Abstract
Background.
Risk factors differentiating methicillin-resistant Staphylococcus aureus (MRSA) from methicillin-sensitive S aureus (MSSA) infections in the pediatric community have been unclear.
Methods.
We performed a prospective case-comparison investigation of clinical, epidemiological, and molecular factors in pediatric community–associated (CA) MRSA and MSSA cases in the San Francisco Bay Area. Chart reviews were conducted in 270 CA-MRSA and 313 CA-MSSA cases. Fifty-eight CA-MRSA (21.4%) and 95 CA-MSSA (30.4%) cases were interviewed. Molecular typing was performed on 111 isolates.
Results.
MSSA represented 53.7% of CA cases and was more likely to cause invasive disease (6.2% vs 1.1%, P = .004). Few potential epidemiologic risk factors distinguished CA-MRSA from CA-MSSA. No differences were found in factors related to crowding, cleanliness, or prior antibiotic use. Compromised skin integrity due to eczema (24.3% vs 13.5%, P = .001) was associated with CA-MSSA. Many exposures to potentially infected or colonized contacts or contaminated objects were assessed; only three were associated with CA-MSSA: having a household contact who had surgery in the past year (18.9% vs 6.0%, P = .02), and regular visits to a public shower (9.1% vs 2.0%, P = .01) or gym (12.6% vs 3.3%, P = .04). Molecular typing identified clonal complex 8 as the predominant genetic lineage among CA-MRSA (96.4%) and CA-MSSA (39.3%) isolates.
Conclusions.
In the context of recent heightened focus on CA-MRSA, the burden of serious disease caused by CA-MSSA among children should not be overlooked. MRSA and MSSA may be growing epidemiologically similar; thus, research, clinical, and public health efforts should focus on S aureus as a single entity.
Keywords: Epidemiology, MRSA, MSSA, Pediatrics, S aureus
Among children, as in adults, the reported numbers and proportion of methicillin-resistant Staphylococcus aureus (MRSA) compared to methicillin-sensitive S aureus infections (MSSA) have dramatically increased [1, 2]. MRSA was previously associated with healthcare risk factors such as recent hospitalization or surgery or presence of an indwelling catheter, but it has emerged in the community among previously healthy individuals without such risk factors [3]. This change in epidemiology has necessitated changes in treatment practices and burdened healthcare resources, not only due to high numbers of skin and soft tissue infections [4], but also because of life-threatening, invasive disease [5].
Understanding the risk factors for community-associated MRSA (CA-MRSA) will help shape public health interventions. Among adults, CA-MRSA has been associated with risk factors including injection drug use [6], homelessness [7], incarceration, and visiting bars and raves [8]. Outbreaks have also occurred among athletes [9], military recruits [10], and men who have sex with men [11].
However, the risk factors for CA-MRSA versus CA-MSSA infections in children are unclear [12, 13]. Surveillance studies and reports from outbreaks suggest that children from low socioeconomic status or racial minority groups, such as black or African-Americans, Pacific Islanders, and Native Americans are at risk [14]. There is documentation of transmission in daycare centers [15], households [16], and sports teams [17]. However, these studies are subject to bias as they are mostly retrospective, with data limited to chart reviews or specific outbreaks. Recognizing that the dynamics of CA-MRSA transmission differ from those in healthcare settings, the Centers for Disease Control (CDC) developed the “Five Cs of CA-MRSA Transmission.” This conceptual model suggests that infection is related to (1) contact with infected or colonized persons; (2) cleanliness; (3) compromised skin integrity; (4) contaminated objects or surfaces; and (5) crowding. A sixth “C” refers to capsules, or prior antibiotic use [18]. A high prevalence of these risk factors has been identified among children with CA-MRSA [19], but a study examining the relationship between these potential risk factor categories and CA-MRSA versus CA-MSSA has not been documented.
We performed a prospective case-comparison investigation among pediatric patients from three medical centers in the San Francisco Bay Area [20]. For patients with a positive S aureus culture, we performed a chart review to evaluate clinical and demographic factors, telephoned parents or guardians of subjects to administer an extensive interview evaluating the 6 “Cs” of potential risk factors, and genotyped isolates for strain characterization.
MATERIALS AND METHODS
Study Population
We identified inpatient and outpatient S aureus isolates from patients 0–18 years of age from the clinical microbiology laboratories at three sites in the San Francisco Bay Area: University of California, San Francisco (UCSF) and San Francisco General Hospital (SFGH) from February 2008 to January 2009, and Children's Hospital Oakland (CHO) from February 2008 to March 2009. The study was approved by the UCSF and CHO Institutional Review Boards.
Data Collection
Demographic and clinical data were collected by chart abstraction, which was performed for all S aureus isolates identified at UCSF and SFGH. Due to the high volume of isolates at CHO (three times the number as UCSF and SFGH combined), one third were randomly selected for chart abstraction. Multiple S aureus isolates from a subject were considered part of the same episode if collected within 1 month of the first isolate [21]. Episodes were included if the following CDC clinical criteria for CA-MRSA were met: did not live in a long-term care facility in the past year, no hospitalization in the past year, no dialysis or surgery in the past year, and no presence of indwelling catheter or other percutaneous device [22]. We also included subjects with MSSA, minor outpatient surgical procedures, history of S aureus infection or colonization, and a positive culture collected after 48 hours of hospitalization if chart review revealed that symptoms began before admission. Although most were born in a hospital, infants were included if the postnatal course was uncomplicated. Other episodes, including patients with underlying cystic fibrosis, were classified as healthcare-associated S aureus. Isolates were excluded if identified as hospital-associated (HA) S aureus infections, or chart review revealed that the culture reflected colonization and not infection. Potential for misclassification was low because HA cases were likely to present at the same hospital where they had records for chart review.
We called each subject with community-associated S aureus infections using contact information in the medical record. HA and CA classification was verified and potential epidemiological risk factors were assessed in a telephone interview with a consenting parent or guardian. In addition, activities and habits were assessed directly and confidentially from subjects older than 12 years after obtaining informed consent from a parent or guardian. Interviewers were blinded to whether subjects had MRSA or MSSA infections. Subjects previously interviewed during the study period were not interviewed again.
Laboratory Methods
Standard methods recommended by the Clinical and Laboratory Standards Institute were used to identify S aureus infection and antibiotic susceptibility (including the D-test for clindamycin-sensitive and erythromycin-resistant isolates) in all 3 clinical laboratories.
For molecular analysis, we collected a convenience sample of CA–S aureus isolates at UCSF from July 2008 to January 2009, SFGH from September 2008 to January 2009, and CHO from February to March 2009. Presence of the mecA [23], Panton-Valentine leukocidin (PVL) [24], and type I arginine catabolic mobile element (ACME) genes [25] were determined by polymerase chain reaction performed in triplicate. Spa sequences were determined by DNA sequence analysis of the protein A gene polymorphic region [25]. Spa types and the associated multilocus sequence types (MLST) were assigned from the Ridom Spa Server database (www.ridom.de/spaserver). Spa types without an associated MLST in the Ridom database were arbitrarily assigned as sequence type (ST) A to I. Spa sequences without a spa type in the database, but differing by three or fewer spa repeat sequence alterations to a spa type in the database, were assigned as related spa and sequence types. Clonal complexes (CC) were assigned according to designated MLST (http://www.mlst.net). Sequence types not belonging to any CC were not assigned a CC.
Statistical Analysis
Univariate summaries of demographic and clinical characteristics were performed for all CA–S aureus subjects and episodes, respectively. Potential epidemiological risk factors were summarized for interviewed subjects only. Data points from UCSF and SFGH subjects were assigned a weight of 1 and those from CHO a weight of 3 in all analyses to account for our stratified sampling scheme. Rao-Scott χ2 test for categorical data (based on the Pearson χ2 statistic with a correction accounting for sampling design effects) and linear regression for continuous data were used. Subjects were categorized as having recurrent infection based on self-report of having had at least 1 infection in the past year that is similar to the current infection (ie, same site, similar size or shape, or similar symptoms). Hypothesis tests were two-tailed and those with P ≤ .05 were considered significant. Analyses were performed using SAS versions 9.1.3 and 9.2 for Windows (Cary, NC).
RESULTS
Study Population
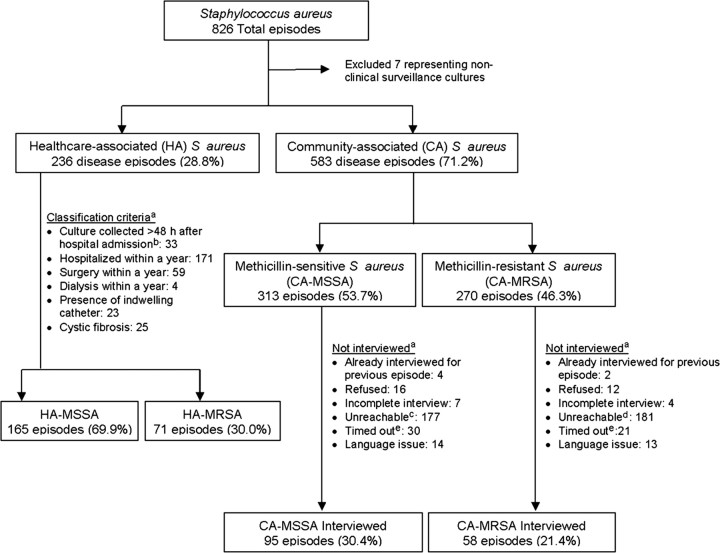
We identified 826 S aureus episodes during the study period. After nonclinical surveillance cultures were excluded, 28.8% were classified as healthcare-associated (HA) infections and 71.2% as CA infections. MSSA predominated in both groups, representing 69.9% of HA infections and 53.7% of CA infections. Among CA-MRSA subjects, 21.4% were interviewed; among CA-MSSA subjects, 30.4% were interviewed. The main reason for noninterview was inability to reach subjects using the telephone numbers in their medical charts (Figure 1). When demographic and select clinical characteristics (ie, hospitalization status, culture site, clinical diagnosis, and underlying disease) between interviewed and noninterviewed subjects were compared, no significant differences were found except that a greater proportion of the interviewed group was Asian or Pacific Islander (13.5% vs 8.5%, P = .01).
Figure 1.
Recruitment and classification. aMore than one reason may apply. bDue to possible delays in incision and drainage, patients with the following clinical diagnoses were not automatically categorized as healthcare-associated cases: osteomyelitis, septic arthritis, pneumonia, empyema, abscess or any skin and soft-tissue infection. cAverage number of unsuccessful call attempts: 2.4 (median 1, min 1, max 9). dAverage number of unsuccessful call attempts: 3.2 (median 1, min 1, max 12). eInitial contact with subject was not made within 4 months of culture date.
Clinical Factors of CA-MSSA Versus CA-MRSA Infections
Compared to CA-MRSA, CA-MSSA infections were more likely to result in non–skin invasive disease (6.2% vs 1.1%, P = .004). CA-MSSA cases had longer hospitalizations (median 3.3 days, 95% confidence interval [CI] 2.5–4.0, vs 2.2 days, 95% CI 1.8–2.5, among CA-MRSA cases) and a longer length of symptoms prior to presentation (4.3 days, 95% CI 3.1–5.5 vs 1.9 days, 95% CI 1.3–2.5). A significantly higher percentage of CA-MRSA infections occurred in the buttock and groin area (33.1% vs 11.0%, P < .001). The median ages of subjects with groin or buttock infections were similar (0.9 years, 95% CI 0.9–0.9, among CA-MSSA subjects and 0.7 years, 95% CI 0.6–0.8, among CA-MRSA subjects, P = .17). CA-MRSA skin infections were diagnosed more often as abscess or cellulitis and CA-MSSA infections were diagnosed more often as superinfected eczema or impetigo. Cultures from CA-MSSA cases were more likely to be polymicrobial (41.5% vs 17.5%, P < .0001) (Table 1). Antibiotic susceptibility profiles are detailed in Supplementary Table 1.
Table 1.
Clinical Factors of Community-Associated Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus
| Clinical Factors | CA-MSSA (N = 313) No. (Weighted %)a |
CA-MRSA (N = 270) No. (Weighted %)a |
P Value |
|---|---|---|---|
| Days of symptoms prior to presentationb | |||
| Median, 95% CI | 4.3 (3.1–5.5) | 1.9 (1.3–2.5) | — |
| Clinical course | |||
| Hospitalized | 70 (29.7%) | 86 (36.8%) | .11 |
| Median length of stay, days (95% CI) | 3.3 (2.5–4.0) | 2.2 (1.8–2.5) | — |
| Deaths | 0 | 0 | — |
| Clinical diagnosisc | |||
| Invasive disease | 16 (6.2%) | 3 (1.1%) | .004 |
| Bacteremia | 5 (1.5%) | 2 (1.0%) | .59 |
| Pneumonia | 4 (1.4%) | 0 | — |
| Osteomyelitis | 10 (3.8%) | 0 | — |
| Abscess–not skin | 4 (1.4%) | 3 (1.1%) | .82 |
| Septic arthritis | 4 (1.4%) | 0 | — |
| Skin infection | 151 (49.1) | 215 (79.2%) | <.0001 |
| Abscess–skin | 39 (13.2%) | 115 (43.5%) | <.0001 |
| Cellulitis | 65 (23.8%) | 131 (47.6%) | <.0001 |
| Folliculitis | 4 (1.0%) | 4 (1.6%) | .56 |
| Impetigo | 21 (7.4%) | 9 (2.4%) | .008 |
| Superinfected eczema | 21 (5.0%) | 3 (0.5%) | <.0001 |
| Other skin infectionsd | 40 (9.3%) | 12 (3.7%) | .06 |
| Othere | 54 (18.8%) | 16 (7.1%) | .003 |
| Unknown clinical diagnosis | 84 (24.3%) | 36 (13.2%) | .002 |
| Culture site | |||
| Sterile | |||
| Blood | 10 (4.1%) | 3 (1.5%) | .10 |
| Bone | 1 (0.2%) | 0 | — |
| Nonsterile | |||
| Wound or pustule | 220 (70.1%) | 182 (75.5%) | .17 |
| Abscess | 34 (8.2%) | 77 (19.8%) | <.0001 |
| Respiratoryf | 13 (6.0%) | 0 | — |
| Other | 30 (9.5%) | 8 (3.5%) | .008 |
| Unknown site | 5 (0.5%) | 3 (0.9%) | .63 |
| Body site for wound, pustule or abscess culturesg | |||
| Extremity | 106 (38.0%) | 84 (32.6%) | .25 |
| Groin/buttock | 22 (11.0%) | 79 (33.1%) | <.0001 |
| Face/head/neck | 73 (28.9%) | 40 (15.4%) | .0010 |
| Trunk | 24 (11.5%) | 38 (13.7%) | .50 |
| Other | 13 (6.0%) | 17 (8.1%) | .43 |
| Polymicrobial culturesh | 129 (41.5%) | 48 (17.5%) | <.0001 |
| Underlying conditionsc | |||
| Eczema | 106 (24.3%) | 48 (13.5%) | .001 |
| Asthma | 40 (10.6%) | 31 (10.5%) | .97 |
| Diabetes | 1 (0.5%) | 0 | — |
| Immunosuppressed | 1 (0.2%) | 0 | — |
| Influenza (last 10 days) | 1 (0.5%) | 0 | — |
| Lupus | 9 (1.9%) | 3 (1.5%) | .70 |
| Malignancy | 0 | 1 (0.5%) | — |
| Obesity | 4 (1.0%) | 7 (1.8%) | .44 |
| Premature birth | 2 (0.3%) | 4 (1.9%) | .02 |
| Substance abuse | 3 (0.6%) | 3 (1.2%) | .26 |
| Other conditions | 38 (8.9%) | 23 (5.9%) | .17 |
Abbreviations: CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus; CA-MSSA, community-associated methicillin-sensitive S aureus; CI, confidence interval.
aPercentages calculated based on weighted data (weight of 3 for Children's Hospital Oakland subjects, 1 for all other subjects).
bObtained from chart abstractions, CA-MSSA n = 95, CA-MRSA n = 58.
cMore than 1 may apply.
dIncludes traumatic wound, surgical incision (2 infections after pilomatricoma removal), nail, carbuncle/furuncle, sebaceous gland, hair, and skin infections not otherwise specified.
eIncludes kidney, eye, urinary tract, lymph node, ear (internal or external), and other infections.
fIncludes nasopharyngeal, throat, sputum, tracheal/endotracheal tube aspirate, and bronchoalveolar lavage.
gSome episodes involved multiple body sites; the total number of body sites is greater than the total number of cultures.
hData based on isolates not episodes, CA-MSSA n = 317, CA-MRSA n = 272.
Demographic Risk Factors for CA-MSSA Compared to CA-MRSA Subjects
CA-MRSA subjects were more likely to be black or African-American (53.2% vs 25.8%, P < .0001) and of lower socioeconomic status as evidenced by income (P = .008) and parental education level (P = .06).
CA-MSSA was more likely to occur among males as well as nonblack or non–African-American ethnic groups. CA-MRSA occurred more commonly in younger subjects (median age 1.5 years, 95% CI 1.1–1.9, vs 5.5 years, 95% CI 4.3–6.7). Homelessness was not statistically significantly associated with CA-MSSA versus CA-MRSA (Table 2).
Table 2.
Demographic Risk Factors for Community-Associated Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus
| Demographics, All Subjects | CA-MSSA (n = 313) No. (Weighted %)a | CA-MRSA (n = 270) No. (Weighted %)a | P Value |
|---|---|---|---|
| Sex | |||
| Male | 173 (55.6%) | 116 (41.7%) | .006 |
| Race/ethnicity | <.0001 | ||
| Native American/Alaska Native | 7 (2.2%) | 1 (0.2%) | <.0001 |
| Asian/Pacific Islander | 52 (13.0%) | 17 (4.4%) | .0003 |
| Black or African-American | 63 (25.8%) | 128 (53.2%) | <.0001 |
| Hispanic | 82 (27.7%) | 54 (19.0%) | .04 |
| White | 50 (16.1%) | 28 (8.4%) | .01 |
| Other | 6 (2.4%) | 7 (3.1%) | .70 |
| Multiethnic | 14 (3.1%) | 11 (4.0%) | .54 |
| Unknown | 39 (9.7%) | 24 (7.7%) | .43 |
| Age | |||
| Mean (years) | 6.7 years | 5.1 years | .003 |
| Median in years (95% CI) | 5.5 (4.3, 6.7) | 1.5 (1.1, 1.9) | |
| < 1 | 52 (16.4%) | 56 (20.8%) | |
| 1–3 | 69 (23.1%) | 97 (37.6%) | |
| 4–9 | 88 (29.4%) | 47 (17.1%) | .003 |
| 10–14 | 59 (15.9%) | 33 (12.2%) | |
| 15–18 | 45 (15.2%) | 35 (15.2%) | |
| Demographics, Interviewed Subjects | CA-MSSA (n = 95) No. (Weighted %)a | CA-MRSA (n = 58) No. (Weighted %)a | P Value |
| Household income | |||
| < $40,000 | 34 (40.7%) | 31 (59.0%) | .008 |
| $40,001–$60,000 | 9 (9.0%) | 6 (9.7%) | |
| $60,001–$80,000 | 10 (9.6%) | 3 (3.5%) | |
| $80,001–$100,000 | 6 (4.8%) | 1 (0.7%) | |
| > $100,000 | 19 (18.6%) | 4 (5.6%) | |
| Refused | 6 (3.6%) | 7 (13.2%) | |
| Unknown | 11 (13.8%) | 6 (8.3%) | |
| Homelessness | |||
| Homeless in year before infection | 2 (2.4%) | 2 (2.9%) | .84 |
| Slept at another person's home in year before infection because had no other place to go | 6 (7.2%) | 9 (14.6%) | .47 |
| Parent education level | .06 | ||
| < High school | 7 (9.0%) | 4 (8.3%) | |
| High school | 19 (22.2%) | 24 (43.1%) | |
| Some college or vocational training | 17 (18.6%) | 14 (25.0%) | |
| Bachelor's degree | 24 (21.6%) | 7 (10.4%) | |
| Postgraduate degree | 20 (20.4%) | 5 (6.3%) | |
| Refused | 3 (1.8%) | 1 (2.1%) | |
| Unknown | 5 (6.6%) | 3 (4.9%) |
Abbreviations: CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus; CA-MSSA, community-associated methicillin-sensitive S aureus; CI, confidence interval.
aPercentages calculated based on weighted data (weight of 3 for Children's Hospital Oakland subjects, 1 for all other subjects).
Epidemiological Risk Factors for CA-MSSA Compared to CA-MRSA Subjects
Using the “6 Cs Framework for CA-MRSA Transmission,” we found few statistically significant differences between potential risk factors for CA-MRSA or CA-MSSA infections (Table 3). There were no statistically significant differences between CA-MRSA compared to CA-MSSA infections for the following: cleanliness, crowded living conditions, exposure to antibiotics, and other high-risk teen behaviors (sexual activity; visits to a bar, rave, or club; smoking or snorting drugs).
Table 3.
Epidemiological Risk Factors for Community-Associated Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus
| Risk Factors | CA-MSSA (n = 95) No. (Weighted %)a | CA-MRSA (n = 58) No. (Weighted %)a | P Value |
|---|---|---|---|
| Exposure to potentially colonized or infected persons | |||
| Visited emergency room or urgent care in year before infection | 32 (29.1%) | 26 (40.8%) | .18 |
| Visited a nursing home in year before infection | 8 (9.6%) | 3 (6.3%) | .52 |
| Lived in a group home in year before infection | 4 (4.8%) | 2 (4.1%) | .86 |
| Incarcerated in the 3 months before infectionb | 0 | 1 (5.9%) | — |
| Attended daycare (<6 years of age only) in year before infection | 11 (25.3%) | 13 (29.8%) | .68 |
| Household contact with similar infection in 6 months before infectionc | 10 (13.6%) | 14 (26.9%) | .17 |
| Healthcare provider diagnosed with staph infection | 5 (55.0%) | 10 (72.2%) | .73 |
| Household contact with any skin infection in 6 months before infectionc | 33 (29.2%) | 19 (35.1%) | .50 |
| Household contact potentially colonized or infected due in year before infectionc | |||
| Working in healthcare setting | 16 (13.3%) | 6 (9.8%) | .54 |
| Working at a long-term care facility | 8 (8.5%) | 4 (8.6%) | .99 |
| Required hospitalization | 9 (7.4%) | 5 (8.7%) | .79 |
| Had surgery | 17 (18.9%) | 5 (6.0%) | .02 |
| Resided in a long-term care facility | 0 | 0 | — |
| Had dialysis | 1 (0.6%) | 1 (2.0%) | .34 |
| Had a percutaneous device or indwelling catheter | 3 (1.7%) | 1 (2.0%) | .89 |
| Working at or attend a daycare | 12 (13.3%) | 7 (10.5%) | .63 |
| Working as a military personnel | 0 | 0 | — |
| Incarcerated | 1 (0.6%) | 4 (7.0%) | .006 |
| Used injection drugs | 1 (0.6%) | 0 | — |
| Pet in household | 41 (39.6%) | 10 (15.0%) | .003 |
| Cleanliness in the 3 months before infection | |||
| Bathing less than once daily | 37 (35.1%) | 28 (48.3%) | .13 |
| Did not use soap while showering or bathing | 9 (7.1%) | 2 (6.2%) | .72 |
| Median number of times wash hands per day | 3.3 (2.6, 4.0) | 3.9 (3.1, 4.7) | |
| Did not use soap while washing hands | 17 (14.8%) | 11 (13.8%) | .99 |
| Wearing a shirt/top more than once before laundering | 14 (14.9%) | 3 (4.8%) | .07 |
| Doing laundry less than once a week | 12 (14.6%) | 13 (24.0%) | .23 |
| Compromised skin integrity | |||
| Skin conditionsd | |||
| Eczema | 106 (24.3%) | 48 (13.5%) | .001 |
| Psoriasis | 6 (1.0%) | 0 | — |
| Other skin conditions | 29 (6.0%) | 13 (4.0%) | .98 |
| Injury or trauma within 1 month before infectione | 41 (36.5%) | 21 (32.6%) | .65 |
| Shaved any parts of body in 3 months before infectionb | 5 (55.0%) | 5 (76.5%) | .39 |
| Median times of lotion use per day in 3 months before infectionf | 0 (0.2, 0.2) | 0.5 (0.4, 0.6) | — |
| Contact with potentially contaminated objects/surfaces in 3 months before infection | |||
| Occasionally wearing clothes worn by someone else before being washed | 8 (5.8%) | 1 (2.1%) | .43 |
| Occasionally using a towel that someone else had used | 21 (18.3%) | 12 (19.2%) | .35 |
| Visited the following at least once a week: | |||
| Public swimming pool | 16 (13.7%) | 5 (7.3%) | .24 |
| Public shower | 8 (9.1%) | 3 (2.0%) | .01 |
| Fitness gym | 12 (12.6%) | 3 (3.3%) | .04 |
| Other public facility (eg, spa, hot tub, sauna, whirlpool) | 7 (8.6%) | 0 | — |
| Participated in contact sportsb | 5 (45.0%) | 1 (17.6%) | .28 |
| Shared razors with othersb | 0 | 3 (53.8%) | — |
| Obtained a tattoob | 0 | 1 (17.6%) | — |
| Crowded living conditions | |||
| Median persons in each bedroom | 1.4 (1.3, 1.6) | 1.5 (1.3, 1.6) | |
| Median number of other children in the home | 0.5 (0.4, 0.6) | 0.1 (0, 0.5) | |
| Exposure to antibiotics in year before infection | 26 (26.8%) | 13 (24.1%) | .74 |
| Number of courses per patient | .64 | ||
| 1 | 14 (47.6%) | 8 (56.3%) | |
| 2 | 7 (31.0%) | 2 (12.5%) | |
| 3+ | 5 (21.3%) | 4 (31.3%) | |
| Other high-risk teenage behaviors in the 3 months before infectionb | |||
| Sexually active | 0 | 2 (23.5%) | — |
| Visited a bar, rave, or club | 0 | 1 (17.6%) | — |
| Smoked cigarettes | 0 | 1 (35.3%) | — |
| Smoked or snorted drugs such as cocaine, crack, or meth | 0 | 0 | — |
Abbreviations: CA-MRSA, community-associated methicillin-resistant Staphylococcus aureus; CA-MSSA, community-associated methicillin-sensitive S aureus.
aAll percentages are calculated based on weighted data (weight of 3 for Children's Hospital Oakland subjects, 1 for all other subjects).
bAdolescents only.
cSubject in contact with household contact at least 3 times a week.
dFrom chart abstraction CA-MSSA n = 313, CA-MRSA n = 270.
eIncludes: insect bite, cut, break or scrape, scratch, blister, burn, piercing from an object, blunt trauma, diaper rash (for children ≤3 years old), or other injury/trauma.
fUse of lotion evaluated due to potential to protect against breaks in the skin.
Compared to CA-MRSA, CA-MSSA was associated with eczema (24.3% vs 13.5% P = .001). There were no significant relationships between other causes of compromised skin integrity and CA-MSSA versus CA-MRSA infection.
Regarding exposure to potentially colonized or infected persons, a significant association was found between recent surgery in a household contact and CA-MSSA infection (vs CA-MRSA), as well as recent incarceration in a household contact and CA-MRSA infection (vs CA-MSSA). Subject visits to an emergency room, nursing home, daycare, group home, or prison were not significantly associated with CA-MRSA versus CA-MSSA infection. Regarding exposure to potential fomites, regular visits to a public shower or fitness gym were associated with CA-MSSA (vs CA-MRSA) infections. Recent similar infection in a household contact was not significantly associated with CA-MRSA versus CA-MSSA infection.
Recurrent Infections Subanalysis
We evaluated risk factors for recurrent infection, defined as self-reported history of at least 1 similar infection in the previous year. Of 153 interviewed subjects, 38 (24.8%) infections were classified as recurrent and 115 (75.2%) as nonrecurrent. Prevalence of skin infection was similar (59.4% vs 58.6%, P = .88), but recurrent cases were less likely to be invasive (0% vs 7.2%) or require hospitalization (5.2% vs 34.5%, P = .001). The prevalence of methicillin resistance was similar (P = .97). Differences in demographic and potential epidemiological risk factors were not statistically significant, with the exceptions being that subjects with recurrent infection had a history of more visits to the emergency room (67.6% vs 24.9%, P < .001), bathed more frequently (P = .003), and were more likely to visit a public swimming pool (20.3% vs 5.9%, P = .03) (Supplementary Table 2).
Among subjects with recurrent infection, we also evaluated potential risk factors for CA-MRSA (34.2%) vs CA-MSSA (65.8%). The only statistically significant difference was homelessness: 28.1% of subjects with recurrent CA-MRSA infection had slept at another person's home in the year before the infection, compared to 2.6% among recurrent MSSA cases (P = .004), though the sample size for this comparison was small.
Molecular Characteristics
Molecular analysis was performed in 111 isolates: 20.4% (55/270) of CA-MRSA strains and 17.9% (56/313) of CA-MSSA strains. These represented 15.3% (9/59) of CA-MRSA and 10.5% (10/95) of CA-MSSA episodes from which an interview was conducted.
Among isolates with spa types present in the Ridom database, CC8 was represented in 80.0% (44/55) of CA-MRSA and 30.4% (17/56) of CA-MSSA isolates (Figure 2). Including isolates with spa types not present in the database, but assigned CC8 according to sequence similarities, 96.4% (53/55) of CA-MRSA and 39.3% (22/56) of CA-MSSA isolates were CC8 (Supplementary Tables 3 and 4). The literature has predominantly used USA300 for molecular characterization. However, we focused on CC8 because recent studies suggest that the virulence of CA-MRSA is related to the CC8 genetic lineage from which the USA300 phenotype originates [26]. Epidemiological data from the dominant CC (CC8) were compared to non-CC8 types and found to be similar (Supplementary Table 5).
Figure 2.
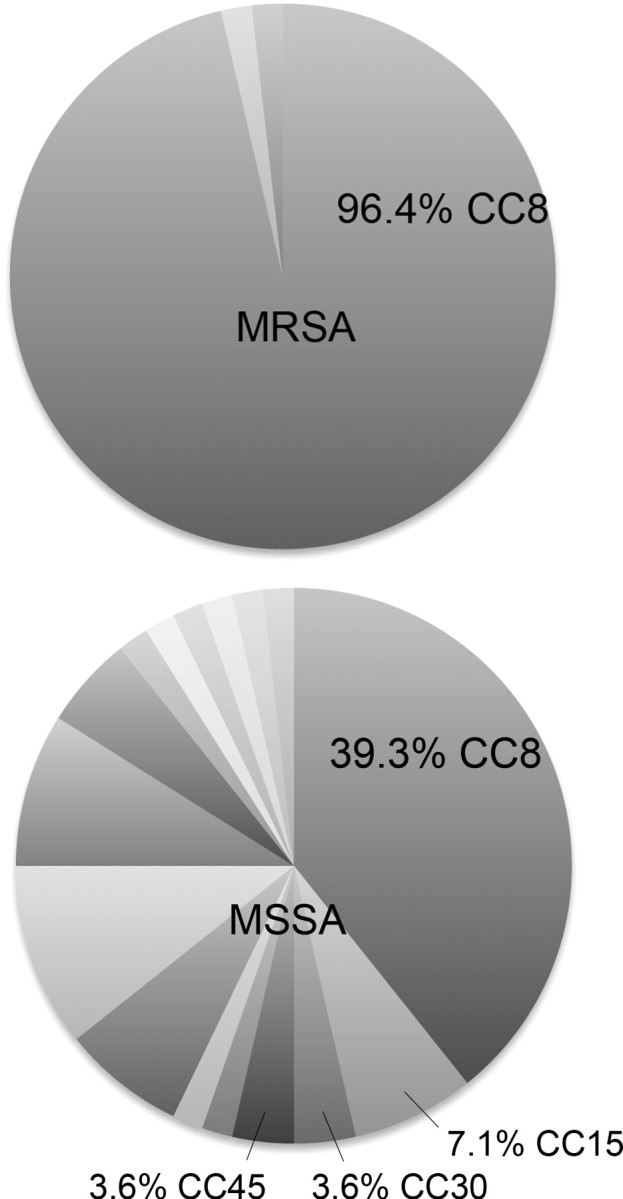
Clonal complex 8 predominated in both community-associated methicillin-resistant and methicillin-sensitive Staphylococcus aureus isolates. Unlabeled pie slices represent sequence types not belonging to any clonal complex (see Supplementary Tables 3 and 4). Abbreviations: MRSA, methicillin-resistant S aureus; MSSA, methicillin-sensitive S aureus; CC, clonal complex.
PVL was identified in 90.9% (50/55) of CA-MRSA isolates and 23.2% (13/56) of CA-MSSA isolates. ACME was identified in 81.8% (45/55) of CA-MRSA isolates and 7.1% (4/56) of CA-MSSA isolates.
DISCUSSION
We prospectively evaluated potential epidemiological risk factors for pediatric CA-MRSA compared to CA-MSSA infection, and found that among a sample of children in the San Francisco Bay Area, there were few differences. We also found genetic similarities in that the predominant genetic lineage of both CA-MRSA and CA-MSSA isolates was CC8. Lastly, despite the concerns about CA-MRSA among the medical community and the public, we found that CA-MSSA infections were more prevalent and invasive among all S aureus infections.
There is a paucity of data comparing the epidemiological risk factors of CA-MRSA and CA-MSSA infection. Most studies are retrospective or generated from outbreaks. One prospective study of hospitalized adults found that CA-MRSA was associated with contact with infected persons, visiting bars and raves, and incarceration, though overall, clinical and epidemiological risk factors could not reliably distinguish between MRSA and MSSA infection [27]. Among children, a prospective study from Texas did not find any significant risk factors, though the assessment was limited to that of healthcare-related risk factors [13]. More recently, a prospective hypothesis-generating study of adult and pediatric subjects from Minnesota found that, compared to CA-MSSA cases and uninfected controls, only antibiotic use in the prior 6 months was associated with CA-MRSA infection, and the assessment of non-healthcare risk factors was unrevealing [28].
In our study, we evaluated the 6 “Cs” of potential risk factors for CA-MRSA and found few differences when compared with CA-MSSA. Similar to previous findings, neither crowding nor cleanliness distinguished CA-MRSA from CA-MSSA infection [28, 29]. While others have found a significant association between prior antibiotic use and CA-MRSA (vs CA-MSSA), we did not [28]. The few significant associations we noted were with CA-MSSA infection (vs CA-MRSA). Of the many exposures to potentially contaminated objects assessed, only regular use of a gym or public shower was associated with CA-MSSA. Compromised skin integrity due to eczema was also associated with CA-MSSA infection. Of the exposures to potentially infected or colonized persons, only having a household contact who had surgery in the past year was associated with CA-MSSA. Others have implicated sexual contact as a possible risk factor for CA-MRSA infections of the groin and buttock [11]. While only two of our pediatric subjects had a history of sexual activity, CA-MRSA was significantly associated with abscess or cellulitis in the buttock and groin areas. We considered whether diaper rash or younger age in patients wearing diapers were associated with CA-MRSA infection, but there were few diaper rashes in our study population and no relationship between age and infections in the groin or buttock area. The association between infections in the buttock or groin area and CA-MRSA may be due to other factors that facilitate colonization of MRSA over MSSA [30].
To our knowledge, there are no prospective studies evaluating the clinical and epidemiological factors in recurrent S aureus infections, which are a growing challenge in pediatrics [31]. Although our sample size was small and relied on self-reported history of prior infection, our analysis was notable in that no invasive disease occurred among recurrent cases. Also, recurrent infections were more commonly due to MSSA rather than MRSA. These findings suggest that, in our region, MRSA may not be the main culprit of recurrent infections, and that recurrent infections may be unlikely to result in invasive disease. Our subjects with recurrent CA-MRSA infections were more likely to have visited the emergency room, a public swimming pool, and bathed more frequently compared to subjects with recurrent CA-MSSA infections; however, in this study, we cannot distinguish whether association of infection with these activities was due to subjects seeking care or following recommendations to decrease MRSA colonization versus exposure to these activities being a potential source of reinfection.
Our molecular analysis demonstrated that CC8 was the predominant genetic lineage of CA-MSSA and CA-MRSA isolates. ST8 has been demonstrated to be the founder of the CC8 lineage and the progenitor for the USA300 phenotype, which in recent years has grown to be the most prevalent phenotype among CA-MRSA isolates [26]. Recent studies suggest that the virulence of CA-MRSA is based on expression of genetic factors intrinsic to the CC8 lineage rather than phenotype or mobile genetic elements [26]. CA-MSSA has historically been heterogeneous [32], but recent studies suggest an increase of USA300 among CA-MSSA isolates in several settings, including among adults and children with invasive and skin infections [1, 33–35]. Our study similarly noted a large proportion of CA-MSSA isolates from CC8.
There were some limitations to our study. First, because our study design focused on identifying risk factors that differed between CA-MRSA and CA-MSSA infections, we could not determine whether any characteristics we evaluated were risk factors for community-associated S aureus infections compared with uninfected controls. Second, we reached less than a third of subjects for interview, likely due to inherent challenges with follow-up telephone interviews (eg, changed telephone numbers, disinterest in returning calls). However, baseline characteristics between subjects who were interviewed and not interviewed were similar, suggesting lack of bias. Strengths of our study include the prospective study design, use of interviews, and detailed assessment of potential epidemiological risk factors, adding important evidence to a sparse pediatric literature and documenting the rapidly evolving epidemiology of S aureus.
In conclusion, we found that among children seen at three major pediatric medical centers in the San Francisco Bay Area, CA-MSSA was more prevalent and caused more invasive disease than CA-MRSA. There were few differences in epidemiological factors between CA-MRSA and CA-MSSA, and the genetic lineage of CA-MSSA may become increasingly similar to CA-MRSA. As more research shows that distinctions of community versus healthcare-associated S aureus infections may be no longer be clinically or epidemiologically relevant, differentiations between MRSA and MSSA may also become less relevant. The contribution of CA-MSSA infections among children with S aureus infections should be recognized, and consideration of S aureus as a single entity will enable the development of rational and more complete prevention, treatment, and research strategies.
Acknowledgments
We thank Kristin Bixel, Ashley Odukoya, Kalyani McCullough, and Erica Winnicki for their assistance in data collection, as well as Rohan Nadarajah, Margaret Wong, Marguerite Roemer, Barbara Haller, and Kristie Vetterli for their assistance in saving and transporting laboratory isolates. We thank Loren Miller for sharing his risk factor survey template and Mark Ayres and Michael Kohn for their information technology support.
Financial support. This work was supported by the San Francisco Department of Public Health; University of California, San Francisco, Resident Research Program Training in Clinical Research grant [M.S.H.]; and National Institutes of Health/National Institute of Allergy and Infectious Diseases [R01 AI070289 to H.F.C].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http:/jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 3.Weber JT. Community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2005;41(Suppl 4):S269–72. doi: 10.1086/430788. [DOI] [PubMed] [Google Scholar]
- 4.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 6.Charlebois ED, Perdreau-Remington F, Kreiswirt B, et al. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;39:47–54. doi: 10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young DM, Harris HW, Charlebois ED, et al. An epidemic of methicillin-resistant Staphylococcus aureus soft tissue infections among medically underserved patients. Arch Surg. 2004;139:947–51. doi: 10.1001/archsurg.139.9.947. discussion 51–3. [DOI] [PubMed] [Google Scholar]
- 8.Miller LG, Quan C, Shay A, et al. A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and -susceptible Staphylococcus aureus skin infection. Clin Infect Dis. 2007;44:483–92. doi: 10.1086/511041. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants—Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. MMWR. 2003:793–5. [PubMed] [Google Scholar]
- 10.Zinderman CE, Conner B, Malakooti MA, LaMar JE, Armstrong A, Bohnker BK. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg Infect Dis. 2004;10:941–4. doi: 10.3201/eid1005.030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 12.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 13.Sattler CA, Mason EO, Jr, Kaplan SL. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr Infect Dis J. 2002;21:910–7. doi: 10.1097/00006454-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 15.Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998;178:577–80. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics. 2004;113:e347–52. doi: 10.1542/peds.113.4.e347. [DOI] [PubMed] [Google Scholar]
- 17.Lindenmayer JM, Schoenfeld S, O'Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998;158:895–9. doi: 10.1001/archinte.158.8.895. [DOI] [PubMed] [Google Scholar]
- 18.Ragan P. Community-acquired MRSA infection: An update. JAAPA. 2006;19:24–9. doi: 10.1097/01720610-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hermos C, Shiau R, Hsiang M, Chambers HF, Pan E. Epidemiology of community-associated methicillin resistant Staphylococcus aureus in San Francisco children. J Pediatr Infect Dis. 2009;4:1305–7693. [Google Scholar]
- 20.McCarthy N, Giesecke J. Case-case comparisons to study causation of common infectious diseases. Int J Epidemiol. 1999;28:764–8. doi: 10.1093/ije/28.4.764. [DOI] [PubMed] [Google Scholar]
- 21.David MZ, Mennella C, Mansour M, Boyle-Vavra S, Daum RS. Predominance of methicillin-resistant Staphylococcus aureus among pathogens causing skin and soft tissue infections in a large urban jail: Risk factors and recurrence rates. J Clin Microbiol. 2008;46:3222–7. doi: 10.1128/JCM.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck JM, Como-Sabetti K, Harriman KH, et al. Community-associated methicillin-resistant Staphylococcus aureus, Minnesota, 2000–2003. Emerg Infect Dis. 2005:1532–8. doi: 10.3201/eid1110.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–61. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 25.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–8. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: A prospective investigation. Clin Infect Dis. 2007;44:471–82. doi: 10.1086/511033. [DOI] [PubMed] [Google Scholar]
- 28.Como-Sabetti KJ, Harriman KH, Fridkin SK, Jawahir SL, Lynfield R. Risk factors for community-associated Staphylococcus aureus infections: Results from parallel studies including methicillin-resistant and methicillin-sensitive S. aureus compared to uninfected controls. Epidemiol Infect. 2011;139:419–29. doi: 10.1017/S0950268810001111. [DOI] [PubMed] [Google Scholar]
- 29.Miller LG, Kaplan SL. Staphylococcus aureus: A community pathogen. Infect Dis Clin N Am. 2009;23:35–52. doi: 10.1016/j.idc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16:425–31. doi: 10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan SL. Commentary: Prevention of recurrent staphylococcal infections. Pediatr Infect Dis J. 2008;27:935–7. doi: 10.1097/INF.0b013e31818632b3. [DOI] [PubMed] [Google Scholar]
- 32.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrillo-Marquez MA, Hulten KG, Hammerman W, Mason EO, Kaplan SL. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr Infect Dis J. 2009;28:1076–80. doi: 10.1097/INF.0b013e3181adbcfe. [DOI] [PubMed] [Google Scholar]
- 34.McCaskill ML, Mason EO, Jr, Kaplan SL, Hammerman W, Lamberth LB, Hulten KG. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr Infect Dis J. 2007;26:1122–7. doi: 10.1097/INF.0b013e31814536e0. [DOI] [PubMed] [Google Scholar]
- 35.Chen AE, Carroll KC, Diener-West M, et al. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatrics. 2011;127:e573–80. doi: 10.1542/peds.2010-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]