Abstract
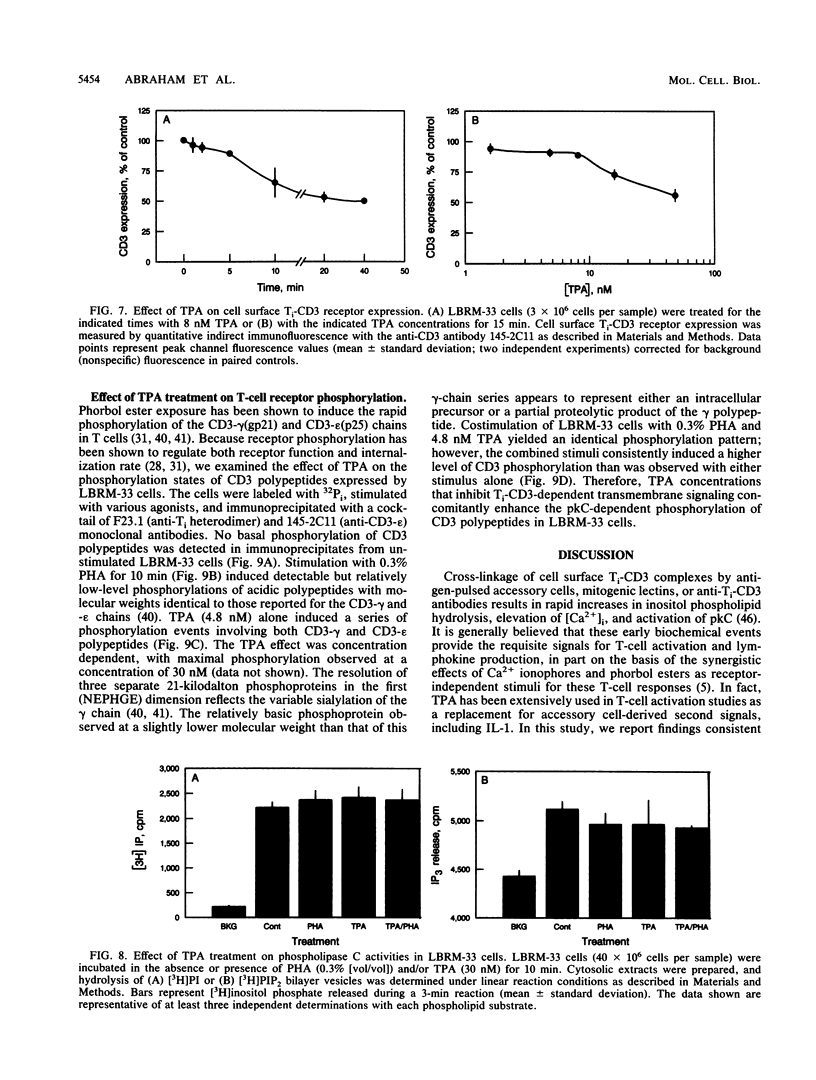
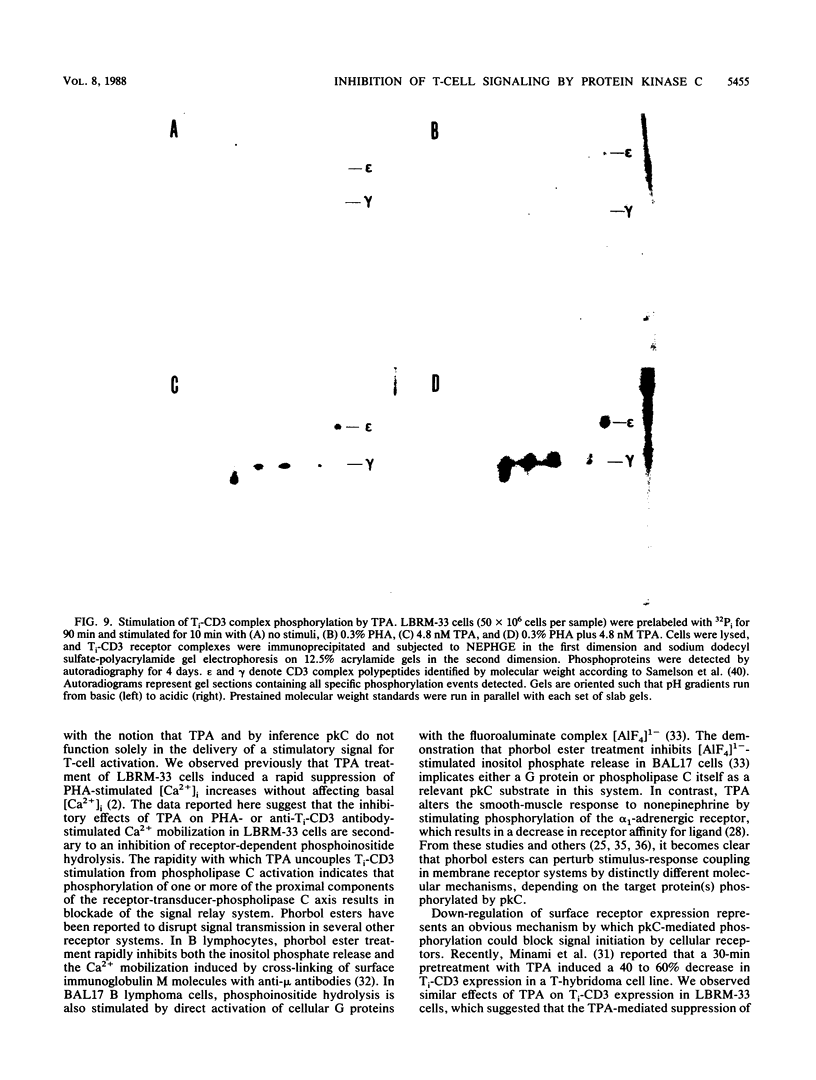
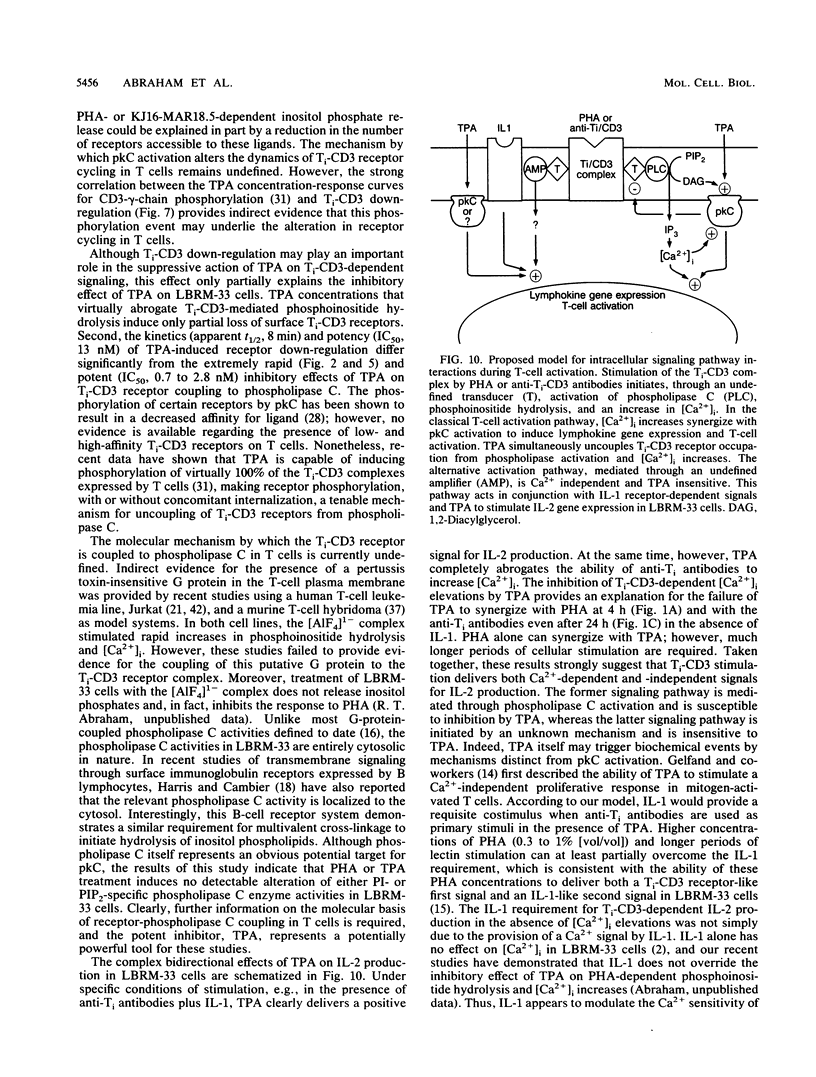
The murine T-lymphoma cell line LBRM-33 is known to require synergistic signals delivered through the antigen receptor (Ti-CD3) complex, together with interleukin 1 (IL-1), for activation of IL-2 gene expression and IL-2 production. Although 12-O-tetradecanoylphorbol-13-acetate (TPA) was capable of replacing IL-1 as an activating stimulus under certain conditions, biologic studies indicated that TPA failed to synergize with Ti-CD3-dependent stimuli under conditions in which IL-1 was clearly active. Acute exposure to TPA and other active phorbol esters resulted in a concentration-dependent inhibition of the increases in phosphoinositide hydrolysis and intracellular free Ca2+ concentration stimulated by phytohemagglutinin or anti-Ti antibodies. TPA treatment induced no direct alteration of phospholipase C enzymatic activities in LBRM-33 cells. In contrast, both Ti-CD3 cross-linkage and TPA rapidly stimulated the phosphorylation of identical CD3 complex polypeptides, presumably via activation of protein kinase C. Exposure of LBRM-33 cells to TPA resulted in a time-dependent, partial down-regulation of surface Ti-CD3 expression. Thus, TPA treatment inhibited the responsiveness of LBRM-33 cells to Ti-CD3-dependent stimuli by inducing an early desensitization of Ti-CD3 receptors, followed by a decrease in membrane receptor expression. These studies indicate that phorbol esters deliver bidirectional signals that both inhibit Ti-CD3-dependent phosphoinositide hydrolysis and augment IL-2 production in LBRM-33 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Abraham R. T., Ho S. N., Barna T. J., McKean D. J. Transmembrane signaling during interleukin 1-dependent T cell activation. Interactions of signal 1- and signal 2-type mediators with the phosphoinositide-dependent signal transduction mechanism. J Biol Chem. 1987 Feb 25;262(6):2719–2728. [PubMed] [Google Scholar]
- Acuto O., Reinherz E. L. The human T-cell receptor. Structure and function. N Engl J Med. 1985 Apr 25;312(17):1100–1111. doi: 10.1056/NEJM198504253121706. [DOI] [PubMed] [Google Scholar]
- Albert F., Hua C., Truneh A., Pierres M., Schmitt-Verhulst A. M. Distinction between antigen receptor and IL 2 receptor triggering events in the activation of alloreactive T cell clones with calcium ionophore and phorbol ester. J Immunol. 1985 Jun;134(6):3649–3655. [PubMed] [Google Scholar]
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter H. R., Smith A. D. Resolution of the phosphoinositide-specific phospholipase C isolated from porcine lymphocytes into multiple species. Partial purification of two isoenzymes. Biochem J. 1987 Jun 15;244(3):639–645. doi: 10.1042/bj2440639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chilson O. P., Boylston A. W., Crumpton M. J. Phaseolus vulgaris phytohaemagglutinin (PHA) binds to the human T lymphocyte antigen receptor. EMBO J. 1984 Dec 20;3(13):3239–3245. doi: 10.1002/j.1460-2075.1984.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Mills G. B., Grinstein S. Mitogens trigger a calcium-independent signal for proliferation in phorbol-ester-treated lymphocytes. 1985 May 30-Jun 5Nature. 315(6018):419–420. doi: 10.1038/315419a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. K., Cambier J. C. B lymphocyte activation. Transmembrane signal transduction by membrane immunoglobulin in isolated cell membranes. J Immunol. 1987 Aug 1;139(3):963–970. [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler J. R., Harden T. K. Guanine nucleotide-dependent pertussis-toxin-insensitive stimulation of inositol phosphate formation by carbachol in a membrane preparation from human astrocytoma cells. Biochem J. 1986 Oct 1;239(1):141–146. doi: 10.1042/bj2390141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Abraham R. T., Gillis S., McKean D. J. Differential bioassay of interleukin 2 and interleukin 4. J Immunol Methods. 1987 Apr 2;98(1):99–104. doi: 10.1016/0022-1759(87)90441-8. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N., Altman A. Human T lymphocyte activation by tumor promoters: role of protein kinase C. J Immunol. 1987 May 15;138(10):3100–3107. [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisaka Y., Toyoshima S., Osawa T. Phosphatidylinositol-specific phospholipase C of murine lymphocytes. Arch Biochem Biophys. 1986 Sep;249(2):569–578. doi: 10.1016/0003-9861(86)90035-4. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Ikeda K., Kozawa O., Takai Y. Modes of inhibitory action of protein kinase C in the chemotactic peptide-induced formation of inositol phosphates in differentiated human leukemic (HL-60) cells. J Biol Chem. 1987 May 15;262(14):6766–6770. [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Martin P. J., Spooner C. E., Hansen J. A., Meier K. E. Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol. 1986 Jun 1;136(11):3945–3952. [PubMed] [Google Scholar]
- Lee K. Y., Ryu S. H., Suh P. G., Choi W. C., Rhee S. G. Phospholipase C associated with particulate fractions of bovine brain. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5540–5544. doi: 10.1073/pnas.84.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for expression of the interleukin 2 receptor. J Immunol. 1985 Mar;134(3):1640–1643. [PubMed] [Google Scholar]
- Minami Y., Samelson L. E., Klausner R. D. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. J Biol Chem. 1987 Sep 25;262(27):13342–13347. [PubMed] [Google Scholar]
- Mizuguchi J., Beaven M. A., Li J. H., Paul W. E. Phorbol myristate acetate inhibits anti-IgM-mediated signaling in resting B cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4474–4478. doi: 10.1073/pnas.83.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Ji Y. Y., Nakabayaschi H., Huang K. P., Beaven M. A., Chused T., Paul W. E. Protein kinase C activation blocks anti-IgM-mediated signaling BAL17 B lymphoma cells. J Immunol. 1987 Aug 15;139(4):1054–1059. [PubMed] [Google Scholar]
- Nau G. J., Moldwin R. L., Lancki D. W., Kim D. K., Fitch F. W. Inhibition of IL 2-driven proliferation of murine T lymphocyte clones by supraoptimal levels of immobilized anti-T cell receptor monoclonal antibody. J Immunol. 1987 Jul 1;139(1):114–122. [PubMed] [Google Scholar]
- O'Flynn K., Russul-Saib M., Ando I., Wallace D. L., Beverley P. C., Boylston A. W., Linch D. C. Different pathways of human T-cell activation revealed by PHA-P and PHA-M. Immunology. 1986 Jan;57(1):55–60. [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Otten G., Wilde D. B., Prystowsky M. B., Olshan J. S., Rabin H., Henderson L. E., Fitch F. W. Cloned helper T lymphocytes exposed to interleukin 2 become unresponsive to antigen and concanavalin A but not to calcium ionophore and phorbol ester. Eur J Immunol. 1986 Mar;16(3):217–225. doi: 10.1002/eji.1830160302. [DOI] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Patel M. D., Weissman A. M., Harford J. B., Klausner R. D. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986 Sep 26;46(7):1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Hasegawa-Sasaki H. Activation of polyphosphoinositide phospholipase C by guanosine 5'-O-(3-thio)triphosphate and fluoroaluminate in membranes prepared from a human T cell leukemia line, JURKAT. FEBS Lett. 1987 Jun 22;218(1):87–92. doi: 10.1016/0014-5793(87)81024-4. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Daine B. Activation of IL 1-dependent and IL 1-independent T cell lines by calcium ionophore and phorbol ester. J Immunol. 1986 Feb 1;136(3):1033–1037. [PubMed] [Google Scholar]
- Zlotnik A., Daine B., Smith C. A. Activation of an interleukin-1-responsive T-cell lymphoma by fixed P388D1 macrophages and an antibody against the Ag:MHC T-cell receptor. Cell Immunol. 1985 Sep;94(2):447–453. doi: 10.1016/0008-8749(85)90269-2. [DOI] [PubMed] [Google Scholar]