Abstract
Recent evidence suggests that processes of inflammation and angiogenesis are interconnected, especially in human pathologies. Newly formed blood vessels enable the continuous recruitment of inflammatory cells, which release a variety of proangiogenic cytokines, chemokines, and growth factors and further promote angiogenesis. These series of positive feedback loops ultimately create a vicious cycle that exacerbates inflammation, transforming it into the chronic process. Recently, this concept of reciprocity of angiogenesis and inflammation has been expanded to include oxidative stress as a novel mechanistic connection between inflammation-driven oxidation and neovascularization. Production of reactive oxygen species (ROS) results from activation of immune cells by pro-inflammatory stimuli. As oxidative stress can lead to chronic inflammation by activating a variety of transcription factors including NF-κB, AP-1, and PPAR-γ, inflammation itself has a reciprocal relationship with oxidative stress. This review discusses the recent findings in the area bridging neovascularization and oxidation and highlights novel mechanisms of inflammation and oxidative stress driven angiogenesis.
Keywords: Angiogenesis, Inflammation, Oxidative stress
Introduction
Although vasculature in adult mammals is mainly quiescent, new blood vessel formation is required for timely tissue repair and remodeling after injury. At the same time, angiogenesis promotes a number of pathophysiological conditions associated with inflammation, solid tumor growth, and metastasis, as well as eye diseases. The process of angiogenesis involves migration, proliferation, differentiation, and adhesion of multiple cell types, including endothelial, mural, and inflammatory cells [1–3]. Whereas developmental angiogenesis mostly depends on conventional angiogenic signals, such as vascular endothelial growth factor (VEGF) and Notch, pathological angiogenesis appears to be closely associated with inflammation and inflammation-generated oxidative stress [4–7]. A number of novel mechanistic components contributing to neovascularization have been recently identified, however, the link coupling inflammation-dependent oxidation or inflammation-independent oxidation to the angiogenic process remains largely unknown. In this review, we summarize recent findings in this area and discuss their implications in the context of antiangiogenic therapeutic strategies.
Angiogenesis and inflammation
Inflammation is a host defense mechanism enabling the body to respond to injury and pathogenic challenges. Acute inflammation provides an immediate response against infectious agents with the so-called “search and destroy” mechanism, and involves rapid recruitment and activation of neutrophils, eosinophils, and natural killer (NK) cells [3, 6, 7]. The main weapon used by these cells against pathogens is the generation of reactive oxygen species, followed by an infiltration of leukocytes to remove remaining pathogens [8, 9]. In contrast to this protective mechanism, chronic inflammation causes substantial tissue damage which might create procarcinogenic conditions [6, 10]. In this situation, pathological angiogenesis promotes a continuous recruitment of inflammatory cells, thereby exacerbating inflammation and damage [11]. A number of inflammatory cells, including neutrophils, eosinophils, mast cells, NK cells, macrophages, and dendritic cells (DCs), are involved in inducing and promoting angiogenesis, while alternatively activated (or M2) macrophages seem to play a crucial role in tumor angiogenesis [3, 12]. Under hypoxia condition, inflammatory cells secrete a plethora of proangiogenic factors, including VEGF, tumor necrosis factor-α (TNF-α), and other cytokines, increasing vascular permeability and facilitating additional recruitment of immune cells [7, 11, 13, 14]. Multiple types of leukocytes and macrophages also contribute to the proteolytic remodeling of the extracellular matrix (ECM) by releasing matrix metalloproteinases (MMPs), cathepsins, plasminogen, and urokinase, thereby allowing blood vessel formation [2, 10]. Angiogenesis is indirectly enhanced by chemokines, such as CXCL12, CXCL8, and CXCL1, especially those derived from neutrophils, which further potentiate inflammation [3, 15]. However, one of the main functions of activated inflammatory cells is the generation of ROS, which serves as an important stimulus of angiogenic signaling [8, 9].
Toll-like receptors in angiogenesis
It has been discovered that TLRs act as guardians of innate immunity, revealing several classes of pathogens either on the plasma membrane or on the endosomal membranes after pathogen phagocytosis [16–18]. TLR expression is not restricted to leukocytes, but has been reported in multiple cell types, including endothelial cells, implying that the role of the TLR pathway may not be limited to immune response [16, 19]. Recent evidence suggests that the TLR pathway significantly contributes to angiogenesis. It has been shown that LPS, a well-known TLR4 ligand, interacts with adenosine A2A receptor (A2AR) agonists through the TLR4 pathway, resulting in a markedly increased production of VEGF in macrophages [20]. LPS is also known to directly stimulate endothelial sprouting in vitro through a TRAF6-mediated activation of NF-κB and JNK [21]. On the other hand, it has been demonstrated that MALP-2, a TLR2/6 ligand, promotes angiogenesis in a TLR2/6 dependent manner by inducing the secretion of a granulocyte-macrophage colony stimulating factor (GM-CSF) [22]. In addition, it has been reported that poly(I:C), a TLR3 ligand, induces the hypoxia inducible factor 1α (HIF-1α) activation and VEGF secretion in a TLR3 dependent manner in vitro [23]. An increasing body of evidence shows that TLRs also recognize endogenously generated molecular patterns, i.e. denatured or death-associated molecular patterns (DAMPs) [24, 25]. Interestingly, recent studies have shown that the high-mobility group B1 (HMGB1) and oxidation-generated ω-(2-carboxyethyl)pyrrole (CEP) activate TLR signaling and promote angiogenesis [19, 26]. Given that TLRs are required to trigger a defensive inflammatory response to infection, the ongoing investigation of the role of the TLR pathway in angiogenesis bridges inflammation and angiogenesis.
Novel mechanisms of oxidation-driven angiogenesis
One of the signature cellular processes observed during inflammation is respiratory burst, which results in the generation and accumulation of extracellular ROS aimed to protect against invading pathogens [27–29]. At the same time, ROS, in a form of superoxide anion or hydrogen peroxide, seem to act as bona fide messengers to control multiple cellular functions such as cell cycle, proliferation, and apoptosis [30–36]. However, excessive formation of ROS creates an imbalance in aerobic cells and tissues known as “oxidative stress,” which is associated with ageing and numerous pathologies, including heart and vascular diseases [8, 37–43]. One of the well established roles of ROS in human pathologies is lipid peroxidation, a main contributor to atherosclerosis [5]. Both intracellular and extracellular ROS have been implicated in the process of angiogenesis in a number of pathophysiological settings [38, 44, 45]. First, intracellular ROS were shown to be involved in VEGF-dependent signaling in endothelial cells [46]. In a tumor microenvironment, the NADPH oxidase-dependent ROS increase HIF-1α-dependent VEGF secretion, which, in turn, promotes angiogenesis and facilitates tumor growth [47]. During the wound healing process, ROS released by inflammatory cells (neutrophils and macrophages) and fibroblasts not only act against invading bacteria, but also induce the basic fibroblast growth factor (bFGF) and VEGF expression and signaling [37]. Thus, increasing evidence suggests that ROS are able to promote angiogenesis through known VEGF- or bFGF-dependent pathways.
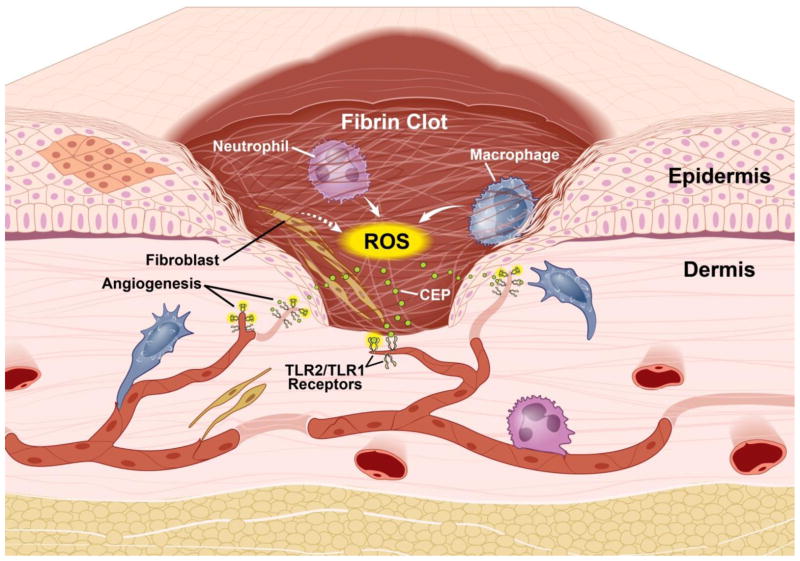
Intriguingly, several recent studies identified ROS as main mediators of novel VEGF-independent pathways of angiogenesis. Newly generated ROS were shown to cause oxidation of readily available polyunsaturated fatty acids present within phospholipid membranes. This process, often observed at the sites of injury or inflammation, leads to local accumulation of carboxyalkyl pyrrole (CAPs) protein adducts [19, 48, 49]. There are three main members of the CAPs family: 2-(ω-carboxyheptyl)pyrrole (CHP), 2-(ω-carboxyethyl)pyrrole (CEP), and 2-(ω-carboxypropyl)pyrrole (CPP) [50]. Among them, CEP has attracted considerable attention as a potential biomarker for advanced age-related macular degeneration [49, 51, 52]. In vitro, exogenously provided CEP had a proangiogenic effect comparable to that of VEGF [19, 53]. During the wound healing process, endogenous CEP accumulated in a transient fashion and its generation was aided by inflammatory cells (neutrophils and macrophages). Molecular patterns of CAPs are recognized by TLR2/1 complex on endothelial cells and trigger MyD88-dependent signaling to promote neovascularization at the wound site, which, in turn, accelerate wound healing and subsequent tissue regeneration (Figure 1). In tumors with substantial inflammation, such as melanomas, CEP seems to continuously accumulate [19].
Figure 1.
Role of oxidation- driven processes in wound angiogenesis. During the inflammatory phase of wound healing, immune cells including neutrophils and macrophages, infiltrate into the injured area and become activated to produce ROS aimed to defend against pathogens. ROS oxidize readily available membrane phospholipids to produce CAPs (exemplified by CEP). CEP adducts are recognized by TLR1/TLR2 complex on endothelial cells to induce neovascularization and accelerate wound healing
It is clear that the cascade of these events occurs in a well-coordinated and interconnected fashion. Inflammation gives rise to ROS, triggering lipid oxidation to generate CEP. In turn, CEP induces angiogenesis leading to enhanced blood flow and additional recruitment of inflammatory cells, which release angiogenic factors at the wound site to further facilitate wound healing or tumor growth. The evidence that CEP neutralization impairs wound healing and diminishes tumor angiogenesis indicates that CEP-TLR2/1-dependent neovascularization is as important as the VEGF pathway in conditions associated with inflammation. Yet, CEP-TLR2/1 induced angiogenesis is independent on VEGF signaling in endothelial cells, but involves Rac1 activation, a key factor that regulates endothelial cell migration and adhesion [19]. Toll-like receptors are known primarily for their role as innate immunity receptors [16–18]; however, CEP-TLR2/1 induced angiogenesis provides the first link between oxidative stress, innate immunity, and vascularization [19].
Another recent finding implicates an alternative mediator of oxidative stress induced pathological angiogenesis, ATM kinase. ATM, a protein kinase from the phosphoinositide 3-kinase-related protein kinase (PIKK) family, is known for its role in the regulation of cell cycle and DNA repair and damage [54, 55]. This essential role of ATM has been recently extended to its function as a redox sensor regulating oxidative stress-associated signaling pathways.
ATM activation by ROS levels was shown to promote pathological neoangiogenesis. Remarkably, ATM seems to be involved uniquely in pathological angiogenesis, such as tumor angiogenesis or ischemia-induced neovascular remodeling, but not in physiological vascularization [56]. ATM activation seems to specifically promote endothelial proliferation, but not any other cell types including pericytes, a-smooth muscle cells, or inflammatory cells. Unexpectedly, ATM activation in endothelial cells does not result in phosphorylation of two main targets of ATM in DNA damage response, p53, or H2AX, but diminishes p38α phosphorylation, a pathway involved in response to oxidative stress. Similar to TLRs, deletion of ATM inhibited angiogenesis even in the presence of VEGF inhibitors, indicating that this process is VEGF independent. Thus, this study establishes another VEGF-independent and oxidation-driven mechanism of angiogenesis.
As discussed above, CEP-TLR2/1 and ATM-p38α seem to have indispensable roles in angiogenesis, from tissue regeneration and remodeling to tumor progression. These two novel VEGF independent signaling pathways establish new targets for treatment of angiogenesis-associated pathologies, such as tumors resistant to anti-VEGF therapy. Furthermore, these findings demonstrate how oxidation and inflammation are able to orchestrate neovascularization. This cross-talk between inflammation-induced oxidative stress and angiogenesis is emerging as an important mechanism underlying vascular complications in humans. However, the detailed mechanistic aspects of this interface remain to be elucidated. For example, it remains ambiguous how exactly CEP triggers TLR2/1 downstream signaling in endothelium versus other TLR expressing cells, leading to neovascularization in injured tissues and tumors. In addition, DAMPs are known to trigger multiple signaling pathways. Thus, it is possible that CAPs can be recognized by other pattern recognition receptors. In this regard, a recent study demonstrated that platelets respond to CAPs, including CEP, via TLR9 activation, thereby promoting platelet hyper-reactivity and thrombosis in pathologies associated with oxidative stress [57]. This evidence implies that CAPs serve as previously unrecognized pathological mediators involved in a number of oxidation and inflammation associated human diseases, now including atherosclerosis and thrombosis. In sum, these novel mechanisms underlying oxidation-driven processes might be applicable to more pathological situations than it was first anticipated. It is still not clear whether there is any cross-talk between oxidation-triggered and conventional signaling for both TLRs and ATM. Further analysis of the molecular mechanism of oxidation and inflammation induced angiogenesis will provide the necessary information for translating these findings into therapeutic approaches.
Therapeutic implications
Since tumor growth and metastasis critically depend on sufficient blood supply, the inhibition of tumor neovasculature is considered to be a crucial anticancer strategy in clinical oncology. A variety of proangiogenic factors, such as VEGF, bFGF, interleukin 8 (IL-8), G-CSF, and GM-CSF, are produced by tumor cells as well as tumor stroma, and aim to promote angiogenesis [2, 8]. Particularly, VEGF and its pathway, which specifically affects endothelial cells proliferation and migration during angiogenesis, serve as primary targets for antiangiogenic therapy [58, 59]. Several of anti-VEGF approaches are already approved for clinical use worldwide, indicating its substantial clinical efficacy [58, 59]. There are, however, several drawbacks for anti-VEGF therapy. First, VEGF blockers do not differentiate between pathological and healthy vasculature, thereby causing undesirable side effects, such as delayed wound healing, pulmonary hemorrhage, gastrointestinal perforation, and thrombolytic events [60–63]. Thus, there is a need to target pathological vasculature while sparing normal blood vessels. In this regard, it is intriguing that ATM is specifically activated by oxidative stress in newly formed pathological vasculature, but not in normal healthy vessels [56]. Second, multiple cancers seem to show considerable resistance to anti-VEGF therapy [64, 65]. It has been shown that an extended VEGF blockade might result in tumor revascularization, which is independent of VEGF signaling [60, 66]. In most cases this resistance to anti-VEGF is associated with inflammation and infiltration of myeloid cells which create substantial oxygen tension. This, in turn, results in the accumulation of CAPs promoting angiogenesis in VEGF independent manner. Therefore, targeting oxidation-driven processes might overcome limitations of anti-VEGF therapy, especially in relation to tumor resistance.
In sum, it is important to continue the search for novel and VEGF-independent proangiogenic pathways, such as CEP/TLR2/MyD88 axis and ROS/ATM/p38α pathway. In this respect, improved understanding of oxidation-driven and inflammation-dependent angiogenesis will pave the way toward improved antiangiogenic therapy.
Acknowledgments
This study was supported by research funding from NIH grant HL071625 to T.V.B. We thank Emelye Crehore for her assistance with manuscript proofreading.
Footnotes
Conflicts of Interests Statement
The authors declare no conflict of interests. A patent describing the role of CEP has been submitted.
References
- 1.Jain RK. Molecular regulation of vessel maturation. Nature medicine. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 2.Ruegg C. Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. Journal of leukocyte biology. 2006;80:682–684. doi: 10.1189/jlb.0606394. [DOI] [PubMed] [Google Scholar]
- 3.Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer metastasis reviews. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 4.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annual review of cell and developmental biology. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 5.Kutuk O, Basaga H. Inflammation meets oxidation: NF-kappaB as a mediator of initial lesion development in atherosclerosis. Trends in molecular medicine. 2003;9:549–557. doi: 10.1016/j.molmed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Current opinion in genetics & development. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free radical biology & medicine. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2003;54:469–487. [PubMed] [Google Scholar]
- 10.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free radical biology & medicine. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konisti S, Kiriakidis S, Paleolog EM. Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:153–162. doi: 10.1038/nrrheum.2011.205. [DOI] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutation research. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Grote K, Schutt H, Schieffer B. Toll-like receptors in angiogenesis. The Scientific World Journal. 2011;11:981–991. doi: 10.1100/tsw.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Akira S. TLR signaling pathways. Seminars in immunology. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. TLR signaling. Cell death and differentiation. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 19.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. The American journal of pathology. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollet I, Opina CJ, Zimmerman C, Leong KG, Wong F, Karsan A. Bacterial lipopolysaccharide directly induces angiogenesis through TRAF6-mediated activation of NF-kappaB and c-Jun N-terminal kinase. Blood. 2003;102:1740–1742. doi: 10.1182/blood-2003-01-0288. [DOI] [PubMed] [Google Scholar]
- 22.Grote K, Schuett H, Salguero G, Grothusen C, Jagielska J, Drexler H, Muhlradt PF, Schieffer B. Toll-like receptor 2/6 stimulation promotes angiogenesis via GM-CSF as a potential strategy for immune defense and tissue regeneration. Blood. 2010;115:2543–2552. doi: 10.1182/blood-2009-05-224402. [DOI] [PubMed] [Google Scholar]
- 23.Paone A, Galli R, Gabellini C, Lukashev D, Starace D, Gorlach A, De Cesaris P, Ziparo E, Del Bufalo D, Sitkovsky MV, Filippini A, Riccioli A. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia. 2010;12:539–549. doi: 10.1593/neo.92106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunological reviews. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Current opinion in immunology. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 26.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 27.Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clinical and experimental immunology. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) The Journal of experimental medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature reviews Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. The Journal of biological chemistry. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 31.Kanayama A, Miyamoto Y. Apoptosis triggered by phagocytosis-related oxidative stress through FLIPS down-regulation and JNK activation. Journal of leukocyte biology. 2007;82:1344–1352. doi: 10.1189/jlb.0407259. [DOI] [PubMed] [Google Scholar]
- 32.Gorlach A, Kietzmann T, Hess J. Redox signaling through NADPH oxidases: involvement in vascular proliferation and coagulation. Annals of the New York Academy of Sciences. 2002;973:505–507. doi: 10.1111/j.1749-6632.2002.tb04691.x. [DOI] [PubMed] [Google Scholar]
- 33.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. The Journal of cell biology. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American journal of physiology Lung cellular and molecular physiology. 2000;279:L1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 35.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. Journal of cell science. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 36.San Martin A, Griendling KK. Redox control of vascular smooth muscle migration. Antioxidants & redox signaling. 2010;12:625–640. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.auf dem Keller U, Kumin A, Braun S, Werner S. Reactive oxygen species and their detoxification in healing skin wounds. The journal of investigative dermatology Symposium proceedings/the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2006;11:106–111. doi: 10.1038/sj.jidsymp.5650001. [DOI] [PubMed] [Google Scholar]
- 38.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovascular research. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer letters. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of carcinogenesis. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Reactive oxygen species promote angiogenesis in the infarcted rat heart. International journal of experimental pathology. 2009;90:621–629. doi: 10.1111/j.1365-2613.2009.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free radical biology & medicine. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 43.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. Journal of dermatological science. 2011;63:1–9. doi: 10.1016/j.jdermsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Molecular and cellular biochemistry. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 46.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. The Journal of biological chemistry. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 47.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer research. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 48.Salomon RG. Structural identification and cardiovascular activities of oxidized phospholipids. Circulation research. 2012;111:930–946. doi: 10.1161/CIRCRESAHA.112.275388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. The Journal of biological chemistry. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 51.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malinin NL, West XZ, Byzova TV. Oxidation as “the stress of life”. Aging. 2011;3:906–910. doi: 10.18632/aging.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 55.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 56.Okuno Y, Nakamura-Ishizu A, Otsu K, Suda T, Kubota Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nature medicine. 2012 doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- 57.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of Platelet Toll-Like Receptor 9 by Novel Endogenous Ligands Promotes Platelet Hyper-Reactivity and Thrombosis. Circulation research. 2012 doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature clinical practice Oncology. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 59.Duda DG, Batchelor TT, Willett CG, Jain RK. VEGF-targeted cancer therapy strategies: current progress, hurdles and future prospects. Trends in molecular medicine. 2007;13:223–230. doi: 10.1016/j.molmed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nature reviews Clinical oncology. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 61.Kerr BA, Byzova TV. The dark side of the oxidative force in angiogenesis. Nature medicine. 2012;18:1184–1185. doi: 10.1038/nm.2881. [DOI] [PubMed] [Google Scholar]
- 62.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. British journal of cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature reviews Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 66.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]