Abstract
This study examined the course of neuropsychological functioning in patients with chronic myelogeous leukemia (n = 91) or myelodysplastic syndrome (n = 15) who underwent standard treatment for their disease or allogeneic hematopoietic stem cell transplantation (HSCT) at baseline, 12 months, and 18 months post-treatment. At baseline, 23% of the participants (n = 75) in the longitudinal sample had Z-scores on at least one of the neuropsychological tests that were <1.4. Participants in the study showed improvement over baseline at the 12 and 18 months assessments. The average Z-scores for the six cognitive domains in the longitudinal data set over the course of the study ranged from −0.89 to 0.59. Significant predictors of change in neuropsychological test scores included age, with older participants showing less improvement over time. Other predictors included baseline cognitive domains (language, memory, and attention), previous cocaine use, disease status, intelligence quotient, and quality of life measures. Findings support previous studies in patients with hematological malignancies who showed cognitive impairments at baseline prior to HSCT. However, there was little evidence for further cognitive decline over the course of 18 months.
Keywords: Cancer, Neuropsychology, Hematological malignancy, Cognition, Cancer treatment, Hematopoietic stem cell transplantation
Introduction
The number of survivors from a variety of cancers continues to increase as the treatment options become more refined and targeted. Many patients and families are concerned about the possible changes in quality of life over time as the cancer is “cured.” These include issues with changes in interpersonal relationships, employment, and physical sequelae, as well as cognition. While many patients experience mild cognitive effects from chemotherapy or other treatment modalities during the acute phase of treatment, some have persisting late cognitive effects.
Hematopoietic stem cell transplantation (HSCT) is an example of a potentially life-saving treatment for those with hematologic malignancies and other immunohematopoietic disorders. There are two different types of HSCT: Autologous (where the recipient is his/her own donor) and allogeneic (where another person is the source of the donor marrow). The consequences of HSCT on cognition are being elucidated. Just as the treatment itself becomes more sophisticated, studies examining the effect of HSCT on cognition have become more informative. Many earlier pioneering studies ascertained neuropsychological outcomes potentially associated with HSCT in a mixed disease population (combining those with hematologic and solid tumor diseases) or did not include both pre- and post-treatment data to allow for evaluation of changes (Andrykowski et al., 1990). Nonetheless, Andrykowski and colleagues (1992) showed impairments prior to HSCT in approximately half of the patients, with memory being a particular area of vulnerability. Those with a history of cranial irradiation or intrathecal chemotherapy were at greater risk for neuropsychological impairments, but follow-up data were not available. Another earlier study that assessed the impact of autologous bone marrow transplant by Ahles, Tope, Furstenberg, Hann, and Mills (1996) found that patients with either hematological disorders or breast cancer exhibited a decline in performance over time (from baseline to within 1–2 days of discharge). However, longer term cognitive effects were not studied.
In the past decade, prospective studies have been conducted in individuals with a variety of cancer diagnoses in order to better understand the cognitive declines that can be apparent in patients over a longer period of follow-up. In patients with hematological malignancies, the results have been variable due to differences in time post-treatment and differences in design and data analysis. Sostak and colleagues (2003) defined abnormal cognition as 1 SD below the mean and observed in a prospective study an increased percentage (58) of patients classified as impaired prior to HSCT, with improvement over time in selected patients. Syrjala, Dikmen, Roth-Roemer, and Abrams (2004) found a generalized cognitive decline at 3 months with improvement at 12 months to pretransplant levels. Harder and colleagues (2005) assessed cognition and quality of life and affective measures prior to treatment with HSCT or other therapies in patients with hematological malignancies and found neuropsychological deficits in 20% of the patients compared with normative data. Beglinger and colleagues (2007) also found impairments prior to transplant with improvements on neuropsychological measures over the course of 3 months. Jacobs, Small, Booth-Jones, Jacobsen, and Field (2007) observed improvement in various cognitive domains except for attention over the course of 12 months in patients who underwent HSCT. Harder and colleagues (2007) assessed patients treated with HSCT compared with a cancer patient group who had standard therapy only in a prospective study. They found mild cognitive impairments in both patient groups only at baseline. At 20-month follow-up, there were no changes in cognition over time, although HSCT patients had poorer performances in attention, executive function, and psychomotor speed. Friedman and colleagues (2009) assessed course of cognitive decline in patients who underwent HSCT in a within-subjects design using the Reliable Change Index over the course of 7 months. They found that a selected number of patients showed impairments prior to treatment, not uncommon in other studies of individuals with cancer, and that a group of patients showed further decline in executive functioning, memory, and psychomotor speed after undergoing HSCT. The follow-up time in their study was approximately 6 months, with both autologous and allogeneic types of HSCT, and a subject population consisting of eight hematological malignancies. In a prospective study of hematological patients treated with HSCT or standard treatment, Chang, Meadows, Jones, Antin, and Orav (2009) showed that memory scores improved over the course of the 18-month time period.
In order to assess a more homogenous cancer sample, our research group has focused on patients with either chronic myelogeous leukemia (CML) or myelodysplastic syndrome (MDS). Neither of these diseases is expected to cause cognitive impairment per se, but their treatment, whether chemotherapy or allogeneic HSCT, may result in cognitive changes. CML is a cancer of the white blood cells and is based on the stage of the disease. CML is a relatively indolent disease that may be controlled, but not cured, with oral chemotherapy. MDS is the name of a group of conditions that occur when the blood forming cells in the bone marrow are damaged so that there is dysplastic and ineffective blood cell formation. There is a risk of transformation to acute leukemia. Allogeneic HSCT is a potential treatment option for either disease. Other possible approaches include standard care chemotherapy and supportive treatments. The purpose of the present paper was two-fold. (a) To describe the neuropsychological status of patients prior to allogeneic HSCT or other treatment at the time of cancer diagnosis, since many studies have now observed that patients can have cognitive impairments prior to initiation of treatment. However, the studies have varied in how they classify cognitive impairment via Z-scores (ranging from Z-scores < 1 to a more stringent criteria of <1.5). and (b) To identify the predictors of change in neuropsychological performance at 12 and 18 months post-treatment in this group. Many previous studies have assessed patient populations in the first 6 months to a year post-treatment, and so, we wanted to extend the follow-up in this group in the patients who survived at least 18 months.
Methods
Individuals with either CML or primary MDS were recruited from the Dana Farber /Brigham and Women's Cancer Care in Boston, MA (84.3%), with others coming from the Massachusetts General Hospital and other local practices (6.5%). The remaining participants responded to web-based and other study advertisements (9.2%). All were to receive either allogeneic transplantation (HSCT) or other treatment as directed by their physicians. Additional details are available elsewhere (Chang et al., 2009).
Eligibility criteria included agreement to participate in three neuropsychological evaluations over the course of 18 months, reading and listening comprehension of English, and diagnosis within the past year or a new treatment plan that included HSCT within the next year. Exclusion criteria included history of significant head injury (resulting in loss of consciousness), stroke, epilepsy, or other central nervous system pathology requiring radiation, surgery, or past/current intrathecal medication and current alcohol or substance abuse or dependence, all of which could independently affect performance on neurocognitive testing.
Participants provided written informed consent. They received an honorarium of $50 for each assessment. This study was reviewed and approved by the Partners Institutional Review Board, which is responsible for the review and approval of all human subject research conducted by the staff of Brigham and Women's Hospital and other Partners affiliated hospitals.
Participants completed a baseline assessment interview and testing, which consisted of the following. (a) A patient profile included education, usual occupation, past medical and psychiatric history, and medication history. Usual occupation was coded according to the 1989 General Social Survey (GSS) prestige scales (Nakao and Treas, 1990); (b) The Shipley Institute of Living Scale was used to obtain a full-scale intelligence quotient (IQ) as an estimate of premorbid intellectual abilities (Zachary, 2006). (c) The Medical Outcomes 36 Item Short Form (SF-36) evaluated the physical and mental health of the participant (McHorney, Ware & Raczek, 1993). (d) The brief Profile of Mood States (POMS) assessed general mood state (Cella et al., 1987) (e) The Alcohol and Drug Modules from the Structured Clinical Interview for DSM-IV assessed current alcohol and drug diagnoses (First, Spitzer, Gibbon & Williams, 1994).
Participants were also administered a battery of neuropsychological tests. Test selection was based on previous research on patients with hematological malignancies and incorporated various measures of attention, executive function, memory, processing speed, language, and motor speed (Harder et al., 2002; Meyers, Albitar, & Estey, 2005). The attentional measures included the Digit Span subtest from the Wechsler Adult Intelligence Scale-III (Wechsler, 1997) and the Trail Making Test, Part A (Reitan, 1995). Executive functioning measures included the Trail Making Test, Part B, Verbal Fluency Test (Benton and Hamsher, 1989), and the interference condition of the Stroop Color-Word Test (Golden and Freshwater, 2002). Anterograde learning and memory were assessed with the six-trial Buschke Selective Reminding Test (Masur et al., 1989). Four measures from the Buschke are emphasized in this study: total recall, long-term storage, consistent long-term retrieval, and delayed recall. Language assessment was limited to category (semantic) fluency (animals). The Processing Speed domain included the WAIS-III Digit-Symbol subtest, and the color and word conditions from the Stroop Color Word Test. Motor speed was assessed by performance on the Grooved Pegboard Test for both the dominant and nondominant hands (Klove, 1963; Psychological Assessment Resources, Inc, 2000). The reliability and validity of these tests are well documented (Lezak, Howieson, & Loring, 2004; Mitrushina et al., 2005; Strauss, Sherman & Spreen, 2006).
Participants completed repeated assessments 12 and 18 months after the baseline evaluation. Each repeat assessment included the neuropsychological test measures as well as the SF-36 and the brief POMS. Two subscales (mental component score [MCS] and physical component score [PCS]) were derived from the SF-36. The scores have a mean of 50 and a standard deviation of 10. In order to mitigate possible learning effects on the neuropsychological testing, participants were randomly assigned alternate forms of the Verbal Fluency Test (Lacy et al., 1996; Ruff, Light, Parker, & Levin, 1996), Trail Making Test (Franzen, Paul, & Iverson, 1996), and Buschke Selective Reminding Test (Hannay & Levin, 1985). Participants were assigned to different forms for the three testing sessions at enrollment based on a computer algorithm.
Neuropsychological tests were scored according to the established procedures and raw scores were converted to Z-scores from age, sex, and/or education-corrected normative data (Larrabee, Trahan, & Levin, 2000; Ruff & Parker, 1993; Tombaugh, 2004, Tombaugh, Kozak, & Rees, 1999) and published test manuals (Golden and Freshwater, 2002; Wechsler 1997). A composite score for each domain was calculated by averaging the neuropsychological tests' Z-scores within their respective domains (e.g., attention, executive, memory, language, processing speed, and motor; see Table 2 for the listing of the individual test scores within the various cognitive domains).
Table 2.
Percent of test scores that were impaired (Z ≤ −1.4) or normal at baseline for the entire sample (N = 106) at baseline by cognitive domain
| Cognitive domain | Impaired (%) | Normal (%) |
|---|---|---|
| Attention (Digit Span, Trail Making Part A) | 5 | 95 |
| Executive Functioning (Trail Making Part B, Verbal Fluency Test, Stroop Color-Word Condition) | 16 | 84 |
| Memory [Buschke Selective Reminding Test (Total Recall, LTS, CLTR, Delayed Recall)] | 34 | 66 |
| Processing Speed (Digit Symbol-Coding, Stroop Word and Color Conditions) | 11 | 89 |
| Language (Animal Fluency) | 10 | 90 |
| Motor (Grooved Pegboard, dominant and nondominant hands) | 32 | 68 |
Data Analysis
All analyses were carried out using the SAS statistical package (version 9.1). Simple descriptive statistics were calculated and are reported as percentages, means, standard deviations (SD), and ranges, as appropriate. A repeated-measures analysis of variance using the Mixed procedure was used for selected statistical analyses. Proc Mixed was used because of its many analytic advantages including use of continuous time-dependent covariates and inclusion of participants with multiple responses that have one or more data values missing at random, among others (High, 2001; Wolfinger & Chang, 1995). Missing data at the 18-month analyses were carried forward for survivors from 12 months.
Changes in the six neurocognitive domains were calculated as the quantitative difference in Z-scores between the 12-month assessment and baseline and the 18-month assessment and baseline. Two separate analyses were run, one assessing baseline predictors of 12-month change and the other assessing predictors of 18-month change. Neurocognitive, psychosocial, and clinical baseline predictors were tested independently using the Pearson correlation coefficients as the measure of association. These included the following: The individual neuropsychological domains as above, estimated IQ, age, PCS, MCS, disease status (accelerated CML vs. stable CML vs. MDS), treatment (HSCT or other), lifetime marijuana use, lifetime cocaine use, brief POMS, and baseline hematocrit levels. Variables were chosen based on previous research in this sample and in other studies with this patient population (Chang et al., 2009, 2010; Jacobsen et al., 2004; Meyers et al., 1994; Meyers, Albitar & Estey, 2005). Substance use was chosen as a predictor based on previous research that lifetime cocaine use disorders are associated with increased risk of mortality. Baseline predictors correlated with any of the six neurocognitive changes (p < .10) were considered as eligible for the final stepwise regression models.
Based on the analyses above, for the 12-month change in neurocognitive function analyses, six final models were run for each neurocognitive domain change. This contained the six baseline neurocognitive measures, four baseline variables (age, estimated IQ, disease status [accelerated CML vs. stable CML; MDS vs. stable CML]) that were associated with change (p < .05), and then a forward selection on five other associated variables (PCS, MCS, marijuana use, cocaine use, and hematocrit levels; p < .1). For the 18-month change in neurocognitive function analyses, six models were run for each neurocognitive change containing the six baseline neurocognitive measures, three variables (age, MCS, and estimated IQ) associated with change (p < .05), and then a forward selection on six other associated variables (PCS, marijuana use, cocaine use, accelerated CML vs. stable CML, MDS vs. stable CML, and hematocrit levels; p < .1).
Results
Each participant completed an initial evaluation at a median of 5.6 months after the participants' diagnosis date or 6.1 months for the 91 people diagnosed with CML and 3.8 months for the 15 diagnosed with MDS. Seventy-seven participants completed the 12-month evaluation (23 individuals died and 6 withdrew or were lost to follow-up) and 67 participants completed the 18-month evaluation (2 died and 8 withdrew or were lost to follow-up).
Table 1 summarizes the demographic and clinical characteristics at enrollment for the 77 participants for whom longitudinal data were available and the entire sample. Both groups were similar in terms of mean age (∼48 years), gender (>50% male), marital status (∼62% married), and educational attainment (∼50% with at least a 4-year college degree). Most were of white, non-Hispanic (∼88%) background. None satisfied diagnostic criteria for current alcohol or substance use disorders, and both groups had similar rates of lifetime substance use disorders.
Table 1.
Demographic and clinical characteristics at enrollment at baseline assessment for the entire and longitudinal samples
| Longitudinal | Entire | |
|---|---|---|
| Demographic variables | n = 77 | n = 106 |
| Age | ||
| Mean (SD) (years) | 48.6 (13.7) | 48.1 (13.4) |
| Gender (%) | ||
| Men | 52 | 55 |
| Women | 48 | 45 |
| Marital status (%) | ||
| Single | 20.8 | 22.7 |
| Married | 63.6 | 57.6 |
| Divorced | 9.1 | 11.3 |
| Widowed | 3.9 | 3.8 |
| Other | 2.6 | 4.6 |
| Race (%) | ||
| White, non-Hispanic | 88.2 | 86 |
| Black, non-Hispanic | 6.6 | 6.6 |
| Asian/Pacific Islander | 1.3 | 2.8 |
| Native American | 1.3 | 0.9 |
| Other | 1.3 | 2.8 |
| Education (%) | ||
| Graduate or professional | 20.8 | 20.8 |
| College, 4 year | 28.6 | 27.4 |
| Partial College | 27.3 | 25.5 |
| High school | 20. 8 | 22.6 |
| Other | 2.6 | 3.7 |
| Occupation (Mean [SD]) | ||
| Range, 0–86.05 | 48.4 (21.02) | 47.6 (21.02) |
| Estimated IQ | 106.9 (11.1) | 104.9 (10.3) |
| Clinical variables | ||
| Disease (%) | ||
| CML, stable | 77 | 67 |
| CML, accelerated | 12 | 19 |
| MDS | 11 | 14 |
| Treatment (%) | ||
| HSCT | 34 | 42 |
| Other treatment | 66 | 58 |
| Baseline hemoglobin (Mean [SD]) | 12.18 (1.52) | 11.7 (1.84) |
| Lifetime substance abuse or dependence (%) | ||
| Alcohol | 27.3 | 28 |
| Cocaine | 6.5 | 9 |
| Marijuana | 13 | 12 |
Notes: CML = chronic myelogenous leukemia; MDS = myelodysplastic syndrome; HSCT = Hematopoietic stem cell transplantation; IQ = intelligence quotient.
However, the groups did differ in other ways, primarily related to attrition due to mortality. Larger proportions of those able to provide longitudinal data had stable phase CML (p = .002), had treatments other than HSCT (p = .008), and higher hemoglobin values at study enrollment (p = .0013). More people with CML received HSCT than those with MDS (47% vs. 20%, p = .053); 94% of the HSCT recipients received total body irradiation (total dose 14 Gy, in 7 fractions). Among those with CML treated in other ways, the majority received imantinib mesylate (84.5%); other treatments included hydroxyurea (10.4%) or interferon (IFN) (4.2%). Treatment other than HSCT for those with MDS included hydroxyurea (17%), supportive treatment (25%), erythropoietin (33%), and azactidine (42%), with some individuals receiving more than one of the treatments simultaneously.
Performances on Neuropsychological Measures
We considered a Z-score ≤−1.4 to reflect a performance below expected values, which is below the 8th percentile for the general population and considered to be in the borderline range from a clinical perspective. This constituted our definition of an impaired performance. In calculating below normal baseline performances, we used the individual Z-scores for each test data point, for a total of 15 scores across the six cognitive domains. We first looked at the percent of test scores that were impaired (Z ≤ −1.4) for each cognitive domain at baseline for the entire sample. As can be seen in Table 2, the majority of the participants had Z-scores within the normal range on the domains of attention, executive functioning, processing speed, and language. The majority of poorer scores were obtained on the memory measures and on the grooved pegboard.
At baseline, 19% of the sample in the longitudinal data set had scores that were all within normal limits (WNLs) on the individual test scores when compared with normative data using calculated Z-scores (Table 3). Twenty-three percent of the sample had at least one score that was below the 8th percentile and 18% had two scores that were considered impaired. The remainder of the sample (39%) had three or more scores that were below the 8th percentile. By the 18-month follow-up, 36% of the sample had scores that were WNLs, which was significant compared with baseline (McNemar's Test = 8.0; df = 1; p < .005). The percent of individuals who had impairment on one test (15%) at 18 months was slightly lower compared with baseline (23%), though this did not reach significance. Those with scores below the 8th percentile on two (19%) or more (31%) tests at the 18-month follow-up were similar compared with the baseline data. The domains of Memory and Motor were the primary areas underlying the abnormal Z-scores in the groups. Fig. 1 presents the data for the participants at the three time points.
Table 3.
Percent of test scores that were Z ≤ −1.4 at baseline for the longitudinal sample (N = 75) and at 18-month post-treatment
| Number of test scores | Baseline (%) | 18 months (%) |
|---|---|---|
| Nonea | 19 | 36 |
| 1 | 23 | 15 |
| 2 | 18 | 19 |
| >3 | 39 | 31 |
ap < .005 (baseline vs. 18 months).
Fig. 1.
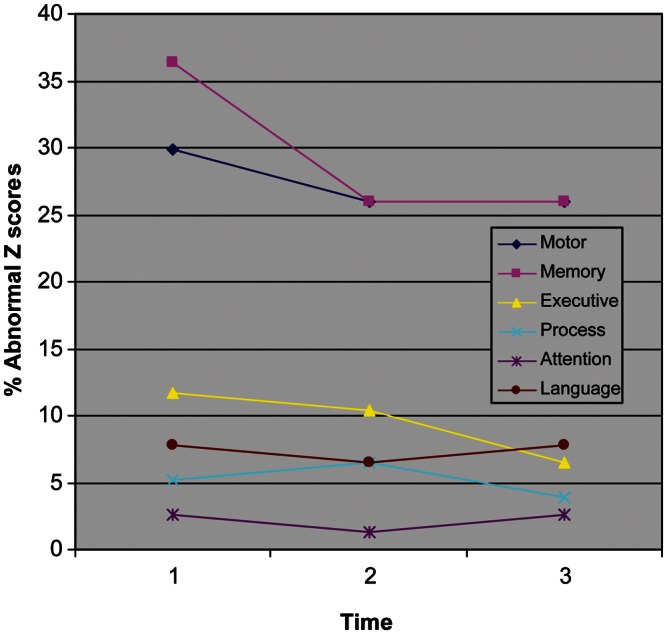
Percent abnormal Z-scores (Z ≤ −1.4) for the six cognitive domains at baseline and 12 and 18 months follow-up in the longitudinal data set.
Table 4 presents the average Z-scores for the six cognitive domains at baseline and 12 and 18 months post-treatment for the longitudinal sample. As indicated, the average Z-scores ranged from −0.89 to 0.59, which is within the normal range. Performance on all of the cognitive domains showed relative improvement over time relative to baseline and were significant for the Executive Function and Memory domains. Z-scores close to −1.0 were most evident for the Memory and Motor domains at baseline. While they improved over time, they were still in the negative direction. Attention and language were areas of relative strength for the sample, with Z-scores above 0 at all time points, and patients overall performed in the average range on Attention at all three time points. Performance on Executive Function, Processing Speed, and Language were close to Z = 0 at all times, with scores that were not significantly different from normal at 18 months.
Table 4.
Performance in the cognitive domains at baseline and 12- and 18-month post-treatment for the longitudinal sample (N = 77)
| Domain | Average Z-score (SD) p-value for normal performance |
p-value for change over time | ||
|---|---|---|---|---|
| Baseline | 12 months | 18 months | ||
| Attention (Digit Span, Trail Making Part A) | 0.43 (0.1), p < .01 | 0.49 (0.09), p < .01 | 0.59 (0.09), p < .01 | p = .12, F = 2.17 |
| Executive Function (Trail Making Part B, Verbal Fluency Test, Stroop Color-Word Condition) | −0.26 (0.13), p = .05 | −0.07 (0.11), p = .53 | 0.06 (0.11), p = .60 | p < .01, F = 7.80 |
| Memory [Buschke Selective Reminding Test (Total Recall, LTS, CLTR, Delayed Recall)] | −0.89 (0.15), p < .01 | −0.61 (0.16), p < .01 | −0.49 (0.16), p < .01 | p < .01, F = 5.02 |
| Processing Speed (Digit Symbol-Coding, Stroop Word and Color Conditions) | −0.20 (0.07), p < .01 | −0.22 (0.07), p = .01 | −0.08 (0.09), p = .37 | p = .06, F = 2.98 |
| Language (Animal Fluency) | 0.10 (0.11), p = .36 | 0.20 (0.13), p = .13 | 0.18 (0.13), p = .15 | p = .65, F = 0.43 |
| Motor (Grooved Pegboard, dominant and nondominant hands) | −0.85 (0.18), p < .01 | −0.73 (0.16), p < .01 | −0.70 (0.19), p < .01 | p = .57, F = 0.57 |
Neuropsychological Predictors of Time Effects
Table 5 presents the analyses for multivariate predictors of performance change at the 12-month assessment. There were improvements over baseline in all of the neuropsychological performance measures, F = 1.91; df = 10, 58; p < .063, but these improvements were small and nonsignificant with the exception of Memory (p = .01). Significant baseline predictors are listed in the table. Baseline predictors that were not significant included type of treatment, lifetime marijuana or cocaine use, brief POMS, baseline hematocrit, and the baseline neuropsychological performances aside from language. Patients with higher baseline Language scores were more likely to improve on all aspects of neuropsychological test performances with the exception of motor skills (and the usual regression to the mean for language). These improvements were significant for Executive Function and Processing Speed. Older patients improved less than younger patients on all measures and reached significance for Executive Function. Compared with patients with stable CML, patients with accelerated CML had less improvement at the 12-month follow-up on all measures, with significance achieved for the Executive Function and Processing Speed domains. Finally, compared with patients with stable CML, patients with MDS showed less improvement on all aspects with the exception of Memory (p = .05). The deficit was significant for Executive Function (p = .03).
Table 5.
Multivariate predictors of change between 12 months and baseline
| Change in performance between 12 months and baseline |
||||||
|---|---|---|---|---|---|---|
| Memory | Attention | Executive Function | Processing | Motor | Language | |
| Average change: 12 months − baseline (SE) | +0.28 (0.11), p = .01 | +0.06 (0.05), p = .29 | +0.19 (0.10), p = .06 | +0.00 (0.04), p = .98 | +0.12 (0.15), p = .42 | +0.09 (0.11), p = .38 |
| Baseline predictors | ||||||
| Language Average | 0.168 (0.146), p = .25 | 0.004 (0.067), p = .95 | 0.202 (0.102), p = .05 | 0.102 (0.048), p = .037 | −0.156 (0.192), p = .42 | −0.299 (0.134), p = .029 |
| Age | −0.014 (0.009), p = .14 | −0.006 (0.004), p = .17 | −0.01 (0.007), p = .04 | −0.003 (0.003), p = .28 | −0.009 (0.012), p = .47 | −0.01 (0.009), p = .26 |
| Estimated IQ | −0.009 (0.015), p = .56 | 0.011 (0.007), p = .11 | −0.007 (0.01), p = .523 | 0.001 (0.005), p = .78 | 0.016 (0.019), p = .40 | 0.011 (0.014), p = .41 |
| Accelerated CML (vs. Stable CML) | −0.706 (0.378), p = .067 | −0.264 (0.174), p = .14 | −0.683 (0.266), p = .013 | −0.297 (0.124), p = .02 | −0.265 (0.481), p = .58 | −0.657 (0.347), p = .06 |
| MDS (vs. Stable CML) | 0.863 (0.439), p = .05 | −0.243 (0.202), p = .24 | −0.672 (0.308), p = .033 | −0.265 (0.144), p = .07 | −0.186 (0.572), p = .75 | −0.335 (0.403), p = .41 |
Notes: Models were adjusted for the variables shown as well as baseline performance on Memory, Executive Function, Processing, Motor, and Attention. Additional potential predictors PCS, MCS, marijuana use, cocaine use, and hematocrit levels; which showed p < .10 on univariate tests, were considered but not included by forward selection. IQ = intelligence quotient; CML = chronic myelogenous leukemia; MDS = myelodysplastic syndrome. Bold values indicate statistical significance.
At 18 months, there were improvements over baseline in all of the cognitive domains, and the increases were significant for Memory, Attention, and Executive Function—F = 1.47; df = 9, 58; p < .182. Table 6 presents the multivariate predictors of change at 18 months compared with baseline. Patients with higher Memory baseline scores were more likely to improve on all other cognitive domains except for the usual regression to the mean for Memory. These improvements were significant for the Attention, Motor, and Language domains. Similarly, patients with higher Attention baseline scores were more likely to improve on all other cognitive domains. This improvement was significant only for the Memory domain. Again, age was a significant predictor of performance as older patients improved less than younger patients on all domains except the Language domain. This reached significance for the Attention and Executive Function domains. Patients with higher IQs improved more on all aspects of neuropsychological measures with the exception of Executive Function. The age effect reached significance for the Attention and Language domains. The SF-36 MCS was a predictor of performance in the Motor domain, with higher scores resulting in improvements in Motor performances. History of past cocaine use was a predictor of improvement in Attention scores at 18 months. Higher baseline PCSs from the SF-36 showed less improvement in Attention at 18 months.
Table 6.
Multivariate predictors of change between 18 months and baseline
| Change in performance between 18 months and baseline |
||||||
|---|---|---|---|---|---|---|
| Memory | Attention | Executive Function | Processing | Motor | Language | |
| Average change: 18 months − baseline (SE) | +0.39 (0.13), p < .01 | +0.16 (0.08), p = .04 | +0.32 (0.08), p < .01 | +0.12 (0.06), p = .051 | +0.15 (0.14), p = .29 | +0.08 (0.10), p = .46 |
| Baseline predictors | ||||||
| Memory Average | −0.301 (0.126), p = .02 | 0.156 (0.06), p = .007 | 0.122 (0.07), p = .09 | 0.092 (0.06), p = .11 | 0.27 (0.123), p = .031 | 0.268 (0.09), p = .004 |
| Attention Average | 0.472 (0.241), p = .055 | −0.648 (0.109), p < .0001 | 0.219 (0.133), p = .1 | 0.126 (0.109), p = .25 | 0.358 (0.241), p = .14 | 0.022 (0.168), p = .90 |
| Age | −0.003 (0.011), p = .77 | −0.01 (0.004), p = .023 | −0.012 (0.006), p = .04 | −0.007 (0.005), p = .131 | −0.019 (0.01), p = .07 | 0.003 (0.007), p = .67 |
| Estimated IQ | 0.012 (0.018), p = .518 | 0.026 (0.008), p = .002 | −0.0007 (0.01), p = .941 | 0.003 (0.008), p = .713 | 0.005 (0.018), p = .80 | 0.033 (0.013), p = .013 |
| MCSa | −0.006 (0.013), p = .654 | −0.0002 (0.005), p = .978 | 0.002 (0.007), p = .824 | 0.001 (0.006), p = .797 | 0.037 (0.013), p = .007 | −0.003 (0.009), p = .773 |
Notes: IQ = intelligence quotient. aMCS = mental component scorefrom SF-36. Bold values indicate statistical significance.
Discussion
Our finding of mild cognitive impairments at baseline testing in selected patients with CML or MDS is similar to other prospective studies, including those with blood cancers (Beglinger et al., 2007; Friedman et al., 2009; Meyers et al., 1994, 2005) as well as other types of malignancies (Wefel, Lenzi, Theriault, Davis, & Meyers, 2004). It is unclear at this point whether or not this represents a normative variation (Schretelen, Testa, Winicki, Perlson, & Gordon, 2008) or if it is secondary to other factors (i.e., biologic, affective) associated with a recent cancer diagnosis or if cytokines, which are cell signaling molecules that regulate the body's inflammatory response, are impacting brain function in selected patients. Increasing attention has been paid to neuropsychological test score variations in normal populations. Our relatively large sample of CML and MDS participants showed some impairments, defined here as Z ≤ 1.4, on selected test scores at baseline. While we limited our analysis to 15 variables, the probability of observing an impaired score increases with an increase in the number of variables. It seems that there are likely other factors involved given that cognitive impairments are found prior to treatment in other cancer populations. With respect to blood cancers, Meyers and colleagues (2005) found that higher interleukin-6 (IL-6) levels, which are cytokines that can have direct effects on brain function and initiate a stress response, were associated with poorer executive functioning in patients with AML/MDS and higher IL-8 levels were associated with improved memory performances. They did not find that hemoglobin levels were related to cognitive performances or fatigue levels. However, Jacobsen and colleagues (2004) found that lower hemoglobin levels were associated with cognitive decline in a mixed group of solid tumor patients. Chang and colleagues (2009) found that higher hemoglobin levels and higher IQ at enrollment were found to be associated with a decreased risk of death over the course of the study (18 months). We did not observe any effect of baseline hemoglobin levels on cognitive performance in the longitudinal analyses. Unfortunately, we did not have IL data available for our sample or hemoglobin levels at the follow-up testing. Future research should focus on additional biological and psychosocial predictors of cognitive changes over time and at the time of cancer diagnosis.
With respect to the neuropsychological predictors, we found that type of treatment,that is, standard versus HSCT, was not predictive of change on any of the neuropsychological measures. Our findings were similar to Syrjala and colleagues (2004) who also found that late-delayed cognitive impairments are rare in HSCT patients tested at 1-year post-treatment. However, Friedman and colleagues (2009) found that a subgroup of HSCT patients showed further cognitive decline at the 28-week follow-up using a within-subjects design. However, it should be noted that our study population all received allogeneic transplants and other studies have included patients with autologous transplants.
Other studies (Pavol et al., 1995; Scheibel, Valentine, O'Brien, & Meyers, 2004) have suggested that higher doses of IFN-α, which exert its treatment effect by inducing cytokines, may also contribute to cognitive impairment in patients undergoing treatment. The pattern of deficits (frontosubcortical impairment) in these patients is similar to that seen in individuals treated with chemotherapy and in other patient populations as well. However, in our study, only a very small percentage (<5%) of our patients received IFN-α.
Cognitive reserve is an issue that is garnering more attention in research studies and in informing clinical practice for neurological and medical conditions, including cancer. It has been found to be a moderator in studies of individuals with dementia (Mortimer, Snowden, & Markesbery, 2007; Stern, 2006) and multiple sclerosis (Benedict, Morrow, Guttman, Cookfair, & Schretlen, 2010). Cognitive reserve is defined in various ways, including reading achievement scores, educational attainment, and IQ measures. We used IQ rather than education in our models as a measure of cognitive reserve. In the present study, IQ was a significant predictor of neuropsychological test performance at the 18-month follow-up, where those with higher IQ showed greater improvement on many of the domains, with the exception of executive functioning. In a breast cancer sample, older individuals with lower cognitive reserve exposed to chemotherapy had lower scores on tests of processing speed compared with controls and a group that did not have chemotherapy (Ahles et al., 2010). This was similar to our finding in that older individuals showed less improvement over time. The fact that baseline language (semantic fluency) was a significant predictor of improved performance in our study sample may be related to the relative stability in language measures in selected clinical populations. It may also be that semantic fluency in our sample was a potential surrogate of cognitive reserve, since IQ and semantic fluency were correlated in our sample (Pearson's correlation = .35; p < .001).
In our data, other mood and quality of life variables were not significant predictors of neurocognitive change over time, aside from the finding that higher MCSs from the SF-36 at baseline was a predictor of improved motor scores at 18 months. The general finding is consistent with many other studies in this patient population in that affective state cannot necessarily account for cognitive changes/impairments (Beglinger et al., 2007; Friedman et al., 2009; Meyers et al., 1994; Poppelreuter, Weis, Kulz, Tucha, Lange & Bartsch, 2004). Harder et al., (2002) found overall general health, fatigue, and educational level as main predictors of cognitive impairment in the long-term survivors after HSCT or stem cell grafts for a variety of hematological diseases (leukemia, lymphoma, MDS, multiple myeloma, anemia). We observed that accelerated CML, which is an indicator of more advanced disease, predicted neuropsychological changes over time. However, we did not assess fatigue directly and our mood measure was an aggregate measure. Future studies should use formal rating scales as a way of measuring fatigue per se, and measuring the various components of fatigue (physical, cognitive, psychosocial) may also be informative.
Syrjala and colleagues (2004) noted that chronic graft versus host disease (GVHD) increased the risk of motor impairment. GVHD occurs when immune cells from a nonidentical donor (graft) recognize the transplant recipient (host) as foreign, thereby initiating an immune reaction that causes disease in the recipient. Sostak and colleagues (2003) also showed that chronic GVHD was a risk factor for cognitive impairment. In our longitudinal sample, the percentage of individuals who underwent HSCT who had GVHD was 40% at the 12-month evaluation and 43% at the 18-month evaluation; these numbers (10 patients) were too small to test whether or not this was a determining factor in the results.
There are some limitations in our study. First, there is a potential bias in our longitudinal sample, as more of these participants had stable CML and started with a higher initial hematocrit, perhaps signaling that their health status was in general better. Many patients did not receive HSCT and so perhaps were considered as “less sick” and thus were treated with other less invasive and intensive treatment. Indeed, we found that disease status (accelerated CML, MDS) was a predictor of neuropsychological changes at the 12-month follow-up, indicating that these patients showed less improvement over this time period. There were also 25 patients who died prior to follow-up, again implicating a relatively healthier group in the follow-up sample. Another bias in our study includes the issue of the normative data used, which varied depending on the individual test. We used a variety of clinical tests that each has their own independent normative samples based on data from test manuals and/or published data. In addition, practice effects cannot be ruled out entirely, though the use of alternate forms for some of the tests helped to mitigate this effect (Calamia, Markon, & Tranel, 2012). These potential limitations notwithstanding, we also note some strengths of our study, which include high rates of follow-up in a rather homogenous sample and the prospective longitudinal design.
While we found that performances actually improved over time, there appears to be a subset of patients who continue to have impairments if the number of abnormalities on individual test scores is considered. Our longitudinal multivariate analysis may have masked what may be a cognitive decline in selected patients. Clinically, there is a subset of patients who continue to have mild cognitive deficits years following treatment for cancer, in particular breast cancer and leukemia survivors. The use of the Reliable Change Index is a way of assessing individual variability as well and is garnering more use in the literature and in clinical practice in determining clinically significant change. Indeed, Vardy, Rourke, and Tannock (2007) in a review of the literature in cognitive functioning and chemotherapy discuss the lack of consistency in defining cognitive impairment, the variety of tests used, and the range of statistical analyses used in attempting to ascertain cognitive outcomes in cancer patients. As more cancer clinical trials incorporate neurocognitive tests as important outcome measures in addition to remission and survival rates, we will have additional data in order to advise patients regarding the risks, if any, of late delayed effects.
A similar pattern of neurocognitive deficits emerges in many studies of cancer patients in that impairments in memory, psychomotor/processing speed, and executive function are the areas most affected, even prior to treatment. Our findings of cognitive impairment in selected patients prior to HSCT or other treatment are consistent with the literature. The pattern of “chemobrain” is thought to resemble frontosubcortical network dysfunction, which can be seen in other patient populations as well, including those with demyelinating disease or small vessel ischemic disease, and is nonspecific. The majority of the patients who survived over the course of our study had relatively stable scores with respect to performances on cognitive tests. However, it is apparent in studies of cancer survivors and in clinical practice that there is a subset of patients who tend to be at risk for cognitive impairments over the course of their disease and treatment.
Future research needs to address which patients may be more vulnerable to neurocognitive impairments and ways to identify them. Differences due to immune reactivity, cognitive reserve, susceptibility to fatigue, genetics, and/or precancer affective state are likely candidates in mediating vulnerability to the manifestation of cognitive deficits following cancer treatment. It may be that interaction between these factors are all contributing to either the presence or the absence of late cognitive effects in cancer survivors. The medical community needs to be more proactive in their treatment of possible cognitive late effects. Interventions aimed at developing compensatory strategies for cognitive issues prior to or during chemotherapy may be helpful and is a current area of interest in breast cancer treatment (Ferguson et al., 2007, 2010). There are also cancer centers that are offering cognitive rehabilitation during and after treatment in order to help patients devise compensatory strategies proactively. Pharmacological treatment is also an active area of research and clinical care in helping to improve cognition in cancer patients while undergoing treatment or following treatment (Kohli et al., 2009; Shaw et al., 2006; Sood, Barton, & Loprinzi, 2006). A combination of both behavioral and pharmacological treatments is likely to result in improved cognitive functioning and perceived improvement in quality of life in cancer survivors.
Funding
This work was supported in part by the American Cancer Society (RSG-01-246-01-PBP to GC) and the National Institute on Alcohol Abuse and Alcoholism (K24 AA 000289 to GC).
Conflict of Interest
None declared.
Acknowledgements
The authors wish to acknowledge the technical assistance of Alyson Lavigne-Dolan and Christine Brieglieb in data collection and data entry.
Appendix
Table A1: Multivariate predictors of change between 18 months and baseline, adjusted for education instead of IQ
| Change in performance between 18 months and baseline |
||||||
|---|---|---|---|---|---|---|
| Memory | Attention | Executive function | Processing | Motor | Language | |
| Baseline predictors | ||||||
| Memory average | −0.38 (0.10) | 0.20 (0.05) | 0.13 (0.06) | 0.11 (0.05) | 0.11 (0.11) | 0.24 (0.08) |
| p < .01 | p < .01 | p = .04 | p = .02 | p = .30 | p < .01 | |
| Attention Average | 0.48 (0.15) | −0.46 (0.08) | 0.20 (0.11) | 0.18 (0.08) | 0.42 (0.18) | −0.01 (0.12) |
| p < .01 | p < .01 | p = .09 | p = .04 | p = .02 | p = .93 | |
| Age | −0.005 (0.01) | −0.007 (0.005) | −0.011 (0.006) | −0.006 (0.005) | −0.023 (0.010) | 0.010 (0.007) |
| p = .65 | p = .203 | p = .05 | p = .171 | p = .02 | p = .18 | |
| Education | −0.12 (0.12) | −0.12 (0.06) | −0.03 (0.07) | 0.01 (0.06) | 0.03 (0.13) | −0.23 (0.09) |
| p = .33 | p = .06 | p = .68 | p = .85 | p = .82 | p = .01 | |
| MCS | −0.002 (0.01) | −0.007 (0.006) | 0.001 (0.007) | 0.001 (0.006) | 0.030 (0.012) | −0.009 (0.008) |
| p = .89 | p = .22 | p = .86 | p = .81 | p = .01 | p = .31 | |
Note: All models adjusted for baseline performance, and Education was used as an ordinal predictor with 1 = Graduate/Professional and 4 = High School. MCS = mental component score. Bold values indicate statistical significance.
References
- Ahles T. A., Saykin A. J., Brenna C., McDonald B. C., Li Y., Furstenberg C. T., et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A., Tope D. M., Furstenberg C., Hann D., Mills L. Psychologic and neuropsychologic impact of autologous bone marrow transplantation. Journal of Clinical Oncology. 1996;14(5):1457–1462. doi: 10.1200/JCO.1996.14.5.1457. [DOI] [PubMed] [Google Scholar]
- Andrykowski M. A., Altmaier E. M., Barnett R. L., Burish T. G., Gingrich R., Henslee-Downey P. J. Cognitive dysfunction in adult survivors of allogeneic marrow transplantation: Relationship to dose of total body irradiation. Bone Marrow Transplant. 1990;6:269–276. [PubMed] [Google Scholar]
- Andrykowski M. A., Schmitt F. A., Gregg M. E., Brady M. J., Lamb D. G., Henslee-Downey P. J. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer. 1992;70(9):2288–2297. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Beglinger L. J., Duff K., VanDer Heiden S., Moser D. J., Bayless J. D., Paulsen J. S., et al. Neuropsychological and psychiatric functioning pre- and posthematopoietic stem cell transplantation in adult patients: A preliminary study. Journal of the International Neuropsychological Society. 2007;13:172–177. doi: 10.1017/S1355617707070208. [DOI] [PubMed] [Google Scholar]
- Benedict R. H. B., Morrow S. A., Guttman B. A., Cookfair D., Schretlen D. J. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. Journal of the International Neuropsychological Society. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- Benton A. L., Hamsher K. deS. Multilingual aphasia examination. Iowa City, IA: AJA Associations; 1989. [Google Scholar]
- Calamia M., Markon K., Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist. 2012;26:543–570. doi: 10.1080/13854046.2012.680913. [DOI] [PubMed] [Google Scholar]
- Cella D. R., Jacobsen P. B., Orav E. J., Holland J. C., Silberfarb P. M., Rafla S. A brief POMS measure of distress for cancer patients. Journal of Chronic Disease. 1987;40:939–942. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- Chang G., Meadows M.-E., Jones J. A., Antin J. H., Orav E. J. Mental status changes after hemaopoietic stem cell transplantation. Cancer. 2009;115:4625–4635. doi: 10.1002/cncr.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G., Meadows M.-E., Jones J. A., Antin J. H., Orav E. J. Substance use and survival after treatment for chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS) American Journal of Drug and Alcohol Abuse. 2010;36:1–6. doi: 10.3109/00952990903490758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. J., Ahles T. A., Saykin A. J., McDonald B. C., Furstenberg C. T., Cole B. F., et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psycho-Oncology. 2007;16:772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. J., McDonald B. C., Rocque M. A., Furstenberg C. T., Horrigan S., Ahles T. A., et al. Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psycho-Oncology. 2010;21(2):176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W., et al. Structured clinical interview for axis I DSM-IV disorders. Patient edition (SCID-I/P, version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Franzen M. D., Paul D., Iverson G. L. Reliability of alternate forms of the Trail Making Test. The Clinical Neuropsychologist. 1996;10(2):125–129. [Google Scholar]
- Friedman M. A., Fernandez M., Wefel J. S., Myszka K. A., Champlin R. E., Meyers C. A. Course of cognitive decline in hematopoietic stem cell transplantation: A within-subjects design. Archives of Clinical Neuropsychology. 2009;24(7):689–698. doi: 10.1093/arclin/acp060. [DOI] [PubMed] [Google Scholar]
- Golden C. J., Freshwater S. M. Stroop Color and Word Test: A manual for clinical and experimental uses. Wood Dale, IL: Stoelting.; 2002. [Google Scholar]
- Hannay J. H., Levin H. S. Selective reminding test: An examination of the equivalence of four forms. Journal of Clinical and Experimental Neuropsychology. 1985;7:251–263. doi: 10.1080/01688638508401258. [DOI] [PubMed] [Google Scholar]
- Harder H., Corenlissen J. J., van Gool A. R., Duivenvoorden W. M., Eijkenboom H, van den Bent M. J. Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer. 2002;95:183–192. doi: 10.1002/cncr.10627. [DOI] [PubMed] [Google Scholar]
- Harder H., Van Gool A. R., Cornelissen J. J., Duivenvoorden H. J., Eijkenboom W. M., Barge R. M., et al. Assessment of pre-treatment cognitive performance in adult bone marrow or haematopoietic stem cell transplantation patients: a comparative study. European Journal of Cancer. 2005;41(7):1007–1016. doi: 10.1016/j.ejca.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Harder H., Van Gool A. R., Duivenvoorden H. G., Cornelissen J. J., Eijkenboom W. M. H., Barge R. M. Y., et al. Case-referent comparison of cognitive functions in patients receiving haematopoietic stem-cell transplantation for haematological malignancies: Two-year follow-up results. European Journal of Cancer. 2007;43:2052–2059. doi: 10.1016/j.ejca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- High R. Should you be using Proc Mixed to analyze continuous data? Computing News. Retrieved 28 November 2007 from http://cc.uoregon.edu/cnews/summer2001/procmixed.html .
- Jacobs S. R., Small B. J., Booth-Jones M., Jacobsen P. B., Field K. K. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer. 2007;110:1560–1567. doi: 10.1002/cncr.22962. [DOI] [PubMed] [Google Scholar]
- Jacobsen P. B., Garland L. L., Booth-Jones M., Donovan K. A., Thors C. L., Winters E., et al. Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. Journal of Pain and Symptom Management. 2004;28:7–18. doi: 10.1016/j.jpainsymman.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical Neuropsychology. In: Forster M., editor. The Medical Clinics of North America. Saunders: New York; 1963. [PubMed] [Google Scholar]
- Kohli S., Fisher S. G., Tra Y., Adams M. J., Mapstone M. E., Wesnes K. A., et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy M. A., Gore P. A., Pliskin N. H., Henry G. K., Heilbronner R. L., Hamer D. P. Verbal fluency task equivalence. The Clinical Neuropsychologist. 1996;10:305–308. [Google Scholar]
- Larrabee G. J., Trahan D. E., Levin H. S. Normative data for a six-trial administration of the verbal selective reminding test. The Clinical Neuropsychologist. 2000;14:110–118. doi: 10.1076/1385-4046(200002)14:1;1-8;FT110. [DOI] [PubMed] [Google Scholar]
- Lezak M. D., Howieson D. B., Loring D. W. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Masur D. M., Guld P. A., Blau S., Thal L. J., Levin H., Aronson M. K. Distinguishing normal and demented elderly with the Selective Reminding Test. Journal of Clinical and Experimental Neuropsychology. 1989;11:615–630. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E., Raczek A. E. The MOS 36-item, short-form health survey (SF-36): psychometric and clinical uses of validity in measuring physical and mental constructs. Medical Care. 1993;331:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Albitar M., Estey E. Cognitive impairment, fatigue and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Weitzner M., Byrne K., Valentine A., Champlin R. E., Przepiorka D. Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. Journal of Clinical Oncology. 1994;12:820–826. doi: 10.1200/JCO.1994.12.4.820. [DOI] [PubMed] [Google Scholar]
- Mitrushina M., Boone K. B., Razani J., D'Elia L. F., et al. Handbook of Normative Data for Neuropsychological Assessment. 2nd edn. New York: Oxford University Press; 2005. [Google Scholar]
- Mortimer J. A., Snowden D. A., Markesbery W. R. Brain reserve and risk of dementia: Findings from the Nun Study. In: Stern Y., editor. Cognitive reserve: Theory and applications. New York: Taylor & Francis; 2007. pp. 237–249. [Google Scholar]
- Nakao K., Treas J. Chicago, IL: NORC; 1990. Computing 1989 prestige scores. GSS Methodological Report No. 70. [Google Scholar]
- Pavol M. A., Meyers C. A., Rexer J. L., Valentine A. D., Mattis P. J., Talpaz M. Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology. 1995;45:947–950. doi: 10.1212/wnl.45.5.947. [DOI] [PubMed] [Google Scholar]
- Poppelreuter M., Weis J., Kulz A. K., Tucha O., Lange K. W., Bartsch H. H. Cognitive dysfunction and subjective complaints of cancer patients: a cross-sectional study in a cancer rehabilitation centre. European Journal of Cancer. 2004;40:43–49. doi: 10.1016/j.ejca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Psychological Assessment Resources, Inc. Lutz, FL: Grooved Pegboard; 2000. [Google Scholar]
- Reitan R. M. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology. 1995;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Ruff R. M., Light R. H., Parker S. B., Levin H. S. Benton Controlled Oral Word Association Test: Reliability and updated norms. Archives of Clinical Neuropsychology. 1996;11:329–338. [PubMed] [Google Scholar]
- Ruff R. M., Parker S. B. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the finger tapping and grooved pegboard test. Perceptual and Motor Skills. 1993;62:407–416. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Scheibel R. S., Valentine A. D., O'Brien S., Meyers C. A. Cognitive dysfunction and depression during treatment with interferon-alpha and chemotherapy. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:186–191. doi: 10.1176/jnp.16.2.185. [DOI] [PubMed] [Google Scholar]
- Schretelen D. J., Testa S. M., Winicki J. M., Perlson G. D., Gordon B. Frequency and bases of abnormal performance by healthy adults on neuropsychological testing. Journal of the International Neuropsychological Society. 2008;14:436–445. doi: 10.1017/S1355617708080387. [DOI] [PubMed] [Google Scholar]
- Shaw E. G., Rosdhal R., D'Agostino R. B., Jr., Lovato J., Naughton M. J., Robbins M. E., Stephen R., Rapp S. R. Phase II Study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- Sood A., Barton D. L., Loprinzi C. L. Use of methylphenidate in patients with cancer. American Journal of Hospice and Palliative Care. 2006;23:35–40. doi: 10.1177/104990910602300106. [DOI] [PubMed] [Google Scholar]
- Sostak P., Padovan C. S., Yousry T. A., Ledderose G., Kolb H. J., Straube A. Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology. 2003;60:842–848. doi: 10.1212/01.wnl.0000046522.38465.79. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20(Supplement 2):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M. S., Spreen O. A Compendium of Neuropsychological Tests: Administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Syrjala K. L., Dikmen S. L., Roth-Roemer S., Abrams J. R. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104:3386–3392. doi: 10.1182/blood-2004-03-1155. [DOI] [PubMed] [Google Scholar]
- Tombaugh T. N. Trail making test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tombaugh T. N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology. 1999;14:167–177. [PubMed] [Google Scholar]
- Vardy J., Rourke S., Tannock I. F. Evaluation of Cognitive Function Associated with Chemotherapy: A review of published studies and recommendations for future research. Journal of Clinical Oncology. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wefel J. S., Lenzi R., Theriault R. L., Davis R. N., Meyers C. A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wolfinger R., Chang M. Comparing the SAS GLM and Mixed procedures for repeated measures. Proceedings of the Twentieth Annual SAS Users Group International Proceedings; Cary, NC: SAS Institute; 1995. [Google Scholar]
- Zachary R. A. Shipley Institute of Living Scale. Western Psychological Services: Los Angeles, CA.; 2006. [Google Scholar]


