Abstract
Background.
Little is known about the incidence and etiology of healthcare-associated infections in immunosuppressed children.
Methods.
Data collected prospectively between 1983 and 2008 were used to analyze changes in the rate, types of infection, and infecting organisms over time in patients treated at a children's cancer hospital. Neutropenia was evaluated as a risk factor.
Results.
Over the 26-year study period, 1986 healthcare-associated infections were identified during 1653 hospitalizations. The infection rate decreased significantly from 5.6 to 2.0 infections per 100 discharges (P < .01) and from 9.0 to 3.7 infections per 1000 patient-days (P < .01). Bloodstream infections were the most common type of infection (32.7% of all infections). Staphylococci (46.4% of Gram-positive bacteria), Escherichia coli (36.7% of Gram-negative bacteria), and Candida spp. (68.7% of fungi) were the most common pathogens isolated. An absolute neutrophil count (ANC) nadir <100 per mm3 was significantly associated (P < .0001) with an increased rate of infections compared with higher ANC nadirs.
Conclusions.
Despite a steady expansion in hospital capacity and patient encounters over the last 3 decades, rates of healthcare-associated infections decreased significantly at our hospital. These data suggest that sustained decreases in the rate of healthcare-associated infections in immunosuppressed children are possible. An ANC <100 per mm3 is a risk factor for healthcare-associated infections in this population.
(See the Editorial Commentary by Coffin and Huskins, on pages 44–6.)
When St. Jude Children's Research Hospital (SJCRH) was founded in 1962, childhood leukemia was a universally fatal disease [1]. Substantial progress has been made in the intervening half-century, with survival rates today surpassing 90% at 5 years for childhood acute lymphoblastic leukemia [2] and 70% at 3 years for acute myeloid leukemia [3]. Infection control is an often overlooked contributor to this success. Due to the severe immunosuppression and myelosuppression that accompany treatment of childhood cancer, infection has historically been an important, preventable cause of death [4]. In particular, it has been appreciated for several decades that low absolute neutrophil counts (ANCs) are associated with an increased risk of infections in this patient population [4]. However, the ANC nadir reached during a hospitalization has not been assessed as a risk factor for healthcare-associated infections (HAIs).
An estimated 1.7 million HAIs occur annually in the United States [5]. These infections account for significant morbidity, mortality, and cost. Although it is acknowledged that immunosuppression and myelosuppression are important risk factors for the development of HAIs, data on the incidence and etiology in persons with cancer are sparse [6]. Reports from the 1980s suggested that the incidence of HAIs in children with cancer was variously 6.3 per 1000 patient-days [7], 9.3 per 100 discharges [8], or 11.7 per 100 admissions [9]. Similar rates ranging from 4.8 to 17.7 HAIs per 1000 patients-days were reported from studies in the 2000s in Europe, although the numbers of patients studied were small [10, 11]. No published studies have assessed changes in the incidence and etiology of HAIs in patients with cancer over time. Here we present a comprehensive assessment of 1986 HAIs prospectively collected over a 26-year period between 1983 and 2008 at a pediatric cancer hospital. Based on the known association of low ANC with infections in general [4], ANC nadir was assessed as a risk factor for the more specific category of HAIs.
METHODS
Infection Control Program
SJCRH is an independent biomedical research facility with a free-standing hospital dedicated to advancing cures and means of prevention for catastrophic diseases. The SJCRH Infection Control Program has continuously operated under the direct supervision of a single person (B. F. W.) with a consistent set of guidelines and definitions since 1983 (Table 1). Continuous, hospital-wide, “whole house” infection surveillance, including daily chart-based review of all inpatient medical records and all positive microbiology laboratory reports, is the primary strategy used to minimize, reduce, or eliminate prioritized infectious risks to patients. Culture-negative HAIs (eg, many surgical-site or respiratory-tract infections) are identified through chart review of daily notes and relevant reports. Data on HAIs are recorded daily for every inpatient, collated, and then reported monthly. Healthcare-associated infections are stratified by service and underlying disease into 5 categories: bone marrow transplant; acute myeloid leukemia; acute lymphoblastic leukemia or lymphoma; solid tumor; and other diagnoses, including sickle cell disease, human immunodeficiency virus infection; and other hematologic disorders.
Table 1.
Timeline of Major Changes to Infection Control at St. Jude.
| Year | Change or Intervention |
|---|---|
| 1962 | St. Jude Children's Research Hospital opens |
| 1975 | Whole house surveillance for infections adopted |
| 1977 | Author B. J. W. joins Infection Control Program; trimethoprim-sulfamethoxazole prophylaxis initiated |
| 1979 | Empiric therapy of neutropenia and fever adopted |
| 1982 | Founding of Infection Control Committee under author W. T. H. |
| 1983 | Prospective data collection for current study begun under author B. J. W. |
| 1985 | First major hand hygiene initiative; annual testing of HEPA filtration systems and air culturing for fungi introduced; flu vaccines offered to all employees |
| 1986 | Incorporation of preservatives into heparin flushes |
| 1989 | Opening of negative-pressure rooms for respiratory isolation |
| 1990 | Use of HEPA filtration masks for neutropenic patients |
| 1995 | Opening of new patient care center |
| 1996 | Opening of new surgical suites and transfer of all surgical care to St. Jude |
| 1997 | Carpeting removed from hospital |
| 1998 | Entire campus is HEPA-filtered |
| 2000 | HEPA filtration of domiciliary care facilities |
| 2001 | Infection control officer position created (author J. A. M.); alcohol hand-gel utilization begins; antifungal and antibacterial prophylaxis of high-risk patients adopted |
| 2003 | Comprehensive air quality and balancing program initiated |
| 2004 | High compliance with flu vaccination achieved through feedback and follow-up program [19] |
| 2005 | Staged introduction of multiple polymerase chain reaction assays for improved pathogen detection begins |
| 2006 | High compliance with hand hygiene achieved |
Abbreviation: HEPA, High-Efficiency Particulate Air.
Data Collection
Data on HAIs were prospectively collected on paper forms as part of routine infection control surveillance on patients admitted to SJCRH between 1 January 1983 and 31 December 2008. Analysis of these data was approved by the Institutional Review Board of SJCRH. Healthcare-associated infections were defined as infections that developed after 48 hours of hospitalization when the interval between admission and onset of symptoms was greater than the incubation period of the disease and when there was no evidence that the infection was present or incubating on admission. The type of HAI was assigned according to Centers for Disease Control and Prevention/National Healthcare Safety Network (NHSN) guidelines [12], with the exceptions that pneumonia and upper and lower respiratory-tract infections were combined as respiratory infections and Clostridium difficile–associated gastroenteritis was only considered an HAI if it developed after 7 days of hospitalization. Data collection on ANCs from patients with HAIs was begun in 1987, but complete, baseline comparative data for admissions not complicated by an HAI were only available for 12 years (1993–96, 1998–99, 2001–02, and 2004–07). Data on the length of hospitalizations were available for the first 7 of these 12 years. All data were entered into an electronic database (by J. A. M.) in 2009 for the purpose of conducting these analyses.
Statistical Analyses
We used a regression model with autoregressive and moving average (ARMA) errors to assess the trend of yearly infection rates. This method accounts for the potential correlations among the time-series data by modeling the error terms with an ARMA structure. Akaike's information corrected criterion was used to select the best models among several ARMA structures, including AR(1), AR(2), MA(1), MA(2), ARMA(1,1) and ARMA(2,2). We did not explore structures higher than ARMA(2,2) due to the relatively small number of years under study. The maximum likelihood method was used to estimate parameters.
We fit binomial regression models with logit link to investigate the association between infection per discharge rate and its explanatory variables, including ANC (dichotomized as < or >≥100 per mm3), length of stay, and duration at ANC nadir. To test whether ANC was an independent risk factor for HAIs, likelihood ratio tests were performed between models with and without an ANC in the presence of length of stay and duration at ANC.
Analyses were performed with the statistical software R 2·12·0. The functions gls() and glm() were used for the time series and binomial model fitting, respectively. Results are reported with 2-tailed P values or 95% confidence intervals (CIs).
RESULTS
Infection Rates
Inpatient days increased steadily between 1983 and 2008 from 10 582 to 14 389, and the number of discharges likewise increased from 1712 to 2652 (Figure 1). This was mirrored by similar trends in numbers of beds (increased from 48 to 62), intensive care beds (increased from 4 to 8), unique patients (increased from 2227 to 6940 per year), and outpatient encounters (increased from 16 799 to 56 976 per year). Despite this expansion in patient volume, the rate of HAIs decreased significantly over that period, from 5.6 infections per 100 discharges and 9.0 infections per 1000 patient-days in 1983 to 2.0 infections per 100 discharges (slope, −0.18; 95% CI, −0.22 to −0.14) and 3.7 infections per 1000 patient-days (slope, −0.30; 95% CI, −0.35 to −0.24) in 2008 (Figure 1).
Figure 1.
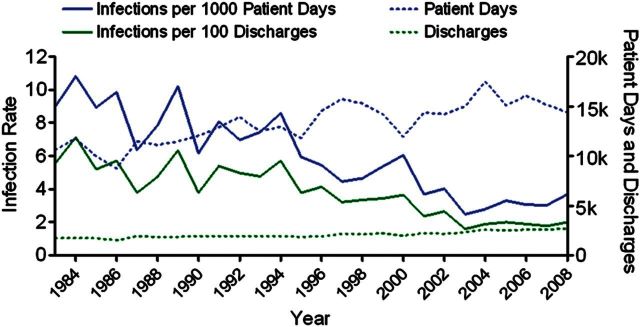
Healthcare-associated infection (HAI) rates, 1983–2008. The rate of HAIs per 100 discharges or 1000 patient-days is plotted (solid lines) and compared to the annual number of discharges and patient-days (dashed lines). The trend is statistically significant for both plots (P < .01).
This sustained decrease in the rate of HAIs was evident across each of the major inpatient services (Figure 2A) despite a general trend of increasing patient numbers treated by each service (Figure 2B). Patients admitted for bone-marrow transplant had the highest rate of infection, ranging from a peak of 100 infections per 100 discharges in 1984 to 8.3 in 2006. The rate of HAIs in patients with acute myeloid leukemia ranged from 25.8 per 100 discharges in 1985 to 0.6 in 2007 and was higher than the rate in patients with acute lymphoblastic leukemia or lymphoma in every year except 2007. The rate of HAIs in patients with solid tumors was similar to that of patients with acute lymphoblastic leukemia or lymphoma.
Figure 2.
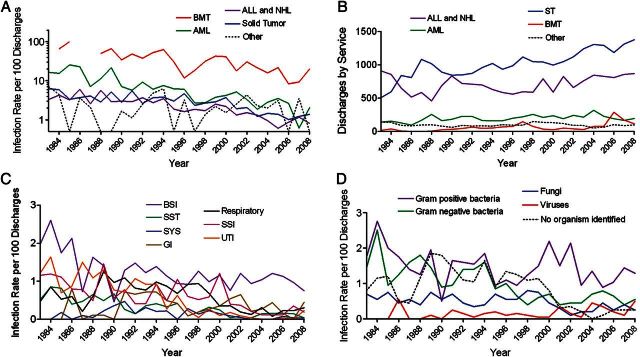
Trends in healthcare-associated infection (HAI) rates, 1983–2008. A, The HAI rate per 100 discharges is stratified by underlying diagnosis or service. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, bone marrow transplant; NHL, non-Hodgkin lymphoma. B, Number of discharges per year is stratified by service. C, The HAI rate per 100 discharges is shown stratified by type of HAI. D, The HAI rate per 100 discharges is stratified by class of organism. Abbreviations: BSI, bloodstream infection; GI, gastrointestinal infection; SSI, surgical-site infection; SST, skin and soft tissue infection; SYS, systemic (disseminated) infection; UTI, urinary-tract infection.
Type of Infections
In the 26 years studied, 1349 patients experienced 1986 HAIs during 1653 hospitalizations. Bloodstream infections were the most common type of HAI identified, accounting for 32.7% of all infections (Table 2). Approximately one-sixth of bloodstream infections were fungemias. Bloodstream infections were more common in transplanted patients and those with acute myeloid leukemia, accounting for more than 40% of infections, whereas surgical-site and urinary-tract infections were most prevalent in patients with solid tumors. Respiratory-tract infections were prominent in the most immunosuppressed and myelosuppressed patients, accounting for 23.0% of all infections in transplanted patients and 19.3% in patients with acute myeloid leukemia. The rates of skin and soft tissue, disseminated, urinary-tract, and surgical-site infections all decreased over time, whereas the rate of bloodstream and gastrointestinal infections did not demonstrate a similar trend (Figure 2C).
Table 2.
Distribution of Healthcare-Associated Infections by Site and Underlying Disease or Service
| Site | Bone Marrow Transplant, No. (%) | Acute Myeloid Leukemia, No. (%) | Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma, No. (%) | Solid Tumor, No. (%) | Other, No. (%) | Total, No. (%) |
|---|---|---|---|---|---|---|
| Bacteremia | 135 (34.1) | 121 (35.0) | 130 (29.9) | 138 (18.4) | 19 (31.7) | 543 (27.3) |
| Fungemia | 27 (6.8) | 20 (5.8) | 23 (5.3) | 33 (4.4) | 4 (6.7) | 107 (5.4) |
| Surgical site | 6 (1.5) | 26 (7.5) | 49 (11.3) | 222 (29.6) | 10 (1.7) | 313 (15.8) |
| Urinary tract | 24 (6.1) | 20 (5.8) | 46 (10.6) | 178 (23.7) | 12 (20.0) | 280 (14.1) |
| Respiratory | 91 (23.0) | 67 (19.3) | 67 (15.4) | 75 (10.0) | 7 (11.6) | 307 (15.5) |
| Skin and soft tissue | 24 (6.1) | 43 (12.4) | 49 (11.3) | 47 (6.3) | 2 (3.3) | 165 (8.3) |
| Gastrointestinal | 65 (16.4) | 29 (8.4) | 36 (8.3) | 39 (5.2) | 6 (10.0) | 175 (8.8) |
| Disseminated bacteria | 4 (1.0) | 2 (0.6) | 4 (0.9) | 2 (0.3) | 12 (0.6) | |
| Disseminated fungus | 18 (4.5) | 9 (2.6) | 22 (5.0) | 8 (1.1) | 57 (2.9) | |
| Othera | 2 (0.5) | 9 (2.6) | 9 (2.0) | 7 (1.0) | 27 (1.3) | |
| Totals | 396 (19.9) | 346 (17.4) | 435 (21.9) | 749 (37.7) | 60 (3.0) | 1986 |
aMeningitis (10), conjunctivitis (6), phlebitis (venous infection) (4), deep abscess (4), myocarditis (2), pericarditis (1).
Etiology of Infections
Gram-positive bacteria were the most commonly identified causes of HAIs in this study, accounting for 39.2% of the 2116 infectious diagnoses (Table 3). Blood and surgical sites were the most common sites for Gram-positive infections, and healthcare-associated C. difficile accounted for the majority of gastrointestinal infections (Supplementary Figure 1). Gram-negative bacteria were common causes of both bloodstream and urinary-tract infections. The majority of systemic infections were due to dissemination of fungal organisms. Viruses were found almost exclusively in respiratory and gastrointestinal infections. No causative organism could be defined for 436 infections, primarily respiratory and surgical-site infections.
Table 3.
Etiology of Healthcare-Associated Infections, 1983–2008
| Organism/Site | Bloodstream | Surgical | Urinary | Respiratory | Skin and Soft Tissue | Gastrointestinal | Disseminated | Othera | Total, No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Coagulase-negative Staphylococci | 143 | 31 | 8 | 1 | 15 | 2 | 200 (25.2) | ||
| Staphylococcus aureus | 49 | 79 | 5 | 5 | 28 | 2 | 1 | 169 (21.2) | |
| Clostridium difficile | 120 | 120 (15.1) | |||||||
| Enterococcus spp. | 52 | 13 | 27 | 1 | 7 | 1 | 101 (12.7) | ||
| Viridans group Streptococci | 63 | 5 | 3 | 2 | 1 | 74 (9.3) | |||
| Other Staphylococcib | 25 | 6 | 1 | 32 (4.0) | |||||
| Corynebacterium spp. | 16 | 5 | 2 | 2 | 2 | 27 (3.4) | |||
| Other Streptococci | 7 | 5 | 5 | 4 | 21 (2.6) | ||||
| Bacillus spp. | 8 | 4 | 1 | 2 | 2 | 17 (2.6) | |||
| Other Gram-positive | 19 | 8 | 3 | 1 | 31 (3.9) | ||||
| Total Gram-positive | 382 | 156 | 46 | 15 | 63 | 121 | 5 | 4 | 792 (39.2) |
| Escherichia coli | 47 | 18 | 121 | 8 | 1 | 195 (36.7) | |||
| Pseudomonas spp. | 40 | 12 | 24 | 7 | 5 | 1 | 2 | 91 (17.2) | |
| Klebsiella spp. | 44 | 7 | 32 | 1 | 4 | 1 | 1 | 90 (17.0) | |
| Enterobacter spp. | 30 | 5 | 14 | 1 | 2 | 52 (9.8) | |||
| Citrobacter spp. | 2 | 13 | 1 | 16 (2.8) | |||||
| Proteus spp. | 3 | 1 | 7 | 2 | 13 (2.5) | ||||
| Stenotrophomonas spp. | 6 | 2 | 1 | 1 | 2 | 1 | 13 (2.5) | ||
| Serratia spp. | 6 | 1 | 2 | 2 | 11 (2.1) | ||||
| Other Gram-negative | 28 | 11 | 2 | 5 | 5 | 2 | 1 | 54 (9.4) | |
| Total Gram-negative | 206 | 57 | 216 | 15 | 29 | 4 | 6 | 2 | 535 (26.1) |
| Candida albicans | 49 | 9 | 12 | 5 | 10 | 1 | 20 | 2 | 108 (39.9) |
| Other Candida spp. | 52 | 2 | 6 | 4 | 1 | 1 | 11 | 1 | 78 (28.8) |
| Aspergillus spp. | 25 | 6 | 9 | 1 | 41 (15.1) | ||||
| Other fungus | 11 | 1 | 8 | 4 | 1 | 19 | 44 (16.2) | ||
| Total fungus | 112 | 11 | 19 | 42 | 21 | 3 | 59 | 4 | 271 (13.4) |
| Rotavirus | 24 | 24 (29.3) | |||||||
| Adenovirus | 2 | 20 | 1 | 23 (28.0) | |||||
| Parainfluenza viruses | 16 | 16 (19.5) | |||||||
| Influenza viruses | 9 | 9 (11.0) | |||||||
| Respiratory syncytial virus | 9 | 9 (11.0) | |||||||
| Other virus | 1 | 1 (1.2) | |||||||
| Total virus | 0 | 0 | 0 | 36 | 0 | 44 | 1 | 1 | 82 (4.1) |
| No organism specified | 2 | 136 | 2 | 201 | 75 | 7 | 1 | 12 | 436 (17.2) |
| Totalsc | 702 | 360 | 283 | 309 | 188 | 179 | 72 | 23 | 2116 |
aMeningitis (10), conjunctivitis (6), phlebitis (venous infection) (4), myocarditis (2), pericarditis (1).
bStaphyloccus haemolyticus (15), Staphylococcus hominis (6), Stapylococcus simulans (5), Staphylococcus intermedius (3), Staphylococcus cohnii (2), Staphlococcus saprophyticus (1).
cFor infections due to multiple organisms, each organism is listed separately.
The rates of Gram-negative bacterial and fungal infections decreased over time, whereas the rate of Gram-positive bacterial infections remained constant (Figure 2D). The decreases were largely due to reductions in the frequency of urinary-tract and disseminated infections (Figure 2C). The rate of infections in which no organism could be identified also decreased, and detection of viruses as causes of infections increased in the last decade due to improved technologies. Most infections in which no organism could be identified were classified as respiratory, surgical site, or skin and soft tissue (Supplementary Figure 2A), and no service was over-represented (Supplementary Figure 2B). Polymicrobial infections accounted for 5.2% of the infections: 2 organisms were detected in 80 cases, 3 in 19, and 4 in 4. Although 9 genera or groupings of bacteria represented 74.2% and 70.9% of Gram-positive and Gram-negative organisms, respectively, numerous rare organisms were identified as causes of HAIs (Supplementary Table 1).
Absolute Neutrophil Counts
The ANC nadir during a hospital admission was a risk factor for developing an HAI. Patients with a lowest-recorded ANC <100 per mm3 during an admission had a rate of infection per 100 discharges more than 3 times higher than those with a higher ANC nadir (Table 4). Length of stay was also greater in patients with an ANC nadir <100 per mm3. The ANC (P < .0001), length of stay (P < .001), and duration at ANC (P < .0001) showed significant associations with the infection per discharge rate. The effect of ANC on infection rate remained significant (P < .0001) after controlling for length of stay and duration at ANC. Further analysis after stratification by ANC <100 per mm3 demonstrated that the ANC nadir predicted significant risk of an HAI for all cancer-related services but not for patients with other diagnoses (Table 5). This risk manifested for all types of infections except other, urinary-tract, and surgical-site infections and was most prominent for disseminated infections. All classes of organisms were identified as causative agents of HAIs more commonly when the ANC nadir was <100 per mm3, although viruses and fungi in particular were rarely found in patients with an ANC >100 per mm3. Thus, ANC nadir is a useful marker for predicting patients at risk for HAIs, and, to some extent, the causative organism.
Table 4.
Rates of Healthcare-Associated Infections (HAIs) Stratified by Absolute Neutrophil Count (ANC) Nadir
| ANC Nadira | Number of HAIsb | Number of Discharges | HAI Rate per 100 Dischargesc | Mean Length of Stayd | HAI Rate per 100 Patient-Days |
|---|---|---|---|---|---|
| <100 | 448 | 6518 | 6.87 | 12.11 | 1.00 |
| 100–499 | 63 | 2881 | 2.19 | 6.56 | 0.39 |
| 500–999 | 41 | 2516 | 1.63 | 5.56 | 0.41 |
| 1000–1499 | 47 | 2445 | 1.92 | 4.93 | 0.51 |
| >1500 | 165 | 10 408 | 1.59 | 4.85 | 0.32 |
aNadir in ANC per mm3 during a hospital admission.
bInfections stratified by ANC in 12 selected years when complete ANC data were available for all admissions (1993–96, 1998–99, 2001–02, and 2004–07).
cRate of healthcare-associated infections per 100 discharges at the specified ANC nadir.
dMean length of stay for all patients who reached the specified ANC nadir during their hospital admissions (data from 7 years; 1993–96, 1998–99, and 2001).
Table 5.
Site, Underlying Disease or Service, and Organism-Specific Rates of Healthcare-Associated Infections Stratified by Absolute Neutrophil Count (ANC) Nadira
| ANC <100 per mm3 | ANC ≥100 per mm3 | Ratio (95% CIs) | |
|---|---|---|---|
| Dischargesb | 6518 | 18250 | |
| Service | |||
| Bone-marrow transplant | 2.81 | 0.13 | 22.28 (14.45–34.34) |
| Acute myelogenous leukemia | 1.14 | 0.12 | 9.87 (6.08–16.01) |
| Acute lymphoblastic leukemia and non-Hodgkin lymphoma | 1.29 | 0.28 | 4.52 (3.2–6.38) |
| Other | 0.17 | 0.10 | 1.71 (0.81–3.62) |
| Solid tumor | 1.47 | 1.11 | 1.33 (1.05–1.69) |
| Site | |||
| Disseminated | 0.41 | 0.01 | 37.80 (8.99–158.91) |
| Gastrointestinal | 1.17 | 0.13 | 8.87 (5.61–14.02) |
| Respiratory | 1.47 | 0.18 | 8.4 (5.67–12.44) |
| Bloodstream | 2.68 | 0.53 | 5.05 (3.95–6.46) |
| Skin and soft tissue | 0.52 | 0.14 | 3.81 (2.27–6.38) |
| Other | 0.06 | 0.02 | 3.73 (.84–16.68) |
| Urinary tract | 0.29 | 0.27 | 1.09 (.64–1.84) |
| Surgical site | 0.26 | 0.47 | 0.56 (.33–.94) |
| Organism | |||
| Virus | 0.61 | 0.02 | 28.00 (10.02–78.23) |
| Fungus | 1.15 | 0.18 | 6.56 (4.34–9.92) |
| No organism specified | 1.37 | 0.40 | 3.41 (2.51–4.63) |
| Gram-positive bacteria | 2.64 | 0.84 | 3.13 (2.52–3.87) |
| Gram-negative bacteria | 1.33 | 0.45 | 2.97 (2.2–3.99) |
Abbreviation: CI, confidence interval.
aRate per 100 discharges of patients who reached the specified ANC per mm3 nadir.
bTotal discharges in 12 selected years stratified by whether patients reached the specified ANC nadir during their hospital admission.
DISCUSSION
Opportunities to acquire HAIs increase as patients live longer and undergo more intensive treatments for cancer. As many of these infections are considered preventable, benchmark data would be valuable as a baseline for intervention studies and to identify opportunities for improvement [13]. However, reliable baseline data on rates of HAIs are not available for immunosuppressed patients [6, 14]. This is likely due to a number of factors: limited numbers of such patients at a single institution, changes in practice over time making longitudinal comparisons difficult, and reluctance to publicize HAI data due to a fear of negative publicity. This report is the largest and most comprehensive description of HAIs in immunosuppressed patients published to date. In addition to providing benchmark data, we demonstrate that the rate of HAIs can be reduced over time through sustained application of infection-control policies, despite increases in both patient volume and the intensity of treatment leading to immunosuppression and myelosuppression.
Our data demonstrate a sustained rate of HAIs in a highly immunosuppressed and myelosuppressed population that is lower than the rate estimated for the general population of the United States. The mean HAI rate in this report for the last 5 years (2004–2008) is 1.9 HAIs per 100 discharges or 3.2 HAIs per 1000 patient-days, whereas the estimated rate for all U.S. hospitals is 4.5 per 100 admissions or 9.3 per 1000 patient-days (from 2002 data) [5]. It is illuminating, in this context, to note that in recent studies the mortality rates from bloodstream infections in cancer patients were lower than comparable data in immunocompetent persons [15, 16]. This has been attributed to the aggressive, protocol-driven approaches of oncologists to prevention and empiric treatment of infection [17, 18].
The reasons for this sustained downward trend in HAIs are likely multifactorial, encompassing changes to the physical structure of the hospital, strong and sustained leadership with support from administration, improvements in the practices of healthcare workers, and provision of prophylactic antifungal and antibacterial agents to high-risk patients (Table 1). The construction of a new patient care center and the transition of all inpatient beds to that facility in August of 1995 is the most notable event that impacted infection control. Improvements to air quality, barrier isolation options, patient, visitor and employee flow within the facility, accessibility of hand hygiene stations, and the ease with which decontamination of rooms could be accomplished were all facilitated through specific design elements of the new building. An important advance was the opening of new surgical suites in 1996, which halted the practice of transporting patients to a local adult hospital for most surgeries. The addition to the institution of dedicated surgical staff, who were subject to our infection-control policies, coupled with changes in the postoperative care of urinary catheters likely contributed to the sustained decrease in urinary-tract infections (Figure 2C) and Gram-negative bacterial infections (Figure 2D) that are evident throughout this period. This downward trend continued despite incremental improvements in blood-culture systems, viral detection methods including polymerase chain reaction, other laboratory diagnostics, and surgical techniques including interventional radiology that have combined to limit in recent years the number of HAIs for which no organism could be determined (Figure 2D).
In general, compliance with infection-control policies has been high at SJCRH. This is evidenced by an annual influenza vaccination rate of 80%–96% in the last 7 years and hand hygiene and personal protective equipment compliance rates that are currently >90% ([19] and data not shown). However, no specific intervention or single policy can be shown to account for the steady, sustained improvement in rate of HAIs that has been demonstrated here. In our opinion, the major factor reducing the HAI rate over this 26-year period was structured implementation of evidence-based, general infection control practices coupled with high compliance. The major finding of this study is that these sustained reductions in HAIs were seen over time. Although no formal analysis was done that related this reduction to the adoption of any specific policies, it did occur during a period of general application of accepted infection-control principles (Table 1). This suggests that our findings are generalizable and that similar results could be obtained at other institutions if currently recommended practices are rigorously adhered to over a sustained period of time.
The ANC of a hospitalized patient with cancer has been recognized as a prognostic marker for the development of infections [4, 20], but a relationship between severe neutropenia and development of HAIs has not previously been assessed. The rate of infections increases below defined thresholds, with <500 neutrophils per mm3 of blood typically accepted as a marker of increased risk requiring empiric therapy of febrile episodes [20]. We found that an ANC <100 per mm3 at any time during a hospitalization was a strong indicator of HAI risk for most types of infection and for all types of organisms (Table 5). This risk was evident for all underlying cancer-related diagnoses and was independent of length of stay.
Previous studies have shown that HAI rates are higher for acute myeloid leukemia than acute lymphoblastic leukemia, and for allogeneic transplants than autologous transplants, and that these differences were related to the duration of neutropenia (using 1000 neutrophils per mm3 as a marker) [7, 11, 21]. Another study found that an ANC <500 per mm3 did not predict an increased risk of HAI, but it did not assess other breakpoints [10]. We propose that severe neutropenia manifest as an ANC <100, but not lesser degrees of neutropenia, be considered a risk factor for the development of HAIs. This may guide future prognostic study of interventions to prevent HAIs in patients with cancer.
Some caveats about the generalizability of our findings can be made. The study population was exclusively children, who were treated at a dedicated facility experienced with immunosuppressed and myelosuppressed patients. There are few extant data about HAIs in children with cancer for comparison. Although our policy to include C. difficile HAIs only if they were detected >7 days after admission may have decreased our overall rates, gastrointestinal infections are a relatively rare cause of HAIs compared with other types of infections [5], and the overall incidence of C. difficile–associated diarrhea at our institution is low (eg, 2.3 total cases per 100 discharges in 2008). It must also be noted that some evolution of NHSN guidelines and definitions occurred over time and that we followed these updates, which may have led to some discontinuities in the longitudinal data. The strengths of the study include the large number of patients and the consistent application of definitions under the direction of a single person over a long study period. We conclude that sustained decreases in the rate of healthcare-associated infections in immunosuppressed and myelosuppressed children are possible through dedicated application of general infection-control principles.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
The authors would like to thank the following infection preventionists who collected primary data: Carla Haley, RN; Ann Cole, RN; Robert Brace, RN; Donna Nance, RN; Sharon Williams, RN; and Mary Anne Giannini, CIC; and we would like to thank Don Sanderlin for providing data on patient encounters.
Authors' contributions. J. A. M. conceived the study, collated and analyzed the data, and wrote the paper. B. F. W. and B. G. W. collected the data. S. W. and M. P. S. provided statistical analysis. B. F. W., S. W., M. P. S., B. G. W., R. T. H., S. C. H., C. H. P., and W. T. H. interpreted the data and assisted with writing the paper.
Financial support. This work was supported in part by the National Cancer Institute at the National Institutes of Health (grant CA21765) and by the American Lebanese Syrian Associated Charities.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Simone JV. A history of St Jude Children's Research Hospital. Br J Haematol. 2003;120:549–55. doi: 10.1046/j.1365-2141.2003.04111.x. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey GP. The changing face of febrile neutropenia—from monotherapy to moulds to mucositis. Fever and neutropenia: the early years. J Antimicrob Chemother. 2009;63(Suppl 1):i3–13. doi: 10.1093/jac/dkp074. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating healthcare-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–66. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–97. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 7.Rotstein C, Cummings KM, Nicolaou AL, Lucey J, Fitzpatrick J. Nosocomial infection rates at an oncology center. Infect Control Hosp Epidemiol. 1988;9:13–19. doi: 10.1086/645727. [DOI] [PubMed] [Google Scholar]
- 8.Robinson GV, Tegtmeier BR, Zaia JA. Brief report: nosocomial infection rates in a cancer treatment center. Infect Control. 1984;5:289–94. doi: 10.1017/s0195941700060355. [DOI] [PubMed] [Google Scholar]
- 9.Ford-Jones EL, Mindorff CM, Langley JM, et al. Epidemiologic study of 4684 hospital-acquired infections in pediatric patients. Pediatr Infect Dis J. 1989;8:668–75. doi: 10.1097/00006454-198910000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. doi: 10.1186/1471-2334-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urrea M, Rives S, Cruz O, Navarro A, Garcia JJ, Estella J. Nosocomial infections among pediatric hematology/oncology patients: results of a prospective incidence study. Am J Infect Control. 2004;32:205–8. doi: 10.1016/j.ajic.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Cardo D, Dennehy PH, Halverson P, et al. Moving toward elimination of healthcare-associated infections: a call to action. Infect Control Hosp Epidemiol. 2010;31:1101–5. doi: 10.1086/656912. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger A, Paul M, Gafter-Gvili A, Rubinovitch B, Leibovici L. Infection-control interventions for cancer patients after chemotherapy: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:97–107. doi: 10.1016/S1473-3099(08)70284-6. [DOI] [PubMed] [Google Scholar]
- 15.Castagnola E, Caviglia I, Pistorio A, et al. Bloodstream infections and invasive mycoses in children undergoing acute leukaemia treatment: a 13-year experience at a single Italian institution. Eur J Cancer. 2005;41:1439–45. doi: 10.1016/j.ejca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 16.van de Wetering MD, de Witte MA, Kremer LC, Offringa M, Scholten RJ, Caron HN. Efficacy of oral prophylactic antibiotics in neutropenic afebrile oncology patients: a systematic review of randomised controlled trials. Eur J Cancer. 2005;41:1372–82. doi: 10.1016/j.ejca.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Hughes WT. Bloodstream infections in cancer patients. Eur J Cancer. 2005;41:1370–1. doi: 10.1016/j.ejca.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Hughes WT. Should prophylactic antibiotics be used in afebrile neutropenic oncology patients? Nat Clin Pract Oncol. 2006;3:130–1. doi: 10.1038/ncponc0431. [DOI] [PubMed] [Google Scholar]
- 19.Hakim H, Gaur AH, McCullers JA. Motivating factors for high rates of influenza vaccination among healthcare workers. Vaccine. 2011;29:5963–9. doi: 10.1016/j.vaccine.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–51. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 21.Carlisle PS, Gucalp R, Wiernik PH. Nosocomial infections in neutropenic cancer patients. Infect Control Hosp Epidemiol. 1993;14:320–4. doi: 10.1086/646750. [DOI] [PubMed] [Google Scholar]


