerism in recipients’ tissues after donor BMDSCs or organ transplantations is at present unclear. Ayala et al. [17] suggest using donor chimerism as a marker of transplantation tolerance that may help to tailor immunosuppressive treatment. Other groups have suggested that microchimerism might contribute to the development of neoplastic epithelial tissues [18,19]. However, this remains unresolved as there are also data arguing against the possibility that BMDSCs represent a direct source of carcinomas in the recipients [20]. There are reports of better BMDSC donor cell engraftment at sites with injury, such as lung after radiation [6], GI inflammation [21], and skin blistering [16]. Regarding the latter injury, Wagner et al. [16] showed histologic evidence of increased amounts of collagen type VII (C7) at the dermal-epidermal junction of children with recessive dystrophic epidermolysis bullosa, a genetic skin disorder caused by a mutation in the C7 gene. They hypothesized that donor BMDSCs mobilized to the skin secreted C7, which resulted in reduced blistering and increased clinical benefit for their patients. Of particular interest with the findings reported here are specific injuries that occur to the salivary glands, such as from therapeutic radiation for head and neck cancers and autoimmune insults in Sjögren’s syndrome. These injuries (either radiation induced or immunologic) might increase donor cell engraftment/microchimerism. A more efficient delivery of BMDSCs into irradiation-damaged salivary glands might be achieved via infusion up the ductal tree [22]. For now, we demonstrate, as a proof-of-concept, that donor cell microchimerism can be identified in human salivary glands.
ACKNOWLEDGMENTS
Roseanne Leakan, Peiman Hematti, David Kleinman, Jane Atkinson, Amalia Dutra, Evgenia Pak, Vidya Sankar, Kathy Kalinyak, and for donating the Na+-K+-2Cl− cotransporter antibody, R. James Turner (NIDCR). This study was supported in part by research funding from the Intramural Research Programs of NCI, NIDCR, and NHLBI, the Canadian Institutes of Health Research, and the Department of Veterans Affairs.
Footnotes
Financial disclosure: The authors declare no competing financial interests.
AUTHORSHIP STATEMENT
S.D.T., A.J.B., S.Z.P., B.J.B., S.R.P., and E.M. designed research; S.D.T., R.S.R., A.J.B., S.Z.P., S.K., Y.L., H.M.N., A.C., Y.S., and E.M. performed or collected data; all the authors analyzed, interpreted data, contributed to the writing of the manuscript; all the authors approved the manuscript.
REFERENCES
- 1.Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall A, Gratwohl A. Adult stem cell transplantation in autoimmune disease. Curr Opin Hematol. 2009;16:285–291. doi: 10.1097/MOH.0b013e32832aacb3. [DOI] [PubMed] [Google Scholar]
- 3.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Alexander SI, Smith N, Hu M, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369–374. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 5.de Weger RA, Verbrugge I, Bruggink AH, et al. Stem cell-derived cardiomyocytes after bone marrow and heart transplantation. Bone Marrow Transplant. 2008;41:563–569. doi: 10.1038/sj.bmt.1705939. [DOI] [PubMed] [Google Scholar]
- 6.Krause DS. Bone marrow-derived lung epithelial cells. Proc Am Thorac Soc. 2008;5:699–702. doi: 10.1513/pats.200803-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaia S, Cappia S, Smedile A, et al. Epithelial microchimerism: consistent finding in human liver transplants. J Gastroenterol Hepatol. 2006;21:1801–1806. doi: 10.1111/j.1440-1746.2006.04675.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto T, Okamoto R, Yajima T, et al. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128:1851–1867. doi: 10.1053/j.gastro.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa F, Yasukawa M, Yoshida S, et al. Human cord bloodand bone marrow-derived CD341 cells regenerate gastrointestinal epithelial cells. FASEB J. 2004;18:1958–1960. doi: 10.1096/fj.04-2396fje. [DOI] [PubMed] [Google Scholar]
- 10.Tran SD, Pillemer SR, Dutra A, et al. Differentiation of human bone marrow-derived cells into buccal epithelial cells in vivo: a molecular analytical study. Lancet. 2003;361:1084–1088. doi: 10.1016/S0140-6736(03)12894-2. [DOI] [PubMed] [Google Scholar]
- 11.Körbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 12.Ikoma T, Kyo S, Maida Y, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201:608.e1–608.e8. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Fox PC. Simplified biopsy technique for labial minor salivary glands. Plast Reconstr Surg. 1985;75:592–593. doi: 10.1097/00006534-198504000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Tóth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55:545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 15.Bratincsak A, Brownstein MJ, Cassiani-Ingoni R, et al. CD45- positive blood cells give rise to uterine epithelial cells in mice. Stem Cells. 2007;25:2820–2826. doi: 10.1634/stemcells.2007-0301. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–639. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala R, Grande S, Albizua E, et al. Long-term follow-up of donor chimerism and tolerance after human liver transplantation. Liver Transpl. 2009;15:581–591. doi: 10.1002/lt.21736. [DOI] [PubMed] [Google Scholar]
- 18.Dubernard G, Oster M, Chareyre F, et al. Increased fetal cell microchimerism in high grade breast carcinomas occurring during pregnancy. Int J Cancer. 2009;124:1054–1059. doi: 10.1002/ijc.24036. [DOI] [PubMed] [Google Scholar]
- 19.Janin A, Murata H, Leboeuf C, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113:1834–1840. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 20.Soldini D, Moreno E, Martin V, Gratwohl A, Marone C, Mazzucchelli L. BM-derived cells randomly contribute to neoplastic and non-neoplastic epithelial tissues at low rates. Bone Marrow Transplant. 2008;42:749–755. doi: 10.1038/bmt.2008.243. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Okamoto R, Yajima T, et al. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128:1851–1867. doi: 10.1053/j.gastro.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 22.Redman RS, Ball WD, Mezey É, Key S. Dispersed donor salivary gland cells are widely distributed in the recipient gland when infused up the ductal tree. Biotechnol. Histochem. 2009;84:253–260. doi: 10.3109/10520290903081377. [DOI] [PMC free article] [PubMed] [Google Scholar]