Abstract
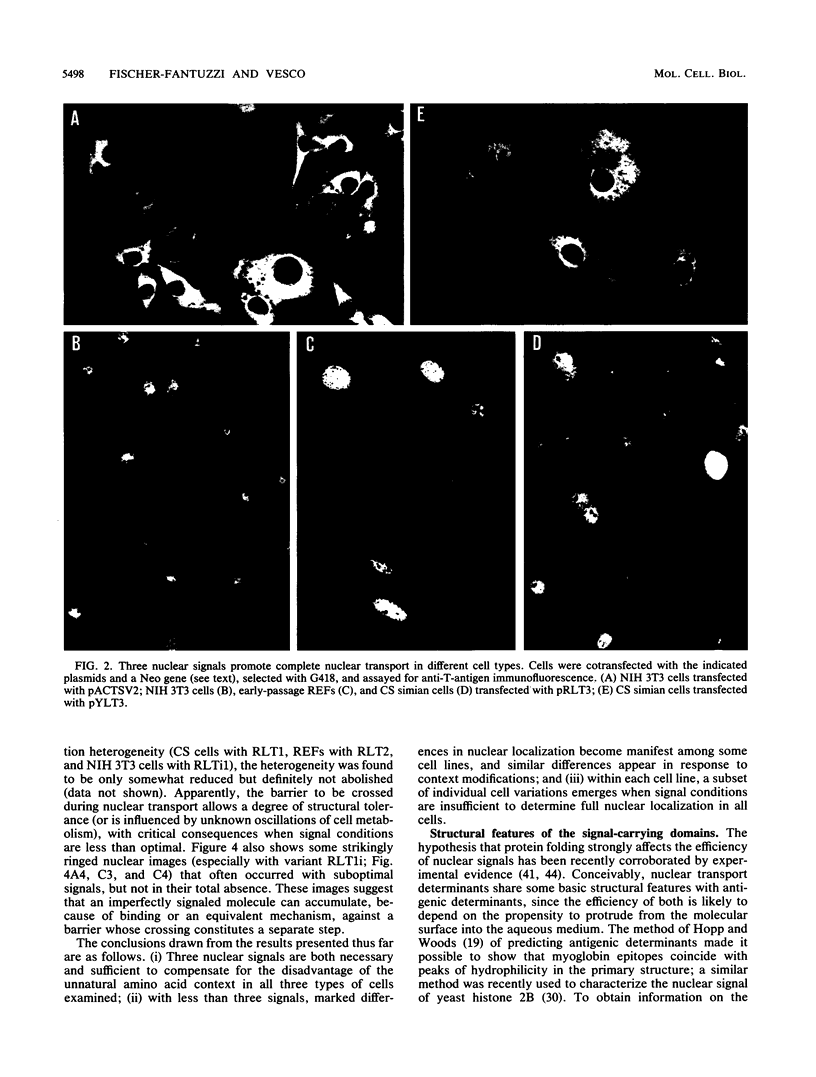
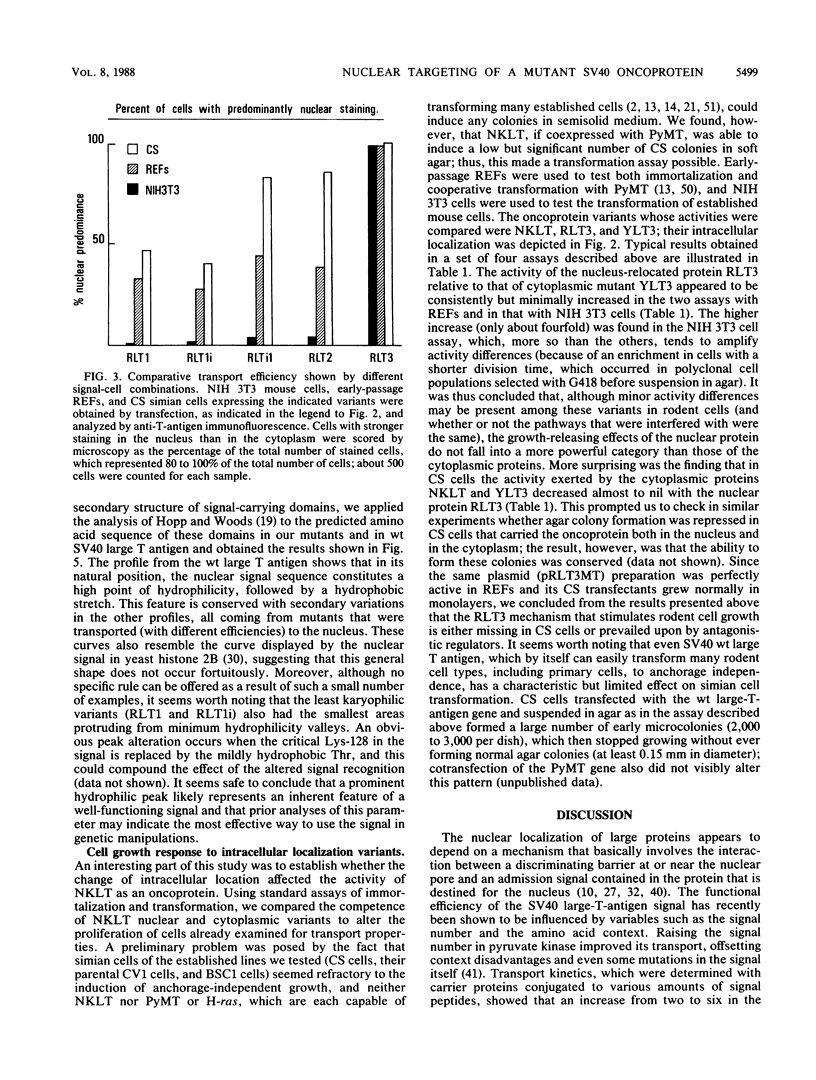
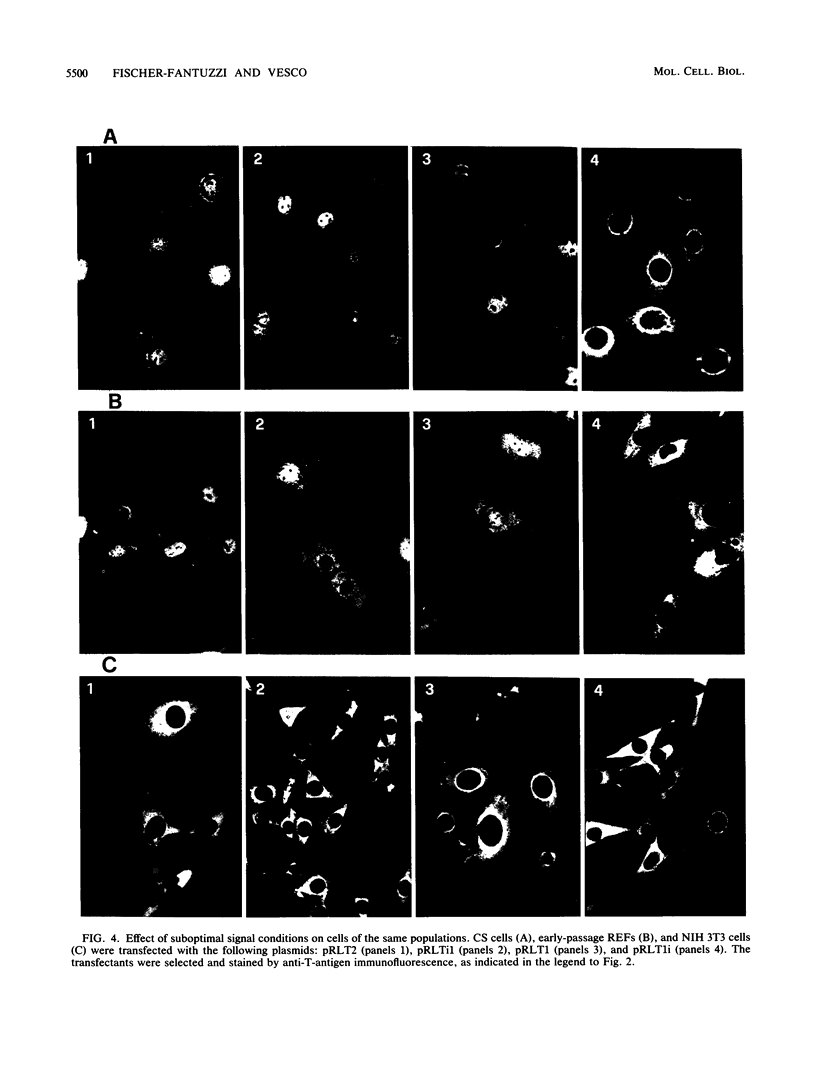
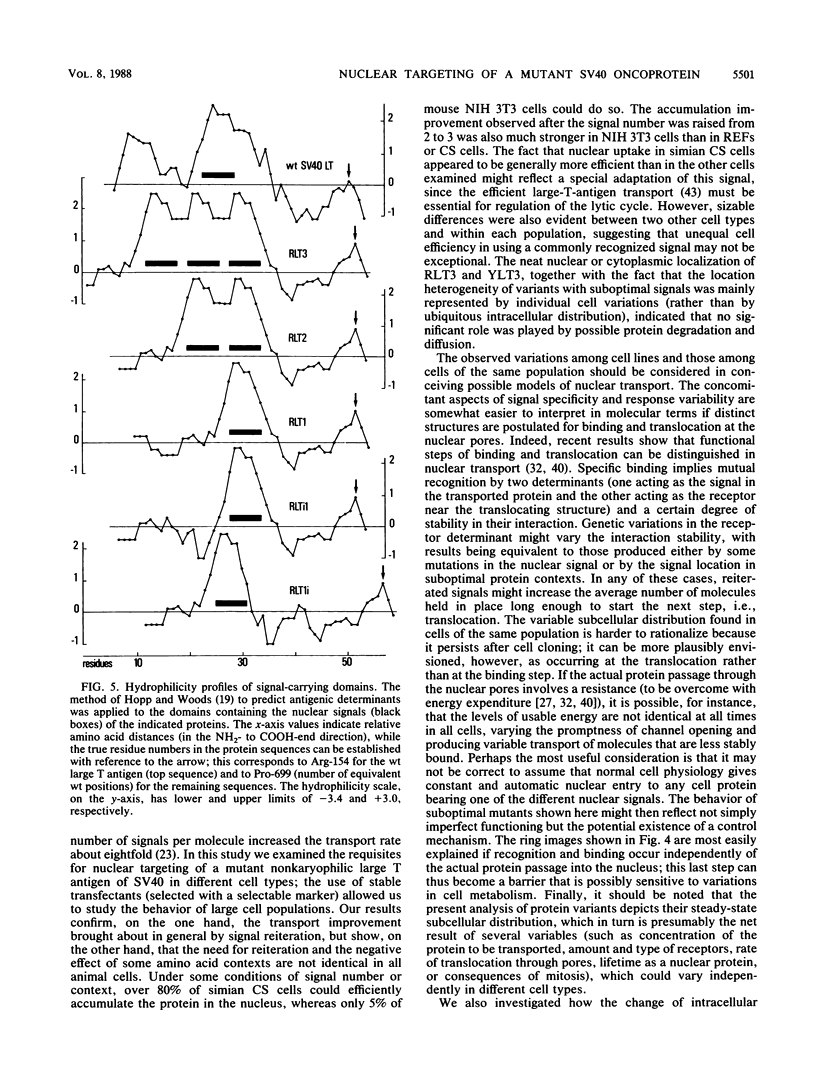
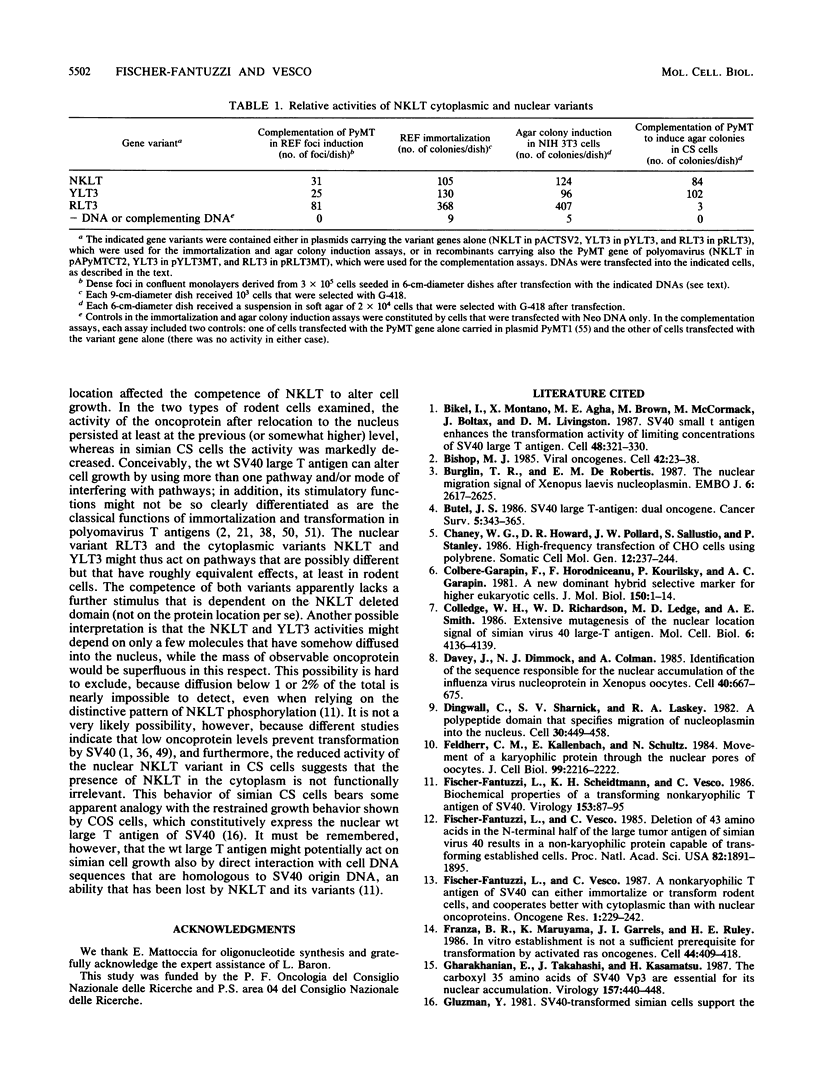
We investigated the requisites for, and functional consequences of, the relocation to the nucleus of a transforming nonkaryophilic mutant of the simian virus 40 large T antigen (a natural deletion mutant lacking an internal large-T-antigen domain that includes the signal for nuclear transport). Synthetic oligonucleotides were used to obtain gene variants with one or more copies of the signal-specifying sequence inserted near the gene 3' end, in a region dispensable for the main large-T-antigen functions. The analysis of stable transfectant populations showed that mouse NIH 3T3 cells, rat embryo fibroblasts, and simian CS cells (a subclone of CV1 cells) differed considerably in their ability to localize some variant molecules into the nucleus. CS cells were always the most efficient, and NIH 3T3 cells were the least efficient. The nuclear localization improved either with reiteration of the signal or with a left-flank modification of the signal amino acid context. Three signals appeared to be necessary and sufficient, even in NIH 3T3 cells, to obtain a nuclear accumulation comparable to that of wild-type simian virus 40 large T antigen; other signal-cell combinations caused a large variability in subcellular localization among cells of the same population, as if the nuclear uptake of some molecules depended on individual cell states. The effect of the modified location on the competence of the protein to alter cell growth was examined by comparing the activity of variants containing either the normal signal or a signal with a mutation (corresponding to large-T-antigen amino acid 128) that prevented nuclear transport. It was found that the nuclear variant was slightly more active than the cytoplasmic variants in rat embryo fibroblasts and NIH 3T3 cells and was notably less active in CS cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Butel J. S. SV40 large T-antigen: dual oncogene. Cancer Surv. 1986;5(2):343–365. [PubMed] [Google Scholar]
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Colledge W. H., Richardson W. D., Edge M. D., Smith A. E. Extensive mutagenesis of the nuclear location signal of simian virus 40 large-T antigen. Mol Cell Biol. 1986 Nov;6(11):4136–4139. doi: 10.1128/mcb.6.11.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Dimmock N. J., Colman A. Identification of the sequence responsible for the nuclear accumulation of the influenza virus nucleoprotein in Xenopus oocytes. Cell. 1985 Mar;40(3):667–675. doi: 10.1016/0092-8674(85)90215-6. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Fantuzzi L., Scheidtmann K. H., Vesco C. Biochemical properties of a transforming nonkaryophilic T antigen of SV40. Virology. 1986 Aug;153(1):87–95. doi: 10.1016/0042-6822(86)90010-3. [DOI] [PubMed] [Google Scholar]
- Fischer-Fantuzzi L., Vesco C. A nonkaryophilic T antigen of SV40 can either immortalize or transform rodent cells, and cooperates better with cytoplasmic than with nuclear oncoproteins. Oncogene Res. 1987 Aug;1(3):229–242. [PubMed] [Google Scholar]
- Fischer-Fantuzzi L., Vesco C. Deletion of 43 amino acids in the NH2-terminal half of the large tumor antigen of simian virus 40 results in a non-karyophilic protein capable of transforming established cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1891–1895. doi: 10.1073/pnas.82.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Gharakhanian E., Takahashi J., Kasamatsu H. The carboxyl 35 amino acids of SV40 Vp3 are essential for its nuclear accumulation. Virology. 1987 Apr;157(2):440–448. doi: 10.1016/0042-6822(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Lee B. A., Maher D. W., Hannink M., Donoghue D. J. Identification of a signal for nuclear targeting in platelet-derived-growth-factor-related molecules. Mol Cell Biol. 1987 Oct;7(10):3527–3537. doi: 10.1128/mcb.7.10.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Smith A. E., Roberts B. L. Signal-dependent translocation of simian virus 40 large-T antigen into rat liver nuclei in a cell-free system. Mol Cell Biol. 1987 Dec;7(12):4255–4265. doi: 10.1128/mcb.7.12.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Fischer-Fantuzzi L., Vesco C., Pipas J. M., Oren M. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J Virol. 1987 Aug;61(8):2648–2654. doi: 10.1128/jvi.61.8.2648-2654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Vesco C., Scheidtmann K. H. Dimers and complexes with p53 are the prevalent oligomeric forms of a transforming nonkaryophilic T antigen of simian virus 40. J Virol. 1987 Mar;61(3):940–944. doi: 10.1128/jvi.61.3.940-944.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Langevin G. L., Singer R. H., Garcea R. L., Hereford L. M. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987 Nov;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Nam H. G., Hereford L. M., Fried H. M. Identification of a nuclear localization signal of a yeast ribosomal protein. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6561–6565. doi: 10.1073/pnas.82.19.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Pannuti A., Pascucci A., La Mantia G., Fisher-Fantuzzi L., Vesco C., Lania L. trans-activation of cellular and viral promoters by a transforming nonkaryophilic simian virus 40 large T antigen. J Virol. 1987 Apr;61(4):1296–1299. doi: 10.1128/jvi.61.4.1296-1299.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecceu F., Komly A., Gardes M., Feunteun J. Properties of simian virus 40 mutants lacking the Asp4-Glu-Asp stretch at the carboxyl-terminus of large T antigen. Virology. 1987 Oct;160(2):485–488. doi: 10.1016/0042-6822(87)90022-5. [DOI] [PubMed] [Google Scholar]
- Pinkert C. A., Brinster R. L., Palmiter R. D., Wong C., Butel J. S. Tumorigenesis in transgenic mice by a nuclear transport-defective SV40 large T-antigen gene. Virology. 1987 Sep;160(1):169–175. doi: 10.1016/0042-6822(87)90057-2. [DOI] [PubMed] [Google Scholar]
- Polvino-Bodnar M., Cole C. N. Construction and characterization of viable deletion mutants of simian virus 40 lacking sequences near the 3' end of the early region. J Virol. 1982 Aug;43(2):489–502. doi: 10.1128/jvi.43.2.489-502.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Roberts B. L., Richardson W. D., Smith A. E. The effect of protein context on nuclear location signal function. Cell. 1987 Jul 31;50(3):465–475. doi: 10.1016/0092-8674(87)90500-9. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickedanz J., Scheidtmann K. H., Walter G. Kinetics of nuclear transport and oligomerization of simian virus 40 large T antigen. Virology. 1986 Jan 15;148(1):47–57. doi: 10.1016/0042-6822(86)90402-2. [DOI] [PubMed] [Google Scholar]
- Schneider J., Schindewolf C., van Zee K., Fanning E. A mutant SV40 large T antigen interferes with nuclear localization of a heterologous protein. Cell. 1988 Jul 1;54(1):117–125. doi: 10.1016/0092-8674(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., de Lange T., Ramsay G., Jakobovits E., Bishop J. M., Varmus H., Lee W. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol. 1987 May;7(5):1697–1709. doi: 10.1128/mcb.7.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Argani P., Mohr I. J., Gluzman Y. Studies on the origin-specific DNA-binding domain of simian virus 40 large T antigen. J Virol. 1987 Oct;61(10):3326–3330. doi: 10.1128/jvi.61.10.3326-3330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Finlay C., Miller D., Marks J., Lozano G., Levine A. J. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J Virol. 1987 Jun;61(6):2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass-Marengo J., Ratiarson A., Asselin C., Bastin M. Ability of a T-antigen transport-defective mutant of simian virus 40 to immortalize primary cells and to complement polyomavirus middle T in tumorigenesis. J Virol. 1986 Sep;59(3):655–659. doi: 10.1128/jvi.59.3.655-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Swimmer C., Cocke T., Shenk T. A second domain of simian virus 40 T antigen in which mutations can alter the cellular localization of the antigen. Mol Cell Biol. 1986 Jun;6(6):2207–2212. doi: 10.1128/mcb.6.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., Benichou D., Girard M. A domain of SV40 capsid polypeptide VP1 that specifies migration into the cell nucleus. EMBO J. 1986 Oct;5(10):2569–2576. doi: 10.1002/j.1460-2075.1986.tb04536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., Benichou D., Girard M. The intranuclear location of simian virus 40 polypeptides VP2 and VP3 depends on a specific amino acid sequence. J Virol. 1987 Dec;61(12):3862–3869. doi: 10.1128/jvi.61.12.3862-3869.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]