Abstract
The mechanism by which incretins and their effect on insulin secretion increase markedly following gastric bypass (GBP) surgery is not fully elucidated. We hypothesized that a decrease in the activity of dipeptidyl peptidase-4 (DPP-4), the enzyme which inactivates incretins, may explain the rise in incretin levels post-GBP. Fasting plasma DPP-4 activity was measured after 10-kg equivalent weight loss by GBP (n = 16) or by caloric restriction (CR, n = 14) in obese patients with type 2 diabetes. DPP-4 activity decreased after GBP by 11.6% (p = 0.01), but not after CR. The increased peak glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) response to oral glucose after GBP did not correlate with DPP-4 activity. The decrease in fasting plasma DPP-4 activity after GBP occurred by a mechanism independent of weight loss and did not relate to change in incretin concentrations. Whether the change in DPP-4 activity contributes to improved diabetes control after GBP remains therefore to be determined.
Keywords: dipeptidyl peptidase inhibitor, GLP-1, gastric bypass, weight loss
Introduction
Concurrent with the rise in obesity and obesity-related metabolic complications such as type 2 diabetes mellitus (T2DM), the number of bariatric surgeries has risen substantially over the last decade. Roux-en-Y gastric bypass (GBP) surgery results in 50% excess weight loss, with resolution of T2DM in up to 80% of the cases [1]. In contrast with the effects of weight loss from caloric restriction (CR) or gastric banding (GB), diabetes remission following GBP occurs rapidly and well before significant weight loss is achieved. Factors other than weight loss play a role in improved glucose metabolism after GBP. Incretins have emerged as potential mediators of the remarkable metabolic improvements following GBP. Circulating glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) concentrations increase significantly in response to meals or oral glucose after GBP [2,3], and the incretin effect on insulin secretion, blunted in patients with T2DM, is restored to normal levels 1 month after GBP [3]. A similar rise in incretin levels was not observed after matched weight loss by diet [4] or after GB surgery [5]. Incretins and other gut hormones, such as peptide YY (PYY), have short half-lives, as they are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) [6]. The mechanisms by which postpran-dial circulating incretins increase significantly after GBP, but not after GB or diet, are not fully elucidated. Both the bypass of the foregut [7] and the rapid exposure of the lower gut to nutrients, or the hindgut hypothesis [8], have been proposed as possible explanations of the rapid rise of incretins after GBP. The goal of this study was to compare the change in fasting plasma DPP-4 activity after GBP and after similar weight loss by CR by GB or diet, in morbidly obese patients with T2DM. We hypothesized increased incretin levels observed after GBP would be accounted for, in part, by decreased DPP-4 activity. Following weight loss by CR, we expect no change in fasting DPP-4 activity, as previous studies have shown no change in incretin levels [4].
Methods
This is a prospective study to investigate the changes in DPP-4 activity in two groups of patients with T2DM. One group was studied before and 1 month after GBP (GBP group) and was compared to a second group studied before and after weight loss by CR by either diet or GB (CR group).
Subjects
Thirty morbidly obese patients with body mass index (BMI) above or equal to 35 kg/m2, eligible candidates for bariatric surgery, age less than 60 years, of all ethnic groups, with T2DM diagnosed for less than 5 years, not on insulin, thiazolidinedione, exenatide or DPP-4 inhibitors, with a glycosylated haemoglobin (HBA1c) less than 8%, participated in the study. Sixteen patients undergoing GBP were studied before and 1 month after surgery. Fourteen patients undergoing CR by GB (n = 3) or diet (n = 11) were studied before and after equivalent ~10-kg weight loss. Both groups were matched for age, weight and BMI, diabetes status and duration at baseline, and for weight loss. All participants signed an informed consent, approved by our institution, before enrolling in the study.
GBP and CR-induced Weight Loss by Diet or GB
The methods of diet, laparoscopic GBP and GB were described previously [4,5]. Briefly, the diet consisted of a meal replacement plan of 1000 kcal/day with weekly visits. The diet for the GBP and the GB patients was based on bariatric standard recommendations. Adjustments of the GB were performed by the bariatric team as needed.
Incretin levels were measured during a 50-g 3-h oral glucose tolerance test (OGTT) and incretin effect by comparing the insulin response after oral and intravenous (IV) isoglycaemic-matched infusion as described before [3].
Total GLP-1 and insulin were measured by RIA and total GIP by enzyme-linked immunosorbent assay (ELISA) (Millipore, St. Charles, MO). DPP-4 activity was assessed kinetically using Gly-Pro-p-nitroaniline (1 mmol/l) as substrate by monitoring the release of p-nitroaniline at 405 nm [9]. The inter- and intra-assay coefficients of variation for DPP-4 were 8 and 5.5%, respectively.
Statistical Analysis
Normal distribution of the variables was verified and therefore parametric analyses were used. Paired t-tests were used to assess within-group changes in outcome measures. Between-group comparisons were performed by independent samples t-test. Data are expressed as mean ± standard deviation. Significance was set at p < 0.05. SPSS 18 was used for data analysis.
Results
Subject characteristics are shown in Table 1. At baseline, BMI and peak GLP-1 and GIP concentrations in response to oral glucose did not differ between GBP and CR. There was a trend for a greater baseline fasting DPP-4 activity in GBP compared to CR (p = 0.058). Weight loss was similar between the groups (−11.1 ± 4.7 vs. −9.9 ± 2.1 kg, p = 0.358), but the duration of weight loss was longer for CR than for GBP (73.5 ± 59.6 vs. 35.9 ± 17.7 days, p = 0.032).
Table 1.
Changes after gastric bypass (GBP) or caloric restriction (CR), *p < 0.001; #p < 0.05.
| GB (n=16)
|
CR (n=14)
|
p of Δ | |||
|---|---|---|---|---|---|
| Baseline | Δ | Baseline | Δ | ||
| Weight (kg) | 111.1 ± 13.6 | −11.1 ± 4.7* | 113.4 ± 11.8 | −10.9 ± 2.1* | 0.358 |
| BMI (kg/m2) | 43.7 ± 5.0 | −4.3 ± 1.5* | 43.9 ± 3.9 | −3.8 ± 0.8* | 0.281 |
| Fasting glucose (mmol/l) | 7.4 ± 1.6 | −1.1 ± 2.0# | 7.8 ± 1.4 | −1.5 ± 0.7* | 0.548 |
| Fasting insulin (pmol/l) | 199.7 ± 160.8 | −81.0 ± 132.0# | 166.1 ± 88.7 | −58.8 ± 81.9# | 0.590 |
| GLP-1 total peak (pmol/l) | 16.4 ± 10.8 | 69.0 ± 42.4* | 16.6 ± 14.1 | −5.1 ± 16.7 | 0.000 |
| GIP peak (ng/l) | 170.7 ± 47.5 | 110.6 ± 72.1* | 186.7 ± 57.80 | 10.1 ± 96.7 | 0.003 |
| Incretin effect on insulin (%) | 20.9 ± 27.6 | 29.1 ± 23.2 | 24.7 ± 17.5 | 10.0 ± 22.5 | 0.033 |
| DPP-4 activity (pmol/min/20 μl) | 529.5 ± 96.4 | −61.5 ± 83.1# | 464.1 ± 83.1 | −5.5 ± 45.0 | 0.032 |
BMI, body mass index; DPP-4, dipeptidyl peptidase-4; GB, gastric banding; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
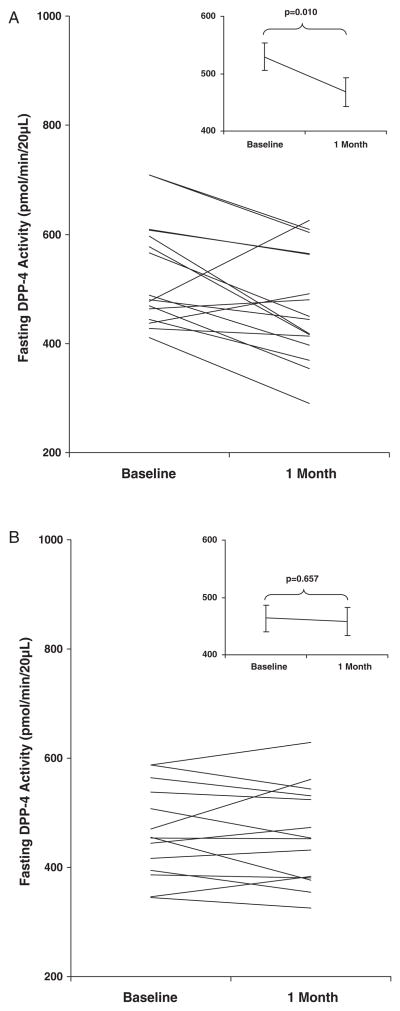
Following similar weight loss, DPP-4 activity decreased significantly after GBP (−61.5 ± 83.1 pmol/min/20 μl; p = 0.010), but not after CR (−5.5 ± 45.0 pmol/min/20 μl; p = 0.657) (Table 1; figure 1). In addition, peak GLP-1 and GIP concentrations increased significantly after GBP by 69.0 ± 42.4 pmol/l and 110.6 ± 72.1 ng/l, respectively (p < 0.001), but remained unchanged after CR (Table 1). The incretin effect on insulin increased significantly after GBP (+29.1 ± 23.2%; p < 0.001), but not after CR (Table 1). Baseline and postweight loss levels of fasting DPP-4 activity did not correlate with weight, BMI, glucose, insulin, GIP or GLP-1 levels or effect.
Figure 1.
Dipeptidyl peptidase-4 (DPP-4) activity at baseline and 1 month after gastric bypass (GBP, A) and after equivalent weight loss by caloric restriction (CR, B). Data are presented as mean ± standard error of the mean.
Conclusions
The present study shows that fasting DPP-4 activity decreases by 11.6% following GBP, in parallel with the rise in incretin levels. The decrease in DPP-4 activity was rather modest compared to the robust increase in incretin levels [3] and to pharmacological inhibition [10]. However, there was no relationship between the change in DPP-4 activity and incretin levels or effect after GBP. Previous studies also failed to show a relationship between DPP-4 activity and incretin response [9]. It is therefore unlikely that decreased plasma DPP-4 activity after GBP contributes significantly to the increase in circulating GLP-1 and GIP concentrations. The decrease in the glomerular filtration rate after weight loss is unlikely to contribute, by decreasing GLP-1 renal clearance, to the higher levels of GLP-1 observed after GBP, as both CR and GBP groups were matched for weight loss. In addition, renal clearance has a limited contribution to the regulation of circulating bioactive incretins compared to the cleavage by DPP-4 [6]. Plasma DPP-4 activity may not account for the total degradation of the incretins by DPP-4 in vivo. DPP-4 is mainly a tissue enzyme, and the relationship between tissue and circulating DPP-4 activity is unknown [9,11]. This could explain the lack of correlation between circulating DPP-4 activity and peak levels of incretins. Future studies should explore tissue DPP-4 activity and its relationship with plasma DPP-4 activity. Our findings contrast with previous data showing no change in DDP-4 activity after biliopancreatic diversion in patients without diabetes [12]. The difference between studies could be secondary to diabetes status, as DPP-4 activity differs according to the degree of glucose tolerance [11], and/or the different types of bariatric surgeries, as biliopancreatic diversion results in greater malabsorption than GBP.
The decrease in DPP-4 activity 1 month after GBP was not seen after matched weight loss by CR. The absence of change in DPP-4 activity parallels the absence of change in incretin levels after GB [5] or diet [4]. It is therefore unlikely that the decrease in DPP-4 activity after GBP is related to CR or weight loss.
In conclusion, our findings show that circulating fasting DPP-4 activity decreases as a result of GBP surgery, in parallel with increased incretin levels. CR alone does neither. The lack of relationship between change in circulating DPP-4 activity and incretin suggests that tissue DPP-4 may be more important in regulating the incretins.
Acknowledgments
The authors thank the volunteer participants; Ping Zhou, Yim Dam and Kristina Andersson for their technical help with assays; Drs J. T., J. M., N. K. and S. B. for referring patients to the study; Antonia Colarusso and Betty Kovack for help with recruitment and the diet-induced weight loss.
This work was funded by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, Program, National Institute of Mental Health Grant MH-48858 and the Swedish Research Council (grant no 6834). B. J. V. is a fellow of the Belgian American Educational Foundation.
Footnotes
Conflict of Interest
M. L. A. helped in data analysis and drafted the manuscript. B. J. V. helped in data analysis and wrote the manuscript. B. A. helped in data collection (performed the DPP-4 assay) and wrote the manuscript. G. C. W., N. J. S. and S. A. helped in data collection. M. B. helped in data collection and analysis. P. G. supervised data analysis. J. T. and J. M. contributed bariatric patients and B. L. designed the study, conducted/collected data, supervised statistical analyses and wrote the manuscript. None of the authors have conflict of interest.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 3.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose M, Machineni S, Olivan B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18:1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab. 2009;23:443–452. doi: 10.1016/j.beem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 10.Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Ryskjaer J, Deacon CF, Carr RD, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155:485–493. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 12.Lugari R, Dei CA, Ugolotti D, et al. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res. 2004;36:111–115. doi: 10.1055/s-2004-814222. [DOI] [PubMed] [Google Scholar]