Abstract
Mammaglobin-A (Mam-A) is a 10 kD secretory protein that is overexpressed in 80% of primary and metastatic human breast cancers. Previous studies from our laboratory demonstrated that Mam-A cDNA vaccine can induce Mam-A specific CD8 T-cell responses and mediate regression of human breast cancer xenografts in NOD/SCID mice. In this report, we present our results on a phase I clinical trial of a Mam-A cDNA vaccination in breast cancer patients with stage-IV metastatic disease, including the impact of vaccination on the expression of the inducible co-stimulator molecule (ICOS) on CD4 T cells. Specimens from seven patients with stage IV metastatic cancer were available for these analyses. Patients were vaccinated with a Mam-A cDNA vaccine on days 0, 28 and 56, and immune responses were assessed at serial time points following vaccination. At six months following the first vaccination, flow cytometric analysis demonstrated a significant increase in the frequency of CD4+ICOShi T cells from 5 ± 2% pre-vaccination to 23 ± 4% (p<0.001), with a concomitant decrease in the frequency of CD4+Foxp3+ T cells (Treg) from 19 ± 6% to 10 ± 5% (p<0.05). ELISpot analysis of CD4+ICOShi sorted T-cells demonstrated that following vaccination the cytokines produced by Mam-A-specific T cells switched from IL-10 (78 ± 21 spm pre-vaccination to 32 ± 14 spm 5 months post-vaccine p<0.001) to IFN-γ (12 ± 6 spm pre-vaccination to 124 ± 31 spm 5 months post-vaccine p<0.001). The ratio of CD4+ICOShi T cells to CD4+Foxp3+ T cells increased from 0.37 ± 0.12 prior to vaccination to 2.3 ± 0.72 (p=0.021) following vaccination. Further, these activated CD4+ ICOShi T-cells induced preferential lysis of human breast cancer cells expressing Mam-A protein. We conclude that mammaglobin-A cDNA vaccination is associated with specific expansion and activation of CD4+ICOShi T cells, with a concomitant decrease in Treg frequency. These encouraging results strongly suggest that Mam-A cDNA vaccination can induce antitumor immunity in breast cancer patients.
Keywords: DNA Vaccine, Mammaglobin-A, Breast Cancer, T-cells, ICOS
Introduction
Breast cancer continues to be a leading cause for cancer related death in women across the globe. Mammaglobin-A (Mam-A), a 10kDa human breast cancer-associated antigen initially identified by Watson and Fleming offers a novel immunotherapeutic target [1]. The Mam-A is expressed almost exclusively in 80% of primary and metastatic breast cancers [1–6]. This specificity as well as wide Mam-A expression in breast cancers, makes it an attractive target for vaccination. We demonstrated that passive transfer of T cells from Mam-A vaccinated HLA-A2/hCD8 double transgenic mice into human breast cancer implanted NOD/SCID mice resulted in significant reduction of tumor size in an HLA-A2 and Mam-A specific manner [7]. Based on these encouraging results, we are currently conducting a phase I clinical trial of a Mam-A cDNA vaccination in breast cancer patients with stage IV metastatic disease.
Enhanced understanding on the role for the innate and adaptive immune systems in controlling the development of malignancies has been established over the past several years [8–10]. Progress in the identification of tumor antigens, elucidation of antigen presenting pathways and effector mechanisms have renewed interest in vaccine therapy for cancer. Immunological analysis following DNA vaccination for the most part has been restricted either towards the induction of antibodies or generation of tumor specific CD8+ T cells [11, 12]. Therefore, currently there is very little information available regarding the impact of cDNA vaccination on tumor specific CD4+ T-cell responses.
Inducible co-stimulator (ICOS) molecules are expressed on newly activated T-cells [13] and in vitro functional studies have demonstrated that ICOS is a potent costimulatory molecule related to B7/CD28 family [14, 15]. Miller et al have demonstrated that tumor-infiltrating CD4+CD25+ regulatory T-cells (Treg) accumulating in prostate tumors express higher levels of ICOS than Treg in the peripheral blood [16]. Recent studies have shown that anti-CTLA-4 treatment of prostate cancer patients can increase the frequency of CD4+ ICOS hi T-cells [17] accompanied by a shift in the ratio of effector to Treg cells and thus improving the clinical outcome. In this report, we provide evidence that following Mam-A cDNA vaccination of breast cancer patients, there is an increased frequency of cytotoxic IFN-γ secreting CD4+ ICOS hi T-cells strongly suggesting that this novel immunotherapeutic approach will be beneficial for treatment of breast cancer.
Experimental Methods
Phase I clinical trial
We have recently initiated a phase I clinical trial of a Mam-A DNA vaccine at Washington University School of Medicine. Seven HLA-A2+ patients with metastatic breast cancer treated with the Mam-A DNA vaccine were available for the correlative studies described in this report. The vaccine was administered on days 0, 28 and 56. Nine normal multiparous women were included in the study following informed consent as a negative control. Another set of patients used as a negative control were the pre-vaccinated patients. As the stage IV metastatic breast cancer has a long disease course a separate cohort of patients for negative control studies were not available during this study. Peripheral blood specimens were obtained prior to vaccination and at serial time points following vaccination. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Uppasala, Sweden) and stored at −135°C until evaluation [18]. The CD4+ T cells were isolated from PBMC by positive selection using a MACS bead isolation kit (Miltenyi Biotec Inc., CA).
ELISpot Assay
Frozen PBMCs were cultured overnight in complete RPMI-1640 and viability was determined by trypan blue exclusion [19]. PBMCs with viability of at least 90% were used for ELISpot analysis. CD4+ T-cells were enriched by MACS bead separation negative selection using immunomagnetic separation cocktails (Stem cell Technologies, Canada). These enriched CD4+ T cells (3×105, >90% purity) were cultured in triplicate in presence of CD3 monoclonal antibody (mAb) (1 µg/mL) and IL-2 (1 µg/mL) for non-specific stimulation or purified Mam-A protein (20µg/ml) on the 96 well ELISpot plates (Multiscreen IP plate, Millipore, MA) pre-coated with IFN-γ mAb (4 µg/mL) or IL-10 mAb (4 µg/mL) in the presence of autologous irradiated CD4 depleted PBMCs as antigen presenting cells (APCs) (3×104) in complete RPMI-1640 medium . Cultures were placed for 48 to 72 hrs in humidified 5% CO2 incubator at 37°C for IFN-γ or IL-10. The plates were washed and developed to detect the number of spots in individual wells using an ImmunoSpot analyzer (Cellular Technology, Shaker Heights, OH). CD4+ T cells plus autologous APCs cultured in medium without antigens was negative control while CD4+ T cells plus autologous APCs cultured with PHA (5 µg/ml) was positive control. Number of spots in negative control subtracted from spots in experimental wells were reported in final results as spots per million cells (spm ± SEM).
Flow cytometry
Abs used for flow cytometry consisted of: CD3-FITC, CD4-PerCP/Cy5.5 (BD PharMingen), CD25-PE, Foxp3-PE (eBiosciences, San Diego, CA), and biotinylated ICOS (eBiosciences) conjugated with streptavidin-APCCy7 (BD Biosciences, San Diego, CA). Intracellular staining for Foxp3, was conducted as per the manufacturer’s guidelines. Samples were analyzed using a FACS Calibur/LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and cell sorting was performed with a Vantage cell sorter (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using BD FACSDiva software. Gates were set according to isotype controls.
RT-PCR
Expression profiles of intracellular signal genes in the CD4+T-cells isolated from PBMCs were analyzed using the FAM-labeled quantitative RT-PCR primers for IL-10, IFN-γ, T-bet and FoxP3 (Applied Biosystems, Foster City, CA) as per the manufacturer’s recommendation. Briefly, total RNA was extracted from 106 cells using TRIzol reagent (Sigma-Aldrich, St. Louis, MO). The RNA was reverse-transcribed and Real-time PCR was performed in a final reaction volume of 20 µL using iCycler 480 Probes Master (Roche Diagnostics, Indianapolis, IN). Each sample was analyzed in triplicate. Cycling conditions consisted of an initial denaturation at 95°C for 15 min, followed by 40 cycles of 95°C for 30s, followed by 61°C for 1 min.
Breast cancer cell lines
The breast cancer cell lines, AU-565 (Mam-A+) and MCF-7 (Mam-A-) [20, 21], were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Breast cancer cell lines were cultured in RPMI-1640 culture medium at 37 °C in 5% CO2 incubator until they reached an optimal 80% confluency. The presence of Mam-A in the cell lines were determined by reverse transcriptase-polymerase chain reaction and western blot analysis.
Cytotoxicity assay
Ability of T cells to lyse the target cells was investigated by LDH cytotoxicity assay (Promega, Madison, WI) [22]. Breast cancer cells (5×103 cells) in 100µl of complete medium were plated in triplicate cultures in round bottom 96-well plates in the presence of varying numbers of CD4+ICOShiT cells (6.25:1 to 50:1) and incubated at 37°C in a humidified 5% CO2 incubator for 4 hrs. Controls were breast cancer target cells alone or CD4+ICOShi T cells isolated from normal subjects. Maximal release was determined by adding Triton X-100 (1%) to the target cells. A colorimetric measurement of the released LDH was developed as per manufacturer’s protocol and measured using UV/Visible spectrophotometer at 450 nm. The percentage specific lysis was calculated using the formula [(experimental LDH release - spontaneous LDH release)/(maximum LDH release - spontaneous LDH release)] × 100. Cold target inhibition analyses were performed to determine the Mam-A specificity with the addition of Mam-A protein (1 to 1000 ng/mL) to culture medium and subsequent LDH release.
Statistical Analysis
Graphpad Prism version 4.03 software (GraphPad Software Inc, CA) was utilized. Mann-Whitney test assessed differences in CD4+ T cell responses specific to individual antigens between two groups (preand post-vaccination). Correlation analysis was performed using Spearman rank test. Two-sided level of significance was set at p<0.05 and results were expressed in mean ± standard error of mean.
Results
Increased frequency of CD4+Foxp3+ T cells in the circulation of breast cancer patients
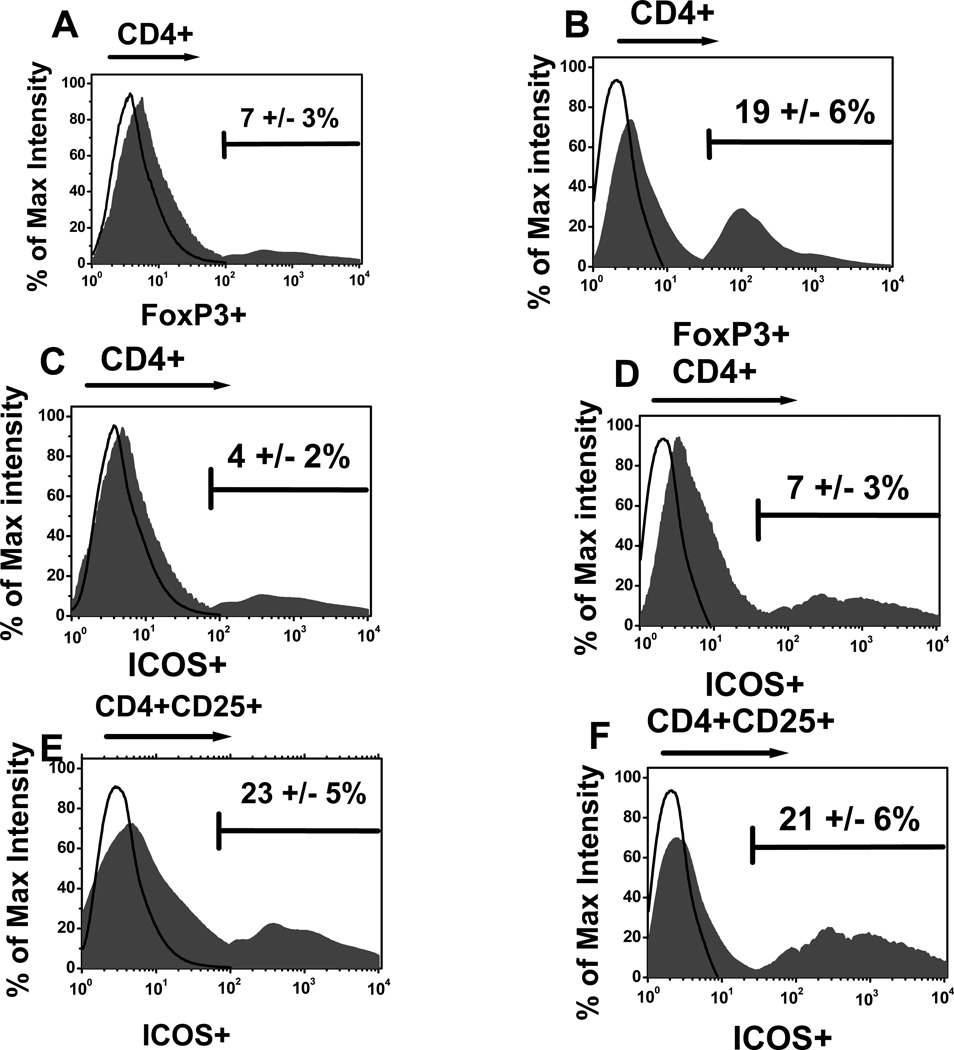
We used the FoxP3 expression on CD4+T-cells to determine the frequency of Treg in Breast cancer patients [23]. As shown in figure 1 the frequency of CD4+Foxp3+ T-cells were significantly increased (p<0.05) in breast cancer patients (n=7) in comparison to normal subjects (19 ± 6% vs 7 ± 3% in normals [n=9]). However, the subset of Tregs which express ICOS in the breast cancer patients (21+5%) remained similar to normals (21±6%). The frequency of ICOS in CD4+T-cells in breast cancer patients (7±3%) was slightly higher than normal subjects (4±2%) which did not reach statistical significance (p=0.67).
Figure 1.
The frequency of CD4+Foxp3+ (A and B), CD4+ ICOShi (C and D) and CD4+CD25+ICOShi (E and F) in normal (n=9) (A, C and E) and breast cancer patients (n=7) (B, D and F).
Increased frequency of CD4+ICOShi T cells following Mam-A vaccination
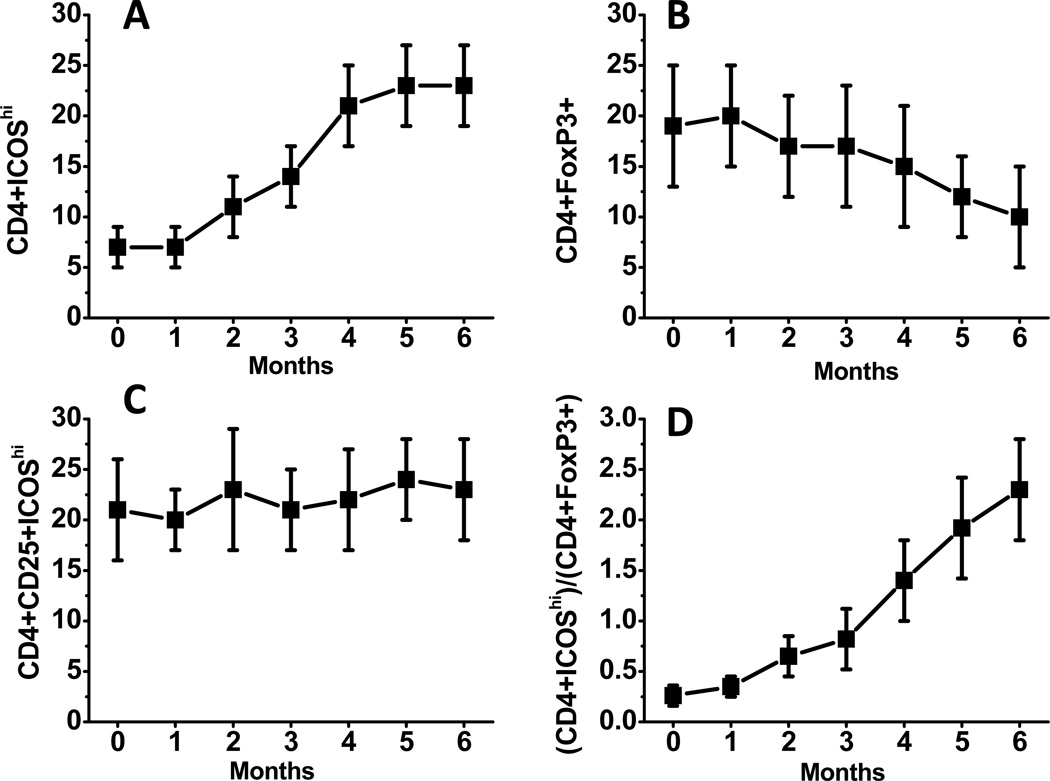
ICOS is expressed in two (Th1 and Th2) subsets of CD4+ T-cells, including Treg [24], and newly activated naïve CD4+ T cells [25]. To evaluate T-cell activation following Mam-A vaccination, the frequency of CD4+ T cells with ICOShi frequency was determined using flow cytometry. As shown in figure 2A, following Mam-A cDNA vaccination within a period of 6 months, the frequency of CD4+ICOShi T cells increased from 7±3% to 23±4%. In contrast, the frequency of Treg (CD4+ Foxp3+) cells decreased (figure 2B) from 19±6% to 10±5%, while the ICOS expression of Treg (CD4+CD25+ICOShi) remained constant (figure 2C) during this period. Conversely, the ratio of CD4+ICOShi to CD4+Foxp3+ cells increased from 0.37±0.12 to 2.3±0.72. These results strongly suggest that following Mam-A vaccination, there was activation of CD4+ T cells with increases in the ICOS expression.
Figure 2.
Serial analysis of the frequency of CD4+ ICOShi (A), CD4+Foxp3+ (B), CD4+CD25+ICOShi (C) and CD4+ICOShi to CD4+Foxp3+ (D) in metastatic breast cancer patients vaccinated with Mam-A cDNA vaccine on days 0, 28 and 56.
Mam-A vaccination resulted in higher frequency of IFN-γ secreting T cells and a decreased frequency of IL-10 secreting CD4+T-helper cells
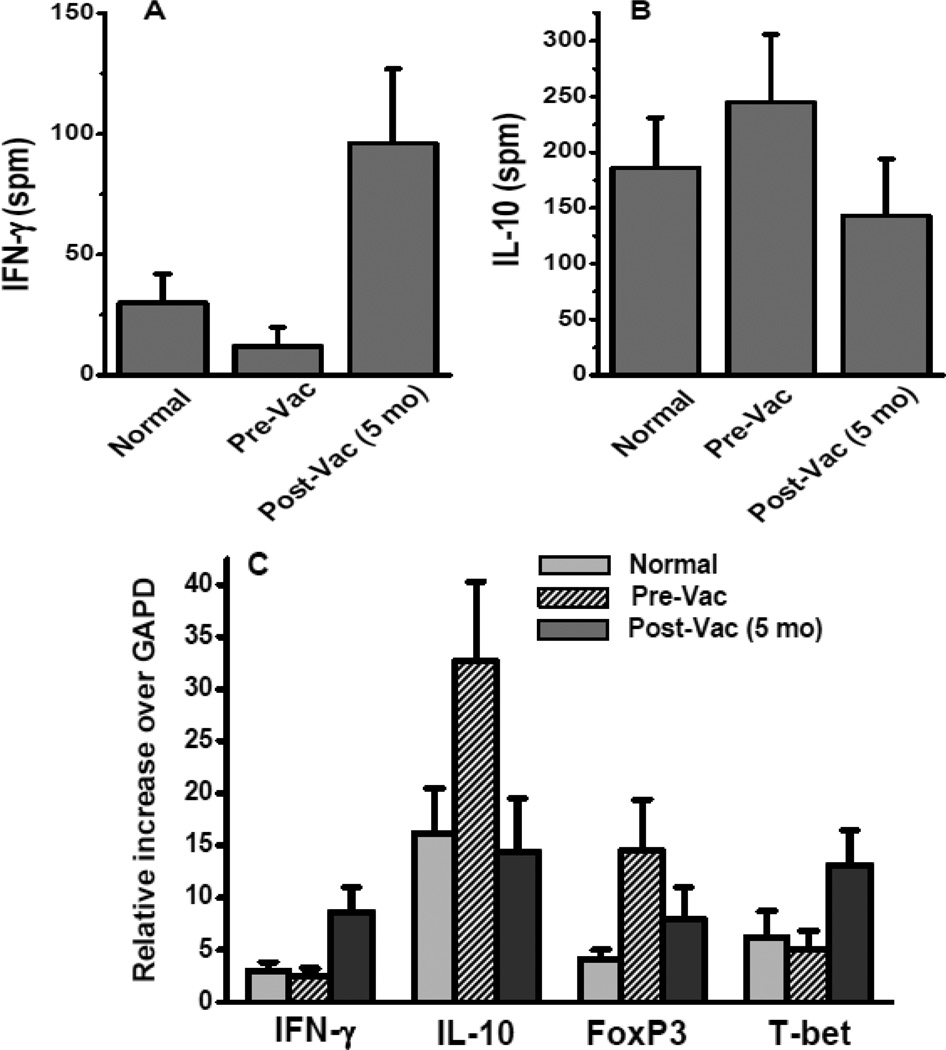
To analyze for a possible switch in the effector cytokine secretion profile of the Th-phenotype induced by Mam-A vaccination, PBMCs from the breast cancer patients who received Mam-A vaccine were analyzed by ELISpot. As shown in Figure 3A, upon non-specific stimulation with anti-CD3 and IL-2, the CD4+T-cells from breast cancer patients induced lower IFN-γ release (12±8 spm) in comparison to the CD4+T-cells from normal subjects (30±12 spm), however, this difference did not achieve statistical significance (p= 0.34). In contrast, following Mam-A vaccination the CD4+T-cells from breast cancer patients demonstrated significantly higher IFN-γ response (96±31 spm, p=0.001). Results shown in Figure 3B demonstrates that upon non-specific stimulation, the CD4+T-cells from breast cancer patients prior to receiving Mam-A vaccination induced significantly higher anti-inflammatory IL-10 release (245±61 spm) as compared with the CD4+T-cells response from normal subjects (186±45 spm, p=0.024). However, following Mam-A vaccination the CD4+T-cells from breast cancer patients demonstrated significantly lower IL-10 response compared to pre-vaccination response(143±51 spm, p=0.012). This demonstrates that Mam-A vaccination of breast cancer patients increased the frequency of IFN-γ producing T cells along with decreased IL-10 response.
Figure 3.
ELISpot assay on CD4+ T-cells collected from normal, breast cancer patients prior to (Pre-Vac) and 5 months following first dose of vaccine (Post-Vac) with non-specific stimulation of CD3 mAb and IL-2 , latter tested for the cytokine release of IFN-γ (A) and IL-10 (B); (C) mRNA level expression of IFN-γ, IL-10, FoxP3 and T-bet from the uncultured CD4+ T-cells collected from normal, breast cancer patients prior to and 5 months following first dose of vaccine.
Furthermore, mRNA expression profile in the unstimulated CD4+T-cells reveals increased expression of IFN-γ (normal: 3±0.8 fold; breast cancer: 2.5±0.7 fold; and following Mam-A vaccine: 8.6±2.4 fold, p=0.003), and effector Th1 lineage marker T-bet (normal: 6.2±2.5 fold; breast cancer: 5.1±1.7 fold; and following Mam-A vaccine: 13.1±3.4 fold, p=0.012). There is also a decreased expression of IL-10 (normal: 16.2±4.3 fold; breast cancer: 32.7±7.6 fold; and following Mam-A vaccine: 14.4±5.1 fold, p=0.001), and FoxP3 (normal: 4.1±0.9 fold; breast cancer: 14.5±4.9 fold; and following Mam-A vaccine: 7.9±3.1 fold, p=0.0048). This confirms our previous results with non-specific stimulation with anti-CD-3 /IL-2 which demonstrated that following Mam-A vaccination there was a shift towards cytotoxic Th-response along with increased IFN-γ secretion.
Induction of Mam-A-specific IFN-γ responses following vaccination
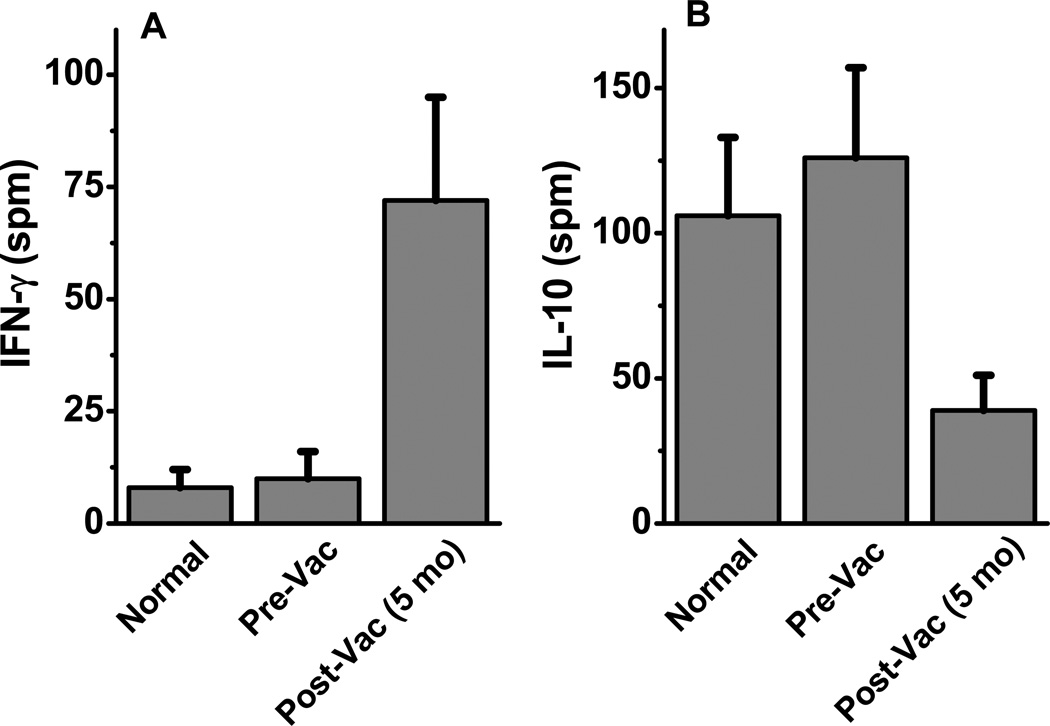
To determine the specificity of IFN-γ responses to Mam-A protein, cytokine responses of CD4+T cells specific for Mam-A protein was determined by ELISpot. The CD4+T-cells were incubated for 48–72 hours in the presence of Mam-A whole protein or human albumin (control) along with autologous irradiated CD4 depleted PBMCs. ELISpot assay was performed to determine the Mam-A specific frequency of IFN-γ and IL-10 producing CD4+T cells. As shown in Figure 4A, following vaccination the breast cancer patients had markedly enhanced IFN-γ responses specific to Mam-A protein (normal/control: 8±4 spm; breast cancer: 10±6 spm; and following Mam-A vaccine: 72±23 spm, p=0.0001). In contrast, the IL-10 responses specific to Mam-A significantly (Figure 4B) decreased (normal/control: 106±27 spm; breast cancer: 126±31 spm and following Mam-A vaccine: 39±12 fold, p=0.0011) following Mam-A vaccination. These results confirm that the increased frequency of IFN-γ+CD4+ T cells observed following vaccination was Mam-A-specific, and suggest that these cells may have cytotoxic capacity.
Figure 4.
ELISpot assay on CD4+ T-cells collected from normal, breast cancer patients prior to (Pre-Vac) and 5 months following first dose of vaccine (Post-Vac) with specific stimulation of Mam-A purified protein, latter tested for the cytokine release of IFN-γ (A) and IL-10 (B).
Increased frequency of IFN-γ+CD4+ICOShi T cells following Mam-A vaccination
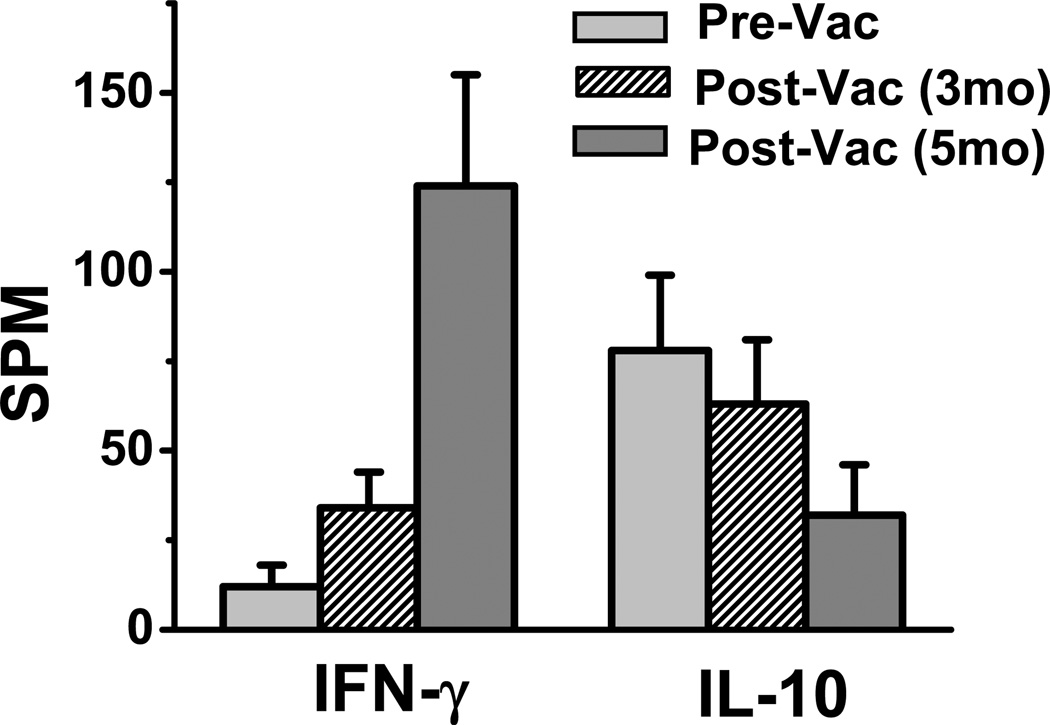
To further analyze the phenotype of the newly activated Mam-A restricted CD4+ ICOShi T-cells, we performed ELISpot analysis on the CD4+ICOShi T-cells collected at serial time points from breast cancer patients following Mam-A vaccination. The CD4+ ICOShi sorted T-cells were incubated for 48–72 hours in the presence of either Mam-A whole protein or human albumin (control) along with autologous irradiated CD4 depleted PBMCs prior to performing ELISpot assay. As shown in figure 5following vaccination the breast cancer patients demonstrated increased IFN-γ response specific to Mam-A protein (normal: 12±6 spm; breast cancer: 34±10 spm; and following Mam-A vaccine: 124±31 spm, p=0.0001), while IL-10 responses specific to Mam-A is significantly decreased (normal: 78±21 spm; breast cancer: 63±18 spm; and following Mam-A vaccine: 32±14 spm, p=0.0027). These results strongly support the hypothesis that the newly activated ICOShi T-helper cells were the cells which were producing higher levels of IFN-γ.
Figure 5.
ELISpot assay on CD4+ ICOShiT-cells collected from breast cancer patients prior to (Pre-Vac), 5 months and 5 months following first dose of vaccine (Post-Vac-3mo, 5mo) with specific stimulation of Mam-A purified protein, latter tested for the cytokine release of IFN-γ (A) and IL-10 (B).
Mam-A vaccination increases the frequency of cytotoxic CD4+ICOShi T cells
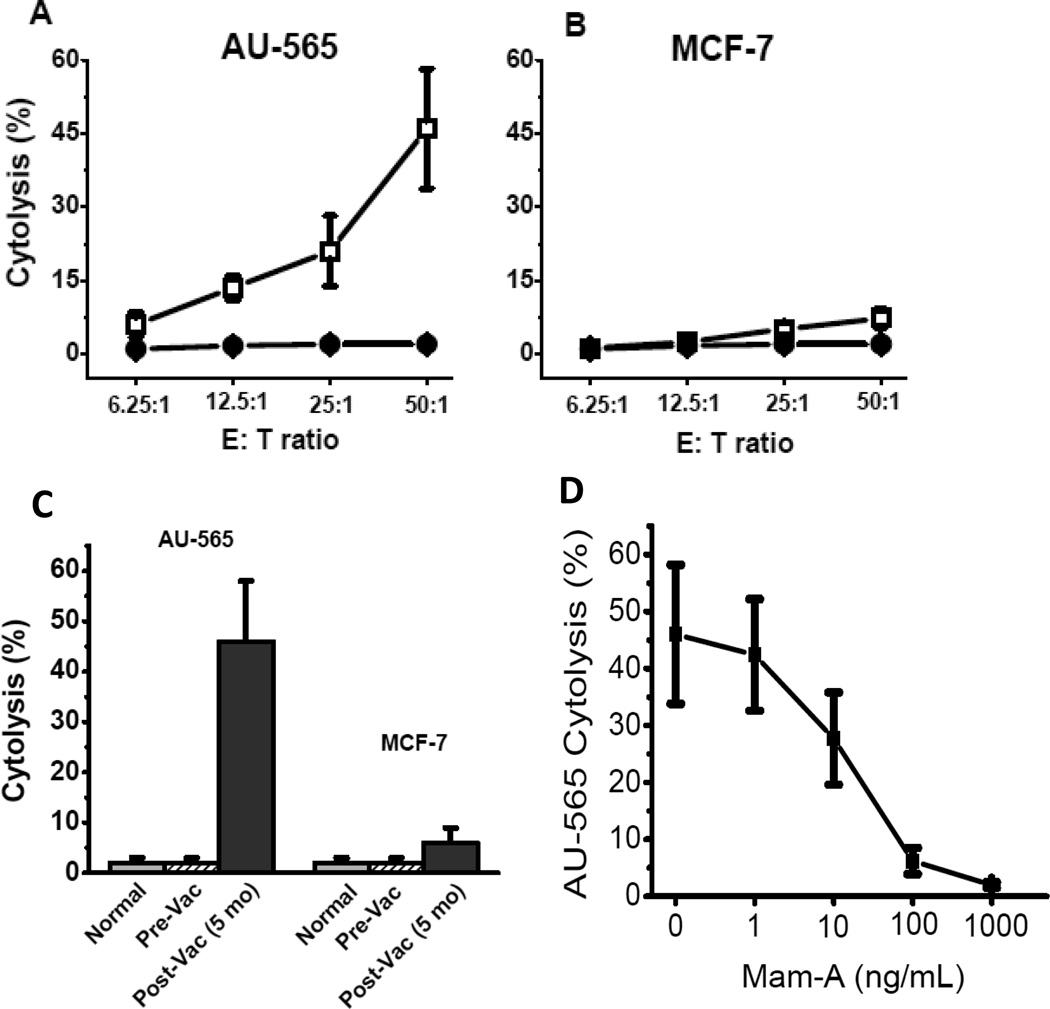
To directly analyze the cytotoxic effect of the CD4+ ICOShi T-cells, we performed cytotoxicity assay on the human breast cancer cell lines AU-565 (Mam-A+) and MCF-7 (Mam-A-) [20, 21]. As shown in figure 6, co-culture of CD4+ ICOShi T-cells (collected from breast cancer patients following vaccination) with AU-565 breast cancer cell line (Mam A+) resulted in significant cytotoxicity (46±12% vs 2±1%, p<0.001). This was specific to Mam-A since no increase in cytotoxicity was noted with a Mam-A(-) MCF-7 breast cancer cell line (6±4% vs 2±1%, p=0.41). Furthermore, addition of Mam-A protein abrogated the cytolytic ability (Figure 6D) of AU-565 (MamA+) by CD4+ ICOShi T-cells in a dose dependent manner (1 to 1000 ng/mL). These results demonstrated that of CD4+ ICOShi T-cells induced following Mam-A vaccination preferentially lyse human breast cancer cells expressing Mam-A protein.
Figure 6.
Cytotixic effect of CD4+ ICOShiT-cells on AU-565 (A) and MCF (B) with CD4+ ICOShiT-cells collected to prior to (open squares) and 5 months following (closed circles) first dose of vaccination with a serial titration of E:T ratio from 6.25:1 to 50:1; (C) Maximum cytotoxic effect of CD4+ ICOShiT-cells at 50:1/E:T ratio on AU-565 and MCF-7 by CD4+ ICOShiT-cells collected from normal, breast cancer patients prior to (Pre-Vac) and 5 months following first dose of vaccination (Post-Vac); (D) Cytotoxic effect of CD4+ ICOShi T-cells from 5 months post-vaccinated patients at 50:1/E:T ratio on AU-565 was inhibited by external addition of Mam-A protein (1 – 1000 ng/mL) in dose-dependent manner.
Discussion
The activation and expansion of immune T cells is tightly controlled by transmembrane ligand/receptor signals [26]. In 1999, Hutloff et al demonstrated the expression of ICOS on CD4+ T-cells using antibodies produced against activated human T cells [27]. Sequence analysis also demonstrated 39% homology to human CD28 [27]. ICOS is a T-cell specific surface molecule related to B7 family along with CD28 and CTLA-4. Although ICOS is expressed at low levels on resting naïve T cells, it is rapidly upregulated following T cell receptor ligation and CD28 costimulation [28]. In vivo studies using ICOS knock-out mice demonstrated that these animals were incapable of controlling viral or parasitic infections owing to impaired Th1 and Th2 responses, respectively [29]. Bladder cancer patients following anti-CTLA-4 monoclonal therapy demonstrated increased frequency of ICOShi in CD4+ T cells in peripheral blood and tumor tissue microenvironment [30]. Furthermore, these ICOShi sub-fraction of CD4+ T cells upon restimulation induced significantly increased levels of IFN-γ, thus suggesting effector functions for the ICOShi expressing T cells.
Successful cancer-specific immunotherapy is based on the identification of tumor-specific antigens and their epitopes for both CD4 and CD8 T cells. The ability of DNA vaccines and CpG oligo-deoxynulceotides to preferentially generate Th1 responses in mouse models has been shown to be useful in prevention and or treatment of intracellular infections [31]. We have previously demonstrated that Mam-A cDNA vaccination of HLA-A/hCD8 double transgenic animals induced specific T cells which upon passive transfer resulted in tumor regression of human breast cancer tumors growing in SCID mice [7]. Based upon this pre-clinical study wehave recently initiated a phase I clinical trail to study the safety and efficacy of Mam-A cDNA vaccination of breast cancer patients with stage IV metastasis. In this report, we present our preliminary results demonstrating that Mam-A cDNA vaccination resulted in eliciting a strong CD4 cellular effector immune responses with antigen specific cytotoxicity.
TICOS has been shown to be expressed on newly activated CD4, CD8 and Treg cell populations [32]. A higher frequency of Treg have been seen in melanoma [33] prostate [16] and bladder cancers [30], and were implicated in evasion of immune system favoring continued growth of the tumors. We demonstrate in this report that Foxp3+ Treg frequency is higher in breast cancer patients (19±6% vs 7±3% in normal subjects, Fig. 1) and more importantly, our results clearly indicate that following Mam-A vaccination there is a significant decrease in the Treg frequency (19±6% vs 10±5%), although not statistically significant possibly due to small number of patients analyzed so far. Our results also demonstrate that ICOS expression on CD4+CD25+Tregs remained constant (approximately 21%) in breast cancer patients prior to and following vaccination similar to that seen in normal subjects. However, the CD4+ICOShi frequency significantly increased following vaccination of breast cancer patients from 7±3% to 23±4%. Furthermore, the ratio of CD4+ICOShi to Tregs also increased from 0.37±0.12 to 2.3±0.72 (Fig. 2). This strongly demonstrates that following Mam-A vaccination there is increased activation of all populations of CD4+T-cells. The ELISpot assay demonstrated that these are Th1/IFN-γ releasing CD4+T-cells (Fig. 3) with significant decrease in Th2/IL-10 frequency. This is also confirmed by mRNA expression analysis, where in increased expression of T-bet a Th1 specific molecule was noted. These results support the conclusion that Mam-A vaccination results in the expansion of Th1 immunity.
The CD4+ T cells were traditionally considered to be a helper cell population which contribute to cytolytic action through augmenting CD8+ T cell activity. However, studies from our laboratory [34] and others [35] have demonstrated direct cytotoxic effector functions for human CD4+ T cells resulting in specific cytotoxicity mediated by perforin and granzyme. Also, there are reports demonstrating that cytotoxic phenotype of T-helper subsets (Th1/IFN-γ) correlates with ICOS expression [36]. Quiroga et al demonstrated that ICOS activation promotes the induction of Th1/IFN-γ cytokine responses against mycobacterium tuberculosis, an intracellular bacterial pathogens [37]. These results are in agreement with our data which demonstrates that newly activated CD4+ICOShi T-cells releasing IFN-γ which were also cytotoxic in vitro to breast cancer cell lines expressing Mam-A protein with HLA class I (HLA-A2) restriction (Fig.5 and Fig. 6). However, whether these cells are involved in vivo in the direct tumor cytotoxic activity to breast cancer cells following Mam-A vaccination needs to be established. Our data is in agreement with the finding of Chen et al, who demonstrated increased frequency cytotoxic IFN-γ producing CD4+ICOShi T-cells in prostate cancer patients following anti-CTLA-4 therapy [17].
In conclusion, data presented in this manuscript demonstrate that following Mam-A DNA vaccination there is a specific expansion of IFN-γ producing CD4+ICOShi T cells with to the ability to specifically lyse breast cancer cells expressing Mam-A. These results are encouraging and support the potential use of Mam-A DNA vaccination as a novel therapeutic strategy for treatment of breast cancer. Our studies, along with other published findings, also suggest that ICOS expression may be employed as a surrogate marker of antitumor response following immune therapy.
Acknowledgement
The authors would like to thank Ms. Billie Glasscock for her assistance in submitting this manuscript.
Funding
This project was funded by DOD/CDMRP-BCRP W81XWH-06-1-0677 (WG). TM is funded by the BJC Foundation.
Abbreviations
- APCs
Antigen presenting cells
- ICOS
Inducible co-stimulatory molecule
- Mam-A
Mammaglobin-A
- mAb
Monoclonal antibody
- PBLs
Peripheral blood lymphocytes
- PBMCs
Peripheral blood mononuclear cells
- Treg
Regulatory T cells
References
- 1.Watson MA, Fleming TP. Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res. 1994;54(17):4598–4602. [PubMed] [Google Scholar]
- 2.Mikhitarian K, Gillanders WE, Almeida JS, Hebert Martin R, Varela JC, Metcalf JS, Cole DJ, Mitas M. An innovative microarray strategy identities informative molecular markers for the detection of micrometastatic breast cancer. Clin Cancer Res. 2005;11(10):3697–3704. doi: 10.1158/1078-0432.CCR-04-2164. [DOI] [PubMed] [Google Scholar]
- 3.Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, Fleming TP. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59(13):3028–3031. [PubMed] [Google Scholar]
- 4.Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci. 2000;923:78–89. doi: 10.1111/j.1749-6632.2000.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 5.Goedegebuure PS, Watson MA, Viehl CT, Fleming TP. Mammaglobin-based strategies for treatment of breast cancer. Curr Cancer Drug Targets. 2004;4(6):531–542. doi: 10.2174/1568009043332862. [DOI] [PubMed] [Google Scholar]
- 6.Gillanders WE, Mikhitarian K, Hebert R, Mauldin PD, Palesch Y, Walters C, Urist MM, Mann GB, Doherty G, Herrmann VM, Hill AD, Eremin O, El-Sheemy M, Orr RK, Valle AA, Henderson MA, Dewitty RL, Sugg SL, Frykberg E, Yeh K, Bell RM, Metcalf JS, Elliott BM, Brothers T, Robison J, Mitas M, Cole DJ. Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlates with traditional predictors of prognosis: an interim analysis of a prospective multi-institutional cohort study. Ann Surg. 2004;239(6):828–837. doi: 10.1097/01.sla.0000128687.59439.d6. discussion 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayanan K, Jaramillo A, Benshoff ND, Campbell LG, Fleming TP, Dietz JR, Mohanakumar T. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J Natl Cancer Inst. 2004;96(18):1388–1396. doi: 10.1093/jnci/djh261. [DOI] [PubMed] [Google Scholar]
- 8.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184(5):1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 10.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson HL. DNA vaccines: basic mechanism and immune responses (Review) Int J Mol Med. 1999;4(5):549–555. doi: 10.3892/ijmm.4.5.549. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 13.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409(6816):97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 15.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13(1):95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 16.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177(10):7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C, Sharma P. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106(8):2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharat A, Benshoff N, Fleming TP, Dietz JR, Gillanders WE, Mohanakumar T. Characterization of the role of CD8+T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res Treat. 2008;110(3):453–463. doi: 10.1007/s10549-007-9741-2. [DOI] [PubMed] [Google Scholar]
- 19.Ilias Basha H, Tiriveedhi V, Fleming TP, Gillanders WE, Mohanakumar T. Identification of immunodominant HLA-B7-restricted CD8+ cytotoxic T cell epitopes derived from mammaglobin-A expressed on human breast cancers. Breast Cancer Res Treat. 2011;127(1):81–89. doi: 10.1007/s10549-010-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer. 2002;102(5):499–506. doi: 10.1002/ijc.10736. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo A, Narayanan K, Campbell LG, Benshoff ND, Lybarger L, Hansen TH, Fleming TP, Dietz JR, Mohanakumar T. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes by CD8+ cytotoxic T lymphocytes from breast cancer patients. Breast Cancer Res Treat. 2004;88(1):29–41. doi: 10.1007/s10549-004-8918-1. [DOI] [PubMed] [Google Scholar]
- 22.Bopp SK, Lettieri T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008;8:8. doi: 10.1186/1471-2210-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ. Regulatory T-cell development: is Foxp3 the decider? Nat Med. 2007;13(3):250–253. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 24.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199(11):1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, Nicolas JF. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol. 2010;126(2):280–289. doi: 10.1016/j.jaci.2010.05.022. 289 e281-287. [DOI] [PubMed] [Google Scholar]
- 26.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 27.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 28.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165(9):5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 29.Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192(1):53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurunathan S, Wu CY, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12(4):442–447. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D. The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur J Immunol. 2007;37(7):1796–1808. doi: 10.1002/eji.200636661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol. 2008;180(5):2967–2980. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 34.Susskind B, Shornick MD, Iannotti MR, Duffy B, Mehrotra PT, Siegel JP, Mohanakumar T. Cytolytic effector mechanisms of human CD4+ cytotoxic T lymphocytes. Hum Immunol. 1996;45(1):64–75. doi: 10.1016/0198-8859(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 35.Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1(1):20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslan N, Yurdaydin C, Wiegand J, Greten T, Ciner A, Meyer MF, Heiken H, Kuhlmann B, Kaiser T, Bozkaya H, Tillmann HL, Bozdayi AM, Manns MP, Wedemeyer H. Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat. 2006;13(8):505–514. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 37.Quiroga MF, Pasquinelli V, Martinez GJ, Jurado JO, Zorrilla LC, Musella RM, Abbate E, Sieling PA, Garcia VE. Inducible costimulator: a modulator of IFN-gamma production in human tuberculosis. J Immunol. 2006;176(10):5965–5974. doi: 10.4049/jimmunol.176.10.5965. [DOI] [PubMed] [Google Scholar]