SUMMARY
The natural history of adult worker honey bees (Apis mellifera) provides an opportunity to study the molecular basis of learning in an ecological context. Foragers must learn to navigate between the hive and floral locations that may be up to miles away. Young pre-foragers prepare for this task by performing orientation flights near the hive, during which they begin to learn navigational cues such as the appearance of the hive, the position of landmarks, and the movement of the sun. Despite well-described spatial learning and navigation behavior, there is currently limited information on the neural basis of insect spatial learning. We found that Egr, an insect homolog of Egr-1, is rapidly and transiently upregulated in the mushroom bodies in response to orientation. This result is the first example of an Egr-1 homolog acting as a learning-related immediate-early gene in an insect and also demonstrates that honey bee orientation uses a molecular mechanism that is known to be involved in many other forms of learning. This transcriptional response occurred both in naïve bees and in foragers induced to re-orient. Further experiments suggest that visual environmental novelty, rather than exercise or memorization of specific visual cues, acts as the stimulus for Egr upregulation. Our results implicate the mushroom bodies in spatial learning and emphasize the deep conservation of Egr-related pathways in experience-dependent plasticity.
KEY WORDS: immediate-early gene, mushroom bodies, honey bee, orientation flight, learning
INTRODUCTION
Honey bees provide an advantageous system for molecular studies of ecologically relevant learning. Honey bees are central place foragers that depend on ephemeral and scattered floral resources. Foraging workers must form robust long-term memories of floral and hive locations, as well as the odor and appearance of preferred floral sources (Robinson and Dyer, 1993; Menzel et al., 2006). Young bees that have not yet initiated foraging behavior (pre-foragers) prepare for the navigational challenges of foraging by performing short learning flights called orientation flights, during which bees acquire information about landmarks and learn to associate position of the sun, time of day and directionality (Winston, 1987; Capaldi and Dyer, 1999). Bees with prior foraging experience will also perform re-orientation flights after the relocation of a colony (Winston, 1987). This information is necessary for later foraging behavior (Becker, 1958), during which bees must accurately navigate between floral sources and the hive. Orientation flights thus offer an exceptional opportunity for molecular analysis because they are discrete, natural learning events driven by innate behavior.
Early growth response protein 1 (Egr-1; also known as zif268, NGFI-A, Krox-24 or zenk) is a canonical immediate-early gene (IEG), a transcription factor whose expression is activity-dependent and associated with learning and novelty detection in many vertebrate systems (Knapska and Kaczmarek, 2004). Egr-1 expression is induced in the hippocampus of rodents engaged in spatial learning tasks (Bozon et al., 2002). Recent investigation of IEG activity in insects has yielded growing evidence for activity- and learning-related expression similar to that in vertebrates (Alaux and Robinson, 2007; Ghosal et al., 2010; Kiya and Kubo, 2011; Kiya et al., 2008; Sen Sarma et al., 2010). However, the insect homolog of Egr-1, here named Egr, has mainly been studied for its role in muscle development (Volk, 1999), though it has been shown to be upregulated in the brains of flies after seizure, suggesting that it may also be induced by neuronal activity (Guan et al., 2005).
Brain regions involved in vertebrate, particularly mammalian, spatial learning have been identified and extensively studied (Mizumori et al., 2004; Moser et al., 2008). Less is known about the neural basis for spatial learning in insects, but there is evidence to suggest that the mushroom bodies, a region of the insect brain involved in sensory integration and memory, support spatial learning in some species (Mizunami et al., 1998; Farris, 2008).
We explored whether honey bee orientation involves a molecular mechanism that is known to be involved in many other forms of learning, characterized by Egr expression. We used this IEG to identify the mushroom bodies as a brain region that is active during orientation flights. To further explore the relationship between Egr expression and orientation flights, we conducted a series of behavioral manipulations designed to isolate various aspects of orientation flight experience. To do this, we tested a set of hypotheses related to the ability of exercise, motor learning, specific visual cues and visual environmental perception to induce upregulation of Egr (Table 1).
Table 1.
Hypotheses tested to determine what aspects of the orientation flight experience are necessary for Egr induction in the honey bee mushroom bodies
MATERIALS AND METHODS
Phylogenetic analyses
We obtained the nucleotide and amino acid sequences for Egr-1, stripe (original Drosophila name for Egr ortholog), GB50091 and >AGAP005288 in Mus musculus, Drosophila melanogaster, Apis mellifera and Anopheles gambiae, respectively, from GenBank. Sequence orthology was examined using CLUSTALW2 alignments (Goujon et al., 2010) and displayed with Boxshade 3.21 (http://www.ch.embnet.org/software/BOX_form.html).
Animals
Single-cohort colonies were created as in Robinson et al. (Robinson et al., 1989). Frames of honeycomb containing pupae were collected from 25–30 colonies with naturally mated queens and stored in a dark, humid incubator at 34°C. Adults were removed from the comb within 24 h of emergence, marked on the thorax with paint (Testors, Rockford, IL, USA) and introduced to an experimental colony. Each colony was composed by placing a laying queen and ~3000 one-day-old adult workers bees into a small Styrofoam hive box with several frames with honeycomb containing nectar and pollen. To ensure that bees were naïve to flight, the entrance to each colony was blocked, and colonies were kept inside at 21–23°C and protected from light for 5–6 days after assembly. In single-cohort colonies composed of young bees, a subset will undergo precocious maturation to produce a foraging workforce (Huang and Robinson, 1996). A previous study found that when flight is restricted for several days, bees on the cusp of foraging become ‘primed’ to make an orientation flight, exiting the colony to do so as soon as the entrance is opened (Capaldi and Dyer, 1999). This process therefore creates a convenient population of young bees ready to perform orientation flights. All experiments, other than the test of Hypothesis II, were performed using young, pre-forager bees. On the day of experimentation, colonies were moved outside and allowed to acclimate for at least 30 min.
Collections and gene expression analyses
Bees that were sampled for molecular analysis were first placed in individual cages and returned to the hive for 30 min (in one experiment, 60 min). Expression of Egr-1 typically peaks 30 min after the initiating stimulus in vertebrate studies (Zangenehpour and Chaudhuri, 2002). Bees were then flash-frozen in liquid nitrogen (for qRT-PCR analysis) or chilled on ice until brain dissection (for in situ analysis).
In situ hybridization was performed as previously described (Velarde et al., 2006). Whole brains were dissected in cold physiological saline (Fahrbach et al., 1995), frozen in OCT medium, and cut in 12 μm frontal sections. Sections were mounted on slides, fixed in 4% paraformaldehyde and hybridized overnight with a digoxigenin (DIG)-labeled RNA probe. After incubation with anti-DIG alkaline phosphatase, slides were developed in NBT/BCIP (nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3′-indolyl phosphate p-toluidine salt) until a clear signal was visible.
qRT-PCR on mushroom body tissue was performed as described in Lutz et al. (Lutz et al., 2012). Mushroom bodies were isolated as described in that study after treatment of flash-frozen heads with RNAlater-ice (Life Technologies, Carlsbad, CA, USA). RNA was extracted from isolated mushroom bodies or other brain regions using Picopure extraction kits (Arcturus, Grand Island, NY, USA). Abundance of transcript was analyzed with a SYBR Green probe, quantified relative to a genomic DNA standard curve, and normalized to eif-s8, a constitutively expressed endogenous control gene used in a previous study (Alaux et al., 2009). In each experiment, expression levels are expressed as ratios, relative to the control or sham-treated group for that experiment. Results shown are an average of two trials (for the initial investigation of orientation flight, three trials; N=6–8 for all groups). Two-factor ANOVAs were performed for multiple trials using PROC MIXED in SAS (SAS Institute, Cary, NC, USA).
Sequences of primers used to generate the probe for in situ hybridization, as well as for qRT-PCR, are listed in Table 2.
Table 2.
Primers used to produce RNA probe for in situ hybridization and qRT-PCR
Is Egr upregulated by orientation flight?
To observe and collect bees performing orientation flights, groups of six to 10 bees were allowed to exit a colony, and an observer confirmed that the flight pattern and duration were typical of orienting bees (Winston, 1987). Control bees were captured while exiting the hive and held for 6–7 min in individual cages next to the hive. The 6–7 min holding period allowed control bees to experience sunlight, outdoor odors and other environmental stimuli for an amount of time roughly equivalent to an orientation flight. Because control bees were collected while exiting the hive, it is presumed that these individuals would have performed an orientation flight had they not been captured.
The same conditions used in this experiment were adapted in order to test specific hypotheses for the upregulation of Egr (Table 1), as described in the following sections.
Hypothesis I – is Egr upregulated by exercise in the absence of flight?
We tested this hypothesis by studying exercise in a familiar environment. Fanning bees (bees exhibiting vigorous, flight-like wing movement while remaining stationary) were collected by setting up a colony as described for naïve orientation flight collections, but in a small glass-walled hive housed indoors; only 1000 worker bees were added. After 7 days, the colony was heated to 40°C for 45 min. This induced some bees to beat their wings vigorously at the hive entrance to provide ventilation (Southwick and Moritz, 1987). Bees that engaged in this fanning behavior for at least 2 min were collected, along with several inactive bees to serve as controls.
Hypothesis II – is Egr upregulated by motor learning associated with flight?
We tested this hypothesis by inducing re-orientation flights by foragers. Foragers, bees with extensive flight experience, perform additional re-orientation flights after a hive is moved, or after moving to a new nest site as part of a swarm (Winston, 1987). These re-orientation flights allow bees to learn their new environment. To induce re-orientation, small colonies with natural age demographies were closed after sunset and transported to a new location at a distance outside the maximum foraging range (Capaldi and Dyer, 1999; Capaldi et al., 2000). This treatment induces bees with foraging experience to perform additional orientation flights in their new environment. The morning after the colony move, orienting foragers and controls were collected as described above, including observations of flight structure and duration. Returning bees were examined to ensure that they had not gathered a pollen or nectar load, indicating that they were performing an orientation flight and not foraging.
Hypothesis III – is Egr upregulated by exposure to specific visual cues?
We tested this hypothesis by studying orientation flights in deprived conditions. Bees will perform orientation flights even in indoor arenas of limited size, devoid of distant landmarks (Brandon and Coss, 1982); such environments are in extreme contrast to the sensorially enriched outdoors. The experimental conditions described above for testing response to a typical first orientation flight were repeated for tethered bees in a small indoor flight enclosure relatively devoid of visual patterning. Temperature in the enclosure was 29.5°C during the day and 24°C at night, and lights were on a 12 h:12 h light:dark cycle.
Bees used in tethered flight experiments came from colonies prepared as for the naïve orientation flight collections. Bees were captured as they exited the colony entrance in preparation for an orientation flight and then immediately placed on ice just until anesthetized. One end of a thread was then fixed to the thorax using superglue; the other end was fixed to the end of a rod. Tethered bees were placed on the floor of a white box and allowed to recover until they were able to walk in a coordinated manner. Flight was induced by lifting the bee's feet from the ground (Feller and Nachtigall, 1989); this action was repeated as often as necessary to sustain flight for 2 min, usually zero to two additional times. Controls were treated in the same manner as foragers, but restrained in an individual plastic cage rather than being induced to fly.
Hypothesis IV – is Egr upregulated by exposure to environmental novelty?
We tested this hypothesis by studying flight in the absence of visual input. The tethered flight experiment described above was repeated using red lighting, which is not visible to bees (Peitsch et al., 1992).
RESULTS
Honey bee Egr is a homolog of the vertebrate Egr gene family
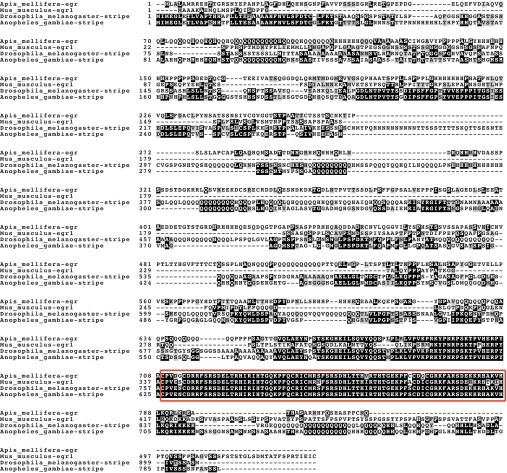
We investigated GB50091, a 2448 bp transcript encoding a putative 815 amino acid protein. This gene is an ortholog of the Drosophila gene stripe; the genes are reciprocal best BLAST hits and share 53.8% sequence identity (e-value: 1e-95). Like stripe, the honey bee gene is a homolog of genes in the Egr gene family in vertebrates, including Egr-1, with which it shares 44.5% sequence identity (e-value: 2e-46). The DNA-binding domain, which consists of three zinc-finger domains (Pavletich and Pabo, 1991), is highly conserved between bee, fly, mouse and mosquito (Fig. 1). The amino acid sequence identity between the honey bee and Drosophila zinc-finger domains is 94%; between the honey bee and mouse zinc-finger domains it is 89%. Because of the apparent functional similarity (see below) to the vertebrate gene Egr-1, we have used the name Egr to refer to the honey bee gene.
Fig. 1.
Alignment of Egr with a vertebrate homolog in mouse (Mus musculus), and orthologs in fruit fly (Drosophila melanogaster) and mosquito (Anopheles gambiae). Conserved residues are highlighted in dark gray. The DNA-binding domain is outlined with a red box.
Egr expression is rapidly and transiently increased in the mushroom bodies of orienting bees
We first assessed the spatial distribution of Egr mRNA in the honey bee brain, using in situ hybridization and qPCR. In situ analysis revealed visible Egr mRNA expression only in scattered Kenyon cells in the mushroom bodies (Fig. 2). Similarly, qRT-PCR analysis showed that Egr expression was enriched in the mushroom bodies compared with the rest of the brain (Fig. 3).
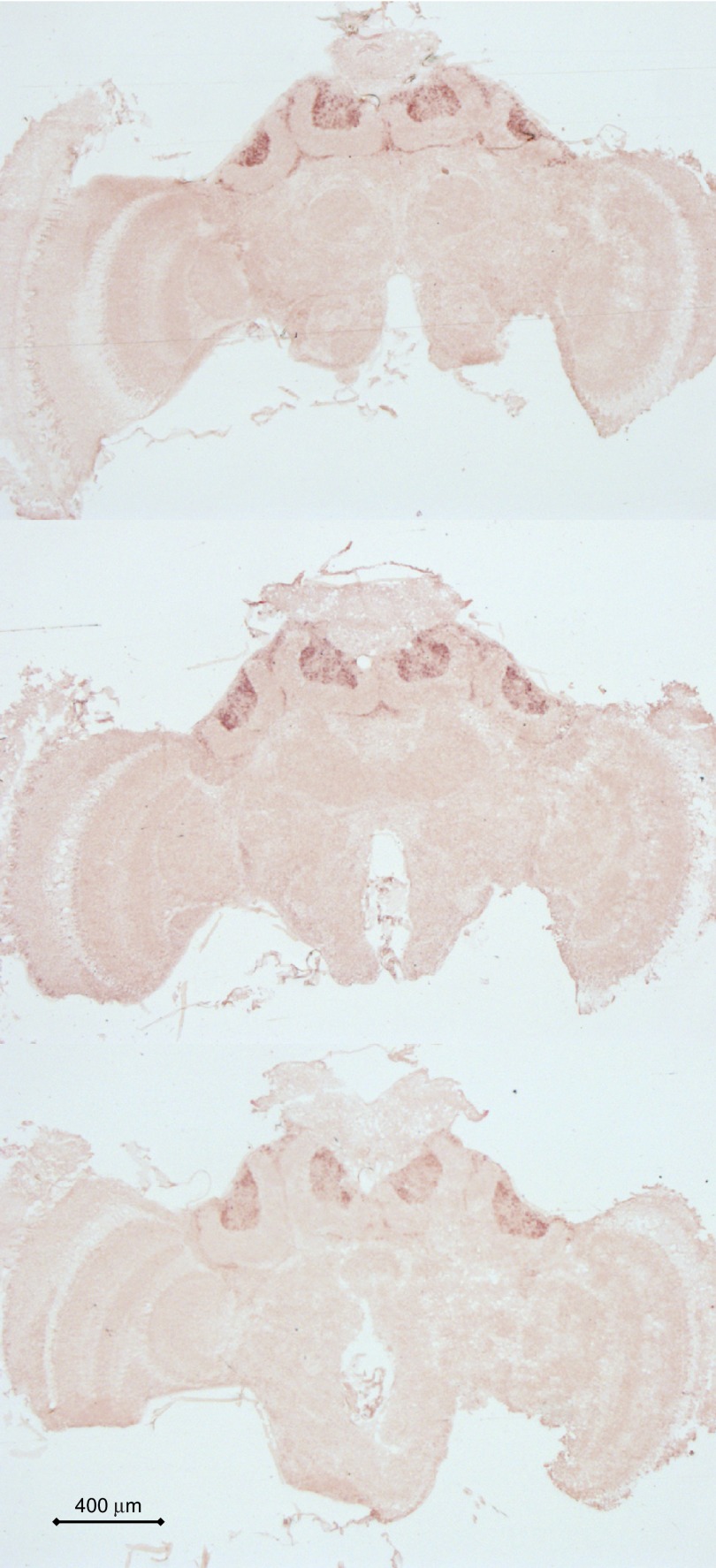
Fig. 2.
Frontal sections of a brain from an orienting bee. Dark red staining indicates Egr expression in mushroom body neuron somata. Staining was not observed in any other brain region.
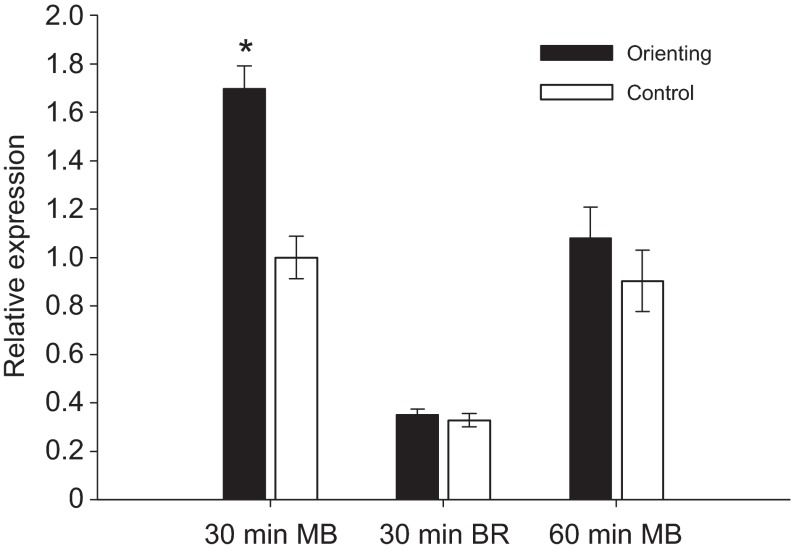
Fig. 3.
Expression of Egr in the brains of orienting and control bees collected either 30 or 60 min post-collection, measured using qRT-PCR. MB, mushroom bodies; BR, all brain tissue other than mushroom bodies. Egr showed nearly twofold upregulation in mushroom bodies 30 min after orientation flight (P<0.0001). Egr was not differentially expressed between orienting bees and controls in BR samples at 30 min, or in MB 60 min after orientation flight.
We next assessed activity-dependent changes in Egr expression in the mushroom bodies of pre-forager bees after a single orientation flight. In many vertebrate species, activity-dependent Egr-1 mRNA expression peaks ~30 min after the relevant stimulus and returns to baseline after 60 min (Zangenehpour and Chaudhuri, 2002). We investigated the temporal dynamics of Egr expression in orienting bees by analyzing transcript abundance in the mushroom bodies of bees collected 30 min and 1 h after flight.
Orienting bees showed an almost twofold upregulation of Egr in comparison to controls (P<0.005; Fig. 3) 30 min after flight. Because control bees were collected as they attempted to perform an orientation flight, and were exposed to sunlight and environmental odors, we hypothesized that increased Egr expression in the mushroom bodies was caused by an aspect of the orientation flight other than preparedness or exposure to stimuli.
The orientation flight, although most significant as an example of spatial learning, can be broken down into several component experiences: exercise, motor learning, exposure to visual cues and exposure to novelty. Any of these components could conceivably induce an IEG response. We performed a series of experiments to test predictions stemming from specific hypotheses on the relationship between the experience of an orientation flight and upregulation of Egr (Table 1).
Egr is not upregulated by exercise in the absence of flight
Because flight in honey bees represents an abrupt increase in metabolic demand, we wondered whether flight mediates an exercise-dependent increase in Egr expression. In rodents, c-fos, arc and Egr-1 expression in the hippocampus increase in response to exercise (Rhodes et al., 2003; Clark et al., 2011). We devised a behavioral manipulation that decoupled the exercise associated with rapid wing movement from real flight. The exercise examined was wing fanning inside the hive entrance to ventilate the hive after heat stress. Pre-forager bees that were collected while fanning experienced a level of activity roughly comparable with that during flight, but in a familiar environment (Yang et al., 2010).
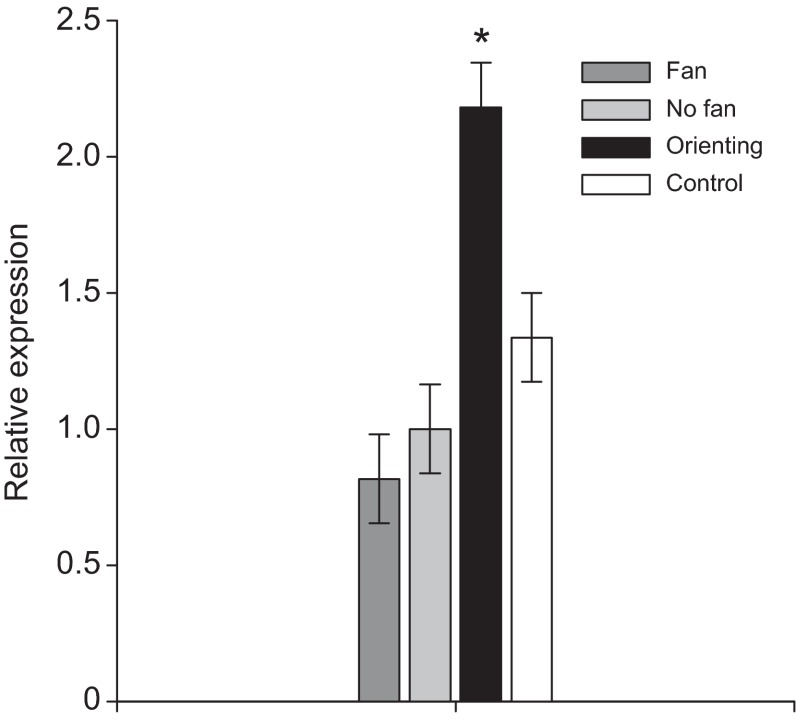
Fanning bees showed no Egr upregulation in response to this form of exercise compared with controls (P=0.859; Fig. 4). As a positive control, bees from the same colony did show the expected Egr upregulation in response to an orientation flight (P<0.001). Although expression in fanning bees appears to be lower than unmanipulated controls from the same colony, this difference is not significant. This experiment suggests that exercise alone cannot induce Egr upregulation in the mushroom bodies.
Fig. 4.
Egr is not upregulated by exercise alone. Expression of Egr in the mushroom bodies of bees vigorously fanning their wings for ~2 min to ventilate the hive, compared with orienting bees and controls. Egr was upregulated in response to flight (P<0.001), but not fanning (P=0.859).
Egr is not upregulated by motor learning associated with flight
Experienced foragers will perform additional orientation flights after participating in a swarm, or after a colony is moved by humans (Winston, 1987). We took advantage of this to examine Egr expression in the mushroom bodies of foragers that performed a re-orientation flight after a colony move. If Egr upregulation was a response to the motor learning aspect of orientation flights performed by pre-foragers, a similar upregulation would not be expected to occur in re-orienting foragers with several days of prior flight experience.
Egr upregulation is not caused by the motor learning aspect of orientation flights, because re-orienting foragers exhibited upregulation of mushroom body Egr expression in comparison to controls (P<0.0005; Fig. 5A). It is thus likely that Egr is upregulated in response to some other experience common to both orientation and re-orientation flights.
Fig. 5.
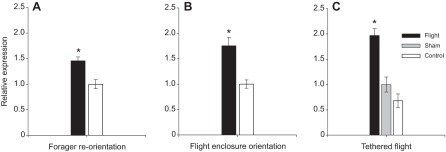
(A) Egr is not upregulated by motor learning associated with flight. Expression of Egr in the mushroom bodies of re-orienting and control foragers. Egr showed upregulation in response to re-orientation by experienced foragers in a novel environment (P<0.0005). (B,C) Egr is not upregulated by exposure to specific visual cues. (B) Expression of Egr in the mushroom bodies of bees orienting in an indoor flight enclosure, compared with controls. Egr was upregulated in response to an orientation flight in this more deprived environment (P<0.0001). (C) Expression of Egr in the mushroom bodies of tethered bees flying for 2 min in a white enclosure. Flight indicates bees that experienced flight while tethered. Sham bees were anesthetized and tethered, but not allowed to fly. Controls were simultaneously collected, unmanipulated bees. Egr was upregulated in response to flight (P<0.0001).
Egr upregulation does not require exposure to specific visual cues
To examine the relationship between feature memorization and Egr upregulation, we investigated the transcriptional response to orientation flight in visually deprived environments. We collected pre-forager bees after they performed an orientation flight in a small indoor flight arena with white floors and bare screen walls and ceiling. Bees that performed an orientation flight in this deprived environment still showed upregulation of mushroom body Egr expression compared with controls (P<0.0001; Fig. 5B).
To examine the effect of even more extreme visual deprivation during flight, pre-forager bees preparing for an orientation flight were tethered to a restraint that allowed freedom of movement and induced to fly in a blank, white arena. Bees that experienced 2 min of tethered flight in a white box devoid of visual cues also showed increased Egr expression compared with tethered and untreated controls (P<0.0001; Fig. 5C). Although tethered bees were exposed to a novel environment during flight, exposure to specific visual cues was not necessary to induce Egr upregulation.
Exposure to environmental novelty is necessary for Egr upregulation
IEG expression in many vertebrate species is upregulated in response to the novelty of a stimulus (Tischmeyer and Grimm, 1999; Clayton, 2000), and orientation flights expose bees to novel stimuli. To test the necessity of visual novelty to induce Egr upregulation, we designed an experiment to give bees the experience of flight without visual novelty. Pre-forager bees experienced tethered flight as described above, but in perceived darkness (Peitsch et al., 1992). These tethered bees showed no upregulation of Egr in comparison with controls (P=0.303; Fig. 6). Orienting bees from the same colony showed upregulation, as in experiments reported above (P<0.005). These results suggest that visual environmental novelty during the orientation flight is necessary to induce Egr upregulation in the mushroom bodies.
Fig. 6.
Egr is upregulated by exposure to environmental novelty, but not by exercise alone. Expression of Egr in the mushroom bodies of tethered bees flying for 2 min in a red light, compared with orienting bees and controls. Egr was upregulated in response to flight (P<0.005), but not to tethered flight in red light (P=0.303).
DISCUSSION
We investigated the transcriptional response of Egr, an insect homolog of Egr-1, in the brains of honey bees after the performance of orientation flights and related behaviors. We found that a single orientation flight upregulates Egr expression exclusively in the mushroom bodies. By contrast, this increased expression was not seen in bees exiting the hive for a flight. Egr expression in the mushroom bodies is thus associated with the experience of, rather than the anticipation of, a naturally occurring learning event.
To our knowledge, we are the first to report that Egr displays activity-dependent expression in an insect, providing evidence for a highly conserved role as an IEG. Despite extensive literature linking the Egr gene family to neuronal activation and learning in vertebrates, there has been little work on the role of this gene in the Drosophila nervous system, and no study linking this gene to novelty or learning.
In vertebrates it has been difficult to distinguish between Egr expression induction resulting from the behavior or stimulus of interest and that resulting from exercise, stress or other confounding factors (Knapska and Kaczmarek, 2004). We found that flight in several contexts and environments was sufficient to cause this upregulation, as long as the environment was novel, but exercise was insufficient to cause Egr upregulation without the visual perception of environmental novelty. These results demonstrate that it is possible for the perception of an experience to trigger Egr expression. Similarly, there are some genes in the mushroom bodies and optic lobes of honey bees that are responsive to changes in the perception of flight distance, rather than the actual distance flown (Sen Sarma et al., 2010).
Egr-1 is hypothesized to play a role in memory consolidation by promoting structural neuroplasticity in the vertebrate brain following exposure to novel or salient stimuli (Knapska and Kaczmarek, 2004; Tischmeyer and Grimm, 1999; Moorman et al., 2011). Many regulatory targets of Egr-1 have been identified, and several are involved in synaptic formation and remodeling. Given this new demonstration of the deep evolutionary conservation of Egr as a mediator of experience-dependent plasticity, it is plausible that Egr may function similarly in the bee brain. A limitation of the present study is that it did not examine potential downstream targets of honey bee Egr. However, because of its homology with vertebrate Egr-1, Egr is a good candidate for a functional link between orientation flights and the resulting dendritic spine remodeling on mushroom body neurons (Brandon and Coss, 1982). Future investigations of downstream targets of neuronal Egr in the honey bees or other insects may yield a more complete picture of how environmental stimuli act through molecular signaling to promote neuroanatomical change. Future studies should also involve determination of the behavioral and downstream molecular consequences of manipulations of Egr signaling through pharmacological manipulations or RNAi knockdown. Such work could provide a more causal link between Egr, downstream signaling and the resulting consolidation of learning that is believed to occur in vertebrates (Bozon et al., 2003; Davis et al., 2003; Pérez-Cadahía et al., 2011).
Our results suggest that the interaction between flight activity and visual novelty is sufficient to stimulate Egr upregulation. To clarify whether flight activity, in combination with visual novelty, is necessary for Egr upregulation, future experiments could examine gene expression or homing behavior after bees are transported mechanically in a manner that imitates flight.
Our molecular and neuroanatomical results support the hypothesis that the mushroom bodies are involved in spatial learning in honey bees, which contrasts with previous research on Drosophila (Neuser et al., 2008; Wolf et al., 1998; Putz and Heisenberg, 2002; Sitaraman et al., 2008; Zars et al., 2000; Ofstad et al., 2011). However, prior work in other insect species besides Drosophila has suggested a possible link between the mushroom bodies and spatial learning. Cockroaches with lesioned mushroom bodies perform poorly on a spatial learning task similar to the Morris water maze (Mizunami et al., 1998). In honey bees, mushroom body neuropil undergoes age-related expansion roughly coincident with orientation flights in both workers and reproductives (Fahrbach et al., 1995; Brandon and Coss, 1982; Withers et al., 1993; Fahrbach et al., 1997), and as mentioned above, one study found rapid remodeling of dendritic spines in the mushroom bodies after an orientation flight (Brandon and Coss, 1982). In addition, Kiya et al. (Kiya et al., 2007) found evidence of neuronal activation in the mushroom bodies of re-orienting foragers. Drosophila have minimal mushroom bodies that receive olfactory input almost exclusively (Heisenberg, 2003), while those of honey bees comprise a substantial portion of total brain volume and receive significant visual and olfactory input. These differences may relate to species differences in foraging ecology, as argued in the following paragraph.
It has been recently suggested that Drosophila spatial learning may not involve the mushroom bodies because this species, in sharp contrast to honey bees, has no need for long-term spatial memories (Zeil, 2012). Hymenopterans (which include bees) and other insects that must navigate a fairly constant environment for days, weeks or months would be predicted to exhibit mushroom body involvement in spatial learning. In addition, insect species whose feeding ecologies depend more heavily on navigation have relatively larger, more elaborate mushroom bodies than Drosophila (Farris, 2008; Mizunami et al., 1998). Given the similarity in mushroom body structure in hymenopterans and the shared ecological pressures that may have shaped them (Farris, 2008), a role in spatial learning is more likely to be shared in bees, ants and wasps, and perhaps other species with elaborate mushroom bodies as well. Our findings suggest that Egr can be a useful tool to understanding the ability of large and small brains to acquire the spatial information needed to navigate successfully.
ACKNOWLEDGEMENTS
The authors thank the following individuals for their assistance: Charley Nye contributed beekeeping expertise; Tom Newman and Scott Kreher provided assistance with molecular techniques; Adam Hamilton, Emma Murdoch and Laura Sligar helped with fieldwork; Morgan Carr-Markell made particularly helpful suggestions to aid in experimental design; and Susan Fahrbach, Seth Ament, members of the Robinson lab and several anonymous reviewers provided feedback that greatly improved the manuscript.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was supported by the National Institutes of Health [OD006416 to G.E.R., HD007333 to C.C.L. (P. I. Janice Juraska)]. Deposited in PMC for release after 12 months.
REFERENCES
- Alaux C., Robinson G. E. (2007). Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J. Chem. Ecol. 33, 1346-1350 [DOI] [PubMed] [Google Scholar]
- Alaux C., Le Conte Y., Adams H. A., Rodriguez-Zas S., Grozinger C. M., Sinha S., Robinson G. E. (2009). Regulation of brain gene expression in honey bees by brood pheromone. Genes Brain Behav. 8, 309-319 [DOI] [PubMed] [Google Scholar]
- Becker L. (1958). Untersuchungen ueber das Heimfindevermoegen der Bienen. Z. Vgl. Physiol. 41, 1-25 [Google Scholar]
- Bozon B., Davis S., Laroche S. (2002). Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus 12, 570-577 [DOI] [PubMed] [Google Scholar]
- Bozon B., Kelly A., Josselyn S. A., Silva A. J., Davis S., Laroche S. (2003). MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos. Trans. R. Soc. Lond. B 358, 805-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon J. G., Coss R. G. (1982). Rapid dendritic spine stem shortening during one-trial learning: the honeybee's first orientation flight. Brain Res. 252, 51-61 [DOI] [PubMed] [Google Scholar]
- Capaldi E. A., Dyer F. C. (1999). The role of orientation flights on homing performance in honeybees. J. Exp. Biol. 202, 1655-1666 [DOI] [PubMed] [Google Scholar]
- Capaldi E. A., Smith A. D., Osborne J. L., Fahrbach S. E., Farris S. M., Reynolds D. R., Edwards A. S., Martin A., Robinson G. E., Poppy G. M., et al. (2000). Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 403, 537-540 [DOI] [PubMed] [Google Scholar]
- Clark P. J., Bhattacharya T. K., Miller D. S., Rhodes J. S. (2011). Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience 184, 16-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F. (2000). The genomic action potential. Neurobiol. Learn. Mem. 74, 185-216 [DOI] [PubMed] [Google Scholar]
- Davis S., Bozon B., Laroche S. (2003). How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav. Brain Res. 142, 17-30 [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Giray T., Robinson G. E. (1995). Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol. Learn. Mem. 63, 181-191 [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Giray T., Farris S. M., Robinson G. E. (1997). Expansion of the neuropil of the mushroom bodies in male honey bees is coincident with initiation of flight. Neurosci. Lett. 236, 135-138 [DOI] [PubMed] [Google Scholar]
- Farris S. M. (2008). Evolutionary convergence of higher brain centers spanning the protostome-deuterostome boundary. Brain Behav. Evol. 72, 106-122 [DOI] [PubMed] [Google Scholar]
- Feller P., Nachtigall W. (1989). Flight of the honey bee. J. Comp. Physiol. B 158, 719-727 [Google Scholar]
- Ghosal K., Naples S. P., Rabe A. R., Killian K. A. (2010). Agonistic behavior and electrical stimulation of the antennae induces Fos-like protein expression in the male cricket brain. Arch. Insect Biochem. Physiol. 74, 38-51 [DOI] [PubMed] [Google Scholar]
- Goujon M., McWilliam H., Li W., Valentin F., Squizzato S., Paern J., Lopez R. (2010). A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695-W699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z., Saraswati S., Adolfsen B., Littleton J. T. (2005). Genome-wide transcriptional changes associated with enhanced activity in the Drosophila nervous system. Neuron 48, 91-107 [DOI] [PubMed] [Google Scholar]
- Heisenberg M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266-275 [DOI] [PubMed] [Google Scholar]
- Huang Z., Robinson G. E. (1996). Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 39, 147-158 [Google Scholar]
- Kiya T., Kubo T. (2011). Dance type and flight parameters are associated with different mushroom body neural activities in worker honeybee brains. PLoS ONE 6, e19301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiya T., Kunieda T., Kubo T. (2007). Increased neural activity of a mushroom body neuron subtype in the brains of forager honeybees. PLoS ONE 2, e371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiya T., Kunieda T., Kubo T. (2008). Inducible- and constitutive-type transcript variants of kakusei, a novel non-coding immediate early gene, in the honeybee brain. Insect Mol. Biol. 17, 531-536 [DOI] [PubMed] [Google Scholar]
- Knapska E., Kaczmarek L. (2004). A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 74, 183-211 [DOI] [PubMed] [Google Scholar]
- Lutz C. C., Rodriguez-Zas S. L., Fahrbach S. E., Robinson G. E. (2012). Transcriptional response to foraging experience in the honey bee mushroom bodies. Dev. Neurobiol. 72, 153-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., De Marco R. J., Greggers U. (2006). Spatial memory, navigation and dance behaviour in Apis mellifera. J. Comp. Physiol. A 192, 889-903 [DOI] [PubMed] [Google Scholar]
- Mizumori S. J., Yeshenko O., Gill K. M., Davis D. M. (2004). Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol. Learn. Mem. 82, 278-298 [DOI] [PubMed] [Google Scholar]
- Mizunami M., Weibrecht J. M., Strausfeld N. J. (1998). Mushroom bodies of the cockroach: their participation in place memory. J. Comp. Neurol. 402, 520-537 [PubMed] [Google Scholar]
- Moorman S., Mello C. V., Bolhuis J. J. (2011). From songs to synapses: molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the ERK/MAPK pathway and synapsins. Bioessays 33, 377-385 [DOI] [PubMed] [Google Scholar]
- Moser E. I., Kropff E., Moser M. B. (2008). Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 31, 69-89 [DOI] [PubMed] [Google Scholar]
- Neuser K., Triphan T., Mronz M., Poeck B., Strauss R. (2008). Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244-1247 [DOI] [PubMed] [Google Scholar]
- Ofstad T. A., Zuker C. S., Reiser M. B. (2011). Visual place learning in Drosophila melanogaster. Nature 474, 204-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. (1991). Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 Å. Science 252, 809-817 [DOI] [PubMed] [Google Scholar]
- Peitsch D., Fietz A., Hertel H., de Souza J., Ventura D. F., Menzel R. (1992). The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 170, 23-40 [DOI] [PubMed] [Google Scholar]
- Pérez-Cadahía B., Drobic B., Davie J. R. (2011). Activation and function of immediate-early genes in the nervous system. Biochem. Cell Biol. 89, 61-73 [DOI] [PubMed] [Google Scholar]
- Putz G., Heisenberg M. (2002). Memories in drosophila heat-box learning. Learn. Mem. 9, 349-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. S., Garland T., Jr, Gammie S. C. (2003). Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 117, 1243-1256 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Dyer F. C. (1993). Plasticity of spatial memory in honey bees: reorientation following colony fission. Anim. Behav. 46, 311-320 [Google Scholar]
- Robinson G. E., Page R. E., Jr, Strambi C., Strambi A. (1989). Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246, 109-112 [DOI] [PubMed] [Google Scholar]
- Sen Sarma M., Rodriguez-Zas S. L., Gernat T., Nguyen T., Newman T., Robinson G. E. (2010). Distance-responsive genes found in dancing honey bees. Genes Brain Behav. 9, 825-830 [DOI] [PubMed] [Google Scholar]
- Sitaraman D., Zars M., Laferriere H., Chen Y. C., Sable-Smith A., Kitamoto T., Rottinghaus G. E., Zars T. (2008). Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA 105, 5579-5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick E. E., Moritz R. F. A. (1987). Social control of air ventilation in colonies of honey bees. J. Insect Physiol. 33, 623-626 [Google Scholar]
- Tischmeyer W., Grimm R. (1999). Activation of immediate early genes and memory formation. Cell. Mol. Life Sci. 55, 564-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde R. A., Robinson G. E., Fahrbach S. E. (2006). Nuclear receptors of the honey bee: annotation and expression in the adult brain. Insect Mol. Biol. 15, 583-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T. (1999). Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 15, 448-453 [DOI] [PubMed] [Google Scholar]
- Winston M. L. (1987). The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; [Google Scholar]
- Withers G. S., Fahrbach S. E., Robinson G. E. (1993). Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238-240 [DOI] [PubMed] [Google Scholar]
- Wolf R., Wittig T., Liu L., Wustmann G., Eyding D., Heisenberg M. (1998). Drosophila mushroom bodies are dispensable for visual, tactile, and motor learning. Learn. Mem. 5, 166-178 [PMC free article] [PubMed] [Google Scholar]
- Yang M. Y., Radloff S., Tan K., Hepburn R. (2010). Anti-predator fan-blowing in guard bees, Apis mellifera capensis Esch. J. Insect Behav. 23, 12-18 [Google Scholar]
- Zangenehpour S., Chaudhuri A. (2002). Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res. Mol. Brain Res. 109, 221-225 [DOI] [PubMed] [Google Scholar]
- Zars T., Wolf R., Davis R., Heisenberg M. (2000). Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn. Mem. 7, 18-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeil J. (2012). Visual homing: an insect perspective. Curr. Opin. Neurobiol. 22, 285-293 [DOI] [PubMed] [Google Scholar]