Abstract
Neurofibrillary tangles (NFT) and amyloid plaques are hallmark neuropathological features of Alzheimer’s disease (AD). There is some debate as to which neuropathological feature comes first in the disease process, with early autopsy studies suggesting that NFT develop first, and more recent neuroimaging studies supporting the early role of amyloid beta (Aβ) deposition. Cerebrospinal fluid (CSF) biomarkers of Aβ42 and hyperphosphorylated tau (p-tau) have been shown to serve as in vivo proxy measures of amyloid plaques and NFT, respectively. The aim of this study was to examine the association between CSF biomarkers and rate of atrophy in the precuneus and hippocampus. These regions were selected because the precuneus appears to be affected early and severely by Aβ deposition, and the hippocampus similarly by NFT pathology. We predicted (1) baseline Aβ42 would be related to accelerated rate of cortical thinning in the precuneus and volume loss in the hippocampus, with the latter relationship expected to be weaker, (2) baseline p-tau181p would be related to accelerated rate of hippocampal atrophy and cortical thinning in the precuneus, with the latter relationship expected to be weaker. Using all ADNI cohorts, we fitted separate linear mixed-effects models for changes in hippocampus and precuneus longitudinal outcome measures with baseline CSF biomarkers modeled as predictors. Results partially supported our hypotheses: Both baseline p-tau181p and Aβ42 were associated with hippocampal atrophy over time. Neither p-tau181p nor Aβ42 were significantly related to cortical thinning in the precuneus over time. However, follow-up analyses demonstrated that having abnormal levels of both Aβ42 and p-tau181p was associated with an accelerated rate of atrophy in both the hippocampus and precuneus. Results support early effects of Aβ in the Alzheimer’s disease process, which are less apparent than and perhaps dependent on p-tau effects as the disease progresses. However, amyloid deposition alone may be insufficient for emergence of significant morphometric changes and clinical symptoms.
Keywords: Biomarkers, Beta Amyloid, Phosphorylated Tau, MRI, Alzheimer’s Disease, Hippocampus, Precuneus
INTRODUCTION
Neurofibrillary tangles (NFT) and amyloid plaques are the hallmark neuropathological features of Alzheimer’s disease (AD). There is some debate about which neuropathological feature comes first in the disease process. Early autopsy studies suggested that NFT develop first (Braak, Braak, Bohl, & Reintjes, 1996), whereas some recent neuroimaging studies, particularly those employing 11C-PiB methods, support the early role of amyloid deposition (see Jack et al., 2010 for review), in line with the amyloid cascade hypothesis (J. A. Hardy & Higgins, 1992; see J. Hardy, 2009 for a critical reappraisal of this hypothesis). CSF biomarkers of phosphorylated tau and Aβ42 have been shown to serve as in vivo proxy measures of NFT and amyloid plaques, respectively (Buerger et al., 2006; Clark et al., 2003; Shaw et al., 2009), and improve diagnostic accuracy for AD (Hampel, Goernitz, & Buerger, 2003; Strozyk, Blennow, White, & Launer, 2003). There is evidence that as amyloid plaques develop, CSF Aβ42 decreases (Shaw, Korecka, Clark, Lee, & Trojanowski, 2007), thus lower CSF Aβ42 suggests increased brain amyloid deposition. Unlike total tau, which may be a general marker of neuronal damage, p-tau is likely to reflect the formation of tangles in AD (Blennow & Hampel, 2003), with increased levels of CSF p-tau thought to reflect increased NFT pathology.
Investigation of the relationship between CSF biomarkers and regional changes on structural and functional MRI may contribute to understanding the pathological mechanisms of AD. Aβ-associated neurodegeneration manifests as cortical thinning in regions vulnerable to early Aβ deposition and this may begin prior to clinically evident cognitive impairment (Becker et al., 2011). The precuneus is a site of preferential amyloid uptake in PiB studies (see Rabinovici & Jagust, 2009 for review), consistently shows hypometabolism in FDG-PET studies of AD and atrophy/cortical thinning in morphometric studies (Buckner et al., 2005), and is a key part of the default network (Buckner, Andrews-Hanna, & Schacter, 2008), which is important for memory function (Sperling et al., 2009). Relationships between CSF Aβ42 or amyloid load as measured by PiB and the precuneus have previously been demonstrated in nondemented older adults and in MCI and AD subjects (Becker et al., 2011; Chetelat et al., 2010; Fjell et al., 2008; Tosun et al., 2010). In contrast to the precuneus, the hippocampus remains relatively free of amyloid deposition during normal aging and early to mid-stage AD (Braak et al., 1996), and there is variability in the literature as to the presence of an association between amyloid load and hippocampal atrophy, with some studies supporting at least a weak relationship (Apostolova et al., 2010; Beckett et al., 2010; Henneman et al., 2009; Mormino et al., 2009; Schuff et al., 2009), and other studies not finding a significant relationship (Becker et al., 2011; Fagan et al., 2009).
A relationship between p-tau and both baseline hippocampal volume and rate of hippocampal atrophy has been demonstrated across several studies (Apostolova et al., 2010; Beckett et al., 2010; de Leon et al., 2006; Hampel et al., 2005; Henneman et al., 2009; Tosun et al., 2010; but also see Schuff et al., 2009), consistent with the well-established finding that the hippocampus is an early site of NFT pathology in the course of AD. While a number of studies have investigated the association of CSF biomarkers and change in selected brain regions, to our knowledge only one study to date has investigated the potential interaction of multiple CSF biomarkers on atrophy. Desikan and colleagues (2011) found an interaction between Aβ42 and p-tau181p status on entorhinal cortex atrophy over time, with elevated atrophy in individuals with abnormal levels of both Aβ42 and p-tau181p. Follow-up analyses further revealed that Aβ42 status was associated with accelerated atrophy in entorhinal cortex only among p-tau181p positive individuals in a nondemented sample. The authors demonstrated this same effect within an “AD-vulnerable” region of interest (ROI) that averaged longitudinal changes in multiple temporal and parietal regions affected subsequently to the entorhinal cortex. They did not examine this effect in the hippocampus and precuneus, and included only nondemented subjects. There has been an increasing emphasis on considering Alzheimer’s disease as a continuum, with the process beginning in otherwise “normal” individuals, and progressing slowly over time, with eventual clinical expression resulting in the diagnostic classifications of MCI and eventually AD dementia. Although the use of diagnostic classification is clinically useful, when studying the effects of CSF biomarkers it is important to examine effects across the entire disease spectrum. This focus separates the current study from recent work that has examined similar questions within diagnostic subgroups (Desikan et al., 2011; Tosun et al., 2010).
The primary aim of this study was to examine the association of CSF biomarkers and rate of atrophy in the precuneus and hippocampus. These regions were selected because the precuneus appears to be affected early and severely by Aβ deposition, and the hippocampus similarly by NFT pathology. A secondary aim was to extend the findings of Desikan and colleagues by assessing the effect of a three-way interaction of Aβ42, p-tau181p and time on rates of atrophy in the precuneus and hippocampus. We predicted (1) baseline Aβ42 would be related to accelerated rate of cortical thinning in the precuneus and volume loss in the hippocampus, with the latter relationship expected to be weaker, (2) baseline p-tau181p would be related to accelerated rate of hippocampal atrophy and cortical thinning in the precuneus, with the latter relationship expected to be weaker and (3) an interaction between low Aβ42 and high p-tau181p would be associated with an accelerated rate of atrophy in both the hippocampus and precuneus. The precentral gyrus was selected as a control region because we did not predict a relationship with either CSF biomarker in this ROI as primary motor regions remain relatively free of Alzheimer’s pathology until late in the disease process.
METHOD
Data used were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), and private pharmaceutical companies and non-profit organizations, as a 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many coinvestigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research, approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years and 200 people with early AD to be followed for 2 years (see www.adni-info.org). This study was approved by each ADNI-affiliated institution. Written informed consent was obtained from all patients or authorized representatives participating in the study.
Participants
ADNI general eligibility criteria are described at www.adni-info.org/Scientists/ADNIGrant/ProtocolSummary.aspx. Briefly, healthy controls (HC) had a Mini-Mental State Exam (MMSE; Folstein, Robins, & Helzer, 1983) score between 24–30 (inclusive), a global Clinical Dementia Rating (CDR; Morris, 1993) score of 0, and did not meet criteria for MCI or dementia (Petersen et al., 2001). MCI participants had MMSE scores between 24–30 (inclusive), a memory complaint, evidence of objective memory loss as measured by education adjusted scores on the Wechsler Memory Scale Logical Memory II, a CDR of 0.5, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. Mildly demented AD participants had MMSE scores between 20–26, global CDR scores of 0.5 or 1.0, and met NINCDS/ADRDA criteria for probable AD (McKhann et al., 1984). The ADNI study collected CSF from approximately 50% of participants at baseline, and from smaller subgroups subsequently. CSF biomarker acquisition procedures for ADNI are described in detail elsewhere (Jagust et al., 2010; Petersen et al., 2010; Trojanowski et al., 2010). A measure derived from the components of the CDR known as “sum of boxes” (CDR-SB) was calculated to further estimate level of clinical impairment. The data used in the current analysis was downloaded on 6/1/2011. Participants with CSF data and baseline and follow-up MRI scans (interval info) that met global quality control criteria were used in the current analysis (see www.loni.ucla.edu/twiki/pub/ADNI/ADNIPostProc/UCSFFreeSrferMethodsSummary.pdf).
MR scanning and brain morphometry
Protocols are described in detail at http://www.loni.ucla.edu/ADNI/Research/Cores/. Two T1-weighted volumes were acquired for each participant. Volumetric (Fischl et al., 2002; Fischl, Salat et al., 2004) and cortical surface reconstruction (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Fischl, van der Kouwe et al., 2004) methods based on FreeSurfer software, optimized for use on large, multi-site datasets, were used. To measure thickness, the cortical surface was reconstructed (Dale et al., 1999) and parcellated into distinct ROIs (Desikan et al., 2006; Fischl, van der Kouwe et al., 2004). Details of the application of these methods to the ADNI data have been described in full elsewhere (Fennema-Notestine et al., 2009). Three a priori selected ROIs were included in the present analyses: precuneus cortical thickness, precentral gyrus cortical thickness and hippocampal volume.
Statistical analyses
We used linear mixed effects (LME) multiple regression (Diggle, Heagerty, Liang, & Zeger, 2002) to model hippocampus, precuneus and precentral gyrus as three separate longitudinal outcomes. Each model included time in months (0, 6th, 12th, 18th and 24th from baseline), baseline CSF biomarkers (Aβ42 and p-tau181p) and their interactions with time as predictors, and baseline age, sex, and apoe4 status as control variables. CSF biomarkers were normalized using the Blom’s rank normalization algorithm (Conover & Inman, 1981) so that estimated effects of these two biomarkers on the outcome could be meaningfully compared. Intercept and time were treated as random effects in all models. All models assumed an unstructured within-subject error covariance structure. Restricted maximum likelihood was used for estimation. Pairwise interactions between CSF biomarkers and time were of substantive interest. That is, we were interested in examining the interplay between CSF biomarkers at baseline and trajectories over time in outcome measures (hippocampal volume, precuneus and precentral gyrus thickness). We first carried out the LME analyses described above on the complete data set (n=342). Next, we applied the same LME models to each diagnostic group separately (normal controls, MCI, and AD) to examine the effect of baseline CSF biomarkers on longitudinal outcomes of interest within each group. Quadratic and higher order time variables did not improve the model fitness indicated by Bayesian information and log-likelihood criteria for any models and is thus not included. The overall fit of the models was examined using a combination of formal fit criteria and visual inspection of residual plots. Results were considered significant when at p < .05; we also provide Bonferroni-adjusted p-values in Table 2.
Table 2.
Results of Mixed Effects Models.
| ALL DX together | Normal | MCI | AD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| coefficient | SE | p-value | coefficient | SE | p-value | coefficient | SE | p-value | coefficient | SE | p-value | |
|
Hippocampal Volume
|
||||||||||||
| Time |
−5.982 | 0.268 | <.0001** | −4.001 | 0.548 | <.0001** | −6.535 | 0.382 | <.0001** | −7.172 | 0.758 | <.0001** |
| Aβ42
|
59.897 | 32.535 | 0.066 | −56.693 | 46.726 | 0.227 | 70.660 | 44.904 | 0.116 | 23.816 | 65.808 | 0.718 |
|
P-tau181p
|
−123.840 | 30.524 | <.0001** | −58.586 | 42.990 | 0.175 | −47.789 | 46.660 | 0.306 | −137.410 | 61.365 | 0.027* |
| Aβ42* time |
0.948 | 0.308 | 0.0021** | 1.135 | 0.547 | 0.040* | 0.288 | 0.437 | 0.510 | 0.858 | 0.733 | 0.245 |
|
P-tau181p* time |
−1.545 | 0.308 | <.0001** | −0.585 | 0.524 | 0.266 | −1.454 | 0.465 | 0.0019** | −1.584 | 0.707 | 0.027* |
|
Precuneus Thickness
|
||||||||||||
| Time |
−0.004 | 0.000 | <.0001** | −0.003 | 0.001 | <.0001** | −0.004 | 0.001 | <.0001** | −0.008 | 0.001 | <.0001** |
| Aβ42
|
0.022 | 0.025 | 0.389 | 0.009 | 0.042 | 0.838 | −0.016 | 0.034 | 0.642 | 0.040 | 0.064 | 0.537 |
|
P-tau181p
|
−0.110 | 0.024 | <.0001** | −0.067 | 0.039 | 0.088 | −0.094 | 0.035 | 0.008* | −0.062 | 0.060 | 0.298 |
| Aβ42* time |
0.001 | 0.000 | 0.232 | 0.000 | 0.001 | 0.739 | 0.001 | 0.001 | 0.203 | −0.002 | 0.001 | 0.226 |
|
P-tau181p* time |
−0.001 | 0.000 | 0.112 | 0.000 | 0.001 | 0.952 | −0.001 | 0.001 | 0.255 | 0.000 | 0.001 | 0.909 |
|
Precentral Gyrus Thickness
|
||||||||||||
| Time |
−0.002 | 0.000 | <.0001** | −0.002 | 0.000 | <.0001** | −0.002 | 0.000 | <.0001** | −0.003 | 0.001 | 0.002** |
| Aβ42
|
0.007 | 0.013 | 0.591 | −0.022 | 0.023 | 0.343 | 0.002 | 0.018 | 0.933 | 0.020 | 0.029 | 0.477 |
|
P-tau181p
|
−0.026 | 0.012 | 0.035* | −0.043 | 0.021 | 0.045* | −0.008 | 0.019 | 0.681 | 0.011 | 0.027 | 0.689 |
| Aβ42* time |
0.000 | 0.000 | 0.955 | 0.001 | 0.000 | 0.237 | 0.000 | 0.000 | 0.876 | −0.001 | 0.001 | 0.495 |
|
P-tau181p* time |
0.000 | 0.000 | 0.116 | 0.000 | 0.000 | 0.687 | 0.000 | 0.000 | 0.611 | −0.001 | 0.001 | 0.056 |
In all models, the following variables are controlled: age at baseline, gender, and Apoe (at least having one e4 vs. none). Tau and a beta are normalized using Blom’s rank normalization algorithm.
p < .05
significant at p < 0.0042, multiple comparison adjusted p-value.
RESULTS
CSF measures were obtained in a subset of ADNI subjects. Out of 819 subjects enrolled in the ADNI I study, 342 subjects had information on Aβ42, p-tau181p, and valid assessment of hippocampal brain volume, precuneus and precentral gyrus measures at baseline. These subjects were included in the current analyses using their follow-up assessments up to 24 months. Demographic characteristics are presented in Table 1.
Table 1.
Baseline characteristics.
| All DX Combined N=342 |
Normal N=103 |
MCI N=163 |
AD N=76 |
Difference among DX+ | |
|---|---|---|---|---|---|
|
| |||||
| N at each follow-up | 0/6/12/18/24 342/330/325/130/236 |
0/6/12/24 103/100/101/81 |
0/6/12/18/24 163/159/151/130/106 |
0/6/12/24 76/71/73/49 |
p-value |
|
Age mean (std)
|
74.84 (6.91) | 75.52 (5.21) | 74.41 (7.39) | 74.86 (7.81) | 0.74 |
|
Female %
|
40.64 | 46.60 | 35.58 | 43.42 | 0.17 |
|
Ethnicity (% Caucasian)
|
95.32 | 93.20 | 94.48 | 100.00 | 0.052 |
|
Years of Education: mean (std)
|
15.66 (2.97) | 15.67(2.81) | 15.86(2.96) | 15.21(3.19) | 0.28 |
|
Apoe4 (at least one e4 allele) %
|
47.95 | 25.24 | 52.15 | 69.74 | <0.001 |
|
MMSE
|
26.83 (2.55) | 29.07 (1.04) | 26.92 (1.80) | 23.59 (1.87) | <0.001 |
|
CDR-SB
|
1.65 (1.72) | 0.02 (0.10) | 1.53 (0.87) | 4.11 (1.42) | <0.001 |
|
Aβ42*
|
0.00 (1.00) | 0.48(0.90) | −0.12(0.97) | −0.48(0.87) | <0.001 |
|
P-tau181p*
|
0.00 (1.00) | −0.54(0.91) | 0.14 (0.92) | 0.48 (0.92) | <0.001 |
|
Hippocampal Volume (mm3)
|
2970.06 (548.28) | 3363.74 (367.42) | 2880.130 (514.50) | 2629.41 (513.24) | <0.001 |
|
Precuneus Thickness (mm)
|
4.04 (0.39) | 4.23 (0.33) | 4.03 (0.36) | 3.83 (0.42) | <0.001 |
|
Precentral gyrus thickness (mm)
|
2.08 (0.21) | 2.14 (0.20) | 2.08 (0.21) | 1.99 (0.20) | <0.001 |
Kruskal-Wallis test for continuous variables and Pearson Chi-Square test for categorical variables; Fisher’s exact test used for ethnicity due to small numbers of non-Caucasian participants.
Normalized values using Blom’s rank normalization algorithm.
Hippocampal volume
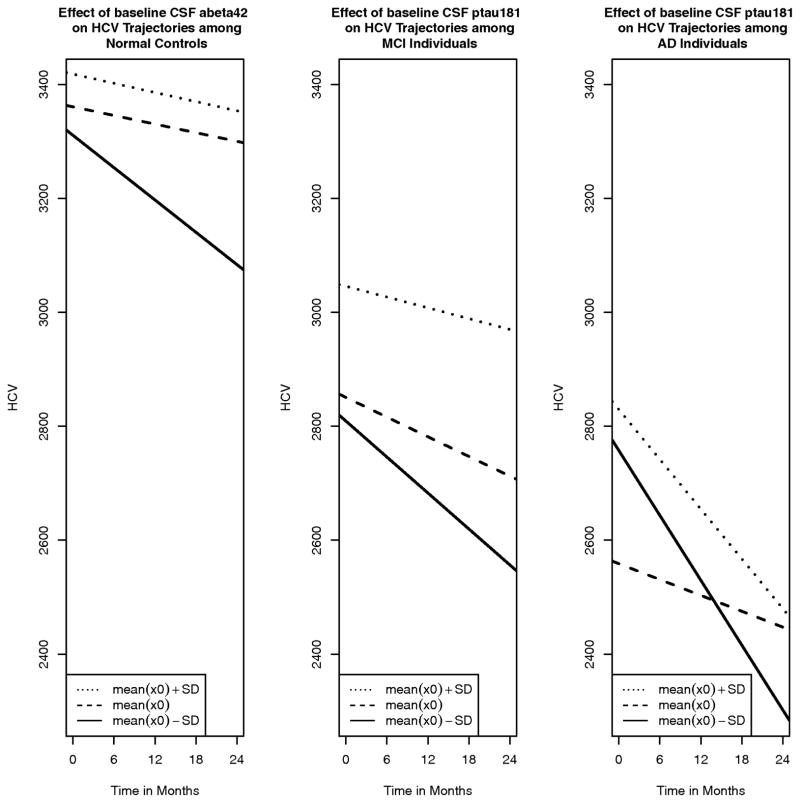
Across all subjects, there was a main effect of time, and this effect persisted within each diagnostic subgroup. There was no main effect of Aβ42, meaning that Aβ42 was not associated with hippocampal volume at baseline, either across all groups or within diagnostic groups. There was a main effect of p-tau181p; higher p-tau181p was associated with lower hippocampal volume, and this effect persisted within the AD group. There was an interaction of time and CSF biomarkers: higher Aβ42was associated with a smaller decline in hippocampal volume over time, and higher p-tau was associated with a larger decline in hippocampal volume over time. When running the model within subgroups, the interaction of Aβ42 and time persisted only within the NC group, suggesting that Aβ42 at baseline does not significantly affect change over time within MCI and AD groups. P-tau181p showed the opposite pattern: baseline p-tau181p does not significantly affect change in hippocampal volume over time in the NC group, whereas within the MCI and AD groups, higher p-tau181p at baseline was associated with greater decline in hippocampal volume over time (Table 2). Figure 1 shows examples of the trajectories of hippocampal volume. We illustrate the effect of high/low CSF biomarkers on hippocampal volume over time for the diagnostic group where significant interactions of biomarkers and time were found using the coefficients obtained in the mixed effects models. For models where hippocampal volume is the outcome, models were ran both with and without controlling for baseline estimated total intracranial vault (eTIV) volume (Buckner et al., 2004) and the same pattern of results was obtained. We present results of models without controlling for eTIV.
Figure 1.
Synergistic effect of CSF (P-tau181p and Aβ42) vs time on the rate of hippocampal atrophy. The plotted lines represent fitted values conditioned upon mean baseline CSF and 1SD above and below mean baseline CSF. Only interactions with an adjusted p-value below 0.10 are plotted.
Precuneus thickness
Across all subjects there was a main effect of time, and this effect persisted within each diagnostic group. There was no main effect of Aβ42, either across all groups or within diagnostic groups. There was a main effect of p-tau181p; higher p-tau181p was associated with lower precuneus thickness at baseline. Within groups, this main effect was significant only within the MCI group. There were no interactions with time, either across all groups or within groups.
Precentral gyrus thickness
Across all subjects there was a main effect of time, and this effect persisted within each individual group. There was no main effect of Aβ42, either across all groups or within individual groups. There was a main effect of p-tau181p for all groups combined; higher p-tau181p was associated with lower precentral gyrus thickness at baseline. Within diagnostic groups, this main effect was significant only within the NC group. There were no interactions with time, either across all groups or within groups.
In a set of secondary analyses, we tested for potential interaction effects of p-tau181p and Aβ42 to determine if having both abnormal p-tau181p and Aβ42 values has an added effect on atrophy over time in our selected ROIs. We dichotomized high and low values of each CSF biomarker for the 3-way interaction to facilitate interpretation of results. Previously defined cutoffs were applied (Shaw et al., 2009): abnormal p-tau181p was defined as p-tau181p > 23pg/ml (−0.34818 using blom normalized scores), and abnormal Aβ42 was defined as Aβ42 < 192pg/ml (0.4395 using blom normalized scores). The proportion of those with both abnormal p-tau181p and Aβ42 was 81.7%, 64.0% and 21.3% among AD, MCI, and normal controls, respectively (Pearson chi-square test, p<0.001). In the entire sample (NC, MCI and AD), there was a 3-way interaction of abnormal p-tau181p and abnormal Aβ42 with time for each ROI (HCV p=0.040, precuneus p=0.0007, precentral gyrus p=0.029), after controlling for gender, Apoe4, and baseline age, p-tau and Aβ42 (continuous variables) and 2-way interactions of p-tau and time, and Aβ42 and time. That is, having the combination of both abnormal p-tau181p and Aβ42 resulted in an additional increased rate of atrophy over time beyond the additive effect of each biomarker. There was also a significant interaction of p-tau and time (p=0.012) when outcome was hippocampal volume (all diagnostic groups combined). Within subgroups, the 3-way interaction remained significant only for the AD group for precuneus (p=0.005) and precentral gyrus (p=0.019) thickness.
DISCUSSION
These results demonstrate that across the Alzheimer’s disease spectrum from normal aging to early dementia, CSF biomarkers exert an influence on rate of atrophy, although this effect varies by region, CSF biomarker, and sample composition.
Baseline association results showed a significant relationship between p-tau181p and hippocampal volume, whereas the relationship between Aβ42 and hippocampal volume was not significant, but a trend was demonstrated. These baseline results supported our prediction that p-tau181p would have a stronger relationship with hippocampal volume than Aβ42. The lack of baseline association of hippocampal volume and markers of amyloid burden has been demonstrated in prior PiB and CSF biomarker studies (Becker et al., 2011; Fagan et al., 2009; Fjell et al., 2010), whereas other studies have demonstrated at least a weak relationship (Apostolova et al., 2010; Henneman et al., 2009; Mormino et al., 2009). A somewhat different pattern of results was revealed for rates of change. Within the entire sample, both p-tau181p and Aβ42 were significantly associated with accelerated rates of hippocampal atrophy. Within-group results demonstrated a relationship between Aβ42 and accelerated rate of hippocampal atrophy within the NC group, but not within the two clinical groups. Although trajectories of change within the NC group may differ since some subjects are destined to develop AD while others are not, the early detection of effects of Aβ42 only in the NC group is consistent with a potential initiating role as suggested by Jack et al. (2010). In contrast, within-group results demonstrated a relationship between p-tau181p and accelerated rate of hippocampal atrophy within the MCI and AD groups, consistent with studies showing that rates of brain atrophy and clinical progression correlate well with pathological indices of NFT (Josephs et al., 2008). Henneman and colleagues (2009) similarly found that CSF p-tau181p predicted accelerated rate of hippocampal atrophy when collapsing across normal and clinical groups, but in that study results did not persist within diagnostic subgroups, potentially due to much smaller sample sizes relative to the current study: In their study, the total sample size combining normal, MCI and AD was 75 subjects. Other studies have demonstrated a significant relationship between p-tau231p or p-tau181p and accelerated hippocampal atrophy in MCI (de Leon et al., 2006; Fjell et al., 2010; Hampel et al., 2005; Tosun et al., 2010).
Considered together, our results demonstrate that lower baseline Aβ42 in the NC group and higher baseline p-tau181p in the MCI and AD groups is associated with an accelerated rate of hippocampal atrophy over time. Our results are consistent with the biomarker model proposed by Hyman, (2011) and further supported by data from Lo et al., (2011) in which Aβ may exert an effect early in the disease, but it has relatively smaller effects later. That is, once there are clinically detectable symptoms warranting a diagnosis of MCI or AD, the downstream effects of Aβ become uncoupled from Aβ itself. Hyman’s model emphasizes a two-stage process in which intervention efforts may be beneficial very early in the disease process, before there is any clinically detectable cognitive symptoms or MRI atrophy, whereas once clinical symptoms emerge, Aβ or another early initiating factor has already instigated the pathological cascade and may be less important for predicting disease progression and ineffective as a treatment target in these later stages of the disease.
Because accumulation of amyloid beta is theorized to occur prior to NFT (Jack et al., 2010), we hypothesized that baseline Aβ42 would be related to accelerated rates of cortical thinning in the precuneus given evidence of early amyloid deposition in this region. However, a pattern opposite to that we predicted was demonstrated: baseline Aβ42 did not predict accelerated rates of cortical thinning in the precuneus, whereas it did predict accelerated rate of hippocampal atrophy as discussed above. In fact, neither CSF biomarker predicted accelerated rates of cortical thinning in the precuneus. A baseline association of p-tau181p and precuneus thickness was demonstrated, whereas a baseline association of Aβ42 and precuneus was not. The precuneus is assumed to be an early site of amyloid deposition based largely on the findings of multiple PiB studies that have demonstrated preferential amyloid uptake in this region (Aizenstein et al., 2008; Mintun et al., 2006; Rowe et al., 2007) and its involvement in the default network (Buckner et al., 2008). A few PiB studies have shown a significant baseline association between amyloid uptake and hippocampal volume (Becker et al., 2011; Chetelat et al., 2010; Fjell et al., 2008), and one study has shown PiB uptake is associated with accelerated hippocampal atrophy in MCI (Tosun et al., 2010). Because PiB and CSF measures of amyloid may not be equivalent, this alone could explain our discrepant findings. Our use of an ROI analysis approach, as opposed to voxelwise analysis that may be more sensitive to localized changes within subregions of the precuneus, may also explain our findings. However, autopsy studies have not demonstrated a predilection for early amyloid plaque accumulation in the precuneus relative to other areas of the neocortex (Nelson et al., 2009), so further investigation of the relationship between amyloid and the precuneus is warranted.
We also predicted a significant relationship between p-tau181p and accelerated rates of precuneus thinning based on a hypothesized indirect relationship. An indirect relationship was expected due to the extensive anatomical and functional connections between posterior cortical regions including the precuneus and medial temporal lobe regions affected early in AD, including the hippocampus (Dorfel, Werner, Schaefer, von Kummer, & Karl, 2009; Kobayashi & Amaral, 2003; Teipel et al., 2010). Decreased resting state functional connectivity between the precuneus and the hippocampus (as well as other regions of the default network) has been demonstrated in patients with early AD, PiB+ normal healthy elderly and PiB− normal healthy elderly APOE4 allele carriers (Sheline, Morris et al., 2010; Sheline, Raichle et al., 2010). Results did not support this hypothesis; we found no baseline or longitudinal associations beween p-tau181p and precuneus thickness. To our knowledge no other studies have directly assessed this relationship.
An interaction between low Aβ42 and high p-tau181p was associated with greater cortical thinning in the precuneus and precentral gyrus and hippocampal atrophy over time, extending the findings of Desikan et al. (2011) to these additional regions in a combined NC, MCI and AD sample. This suggests that having both abnormal Aβ42 and p-tau181p leads to accelerated atrophy. The significance of this effect in our “control” region (precentral gyrus) was surprising and may point toward more diffuse effects of these combined biomarkers. Inconsistent with the results of Desikan et al. (2011), this 3-way interaction persisted only within the AD group for cortical thickness measures. Although the analyses performed on the entire sample demonstrated this effect in all regions studied, the 3-way interaction was not significant within NC or MCI groups when examined individually.
There are several limitations that must be considered when interpreting these results. First, we selected a small number of potential biomarkers to focus on in this study to maintain a narrow focus. We did not include t-tau or ratio values of Aβ42 and tau. We also included only a small number of a priori ROIs, instead of performing exploratory voxelwise analyses. Because ROIs average across an entire region, subtle group difference that may be detected within such a region by voxelwise analysis may be missed; in other words, ROI analyses may be less sensitive. However, examining these effects using ROIs is important, as this is likely to be a widely used approach in large clinical trials applying automated neuroimaging processing techniques. Second, the primary goal of the ADNI is to optimize clinical trials, and the sample is not representative of the general population (e.g., highly educated); therefore the generalizability of the current results is limited. Third, we did not include longitudinal CSF data, thus the timing of biomarker effects cannot be fully disentangled with the current set of analyses. A longer duration of follow-up would likely be necessary to optimally complete such analyses, as there is evidence that little change in CSF biomarkers can be measured over relatively short intervals such as those included in this study (Vemuri et al., 2010). This is particularly notable for Aβ42, as change in this CSF biomarker has not been significantly associated with annual decline in cognitive and functional scores in MCI and AD groups despite evidence of clear cognitive and functional decline (Vemuri et al., 2010), leading some to propose that CSF load is nearly disconnected from the disease stage (Caroli & Frisoni, 2010). Finally, the sample size varied across the diagnostic subgroups, with nearly twice as many subjects in the MCI sample relative to the NC and AD subgroups. This limits the extent to which we can make conclusions regarding the significance or lack thereof of effects across subgroups. Again, this is why we chose to focus primarily on the results collapsed across groups.
In summary, the current results provide at least partial support for the Jack et al. (2010) dynamic biomarker model, although the current analyses alone are insufficient to fully test this model. Results suggest that there may be an early affect of amyloid in the Alzheimer’s pathological cascade process, which appears to be most detectable in its effects on the rate of hippocampal atrophy in normal older people. Despite this early effect, amyloid does not appear to directly affect atrophy in later disease stages. Results also raise questions about how the precuneus is affected by AD since evidence of relationships between Aβ and rates of atrophy are weak. Finally, in conjunction with results from Desikan and colleagues (2011), these results highlight the importance of considering the additive effect of Aβ42 and p-tau181p in the progression of atrophy over time across the Alzheimer’s disease spectrum, and provide further support of the possibility that amyloid deposition alone may be insufficient for emergence of significant morphometric changes and clinical symptoms.
Acknowledgments
This manuscript was a collaborative effort from the 2011 Friday Harbor Advanced Psychometrics Workshop, funded by the National Institute on Aging R13 AG030995. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-LaRoche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, P30 AG008017, R01 AG029672-01A1 and the Dana Foundation.
Footnotes
Disclosures: There were no actual or potential conflicts of interest for any of the authors.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31(8):1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett LA, Harvey DJ, Gamst A, Donohue M, Kornak J, Zhang H, et al. The Alzheimer’s Disease Neuroimaging Initiative: Annual change in biomarkers and clinical outcomes. Alzheimers Dement. 2010;6(3):257–264. doi: 10.1016/j.jalz.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2(10):605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, A beta-amyloid and the onset of Alzheimer’s disease. Neurosci Lett. 1996;210(2):87–90. doi: 10.1016/0304-3940(96)12668-9. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Caroli A, Frisoni GB. The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31(8):1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60(12):1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Inman RL. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician. 1981;35:124–129. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27(3):394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, et al. Amyloid-beta associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011;70(4):657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2. Oxford: Oxford University Press; 2002. [Google Scholar]
- Dorfel D, Werner A, Schaefer M, von Kummer R, Karl A. Distinct brain networks in recognition memory share a defined region in the precuneus. Eur J Neurosci. 2009;30(10) doi: 10.1111/j.1460-9568.2009.06973.x. 1947–1959. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1(8–9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, et al. Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum Brain Mapp. 2009;30(10):3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Amlien I, Bjornerud A, Reinvang I, Gjerstad L, et al. Morphometric changes in the episodic memory network and tau pathologic features correlate with memory performance in patients with mild cognitive impairment. AJNR Am J Neuroradiol. 2008;29(6):1183–1189. doi: 10.3174/ajnr.A1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci. 2010;30(6):2088–2101. doi: 10.1523/JNEUROSCI.3785-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Hampel H, Burger K, Pruessner JC, Zinkowski R, DeBernardis J, Kerkman D, et al. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch Neurol. 2005;62(5):770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- Hampel H, Goernitz A, Buerger K. Advances in the development of biomarkers for Alzheimer’s disease: from CSF total tau and Abeta(1–42) proteins to phosphorylated tau protein. Brain Res Bull. 2003;61(3):243–253. doi: 10.1016/s0361-9230(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Henneman WJ, Vrenken H, Barnes J, Sluimer IC, Verwey NA, Blankenstein MA, et al. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology. 2009;73(12):935–940. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68(8):1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, et al. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6(3):221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63(2):204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, et al. Longitudinal Change of Biomarkers in Cognitive Decline. Arch Neurol. 2011;68(10):1257–1266. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, et al. Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 2009;450(3):336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21(1):117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(Pt 4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6(4):295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, et al. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49(3):2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, et al. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31(8):1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, et al. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75(2):143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]