Abstract
The incidence of Clostridium difficile infection (CDI) in children has increased over the past decade. In recent years, new and intriguing data on pediatric CDI have emerged. Community-onset infections are increasingly recognized, even in children who have not previously received antibiotics. A hypervirulent strain is responsible for up to 20% of pediatric CDI cases. Unique risk factors for CDI in children have been identified. Advances in diagnostic testing strategies, including the use of nucleic acid amplification tests, have raised new questions about the optimal approach to diagnosing CDI in children. Novel therapeutic options are available for adult patients with CDI, raising questions about the use of these agents in children. Updated recommendations about infection prevention and control measures are now available. We summarize these recent developments in pediatric CDI in this review and also highlight remaining knowledge gaps that should be addressed in future research efforts.
Keywords: Clostridium difficile, Pediatrics, Pseudomembranous colitis, Toxic megacolon, Diarrhea
Changes in CDI in children over the past decade have included rising numbers of patients with severe disease, the emergence of strains that hypersporulate, and an increasing incidence of community-onset infections [1–3]. C difficile is an anaerobic, Gram-positive bacillus that can survive in spore form in the environment for many months even in the presence of heat, acid, antibiotics, and most disinfectants [4]. After spores are ingested, they convert to the vegetative toxin-producing form upon entry into the colon, where the bacterium exerts its effects on the host. This review will provide an overview of the pathogenesis, diagnosis, and management of CDI in children.
ASYMPTOMATIC CARRIAGE
In contrast to adults where the rate of colonization in community and hospital settings is 3% and 20%, respectively, the prevalence of C difficile colonization in neonates ranges from 2% to 50% with colonization often occurring within the first week of life [5–7]. In studies from the early 1980s, C difficile was shown to inhabit the intestines of up to 70% of infants by the end of the first year of life [7]. More recently, although 34% of 294 French infants were colonized with C difficile, only 7% were colonized with a toxigenic strain [8]. By approximately 2 years of age, colonization rates are similar to those in adults [9]. Variation in infant carriage rates can be explained by differences in laboratory testing methods and by patient factors. The risk of colonization increases linearly with the duration of hospital stay [10]. Exclusively breast-fed infants are less likely to be colonized than infants receiving formula (16% vs 62%), possibly as a result of formula contamination with spores [11]. Cross-infection from other infants and environmental contamination are the most likely sources of transmission, because mothers are rarely identified as the source of infection [7]. However, asymptomatically colonized infants represent a potential reservoir for transmission to other family members [12–13].
Despite higher rates of colonization, clinically apparent disease in infants remains rare. Infants with and without diarrhea have similar rates of C difficile colonization and toxin production [14–18]. Absence of toxin receptors has been proposed as a reason for asymptomatic colonization during the first year of life [19]. Studies using juvenile rabbits have demonstrated the relative absence of receptors for toxin A on immature enterocytes [20]. However, in neonatal pigs, the number of toxin A receptors on enterocytes is sufficient to cause disease [21]. Other investigators have proposed that the resistance to disease in infants may relate to other factors such as differences in intestinal mucus that prevents toxin binding, or lack of recruitment and activation of neutrophils by the immature immune system [22]. Limitations in the literature on CDI in infants include a focus on hospitalized infants and a paucity of studies using newer molecular diagnostics. In our opinion, definitive conclusions about the pathogenic potential of C difficile in infants cannot yet be made based on available data.
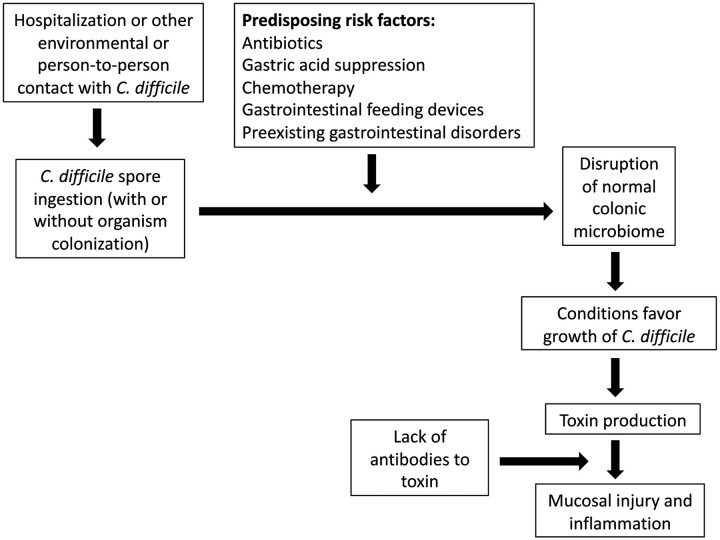
PATHOGENESIS
Three factors place children at risk for CDI (Figure 1): (1) exposure to C difficile spores, (2) disturbance of the normal colonic flora, and (3) impairment of host defenses known to be effective in preventing severe illness and recurrence [23–24]. Only exposure to C difficile is absolutely necessary, and none of the 3 factors alone are sufficient to result in CDI. It remains unclear why some children who ingest toxin-producing C difficile develop CDI and others simply remain colonized.
Figure 1.
Schematic of factors contributing to Clostridium difficile infection.
Toxin Production
The best-described virulence factors are toxins A and B; strains lacking toxins are not pathogenic [25–26]. The toxins are internalized in intestinal epithelial cells and cause cell death and subsequent inflammation [24]. It was initially believed that toxin A was most important for CDI, but more recent data suggest that toxin B may be the more potent toxin, because strains that are negative for toxin A but positive for toxin B are significantly more likely to be associated with severe and recurrent disease [25–27].
Immune Responses
Adaptive immune responses to toxins A and B influence CDI outcomes. The protection afforded by prolonged colonization may be partly mediated by the boosting of serum antibody levels against C difficile toxins, resulting in both decreased severity of infection and fewer recurrences of CDI [28–31]. Approximately 60% of adolescents and adults have detectable serum antitoxin antibodies to C difficile, even in the absence of colonization or infection [32–35]. It is likely that antibody production is stimulated during infancy or through environmental exposure to C difficile or other Clostridium species containing cross-reactive antigens [32].
Hypervirulent Strains
In the early 2000s, outbreaks of severe and recurrent CDI were noted in hospitals throughout North America [36–37]. Subsequent investigation revealed that Canadian and US outbreaks were caused by nearly identical strains of C difficile [36, 38]. This strain was designated as NAP1/BI/027 [36]. The NAP1 strain appears to be transmitted more efficiently than other C difficile strains [38]. In addition to toxins A and B, it possesses a third unrelated toxin, the binary toxin, which mediates cell surface binding and intracellular translocation; however, its clinical significance remains unclear [23]. NAP1 strains have an altered tcdC gene, which modifies the TcdC protein, a negative regulator of toxin A and B production, producing toxin levels 16-fold to 23-fold greater than wild-type strains [39]. Recent isolates have expressed high-level fluoroquinolone resistance, possibly providing a survival advantage in healthcare facilities where fluoroquinolone use is abundant [36]. The NAP1 strain represents 10%–19% of toxigenic C difficile strains in children with CDI, and in 1 small study it was associated with more than 4 times the risk of serious complications compared with other C difficile strains [3, 40–41].
TRENDS IN THE EPIDEMIOLOGY OF CDI IN CHILDREN
Clostridium difficile infection rates are increasing among children in both community and hospital settings [1–2]. Data from 22 children's hospitals in the United States demonstrated a 53% increase in rates from 2001 to 2006 [1]. Despite this trend, rates of colectomy and in-hospital mortality have not increased in children, in contrast to adults [38, 42]. Although hospitalization is a traditional risk factor for CDI, recent evidence suggests that an increasing proportion of individuals with CDI have community-associated (CA) disease [2, 43]. Surveillance in New York in 2008 revealed that 18% of adult CDI cases were CA; 24% of patients had no antibiotic exposure in the prior 12 weeks, but 83% had an outpatient medical visit [44]. Similarly, in a study of 513 children with CDI from 2001 to 2006, the incidence rate among outpatients increased by 11%; 43% of the children had no documented antibiotic exposure before CDI onset [2]. The increase in CDI does not appear related to testing method, because changes were described before the wider use of molecular diagnostics. A more likely explanation is that the increased prevalence of hypervirulent strains promotes disease in younger and healthier individuals even in the absence of traditional risk factors such as antibiotic use. However, this hypothesis may only partly explain the changing epidemiology; however, in the New York study referenced above, only 32% of CA-CDI cases were caused by the NAP1 strain [44]. Other potential sources of exposure in community settings (such as contaminated food or exposure to colonized animals or individuals with CDI) will need to be explored to better understand the rising trend of CA-CDI.
RISK FACTORS FOR INFECTION
Table 2 outlines risk factors associated with CDI in children. Prior antimicrobial use remains the most important risk factor. Virtually all antimicrobials increase the propensity for CDI, but fluoroquinolones, clindamycin, cephalosporins, and penicillins have been most commonly implicated [37, 45–47]. Minimizing antibiotic exposure is imperative to reducing CDI risk [37, 45–47]. Even limited exposure, such as surgical antibiotic prophylaxis, increases a patient's risk of CDI [48–49]. In an analysis of 22 children's hospitals, children diagnosed with CDI within 30 days after an operation had over 18 times the odds of having received perioperative antibiotic prophylaxis compared with children who did not develop CDI [49].
Table 2.
Risk Factors Associated with Clostridium difficile Infection in Children
| Modifiable Risk Factors | ||
| Factor | Evidence | Potential Explanation |
| Antibiotic exposure | Case-control study of 95 children with CDI: cases more likely to have received fluoroquinolones (OR, 17.04; 95% CI, 5.86–49.54) or nonquinolone antibiotics (OR, 2.23; 95% CI, 1.18–4.20) in past 4 weeksa | Antibiotics suppress normal colonic flora, creating a niche for C difficile to flourish |
| Gastric acid suppression | Case-control study of 68 children with CDI: use of proton-pump inhibitors significantly more frequent in cases (OR, 4.5; 95% CI, 1.4–14.4)b; adult studies have found conflicting results; role of acid-suppressing agents in the development of CDI remains controversial | Breaches in protective effect of stomach acid due to elevated gastric pH facilitate bacterial entry and survival of C difficile in the upper gastrointestinal tract |
| Gastrointestinal feeding devices (eg, gastrostomy or jejunostomy tubes) | Case-control study of 95 children with CDI: increased odds of having gastrostomy or jejunostomy tube in cases (OR, 3.32; 95% CI, 1.71–6.42)a | Placement of feeding devices causes mucosal disruption and subsequent tube feeding may introduce C difficile spores on hands of healthcare providers; contamination of formulas with C difficile may occur |
| Nonmodifiable Risk Factors | ||
| Factor | Evidence | Potential Explanation |
| Malignancy and/or chemotherapy | Retrospective cohort study of 4051 cases of pediatric CDI: CDI incidence higher among children with cancer than those without cancer (17.7 vs 1.1 cases per 1000 discharges)c | Increased risk may be attributable to underlying malignancy, antimicrobial activity of chemotherapeutic agents, prolonged receipt of broad-spectrum antimicrobials, impairment of intestinal mucosa from chemotherapy-related toxicities, or long hospitalizations |
| Hypogammaglobulinemia | Limited to observational datad,e | Lack of protective antitoxin antibodies associated with hypogammaglobulinemia, whether congenital or acquired |
| Solid organ transplant | Case-control study of 95 children with CDI: cases had increased odds of prior solid organ transplant (liver, heart, lung, kidney) (OR, 8.09; 95% CI, 2.10–31.12)a; consistent with studies in adults, with highest risk within first 3 months after transplantationf | Increased risk likely due to prolonged hospitalizations, intense immunosuppression, and frequent antimicrobial exposure |
| Hirschsprung's disease | Limited to case reports and case series identifying pseudomembranous colitis on autopsy specimens from children with Hirschsprung's diseaseg,h | Unclear if association exists; if so, contributing mechanisms unknown |
| Inflammatory bowel disease | Retrospective study of 193 children: prevalence of CDI higher in children with IBD than those without IBD (OR, 3.3; 95% CI, 1.5–7.6); in children with IBD, prevalence of active disease significantly greater in CDI patients than uninfected patientsi | Contributing mechanisms unclear- association does not appear to be related to antibiotic use, immunomodulator therapy, or gastric acid suppression |
| Cystic fibrosis | C difficile toxins detected in 47% of 30 CF patients (mean age, 10.5 years)j | Unclear if related to underlying disease or receipt of antibiotic therapy |
Abbreviations: CDI, Clostridium difficile infection; CF, cystic fibrosis; CI, confidence interval; OR, odds ratio.
aSandora T et al. Pediatr Infec Dis J 2011; 30:580; bTurco R et al. Alimet Pharmacol Ther 2010; 31:754; cTai E et al. Pediatr Infec Dis J 2011; 30:610; dGryboski J et al Am J Gastroenterol 1991; 86:685; ePerlmutter D et al. Dig Dis Sci 1985; 30:1149; fRiddle D et al. Curr Opin Org Transp 2008; 12:592; gQualman S et al. Am J Clin Path 1990; 94:410; hPozo F et al. Clin Infect Dis 1994; 19:1160; iPascarella F et al. J Pediatr 2009; 154:854; jYahav J et al. Di Dis Sci 2006; 51:2274.
Although symptoms of CDI typically occur during or shortly after antibiotic use, an increased risk can persist for as long as 8–12 weeks after cessation of antimicrobial therapy [50–51]. In some studies, >85% of adults with CDI have had previous exposure to antimicrobial agents, particularly fluoroquinolones [52–53]. Pediatric studies have reported antibiotic exposure in 35%–75% of children with CDI [2, 54–55]. These studies are limited by their retrospective designs and limited data on prior antibiotic use.
CLOSTRIDIUM DIFFICILE INFECTION IN IMMUNOCOMPROMISED PATIENTS
The role of CDI in immunocompromised children, including those with malignancies, hematopoietic stem cell transplants, solid organ transplants, and other humoral and cellular immunodeficiencies, has been increasingly recognized (Table 2). Impairment of humoral immunity is associated with both severity and recurrence of CDI [27, 30, 56]. Malignancy is the most common underlying chronic comorbidity in children with CDI [1, 57–58]. Many factors may explain the increased risk of CDI in children with cancer, including repeated exposure to broad-spectrum antibiotics, the inherent antimicrobial activity of some chemotherapy regimens, extensive exposure to healthcare facilities, or immunosuppressive effects of chemotherapy, including neutropenia [59–62]. Several studies have highlighted the increased incidence of CDI after hematopoietic stem cell transplantation, particularly in the setting of graft-versus-host disease given the potential for damage to the gut luminal mucosa and the need for additional immunosuppression [63–64].
Other conditions associated with impaired immune systems in children convey similar risks. In a retrospective study of 95 children with CDI, cases had greater than 8 times the odds of prior solid organ transplant compared with controls, a finding in accord with adult studies [55, 65–68]. The incidence of CDI is highest in the initial 3 months after organ transplantation as well as after intensification of immunosuppression to treat graft rejection [69].
The lack of protective antitoxin antibodies associated with hypogammaglobulinemia, whether congenital or acquired, predisposes to more frequent and severe CDI [70]. A retrospective cohort study of over 20 000 children with CDI found that children with human immunodeficiency virus infection had over 4 times the odds of developing CDI compared with children without acquired immunodeficiencies [63]. Available data from adults suggest that severity of CDI presentation and rates of recurrence in immunocompromised patients do not consistently differ from the general population [71]. Whether true differences in CDI outcomes exist for immunocompromised children remains unclear, particularly because complication rates in pediatric CDI are low [3]. There are no consistent data indicating that children with compromised immune systems require more aggressive treatment of CDI [71–72]. Therefore, we advocate using a similar approach to CDI therapy as would be used for immunocompetent children.
APPROACH TO DIAGNOSIS
Case Definition
The case definition of CDI in adults includes diarrhea (3 or more unformed stools in a 24-hour period) and either a positive stool test for C difficile toxin or endoscopic findings compatible with pseudomembranous colitis [73]. There is no distinct case definition for pediatric CDI. Ancillary test results that can be useful for diagnosis and management decisions include leukocytosis, fecal occult blood, hypoalbuminemia, acidosis, and elevated serum creatinine. Radiographic signs such as thickened colonic wall and air-fluid levels are also suggestive but not specific. Although criteria for severe disease in adults have been developed based on expert opinion (peripheral white blood cell count >15 000 cells/mm3 or serum creatinine ≥1.5 times baseline), analogous definitions for severe CDI in children do not exist [73].
Stool Testing
Only unformed stool should be tested for C difficile. Repeat testing after an initial negative result is not recommended given the minimal increase in yield and the substantial increase in cost [74]. The practice of sending 3 tests in rapid succession remains common but is not recommended. In addition, tests of cure are not recommended because ∼25% of patients test positive for several weeks after successful therapy [75–77]. Table 1 reviews the available diagnostic assays for CDI. Although detection of C difficile toxin is necessary to establish infection (in conjunction with appropriate clinical findings), use of the enzyme immunoassay as a stand-alone test is no longer recommended by the American Society for Microbiology due to its poor sensitivity (http://www.asm.org/images/pdf/Clinical/clostridiumdifficile9-21.pdf). One strategy commonly used to overcome this problem is the use of a 2-step approach as described in Table 1. Finding C difficile or its toxin in stool can represent colonization, particularly in infants and younger children, and therefore routine testing of infants should be avoided unless there is a strong clinical suspicion for CDI (for example, CDI may be considered if an infant with antibiotic exposure has persistent diarrhea and concerning abdominal findings that persist despite supportive care and typical viral and bacterial pathogens [eg, norovirus, rotavirus, Salmonella, Campylobacter, etc] have been excluded, or if endoscopy or surgery reveals pseudomembranous colitis).
Table 1.
Comparison of Various Stool Diagnostic Assays to Identify Clostridium difficile Infectiona
| Assay | Target | Advantages | Disadvantages |
|---|---|---|---|
| Toxin EIA | Toxins A and B | Results ≤4 hours; easy to perform, inexpensive; specific (75%–100%) | Least sensitive technique (63%–94%) |
| Cytotoxin assay (or tissue culture assay) | Toxin B > toxin A | Sensitive (up to 100%) and specific (>95%) | Results take up to 48 hours; labor intensive |
| Selective anaerobic culture with confirmation of toxin production (“toxigenic culture”) | Organism and toxin | Most sensitive test; ability to subsequently perform molecular typing and antibiotic susceptibilities | Results take ≥3 days; labor intensive; requires separate assay to identify toxin production |
| ELISA for GDH | Organism only | Results ≤1 hour; sensitive (>94%); inexpensive; good initial screening test | Least specific technique (58%–68%); does not identify toxin production |
| Nucleic acid amplification testb | Gene for toxin A or toxin B | Results ≤3 hours; sensitive (85%–95%) and specific (89%–99%) | Expensive; requires trained personnel; inability to detect future emergence of virulent strains with novel genotypes |
| Two-step algorithm: ELISA for GDH followed by cytotoxin assay or toxin EIA | Organism and toxin | Sensitivity (>94%) and specificity (75%–100%) | Delay in final result for subset of patients in whom results in steps 1 and 2 are discordant |
Abbreviations: EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; GDH, glutamate dehydrogenase.
aSensitivities and specifies derived from Cohen S et al. Infect Control Hosp Epidemiol 2010; 31:431.
bPolymerase chain reaction or transcription-mediated amplification.
SEVERE CDI
Complications that have been associated with CDI include severe dehydration and electrolyte disturbances, toxic megacolon and subsequent bowel perforation, renal failure, hypotension, and death [78]. In a Canadian study of 97 children with CDI there were 2 deaths, and 1 child required a colectomy [41]. In a US study of over 4500 children with CDI, 61 patients (1.2%) underwent colectomy and the all-cause mortality was 4% [1]. Kim et al [3] developed a definition for severe disease in children, which includes severe gastrointestinal complications and laboratory and vital sign abnormalities adapted from adult studies. Of the 48 children they identified with severe disease, 4 had evidence of toxic megacolon, pneumatosis intestinalis, or gastrointestinal perforation. Although severe illness in infants is uncommon, sporadic case reports identifying pseudomembranous colitis on autopsy suggest that severe CDI occasionally occurs in this age group, particularly in those with underlying intestinal pathology such as Hirschsprung's disease [79–80]. A validated definition of severe CDI in children could help guide antimicrobial selection and decisions regarding surgical management.
INITIAL ANTIMICROBIAL TREATMENT
General Principles
Empiric therapy in the absence of diagnostic testing is not recommended if tests are available, because even in a CDI epidemic, only approximately one third of hospitalized patients with antibiotic-associated diarrhea have CDI [81]. Antecedent antimicrobial therapy should be discontinued; if not possible, the narrowest appropriate antibiotic should be used. Antiperistaltic agents should be avoided because they may precipitate toxic megacolon.
Metronidazole
Table 3 outlines the typical approach to treatment of CDI in children. Metronidazole is the initial treatment of choice for children with mild or moderate CDI [1, 54]. Although the minimum inhibitory concentrations of C difficile to metronidazole are increasing, frank resistance has not been documented [82]. When administered orally, metronidazole is absorbed rapidly and almost completely, with only 6%–15% of the drug excreted in stool [83]. In contrast, vancomycin is poorly absorbed, and fecal concentrations after oral administration reach high levels [84].
Table 3.
Available Therapies for Clostridium difficile Infection in Children
| Episode | Recommended Therapy | Alternate Therapies |
|---|---|---|
| Initial episode | Asymptomatic carriage: No therapy | |
| Mild or moderate: Metronidazole (30 mg/kg per day in 4 divided doses orally for 10–14 days; adult dose 500 mg 3 times per day orally) | ||
| Severe: Oral vancomycin (40 mg/kg per day in 4 divided doses for 10–14 days; adult dose 125 mg 4 times per day); consider surgical consultation | ||
| Severe complicateda: Oral vancomycin (40 mg/kg per day in 4 divided doses for 10–14 days; adult dose 500 mg 4 times per day) plus intravenous metronidazole (30 mg/kg per day in 4 divided doses for 10–14 days; adult dose 500 mg 3 times per day); surgical consultation | ||
| Complete ileus: rectal vancomycin (500 mg/100mL normal saline as retention enema 4 times daily)b; plus intravenous metronidazole (30 mg/kg per day in 4 divided doses; adult dose 500 mg 3 times per day) | ||
| First recurrence | Reconfirm diagnosis and repeat therapy based on severity of illness according to recommendations for therapy for initial episode | |
| Second recurrence | Consider tapering and/or pulsed regimen of oral vancomycin3(10 mg/kg 4 times per day for 10–14 days (adult dose 125 mg 4 times per day), 10 mg/kg 2 times per day for 7 days (adult dose 125 mg 2 times per day), 10 mg/kg once per day for 7 days (adult dose 125 mg once daily), 10 mg/kg every 2–3 days for 2–8 weeks (adult dose 125 mg every 2–3 days) | Fidaxomicind (adult dose 200 mg 2 times per day) Nitazoxanidee (1–3 years: 100 mg 2 times per day, 4–11 years: 200 mg 2 times per day, ≥12 years: 500 mg 2 times per day) Rifaximinf (400 mg 3 times per day) |
| Third recurrence and beyond | Fecal microbiota transplantation | Intravenous gamma globuling (400 mg/kg every 3 weeks) Monoclonal antibodiesh |
Abbreviations: CDI, Clostridium difficile infection; ICU, intensive care unit; IVIG, intravenous immunoglobulin; FDA, US Food and Drug Administration.
aGenerally defined as hypotension, toxic megacolon, perforation, need for colectomy, ICU admission for severe disease.
bOptimal dose and volume for rectal vancomycin have not been established, but some experts recommend 50 mL for ages 1–3 years, 75 mL for ages 4–9 years, and 100 mL for ages ≥10 years.
cEvidence limited to uncontrolled studies in adults; optimal dosing and interval in children not established.
dApproved by the FDA for the treatment of CDI in adults ≥18 years, with trials in children ongoing.
eApproved by the FDA for the treatment of Cryptosporidium and Giardia infection in children ≥1 year of age; in randomized controlled trials in adults, produced cure and relapse rates similar to metronidazole and vancomycin for CDI with phase III trials in progress; dosing based on FDA-approved doses for Cryptosporidium and Giardia.
fApproved by the FDA for traveler's diarrhea in children ≥12 years of age; observational data demonstrate benefit for CDI; dosing based on Garey et al. J Antimicrob Chemother 2011; 66:2850.
gEvidence limited to uncontrolled studies in children and adults; optimal number of IVIG doses unknown.
hIn phase II trials in adults, human monoclonal antibodies to C difficile toxin A and B reduced the risk of CDI relapse by 70%. Lowy et al. N Engl J Med 2010; 362:197; no studies to date in children.
Vancomycin
A randomized, placebo-controlled trial of 150 adults comparing a 10-day course of vancomycin to oral metronidazole found no difference in outcomes for mild disease. However, in severe disease, treatment with metronidazole or vancomycin resulted in clinical cure in 76% and 97% of patients, respectively [85]. Although similar studies have not been conducted in children, vancomycin is recommended for severe CDI. Resistance to vancomycin has not been reported [82]. The use of vancomycin as first-line therapy for mild CDI is discouraged because of higher cost compared with metronidazole and concern about the potential for promoting vancomycin-resistant enterococci (VRE), although studies suggest that both antibiotics carry this risk [86–87].
RECURRENT CDI AND ALTERNATIVE THERAPIES
Recurrent CDI is defined as an episode of CDI that occurs ≤8 weeks after the onset of a previous episode, provided that symptoms resolved in the interim [88]. Approximately 30% of children experience a recurrence of CDI, and rates are similar after metronidazole or vancomycin use [54, 89]. Recurrences represent either relapse of infection with the original strain or exposure to new strains [90]. Although the optimal management of recurrent CDI has not been established in either adults or children, Table 3 outlines possible approaches. For the first recurrence, after reconfirming the diagnosis, we recommend the initial therapy be repeated for 10 days. If severe symptoms are present with the recurrence, it is reasonable to escalate from metronidazole to vancomycin. If a child has a second recurrence, we recommend a tapering vancomycin regimen as outlined in Table 3. A similar tapering approach with metronidazole is discouraged because prolonged metronidazole therapy poses a risk of neurotoxicity [91].
Rifaximin
Rifaximin is a non-absorbed rifamycin that has been used as an adjunct agent to treat patients with multiple CDI recurrences [92]. In a randomized, double-blind trial of 68 adults with CDI, patients receiving rifaximin for 20 days immediately after completing standard therapy experienced significantly fewer recurrences than patients receiving placebo (21% vs 49%) [93]. One downside is the potential for isolates to develop high-level resistance (minimum inhibitory concentration values >256 µg/mL) during treatment, with reported rates of resistance as high as 30% [92]. Rifaximin is Food and Drug Administration (FDA)-approved in the United States for the treatment of traveler's diarrhea in individuals ≥12 years of age.
Nitazoxanide
Nitazoxanide is an FDA-approved antimicrobial agent for the treatment of Cryptosporidium parvum and Giardia lamblia infections in children ≥1 year of age. Trials in adults have shown nitazoxanide to produce cure and relapse rates similar to metronidazole or vancomycin for the treatment of CDI [94–96].
Fidaxomicin
Fidaxomicin was approved by the FDA in 2011 for the treatment of CDI in patients ≥18 years of age. In phase 3 trials, fidaxomicin was shown to be non-inferior to oral vancomycin and associated with a significant reduction in CDI recurrence [97]. Fidaxomicin has a narrow spectrum of activity, sparing most members of the colonic microbiome [98]. Cost-effectiveness studies comparing fidaxomicin to other available therapies are needed (approximate costs for a 10-day treatment course in adults: fidaxomicin, $2700; oral metronidazole, $5; vancomycin suspension, $25).
Non-Antimicrobial Treatments
Probiotics
There is no compelling evidence that probiotics can prevent or ameliorate the symptoms of CDI [99]. Currently, probiotics are not recommended for CDI prevention [73].
Passive Immunotherapy
Studies demonstrating the absence of severe CDI in people with preexisting antitoxin antibodies provide the scientific rationale for the development of antibody products against C difficile [28, 30]. Only observational data have been published for pooled intravenous immunoglobulin (IVIG) [100–103]. IVIG was initially reported as effective for immunoglobulin (Ig)-deficient children with chronic recurrent CDI [104]. Because most of the population has detectable IgG to toxins A and B, IVIG may serve to neutralize C difficile toxins [105].
In addition, 2 neutralizing, human monoclonal antibodies against C difficile toxins A and B have shown efficacy in reducing CDI recurrence when used in combination with antibiotic treatment [106]. A randomized, controlled, phase 2 trial involving 200 adults demonstrated that administration of monoclonal antibodies reduced the risk of recurrence by 70% [106]. Additional studies are needed to determine whether monoclonal antibodies will be safe, effective, and cost-effective in children.
Active Immunotherapy
Active immunization is an attractive goal for effective and prolonged protection against CDI. Preventing colonization through vaccination may reduce environmental contamination. DNA vaccines targeting the C difficile receptor-binding domain of TcdA induce neutralizing antibodies to C difficile toxins in mice and protect them from death [107]. Preliminary trials of vaccines containing toxoids A and B have been successful in inducing vigorous serum antibody responses in healthy adults [26, 108–109].
Fecal Microbiota Transplantation
When traditional medical management has failed, fecal microbiota transplantation (FMT) may be considered. Instillation of stool from an uninfected donor has been highly successful in treating CDI in several case reports and uncontrolled case series in both children and adults [110–112]. Complications of FMT are generally associated with the insertion of a nasogastric tube or colonoscope. Additional risks include the possibility of transmissible infectious agents in donor stool. Currently, there is no consensus about the optimal protocol for performing FMT [113].
Colectomy
In adults with CDI, serum lactate levels >5 mmol/L and white blood cell counts of approximately 50 000 cells/mL have been associated with ominous outcomes and warrant surgical consultation for consideration of colectomy [73]. It is not known whether analogous laboratory criteria predict similar risks in children, but in the presence of toxic megacolon, worsening acidosis, and a declining clinical course, surgical consultation should be prioritized.
INFECTION PREVENTION AND CONTROL ISSUES
The rate of acquisition of C difficile during hospitalization is proportional to the duration of hospitalization and can be as high as 40% after 1 month [114]. Healthcare facilities should perform surveillance for healthcare facility-onset, healthcare facility-associated disease (at a minimum), with rates expressed as number of cases per 10 000 patient-days [88]. Rising rates should prompt further investigation and control measures. Identification of asymptomatic carriers is not recommended; however, when CDI is identified, stringent infection control practices should be implemented.
Hand Hygiene
C difficile spores are highly resistant to killing by alcohol [115]. Use of alcohol-based hand hygiene products may simply displace spores over the skin, instead of physically removing spores as occurs with handwashing using soap and water [73]. The decreased effectiveness of alcohol-based hand rubs may be offset by increased hand hygiene compliance with these products and by increased effectiveness against other nonspore-forming, multidrug-resistant pathogens such as methicillin-resistant Staphylococcus aureus and VRE. For these reasons, preferential use of soap and water is recommended only in outbreak settings [73].
Contact Precautions
The use of gowns and gloves (in conjunction with hand hygiene) is recommended as a strategy to decrease the concentration of C difficile on the hands and clothing of healthcare workers [116]. The appropriate use of gloves when caring for a patient with CDI minimizes the risk of hand contamination, further lessening the theoretical risk of incomplete disinfection of hands with alcohol hand rub. Contact precautions are recommended for the duration of C difficile–associated diarrhea [73]. However, if rates of CDI are unacceptably high in an institution, extending the duration of contact precautions until discharge is an alternative strategy, because spores may be shed in the stool for several weeks after diarrhea resolves [117–118]. Although single rooms are preferred, cohorting of infected patients has also been demonstrated to decrease nosocomial CDI [10].
Environmental Disinfection
C difficile spores can survive in the environment for months or years and can be found on multiple surfaces in the healthcare setting [10, 119-121]. Sodium hypochlorite (bleach) solutions kill spores and reduce environmental contamination with C difficile and have been used successfully to control CDI outbreaks [122–126]. Hydrogen peroxide vapor disinfection has been shown to significantly decrease environmental contamination with C difficile after a single 3-hour cycle, even reducing contamination in rooms that had previously been disinfected with sodium hypochlorite solutions [127]. A complete discussion of environmental disinfection is beyond the scope of this review. For further guidance, please refer to a more thorough review on the subject by Dubberke et al [117].
Antimicrobial Stewardship
Judicious antimicrobial use is critical to reduce the burden of CDI [128]. Restriction of antibiotics known to place patients at high risk for CDI, such as clindamycin, has been demonstrated to decrease CDI rates [129–130]. A reduction in overall antimicrobial use played an important role in controlling at least 2 large institutional outbreaks of CDI caused by the NAP1 strain [131–132].
PRIORITIES FOR FUTURE RESEARCH
Although the increasing incidence and severity of CDI in children have led to a resurgence of studies to better understand epidemiology, pathogenesis, treatment, and prevention, many questions remain unanswered. To what extent is C difficile pathogenic among infants, and how does true disease present in that age group? What is the role of toxin A versus toxin B and associated receptors in infants, and is the rarity of disease related to age-dependent receptor concentration or to other factors such as an immature immune response? Is gastric acid suppression a risk factor for CDI in children? What defines severe disease in children, and how can we more easily identify children at increased risk of poor outcomes? What is the optimal therapy for primary and recurrent CDI in children (using clinical trials with adequate power and including children in the evaluation of new therapeutic agents)? What is the role of treatment with passive antibodies in severe infection? Can vaccination effectively prevent CDI in children, and what would be the optimal timing and route of administration? With the renewed interest in pediatric CDI in recent years, we remain hopeful that these knowledge gaps will soon be addressed.
Acknowledgments
We thank Alice J. Hsu and Aaron M. Milstone for assistance with the preparation of this manuscript.
Financial support. This work was supported by the National Institutes of Health (grant number KL2RR025006 to P. D. T.)
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kim J, Smathers SA, Prasad P, et al. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001–2006. Pediatrics. 2008;122:1266–70. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 2.Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007;28:1233–5. doi: 10.1086/520732. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J. 2012;31:134–8. doi: 10.1097/INF.0b013e3182352e2c. [DOI] [PubMed] [Google Scholar]
- 4.Weaver L, Michels HT, Keevil CW. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J Hosp Infect. 2008;68:145–51. doi: 10.1016/j.jhin.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Bolton RP, Tait SK, Dear PR, Losowsky MS. Asymptomatic neonatal colonisation by Clostridium difficile. Arch Dis Child. 1984;59:466–72. doi: 10.1136/adc.59.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson HE, Barclay FE, Honour P, Hill ID. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146:727–33. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- 7.Al-Jumaili IJ, Shibley M, Lishman AH, Record CO. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984;19:77–8. doi: 10.1128/jcm.19.1.77-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau C, Lemee L, Le Monnier A, et al. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol. 2011;60(Pt 8):1112–8. doi: 10.1099/jmm.0.029736-0. [DOI] [PubMed] [Google Scholar]
- 9.Ellis ME, Mandal BK, Dunbar EM, Bundell KR. Clostridium difficile and its cytotoxin in infants admitted to hospital with infectious gastroenteritis. Br Med J (Clin Res Ed) 1984;288:524–6. doi: 10.1136/bmj.288.6416.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 11.Cooperstock M, Riegle L, Woodruff CW, Onderdonk A. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J Clin Microbiol. 1983;17:830–3. doi: 10.1128/jcm.17.5.830-833.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecker MT, Riggs MM, Hoyen CK, et al. Recurrent infection with epidemic Clostridium difficile in a peripartum woman whose infant was asymptomatically colonized with the same strain. Clin Infect Dis. 2008;46:956–7. doi: 10.1086/527568. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox MH, Mooney L, Bendall R, et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62:388–96. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 14.Boenning DA, Fleisher GR, Campos JM, et al. Clostridium difficile in a pediatric outpatient population. Pediatr Infect Dis. 1982;1:336–8. doi: 10.1097/00006454-198209000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Cerquetti M, Luzzi I, Caprioli A, et al. Role of Clostridium difficile in childhood diarrhea. Pediatr Infect Dis J. 1995;14:598–603. doi: 10.1097/00006454-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Svedhem A, Kaijser B, MacDowall I. Intestinal occurrence of Campylobacter fetus subspecies jejuni and Clostridium difficile in children in Sweden. Eur J Clin Microbiol. 1982;1:29–32. doi: 10.1007/BF02014137. [DOI] [PubMed] [Google Scholar]
- 17.Holst E, Helin I, Mardh PA. Recovery of Clostridium difficile from children. Scand J Infect Dis. 1981;13:41–5. doi: 10.1080/00365548.1981.11690365. [DOI] [PubMed] [Google Scholar]
- 18.Emeruwa AC, Oguike JU. Incidence of cytotoxin producing isolates of Clostridium difficile in faeces of neonates and children in Nigeria. Microbiologica. 1990;13:323–8. [PubMed] [Google Scholar]
- 19.Borriello SP 12th C. L. Oakley lecture. Pathogenesis of Clostridium difficile infection of the gut. J Med Microbiol. 1990;33:207–15. doi: 10.1099/00222615-33-4-207. [DOI] [PubMed] [Google Scholar]
- 20.Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest. 1992;90:822–9. doi: 10.1172/JCI115957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keel MK, Songer JG. The distribution and density of Clostridium difficile toxin receptors on the intestinal mucosa of neonatal pigs. Vet Pathol. 2007;44:814–22. doi: 10.1354/vp.44-6-814. [DOI] [PubMed] [Google Scholar]
- 22.Rolfe RD, Song W. Purification of a functional receptor for Clostridium difficile toxin A from intestinal brush border membranes of infant hamsters. Clin Infect Dis. 1993;16(Suppl 4):S219–27. doi: 10.1093/clinids/16.supplement_4.s219. [DOI] [PubMed] [Google Scholar]
- 23.Kelly CP, LaMont JT. Clostridium difficile––more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 24.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–9. doi: 10.1099/jmm.0.030015-0. (Pt 8) [DOI] [PubMed] [Google Scholar]
- 25.Lyras D, O'Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis. 2007;11:5–10. doi: 10.1016/j.ijid.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–9. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 28.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 29.Shim JK, Johnson S, Samore MH, et al. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 1998; 351:633–6. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 30.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 31.Sambol SP, Tang JK, Merrigan MM, et al. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J Infect Dis. 2001;183:1760–6. doi: 10.1086/320736. [DOI] [PubMed] [Google Scholar]
- 32.Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Kelly CP, Pothoulakis C, Orellana J, LaMont JT. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura S, Mikawa M, Nakashio S, et al. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol Immunol. 1981;25:345–51. doi: 10.1111/j.1348-0421.1981.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Hurtado K, Corretge M, Mutlu E, et al. Systemic antibody response to Clostridium difficile in colonized patients with and without symptoms and matched controls. J Med Microbiol. 2008;57:717–24. doi: 10.1099/jmm.0.47713-0. (Pt 6) [DOI] [PubMed] [Google Scholar]
- 36.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 37.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 38.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 39.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 40.Toltzis P, Kim J, Dul M, et al. Presence of the epidemic North American Pulsed Field type 1 Clostridium difficile strain in hospitalized children. J Pediatr. 2009;154:607–8. doi: 10.1016/j.jpeds.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Suh KN, Gravel D, Mulvey MR. Orlando, FL: The Society for Healthcare Epidemiology of America; 2008. Clostridium difficile-associated infections in children admitted to acute care hospitals participating in the Canadian Nosocomial Infections Surveillance Program (CNISP), 2004–2005 [abstract 306] In: Program of the 18th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America (April 2008) [Google Scholar]
- 42.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–72. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surveillance for community-associated Clostridium difficile–Connecticut, 2006. Morb Mortal Wkly Rep. 2008;57:340–3. [PubMed] [Google Scholar]
- 44.Dumyati G, Stevens V, Hannett GE, et al. Community-associated Clostridium difficile infections, Monroe County, New York. Emerg Infect Dis. 2012;18:392–400. doi: 10.3201/eid1803.102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678–84. doi: 10.1093/infdis/162.3.678. [DOI] [PubMed] [Google Scholar]
- 46.Wistrom J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40:1–15. doi: 10.1016/s0195-6701(98)90019-6. [DOI] [PubMed] [Google Scholar]
- 48.Yee J, Dixon CM, McLean AP, Meakins JL. Clostridium difficile disease in a department of surgery. The significance of prophylactic antibiotics. Arch Surg. 1991;126:241–6. doi: 10.1001/archsurg.1991.01410260131019. [DOI] [PubMed] [Google Scholar]
- 49.Rangel SJ, Fung M, Graham DA, et al. Recent trends in the use of antibiotic prophylaxis in pediatric surgery. J Pediatr Surg. 2011;46:366–71. doi: 10.1016/j.jpedsurg.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Anand A, Bashey B, Mir T, Glatt AE. Epidemiology, clinical manifestations, and outcome of Clostridium difficile-associated diarrhea. Am J Gastroenterol. 1994;89:519–23. [PubMed] [Google Scholar]
- 51.Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 52.Chang HT, Krezolek D, Johnson S, et al. Onset of symptoms and time to diagnosis of Clostridium difficile-associated disease following discharge from an acute care hospital. Infect Control Hosp Epidemiol. 2007;28:926–31. doi: 10.1086/519178. [DOI] [PubMed] [Google Scholar]
- 53.Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–60. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 54.Morinville V, McDonald J. Clostridium difficile-associated diarrhea in 200 Canadian children. Can J Gastroenterol. 2005;19:497–501. doi: 10.1155/2005/326296. [DOI] [PubMed] [Google Scholar]
- 55.Sandora TJ, Fung M, Flaherty K, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011;30:580–4. doi: 10.1097/INF.0b013e31820bfb29. [DOI] [PubMed] [Google Scholar]
- 56.Warny M, Vaerman JP, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384–9. doi: 10.1128/iai.62.2.384-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. doi: 10.1186/1471-2334-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tai E, Richardson LC, Townsend J, et al. Clostridium difficile infection among children with cancer. Pediatr Infect Dis J. 2011;30:610–2. doi: 10.1097/INF.0b013e31820970d1. [DOI] [PubMed] [Google Scholar]
- 59.Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17:109–13. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 60.Morales Chamorro R, Serrano Blanch R, Mendez Vidal MJ, et al. Pseudomembranous colitis associated with chemotherapy with 5-fluorouracil. Clin Transl Oncol. 2005;7:258–61. doi: 10.1007/BF02710173. [DOI] [PubMed] [Google Scholar]
- 61.Bilgrami S, Feingold JM, Dorsky D, et al. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1039–42. doi: 10.1038/sj.bmt.1701773. [DOI] [PubMed] [Google Scholar]
- 62.Gorschluter M, Glasmacher A, Hahn C, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2001;33:786–91. doi: 10.1086/322616. [DOI] [PubMed] [Google Scholar]
- 63.Nylund CM, Goudie A, Garza JM, et al. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med. 2011;165:451–7. doi: 10.1001/archpediatrics.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubberke ER, Reske KA, Srivastava A, et al. Clostridium difficile-associated disease in allogeneic hematopoietic stem-cell transplant recipients: risk associations, protective associations, and outcomes. Clin Transplant. 2010;24:192–8. doi: 10.1111/j.1399-0012.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma AK, Holder FE. Clostridium difficile diarrhea after use of tacrolimus following renal transplantation. Clin Infect Dis. 1998;27:1540–1. doi: 10.1086/517744. [DOI] [PubMed] [Google Scholar]
- 66.Gunderson CC, Gupta MR, Lopez F, et al. Clostridium difficile colitis in lung transplantation. Transpl Infect Dis. 2008;10:245–51. doi: 10.1111/j.1399-3062.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 67.Wong NA, Bathgate AJ, Bellamy CO. Colorectal disease in liver allograft recipients—a clinicopathological study with follow-up. Eur J Gastroenterol Hepatol. 2002;14:231–6. doi: 10.1097/00042737-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Theunissen C, Knoop C, Nonhoff C, et al. Clostridium difficile colitis in cystic fibrosis patients with and without lung transplantation. Transpl Infect Dis. 2008;10:240–4. doi: 10.1111/j.1399-3062.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 69.Albright JB, Bonatti H, Mendez J, et al. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int. 2007;20:856–66. doi: 10.1111/j.1432-2277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 70.Perlmutter DH, Leichtner AM, Goldman H, Winter HS. Chronic diarrhea associated with hypogammaglobulinemia and enteropathy in infants and children. Dig Dis Sci. 1985;30:1149–55. doi: 10.1007/BF01314049. [DOI] [PubMed] [Google Scholar]
- 71.Collini PJ, Bauer M, Kuijper E, Dockrell DH. Clostridium difficile infection in HIV-seropositive individuals and transplant recipients. J Infect. 2012;64:131–47. doi: 10.1016/j.jinf.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 72.Castagnola E, Battaglia T, Bandettini R, et al. Clostridium difficile-associated disease in children with solid tumors. Support Care Cancer. 2009;17:321–4. doi: 10.1007/s00520-008-0507-0. [DOI] [PubMed] [Google Scholar]
- 73.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 74.Aichinger E, Schleck CD, Harmsen WS, et al. Nonutility of repeat laboratory testing for detection of Clostridium difficile by use of PCR or enzyme immunoassay. J Clin Microbiol. 2008;46:3795–7. doi: 10.1128/JCM.00684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wenisch C, Parschalk B, Hasenhundl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–8. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 76.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043–6. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 77.Wullt M, Odenholt I. A double-blind randomized controlled trial of fusidic acid and metronidazole for treatment of an initial episode of Clostridium difficile-associated diarrhoea. J Antimicrob Chemother. 2004;54:211–6. doi: 10.1093/jac/dkh278. [DOI] [PubMed] [Google Scholar]
- 78.Kyne L, Merry C, O'Connell B, et al. Factors associated with prolonged symptoms and severe disease due to Clostridium difficile. Age Ageing. 1999;28:107–13. doi: 10.1093/ageing/28.2.107. [DOI] [PubMed] [Google Scholar]
- 79.Qualman SJ, Petric M, Karmali MA, et al. Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am J Clin Pathol. 1990;94:410–6. doi: 10.1093/ajcp/94.4.410. [DOI] [PubMed] [Google Scholar]
- 80.Singer DB, Cashore WJ, Widness JA, et al. Pseudomembranous colitis in a preterm neonate. J Pediatr Gastroenterol Nutr. 1986;5:318–20. [PubMed] [Google Scholar]
- 81.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–9. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 82.Pelaez T, Alcala L, Alonso R, et al. In vitro activity of ramoplanin against Clostridium difficile, including strains with reduced susceptibility to vancomycin or with resistance to metronidazole. Antimicrob Agents Chemother. 2005;49:1157–9. doi: 10.1128/AAC.49.3.1157-1159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169–72. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keighley MR, Burdon DW, Arabi Y, et al. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. Br Med J. 1978;2:1667–9. doi: 10.1136/bmj.2.6153.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 86.Al-Nassir WN, Sethi AK, Li Y, et al. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin–resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008;52:2403–6. doi: 10.1128/AAC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerding DN. Is there a relationship between vancomycin-resistant enterococcal infection and Clostridium difficile infection? Clin Infect Dis. 1997;25(Suppl 2):S206–10. doi: 10.1086/516247. [DOI] [PubMed] [Google Scholar]
- 88.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–5. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 89.Johnson S, Adelmann A, Clabots CR, et al. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;159:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 90.Barbut F, Richard A, Hamadi K, et al. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Product Information. New York, NY: Searle LLC; 2010. Flagyl(R) oral tablets, metronidazole oral tablets. G.D. [Google Scholar]
- 92.Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846–8. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 93.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2850–5. doi: 10.1093/jac/dkr377. [DOI] [PubMed] [Google Scholar]
- 94.Musher DM, Logan N, Mehendiratta V, et al. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007;59:705–10. doi: 10.1093/jac/dkl553. [DOI] [PubMed] [Google Scholar]
- 95.Musher DM, Logan N, Hamill RJ, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43:421–7. doi: 10.1086/506351. [DOI] [PubMed] [Google Scholar]
- 96.Musher DM, Logan N, Bressler AM, et al. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009;48:e41–6. doi: 10.1086/596552. [DOI] [PubMed] [Google Scholar]
- 97.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 98.Venugopal AA, Johnson S. Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile Infection. Clin Infect Dis. 2012;54:568–74. doi: 10.1093/cid/cir830. [DOI] [PubMed] [Google Scholar]
- 99.Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012–7. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 100.Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53:882–4. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- 101.Juang P, Skledar SJ, Zgheib NK, et al. Clinical outcomes of intravenous immune globulin in severe Clostridium difficile–associated diarrhea. Am J Infect Control. 2007;35:131–7. doi: 10.1016/j.ajic.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 102.Abougergi MS, Broor A, Cui W, Jaar BG. Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature. J Hosp Med. 2010;5:E1–9. doi: 10.1002/jhm.542. [DOI] [PubMed] [Google Scholar]
- 103.McPherson S, Rees CJ, Ellis R, et al. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49:640–5. doi: 10.1007/s10350-006-0511-8. [DOI] [PubMed] [Google Scholar]
- 104.Leung DY, Kelly CP, Boguniewicz M, et al. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118(4 Pt 1):633–7. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 105.Abougergi MS, Kwon JH. Intravenous immunoglobulin for the treatment of Clostridium difficile infection: a review. Dig Dis Sci. 2011;56:19–26. doi: 10.1007/s10620-010-1411-2. [DOI] [PubMed] [Google Scholar]
- 106.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 107.Gardiner DF, Rosenberg T, Zaharatos J, et al. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine. 2009;27(27):3598–604. doi: 10.1016/j.vaccine.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aboudola S, Kotloff KL, Kyne L, et al. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003;71:1608–10. doi: 10.1128/IAI.71.3.1608-1610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotloff KL, Wasserman SS, Losonsky GA, et al. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001;69:988–95. doi: 10.1128/IAI.69.2.988-995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126:e239–42. doi: 10.1542/peds.2009-3363. [DOI] [PubMed] [Google Scholar]
- 111.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM. 2009;102:781–4. doi: 10.1093/qjmed/hcp118. [DOI] [PubMed] [Google Scholar]
- 112.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–70. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 113.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clabots CR, Johnson S, Olson MM, et al. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166:561–7. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 115.Wullt M, Odenholt I, Walder M. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect Control Hosp Epidemiol. 2003;24:765–8. doi: 10.1086/502129. [DOI] [PubMed] [Google Scholar]
- 116.Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med. 1990;88:137–40. doi: 10.1016/0002-9343(90)90462-m. [DOI] [PubMed] [Google Scholar]
- 117.Dubberke ER, Gerding DN, Classen D, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S81–92. doi: 10.1086/591065. [DOI] [PubMed] [Google Scholar]
- 118.Sethi AK, Al-Nassir WN, Nerandzic MM, et al. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010;31:21–7. doi: 10.1086/649016. [DOI] [PubMed] [Google Scholar]
- 119.Fekety R, Kim KH, Brown D, et al. Epidemiology of antibiotic-associated colitis; isolation of Clostridium difficile from the hospital environment. Am J Med. 1981;70:906–8. doi: 10.1016/0002-9343(81)90553-2. [DOI] [PubMed] [Google Scholar]
- 120.O'Neill G, Adams JE, Bowman RA, Riley TV. A molecular characterization of Clostridium difficile isolates from humans, animals and their environments. Epidemiol Infect. 1993;111:257–64. doi: 10.1017/s095026880005696x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981;143:42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 122.McMullen KM, Zack J, Coopersmith CM, et al. Use of hypochlorite solution to decrease rates of Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2007;28:205–7. doi: 10.1086/511791. [DOI] [PubMed] [Google Scholar]
- 123.Eckstein BC, Adams DA, Eckstein EC, et al. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31:995–1000. doi: 10.1086/318149. [DOI] [PubMed] [Google Scholar]
- 125.Wilcox MH, Fawley WN, Wigglesworth N, et al. Comparison of the effect of detergent versus hypochlorite cleaning on environmental contamination and incidence of Clostridium difficile infection. J Hosp Infect. 2003;54:109–14. doi: 10.1016/s0195-6701(02)00400-0. [DOI] [PubMed] [Google Scholar]
- 126.Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet. 2000;356:1324 doi: 10.1016/S0140-6736(00)02819-1. [DOI] [PubMed] [Google Scholar]
- 127.Shapey S, Machin K, Levi K, Boswell TC. Activity of a dry mist hydrogen peroxide system against environmental Clostridium difficile contamination in elderly care wards. J Hosp Infect. 2008;70:136–41. doi: 10.1016/j.jhin.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 128.Carling P, Fung T, Killion A, et al. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24:699–706. doi: 10.1086/502278. [DOI] [PubMed] [Google Scholar]
- 129.Climo MW, Israel DS, Wong ES, et al. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann Intern Med. 1998;128(12 Pt 1):989–95. doi: 10.7326/0003-4819-128-12_part_1-199806150-00005. [DOI] [PubMed] [Google Scholar]
- 130.Pear SM, Williamson TH, Bettin KM, et al. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann Intern Med. 1994;120:272–7. doi: 10.7326/0003-4819-120-4-199402150-00003. [DOI] [PubMed] [Google Scholar]
- 131.Valiquette L, Cossette B, Garant MP, et al. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis. 2007;45(Suppl 2):S112–21. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 132.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]