In the United States, invasive meningococcal disease occurs at the highest rates in infants, with a second peak in adolescents. Early symptoms are nonspecific and may resemble viral infection, which poses a diagnostic challenge for clinicians. Moreover, the stakes of diagnostic confusion may be high because the disease may become life threatening within hours. Sequelae among survivors may be profound. In adolescent age groups, control of meningococcal disease caused by serogroups A, C, W-135, and Y rests upon universal vaccination beginning at 11–12 years of age; vaccines for serogroup B are not currently available. One 4-valent vaccine is licensed for use in infants beginning at 9 months of age but is only recommended for those at high risk. Licensure of new vaccines for infants is expected soon, and a universal immunization program is being debated.
A wide range of bacteria can cause meningitis in infants and young children. In neonates aged <3 months, group B streptococcus causes most bacterial meningitis in many developed countries, followed by Escherichia coli and other Gram-negative enteric bacilli. Listeria monocytogenes can also be seen in this age group [1]. Historically, for infants and children aged <5 years, the 3 most common causes of bacterial meningitis and sepsis were Haemophilus influenzae type b (Hib), Streptococcus pneumoniae, and Neisseria meningitidis [1]. Annual disease cases due to Hib in US children aged <5 years have been reduced by 99%, and there has been a substantial decline in pediatric invasive pneumococcal disease due to routine infant immunization programs introduced in the 1990s and 2000s [2, 3]. This leaves N. meningitidis as one of the most important causes of bacterial meningitis in infants and young children. Since 2005, the Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices (ACIP) has recommended routine vaccination against N. meningitidis for all adolescents; recommendations for vaccination of children aged 9 months–10 years are restricted to high-risk groups [4–10]. At the present time, there are no meningococcal conjugate vaccines licensed for use in infants aged <9 months, although there are vaccines currently under review by the Food and Drug Administration (FDA). This article discusses the burden of meningococcal disease in infants in order to provide a backdrop for considering prevention strategies.
Risk of Disease in Young Infants
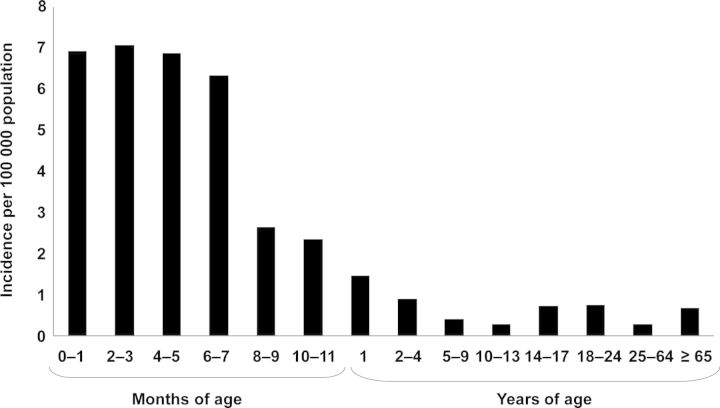
The incidence of invasive meningococcal disease in the United States is highest in infants [11]. From 1998 to 2007, the incidence of culture-confirmed meningococcal disease in those aged <1 year was 5.38 per 100 000 population—more than 10 times higher than the overall rate of 0.53 per 100 000 population and over 7 times higher than the rate in adolescents and young adults (approximately 0.75 per 100 000 population). The highest rates occurred in infants aged <8 months of age, ranging from 6.33 per 100 000 in infants aged 6–7 months to 7.08 in infants aged 2–3 months [11] (Figure 1).
Figure 1.
Age-stratified incidence of culture-confirmed meningococcal disease in the United States, 1998–2007 [11].
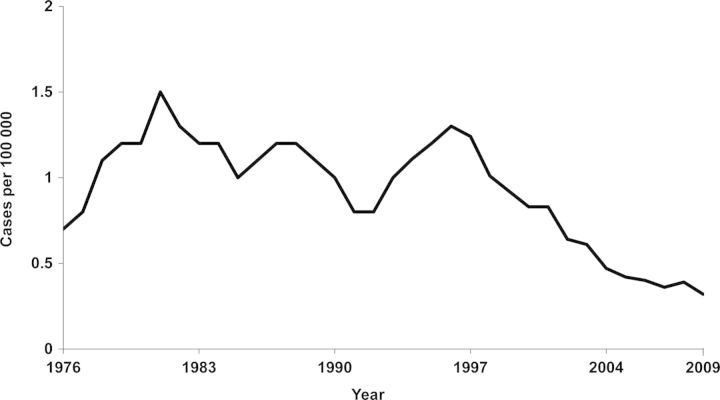
In the United States, between 1976 and 2009, the number of cases of meningococcal disease reported annually ranged from approximately 1000 to 3500. The incidence appears to increase and decrease in a cyclic fashion [12, 13] (Figure 2). The United States is currently experiencing a low number of cases, despite the fact that the overall incidence rate in infants is high. Between 1998 and 2007, the annual estimated number of culture-confirmed meningococcal cases was 1525; of these, 14% (n = 215) occurred in infants aged <1 year. This was similar to the total number of cases in adolescents aged 10–17 years (n = 173) and in older adolescents and young adults aged 18–24 years (n = 213) [11]. Since then, the total number of meningococcal cases reported to the CDC decreased from 1172 in 2008 to 833 in 2010 [12, 14].
Figure 2.
Historical incidence of meningococcal disease in the United States, 1976–2009 [12, 13]. Credit: Courtesy of Centers for Disease Control and Prevention.
The incidence of meningococcal disease in infants may be underreported because of a reliance on culture confirmation [15]. Detection of N. meningitidis in cultures from the cerebral spinal fluid, blood, or other sterile site is the typical method of confirming a meningococcal disease diagnosis. The sensitivity of this test is low, however, if performed after the patient has been given antibiotics [16]. In a study from Ireland investigating the utility of DNA detection with polymerase chain reaction (PCR) assays to diagnose meningococcal disease in children, blood cultures were positive in only 8 of 39 PCR-confirmed cases (21%), due in part to antibiotics being administered before the cultures were obtained [17]. If these results are any indication of the potential discrepancy between culture-confirmed and actual cases, the US incidence in infants and other age groups may be higher than current estimates. In fact, epidemiological surveys performed in countries in Europe and South America have found that a large proportion of cases would have been missed without the use of PCR testing [15]. The use of PCR in diagnosing bacterial meningitis was evaluated in 12 hospitals in São Paulo State, Brazil (February 2007–April 2009). The use of PCR increased the number of identified meningococcal disease cases by 92% compared with culture-confirmed cases alone (90 culture-positive cases; 83 culture-negative, PCR-positive cases) [18]. In the United Kingdom (2009), 1046 meningococcal cases were confirmed, of which 58% were confirmed by PCR alone (approximately 600 cases) [19].
Why are infants at increased risk of meningococcal disease? One factor is the catabolism of transplacentally acquired antibodies, which may be present in the mother because of prior colonization or cross-reactions from related bacteria [20–22]. The infant's immune system is also immature, conferring a physiological immunodeficiency that involves all aspects of the host response to infection [23].
More research is required in specific factors that increase the vulnerability of infants; however, some factors increase the risk of N. meningitidis infection in general, regardless of age. Individuals with immune system deficiencies, such as complement system deficiencies or functional or anatomical asplenia, are at higher risk for meningococcal infection [9]. Furthermore, genetic polymorphisms have been associated with susceptibility to meningococcal infection as well as severity and mortality of meningococcal disease [24, 25]. For example, polymorphisms in IL1RN and IL1B, genes involved in the cytokine pathway, are associated with mortality [24]. Mutations in the gene for mannose-binding lectin (MBL), a component of the innate immune system that activates complement and enhances phagocytosis and killing of bacteria [26], increase susceptibility to meningococcal disease. In an analysis of patients with meningococcal disease, the prevalence of MBL structural variants was more pronounced in patients aged <1 year (57.1%) compared with patients aged <2 years (39.3%) versus healthy controls (8.2%) [27].
Other factors that are generally associated with an increased risk of N. meningitidis infection include coming in close contact with a case, living in crowded conditions, and being exposed to cigarette smoke and respiratory tract infections that cause nasopharyngeal irritation [4]. In a study of 129 case patients and 274 age-matched controls in Oregon and Washington in 1994, children aged <5 years with meningococcal disease were shown to be approximately 8 times more likely to have a mother who smokes than matched controls [28]. The most important risk factor for infants may be exposure to adolescents, such as a sibling or possibly a babysitter. Adolescents are the primary population that carries N. meningitidis bacteria in the upper respiratory tract. Based on a meta-analysis of data from 89 studies in 28 countries, the peak prevalence of meningococcal carriage (23.7%) is at age 19 years [29]. Invasive meningococcal disease occurs only after exposure to a pathogenic strain of meningococcus that colonizes the nasopharyngeal mucosa. Although risk factors for meningococcal disease have been identified, most cases of meningococcal disease occur in otherwise healthy persons.
Rapid Disease Course and Mortality
Neisseria meningitidis is exquisitely susceptible to routinely used antibiotics. Despite this, and despite the availability of intensive supportive care, the overall case fatality rate (CFR) from invasive meningococcal disease in the United States is approximately 12% [11, 20]. Case fatality rates are generally higher in older age groups. In the United States, from 1998 to 2007, the CFR was 6% in infants aged <1 year, 9.7% in adolescents aged 14–17 years, and 13.6% in those aged 18–24 years. The estimated annual number of deaths in infants was 13 compared with 41 deaths among persons aged 14–24 years [11].
Diagnosing meningococcal disease can be challenging, and the window for doing so before the condition of the patient deteriorates is often narrow. Early diagnosis can be difficult because initial symptoms are nonspecific and resemble those of other common, mild infections, most of which are caused by viruses [30–32]. In infants, symptoms include fever, irritability, poor feeding, vomiting, and drowsiness. Signs of sepsis, such as abnormal skin color or cold extremities, may not appear until 5–9 hours later. The hallmark signs of meningitis—bulging fontanel and neck stiffness—may not appear until 8–15 hours into the course of illness, if at all [32]. Petechial or purpuric rash, often the symptom that prompts admission to the emergency room, may begin as a nonspecific rash and can appear later in the course of illness or may not appear at all. In a prospective study of all patients with meningococcal disease in the Netherlands from 2003 to 2005 (N = 752 total reported cases), petechial or purpuric rash was present at hospital admission in 74% of children aged 1–18 years but only in 48% of infants aged <1 year [30].
The consequences of a delay in hospital admission can be grave for anyone with meningococcal disease due to its rapid, aggressive, unpredictable, and potentially fatal course. The disease can progress from early symptoms to death in 24–48 hours [30, 32]. In the study in the Netherlands, the time from the onset of symptoms to death was known for 26 of the 48 patients who died (28 of the total deaths occurred in children aged <5 years). All but 3 individuals died either before or within 48 hours of hospital admission, and nearly 30% of the individuals who died were admitted to the hospital after being ill for >18 hours [30].
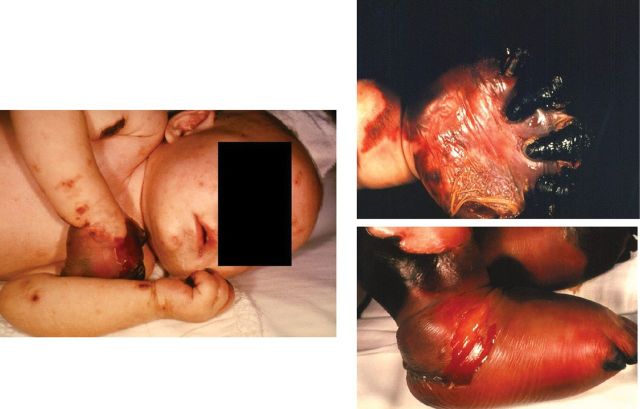
In the pediatric population, invasive meningococcal disease most commonly manifests as meningitis or bacteremia [11, 33]. These syndromes can occur separately but often present together [34]. In about 5%–20% of cases, meningococci multiply rapidly in the bloodstream, sending the patient into severe shock (fulminant meningococcal septicemia) and causing multiple organ failure [16, 34]. The rapid and massive intravascular inflammatory response to infection causes severe hypotension, circulatory collapse, and disseminated intravascular coagulation, with thrombotic lesions in the skin, hands and arms, feet and legs, kidneys, adrenal glands, and choroid plexus. Along with thrombosis, patients with fulminant meningococcal septicemia may develop gangrene in their extremities [34] (Figure 3).
Figure 3.
Meningococcal septicemia with gangrene of the hands and feet in a female infant aged 4 months [35]. Credit: Courtesy of Centers for Disease Control and Prevention.
Disease Burden: Physical and Psychological
Nearly all individuals with meningococcal disease are hospitalized. From 1999 to 2008 in the United States, the median hospital stay for infants with meningococcal disease was 7 days (0–373 days) [36]. Approximately 11%–19% of patients who survive meningococcal disease suffer from significant sequelae, including amputations, deafness, learning/neurodevelopmental deficits, and organ failure (eg, failure of the renal, adrenal, pulmonary, and immune systems). Other sequelae include skin necrosis, seizures, ataxia, and hemiplegia [20, 37]. Although there are few specific data on long-term sequelae in infants, some studies that include infants, toddlers, and young children have shown significant residual disease burden. In one study from 10 US pediatric hospitals from January 2001 to March 2005, 159 cases of meningococcal disease in children were identified (41 cases in children aged <12 months; 22 cases in children aged 12–24 months; 39 cases in children aged 2– < 5 years; 57 cases in children aged 5–19 years). Among the 146 patients who survived, 14 children had skin necrosis (9 of whom were aged ≤4 years), 14 children suffered either unilateral or bilateral deafness, and 2 children underwent amputations, 1 of which was of all 4 extremities. Notably, hearing loss was more common in infants and toddlers aged ≤2 years (8 of 14 children) than in older children [33].
Sequelae such as musculoskeletal disorders, learning deficits, and renal failure may appear years or even decades after infection [38, 39]. Patients who survive meningococcal septicemia may develop progressive orthopedic conditions, most frequently growth plate injuries that result in growth reductions in limbs and asymmetric limb growth. In infants and toddlers, these problems may not emerge for ≥10 years after the initial infection, when the child goes through the rapid adolescent growth period [38–40]. A prospective, case-control study of infants aged <1 year (N = 1717) who had survived meningitis (including bacterial and viral infections) in Wales and England between 1985 and 1987 found that the long-term consequences of meningitis were significant at 5-year follow-up. Of infants who had survived meningococcal meningitis specifically (n = 402), the rates of moderate and severe disability were 7% and 3%, respectively. Moderate disabilities included mild neuromotor disabilities, intellectual impairment, moderate hearing loss, mild or moderate visual impairment, epilepsy controlled with treatment, and hydrocephalus without complications. Severe disabilities included severe neuromotor impairment, intellectual impairment, seizure disorders, and visual or auditory impairment [41].
A retrospective survey study in Quebec, Canada (1990–1994), examined 471 cases of meningococcal serogroup B and C disease to identify the frequency of complications and sequelae. Of the 471 cases, 98 occurred in infants aged <1 year and 89 occurred in toddlers aged 1–4 years. On average, sequelae among meningococcal disease survivors of all ages (n = 420) included scarring (8%), amputations (3%), sensorineural hearing loss (2%), and renal failure (1%). Among serogroup C cases occurring in infants aged <1 year (n = 26), approximately 11% were fatal, 6% had major complications, and 26% had minor complications. In comparison, among serogroup C cases in adolescents aged 11–19 years (n = 101), approximately 14% were fatal, 19% had major complications, and 30% had minor complications [42].
The lingering psychological and emotional effects on survivors and parents of children who have died may be less obvious than the physical sequelae but are also an important part of the burden of meningococcal disease. In a UK study of 29 children aged 2–16 years who had been treated for meningococcal disease at a hospital pediatric intensive care unit, posttraumatic stress disorder symptoms, which were correlated with the severity of the child's illness and behavioral problems at follow-up, occurred in 48% of the mothers of these children [43]. These findings are similar to findings from a study in Amsterdam, which demonstrated psychological distress in 192 mothers and fathers 3 months–7 years after their children (aged 1–18 years) had survived meningococcal disease. Between 31% and 69% of mothers and 29%–58% of fathers reported psychological distress, depending on the time of follow-up. These proportions were significantly higher than could be expected in the general population [44].
Serogroup Dynamics
Most invasive meningococcal disease in the world is caused by serogroups A, B, C, W-135, and Y, although new serogroups can emerge (eg, serogroup X in sub-Saharan Africa) [45]. Protein-polysaccharide conjugate vaccines are only available for A, C, W-135, and Y, because humans do not produce antibodies to the B polysaccharide. Serogroup distribution varies widely between regions and countries. Most invasive meningococcal disease in the United States is caused by serogroups B, C, W-135, and Y [11]. In comparison, the greatest proportion of disease in Africa is caused by serogroups A, C, and W-135 (with a small proportion caused by serogroup X). In Finland, an increase in serogroup Y disease was observed from 1995 to 2010 [46]. In Europe as a whole, invasive meningococcal disease is mostly due to serogroups B and C, although the widespread use of monovalent vaccines targeting serogroup C has decreased the predominance of serogroup C in this region [15].
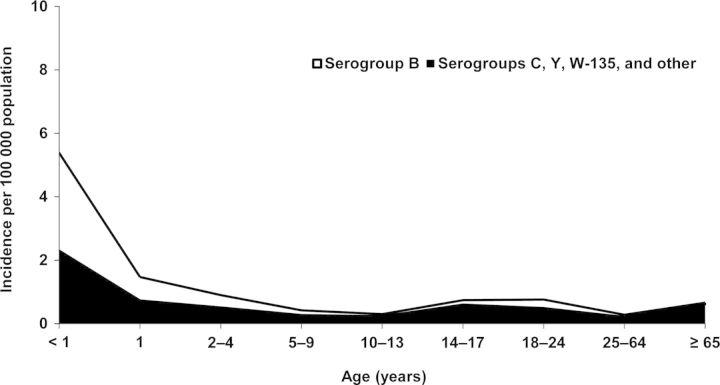
Meningococcal vaccines need to be multivalent because of the fluid and unpredictable nature of serogroup epidemiology. In the United States, for example, the proportion of disease attributable to serogroup Y increased from 2% in 1989 to 37% in 2009, although it is important to point out that at the same time the absolute incidence of disease decreased [47, 48]. Serogroup distribution also differs by age group. For US infants aged <1 year, approximately 57% of invasive meningococcal disease cases are caused by serogroup B, and the remaining 43% are caused by C, W-135, and Y (Figure 4). In children and adolescents aged 10–17 years, 80% of disease is caused by serogroups C, Y, and other (including W-135), and the remaining 20% is caused by serogroup B. Serogroup epidemiology may change from year to year in specific age groups; for example, in 1998–1999, the incidence of serogroups C, W-135, and Y disease in infants exceeded serogroup B disease, whereas the latter was more common in all subsequent years. For persons aged 11–19 years, the incidence of invasive meningococcal disease caused by serogroups C, W-135, and Y, taken collectively, has consistently exceeded that caused by serogroup B [11].
Figure 4.
Distribution of serogroup B versus other serogroups in culture-confirmed meningococcal disease in the United States, 1998–2007 [11].
Neisseria meningitidis serogroup epidemiology is also influenced by the genetic diversity and adaptability of the organism. Horizontal transfer of genes can result in strains that are highly related but which have switched serogroups (eg, from B to C), thus allowing the new strain to escape immune responses targeting the original serogroup; this can contribute to disease outbreaks [49]. An increasingly mobile population also increases the potential for dispersal of serogroups from one region of the world to another [50]. Individuals do not need to travel themselves to be at risk of disease from serogroups that are not prevalent in their home regions, as is illustrated by the case of an Australian infant diagnosed with serogroup W-135 meningococcal disease 6 months after 2 uncles living next door had returned from the Hajj pilgrimage to Mecca (children in Australia receive routine vaccination at age 12 months against serogroup C disease only) [51]. In England, 4 young children (aged 4, 10, 19, and 27 months) were diagnosed with serogroup W-135 disease after their household contacts and/or family members had recently returned from Mecca. All 4 children had been recently vaccinated with a meningococcal serogroup C vaccine [52].
Infant Vaccination
Two meningococcal conjugate vaccines are licensed for use in the United States, MCV4-D (Menactra, sanofi pasteur) [53] and MCV4-CRM (Menveo, Novartis Vaccines) [54], each of which contains polysaccharides from serogroups A, C, W-135, and Y conjugated to a protein. A polysaccharide vaccine (Menomune—A/C/Y/W-135, sanofi pasteur) [55] is also licensed for use in persons aged ≥2 years, but age-appropriate use of conjugate vaccines is preferred.
In the case of MCV4-D, the conjugate protein is diphtheria toxoid; in the case of MCV4-CRM, it is CRM197, a mutant diphtheria toxin that is also used in PCV13. Both MCV4-D and MCV4-CRM are licensed for use as a single dose in persons aged 2–55 years (the MCV4-CRM indication allows for a 2-dose series in high-risk children aged 2–5 years). ACIP recommendations that apply to either product include universal vaccination of adolescents at age 11–12 years, routine revaccination at age 16 years, a 2-dose primary series for persons who may not respond well to vaccination, and repeated revaccination of certain high-risk persons [4–9]
In June of 2011, after extension of the MCV4-D label to include infants and toddlers aged 9–23 months, the ACIP recommended use of MCV4-D for children in this age group with persistent complement deficiency, those traveling to or living in countries where invasive meningococcal disease is hyperendemic or epidemic, and those in defined risk groups during community or institutional outbreaks [10]. Functional or anatomic asplenia was not included because of concerns about potential interference with PCV13 and the fact that pneumococcal disease is a bigger concern for these patients. MCV4-D is not approved for use in infants aged <9 months. Available data show that infants administered 3 doses of MCV4-D (at age 2, 4, and 6 months) had lower responses to each meningococcal vaccine serogroup compared with older children given 2 doses at age 9 and 12 months [53].
The FDA is currently reviewing indications for MCV4-CRM that could extend use to infants aged as young as 2 months [56, 57]. Licensure of HibMenCY-TT (MenHibrix, GlaxoSmithKline Biologicals), a combination of meningococcal serogroups C and Y polysaccharides and Hib polysaccharide, each conjugated to tetanus toxoid, is also being considered [58–60]. Protein-based serogroup B vaccines, at least 1 of which was developed through reverse vaccinology [61, 62], are further down the line.
The decision about whether or not to recommend meningococcal vaccines routinely in infants will be a difficult one. The first vaccines to come along, which will not include serogroup B, will be capable of preventing only about half of cases. These vaccines will need to provide protection early on, perhaps after the second dose, in order to prevent the majority of infant cases. Deliberations on this issue will be occurring at a time when the overall incidence of invasive meningococcal disease in the United States is at its lowest point in decades and the absolute number of preventable cases and deaths will be low. All of this will affect the cost per quality-adjusted life year of a 4-dose universal program.
On the other hand, the incidence in infants far exceeds that in adolescents, an age group for which there is now a 2-dose universal immunization program. Vaccinology has reached a point where the low-hanging fruit—Hib and S. pneumoniae, in this instance—has been picked. The debate over infant meningococcal vaccination will raise a new question: Is it time to invest in a universal program to prevent a very serious, albeit rare, disease in infants?
Acknowledgments
Editorial assistance was provided by Stephanie Brewer, PhD, of International Meetings & Science Inc., which received support from Novartis Vaccines and Diagnostics.
Potential conflicts of interest. R. J. received honoraria from MedImmune, GlaxoSmithKline Vaccines, and Novartis Vaccines and Diagnostics for lectures and service on advisory boards. G. S. M. is a principal investigator in studies funded by GlaxoSmithKline Biologicals, Merck, Novartis, and sanofi pasteur, and has received honoraria from these companies for lectures and service on advisory boards.
All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sáez-Llorens X, McCracken GH., Jr. Bacterial meningitis in children. Lancet. 2003;361:2139–48. doi: 10.1016/S0140-6736(03)13693-8. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children—United States, 1998–2000. MMWR Morb Mortal Wkly Rep. 2002;51:234–7. [PubMed] [Google Scholar]
- 3.Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 4.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11–18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794–5. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2–10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2007;56:1265–6. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042–3. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018–9. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72–6. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391–2. [PubMed] [Google Scholar]
- 11.Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Summary of notifiable diseases—United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1–100. [PubMed] [Google Scholar]
- 13.McNabb SJ, Jajosky RA, Hall-Baker PA, et al. Summary of notifiable diseases—United States, 2006. MMWR Morb Mortal WkIy Rep. 2008;55:1–92. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Final 2010 reports of nationally notifiable infectious diseases. MMWR Morb Mortal Wkly Rep. 2011;60:1088–115. [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison LH, Pelton SI, Wilder-Smith A, et al. The Global Meningococcal Initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29:3363–71. doi: 10.1016/j.vaccine.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Meningococcal disease. In: Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine–Preventable Diseases. 12th. Washington, DC: Public Health Foundation; 2011. pp. 193–203. [Google Scholar]
- 17.Dunlop KA, Coyle P, Mitchell S, et al. Molecular testing of respiratory swabs aids early recognition of meningococcal disease in children. Diagn Microbiol Infect Dis. 2011;70:427–34. doi: 10.1016/j.diagmicrobio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Sacchi CT, Fukasawa LO, Goncalves MG, et al. Incorporation of RT-PCR into routine public health surveillance for meningococcal meningitis in São Paulo State, Brazil. In: IPNC 2010 17th International Pathogenic Neisseria Conference program and abstract guide [abstract P042]. Alberta, Canada: International Pathogenic Neisseria Conference, 2010:98. [Google Scholar]
- 19.Gray S, Campbell H, Marsh J, et al. The epidemiology and surveillance of meningococcal disease in England and Wales. In: IPNC 2010 17th International Pathogenic Neisseria Conference program and abstract guide [abstract P041]. Alberta, Canada: International Pathogenic Neisseria Conference, 2010:98. [Google Scholar]
- 20.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 21.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trotter C, Borrow R, Andrews N, Miller E. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine. 2003;21:1094–98. doi: 10.1016/s0264-410x(02)00630-8. [DOI] [PubMed] [Google Scholar]
- 23.Jaspan HB, Lawn SD, Safrit JT, Bekker LG. The maturing immune system: implications for development and testing HIV-1 vaccines for children and adolescents. AIDS. 2006;20:483–94. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- 24.Brouwer MC, Read RC, van de Beek D. Host genetics and outcome in meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:262–74. doi: 10.1016/S1473-3099(10)70045-1. [DOI] [PubMed] [Google Scholar]
- 25.Wright V, Hibberd M, Levin M. Genetic polymorphisms in host response to meningococcal infection: the role of susceptibility and severity genes. Vaccine. 2009;27(Suppl 2):B90–102. doi: 10.1016/j.vaccine.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 27.Faber J, Schuessler T, Finn A, et al. Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr Infect Dis J. 2007;26:243–46. doi: 10.1097/01.inf.0000256751.76218.7c. [DOI] [PubMed] [Google Scholar]
- 28.Fischer M, Hedberg K, Cardosi P, et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16:979–83. doi: 10.1097/00006454-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Christensen H, May M, Bowen L, Hickman M, Trotter C. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 30.de Greeff SC, de Melker HE, Schouls LM, Spanjaard L, van Deuren M. Pre-admission clinical course of meningococcal disease and opportunities for the earlier start of appropriate intervention: a prospective epidemiology study on 752 patients in the Netherlands, 2003–2005. Eur J Clin Microbiol Infect Dis. 2008;27:985–92. doi: 10.1007/s10096-008-0535-1. [DOI] [PubMed] [Google Scholar]
- 31.Hameed R, Riordan FA. Meningococcal disease presenting as bronchiolitis. J Infect Dis. 2002;44:94–5. doi: 10.1053/jinf.2001.0956. [DOI] [PubMed] [Google Scholar]
- 32.Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;67:397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan SL, Schutze GE, Leake JA, et al. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics. 2006;118:e979–84. doi: 10.1542/peds.2006-0281. [DOI] [PubMed] [Google Scholar]
- 34.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Four-month-old female with gangrene of the feet and hands due to meningococcemia. http://www.vaccineinformation.org/menin/photos.asp . Accessed 1 August 2011. [Google Scholar]
- 36.MacNeil J. Epidemiology of meningococcal disease in infants and young children. Meeting of the Advisory Committee on Immunization Practices. 21–22 October 2009. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/min-archive/min-oct09.pdf . 91–94. [Google Scholar]
- 37.Granoff DM, Gilsdorf JR. Neisseria meningitidis (meningococcus) In: Kliegman RM, Stanton BF, Geme JS, Schor NF, Behrman RE, editors. Nelson Textbook of Pediatrics. 19th. Philadelphia: WB: Saunders Co; 2011. pp. 929–35. [Google Scholar]
- 38.Lowe KG, Boyce JM. Rehabilitation of a child with meningococcal septicemia and quadrilateral limb loss: a case report. Arch Phys Med Rehabil. 2004;85:1354–57. doi: 10.1016/j.apmr.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Allport T, Read L, Nadel S, Levin M. Critical illness and amputation in meningococcal septicemia: is life worth saving? Pediatrics. 2008;122:629–32. doi: 10.1542/peds.2007-2355. [DOI] [PubMed] [Google Scholar]
- 40.Lucaya J, Garel L, Piqueras J. Sequelae of meningococcal sepsis. Pediatr Radiol. 2004;34:441–2. doi: 10.1007/s00247-003-0995-5. [DOI] [PubMed] [Google Scholar]
- 41.Bedford H, de Louvois J, Halket S, Peckham C, Hurley R, Harvey D. Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ. 2001;323:533–6. doi: 10.1136/bmj.323.7312.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson L, De Wals P. Complications and sequelae of meningococcal disease in Quebec, Canada, 1990–1994. Clin Infect Dis 1998. 26:1159–64. doi: 10.1086/520303. [DOI] [PubMed] [Google Scholar]
- 43.Judge D, Nadel S, Vergnaud S, Garralda ME. Psychiatric adjustment following meningococcal disease treated on a PICU. Intensive Care Med. 2002;28:648–50. doi: 10.1007/s00134-002-1237-2. [DOI] [PubMed] [Google Scholar]
- 44.Ehrlich TR, von Rosenstiel IA, Grootenhuis MA, Gerrits AI, Bos AP. Long-term psychological distress in parents of child survivors of severe meningococcal disease. Pediatr Rehabil. 2005;8:220–4. doi: 10.1080/13638490400022246. [DOI] [PubMed] [Google Scholar]
- 45.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;(27Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 46.Toropainen M, Vainio A, Saarinen L, Kuusi M, Kayhty H, Virolainen A. Vol. 51. Ljubljana, Slovenia: The European Meningococcal Disease Society; 2011. Epidemiology of invasive meningococcal disease in Finland, 1995–2010. In: EMGM 2011 abstract book [abstract P009] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC) Active bacterial core surveillance report, emerging infections program network, Neisseria meningitidis. 2009 http://www.cdc.gov/abcs/reports-findings/surv-reports.html . Accessed 31 July 2011. [Google Scholar]
- 48.Jackson LA, Wenger JD. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989–1991. MMWR Surveill Summ. 1993;42:21–30. [PubMed] [Google Scholar]
- 49.Harrison LH, Shutt KA, Schmink SE, et al. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre–meningococcal conjugate vaccine era—United States, 2000–2005. J Infect Dis. 2010;201:1208–24. doi: 10.1086/651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Memish ZA, Goubeaud A, Bröker M, Malerczyk C, Shibl AM. Invasive meningococcal disease and travel. J Infect Public Health. 2010;3:143–51. doi: 10.1016/j.jiph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 51.McMullan B. An infant with meningococcal arthritis of the hip. J Paediatr Child Health. 2009;45:762–3. doi: 10.1111/j.1440-1754.2009.01607.x. [DOI] [PubMed] [Google Scholar]
- 52.Bolt P, Britto J, Nadel S, Levin M. Meningococcal disease due to W135: fresh public health concerns. Arch Dis Child. 2001;84:90–1. doi: 10.1136/adc.84.1.89e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menactra [prescribing information] Swiftwater, PA: Sanofi Pasteur, Inc; 2011. [Google Scholar]
- 54.Menveo [prescribing information] Cambridge, MA: Novartis Vaccines and Diagnostics, Inc; 2011. [Google Scholar]
- 55.Menomune [prescribing information] Swiftwater, PA: Sanofi Pasteur, Inc; 2009. [Google Scholar]
- 56.Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186–93. doi: 10.1097/INF.0b013e31818e037d. [DOI] [PubMed] [Google Scholar]
- 57.Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299:173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 58.Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H. influenzae type b–N. meningitidis C/Y conjugate vaccine in infants. Pediatrics. 2011;127 doi: 10.1542/peds.2009-2992. [DOI] [PubMed] [Google Scholar]
- 59.Marchant CD, Miller JM, Marshall GS, et al. HibMenCY-TT 005 Study Group. Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatr Infect Dis J. 2010;29:48–52. doi: 10.1097/INF.0b013e3181c3ce88. [DOI] [PubMed] [Google Scholar]
- 60.Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469–71. doi: 10.1097/INF.0b013e3181cdd379. [DOI] [PubMed] [Google Scholar]
- 61.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toneatto D, Oster P, Deboer AC, et al. Early clinical experience with a candidate meningococcal B recombinant vaccine (rMenB) in healthy adults. Hum Vaccin. 2011;7:781–91. doi: 10.4161/hv.7.7.15997. [DOI] [PubMed] [Google Scholar]