Abstract
Objective.
Invasive aspergillosis (IA) can cause significant morbidity and mortality in immunocompromised children. The galactomannan (GM) enzyme immunoassay (EIA) has been shown in adult studies to be a useful adjunct in diagnosing IA. Data on this assay in children are limited by small sample sizes and conflicting results; false-positive assays were a concern in historical studies. We sought to evaluate the GM EIA in a large cohort of children who received intensive chemotherapy and/or hematopoietic stem cell transplant. A focus was placed on evaluating the assay specificity, and the potential of measuring GM antigen in urine.
Methods.
A multicenter prospective observational study in children with anticipated prolonged neutropenia was performed. Serum specimens were collected twice weekly, and urine was collected once weekly during neutropenic periods. Operating characteristics were calculated using the GM EIA optical density index cutoffs of 0.5 and 1.0 for both serum and urine specimens.
Results.
At least one serum or urine specimen was tested from 198 patients. Ten patients had one or more repeatedly positive serum specimens, while 37 patients had one or more repeatedly positive urine specimens. The specificity of serum and urine testing was 95% and 80%, respectively. Although the urine test resulted in a higher false positivity rate, it successfully identified the only case of probable IA.
Conclusions.
Data suggest that the serum GM EIA does not provide frequent false-positive results as previously reported. Screening for galactomannan, or a related antigen in urine, needs to be further evaluated as it may be amenable to development of surveillance strategies.
(See the Editorial Commentary by Lehrnbecher and Groll, on pages 112–5.)
Invasive fungal infections (IFI) are a major cause of morbidity and mortality amongst pediatric hematopoietic stem cell transplant (HSCT) recipients and in some pediatric oncology patients receiving intensive chemotherapy. The frequency and severity of such infections in immunocompromised patients has risen steadily over the past few decades [1–4]. Specifically, infections with Aspergillus spp. account for the vast majority of non-Candida IFI in children with cancer and result in a devastatingly high case fatality rate. In the Children's Cancer Group (CCG) Phase III AML chemotherapy trial CCG 2961, the incidence of IFI in children with AML was 13% per treatment phase. Nearly one-third of the documented IFI were caused by Aspergillus and the case fatality rate of invasive aspergillosis (IA) ranged from 15% to 57%, depending on the phase of chemotherapy. Importantly, 31% of all infection-related deaths in this trial were directly attributable to IA [5].
A major contributing factor for the dismal outcomes of IA is the lack of a diagnostic test that can be used to accurately identify IA in a timely manner. The traditional approach to diagnosing IA includes a combination of clinical signs (prolonged fever in the setting of neutropenia), radiographic evidence (eg, “halo sign” on chest computed tomography [CT] scan), and attempts to isolate the mold by tissue procurement via invasive procedures. These modalities are either invasive, nonspecific, or lack sensitivity.
The advent of biomarker tests such as the galactomannan (GM) enzyme immunoassay (EIA) offers a potential adjunct for noninvasive diagnosis of IA. GM is a polysaccharide component of the Aspergillus cell wall that is released from growing Aspergillus hyphae. The Platelia Aspergillus enzyme immunoassay is a commercially available kit from Bio-Rad Laboratories that utilizes the EB-A2 monoclonal antibody in a double-sandwich assay for detection of GM. Results of the test are interpreted based on the optical density (OD) ratio of the sample divided by a threshold control provided in the kit, referred to as the OD index. The test received FDA clearance for use in adult immunocompromised patients using a low index to define positivity, after results of a prospective study found that an OD index of 0.5 resulted in improved sensitivity and earlier indication of infection compared with prior recommendations (OD index 1.0) [6].
There have been limited pediatric studies focused on use of the GM EIA. Earlier studies evaluating GM EIA in adults and children suggested much higher false-positive rates (up to 44%) in children as compared to adults [7]. Pediatric-focused studies revealed much lower false-positive rates; however, each of these studies is limited by small sample sizes and/or a lack of a standardized definition for IA [8–12]. We performed a prospective study in a large cohort of pediatric patients with leukemia or solid tumors who had received intensive chemotherapy or undergone HSCT to evaluate the utility of using the GM EIA for the prospective monitoring of IA. A focus was placed on evaluating the specificity and false-positive rate of the serum assay, and the potential utility of evaluating antigen levels in a noninvasive compartment (urine).
METHODS
Study Design
We performed a multicenter prospective observational study to define the operating characteristics of serum and urine specimen GM EIA testing for identifying IA in children less than 19 years of age with anticipated prolonged neutropenia secondary to chemotherapy or a conditioning regimen for HSCT.
Patient Population
The following 4 patient types were considered for cohort inclusion: (1) patients receiving chemotherapy for leukemia with anticipated neutropenia (ANC <1000/µL) for a minimum of 3 weeks, or 2 weeks if the chemotherapy regimen included concurrent steroids; (2) patients receiving repetitive cycles of intensive chemotherapy supported with autologous peripheral blood stem cells for the treatment of solid tumors; (3) patients receiving intensive chemotherapy for a solid tumor with an anticipated period of neutropenia of at least 3 weeks or at least 1 week if hospitalization for mucositis or gastrointestinal toxicity was anticipated; (4) patients undergoing myeloablative allogeneic or autologous bone marrow, cord blood, or peripheral blood stem cell transplantation for treatment of malignant or nonmalignant conditions. Patients were only eligible to participate during one neutropenic period following chemotherapy, but were eligible for sequential enrollment following HSCT.
The following exclusion criteria were used: weight less than 5 kg at time of study enrollment, weekly requirement of packed RBC transfusions in excess of 100 mL/kg, documented proven or probable IA within 3 months of study enrollment, or anticipation that the patient would be unavailable for the 30- and 60-day follow-up contact.
Specimen Collection, Processing, and Analysis
For patients receiving intensive chemotherapy without HSCT, baseline blood and urine specimens were obtained at the time of admission for chemotherapy. Upon the onset of neutropenia (ANC <1000/µL), blood was collected twice weekly, and first morning urine was collected once weekly until neutrophil recovery. This schedule was continued until the neutrophil count (ANC ≥1000/µL) had been recovered for one week. For those patients undergoing an HSCT, baseline blood and urine specimens were collected at the onset of conditioning therapy. Upon the onset of neutropenia (ANC <1000/µL), blood was collected twice weekly, and first morning urine was collected once weekly until the patient was discharged from the inpatient service. Those patients determined to have IA were scheduled to have both serum and urine samples collected until there were clinical signs and/or radiographic evidence of improvement. In the event that a bronchoalveolar lavage (BAL) was performed for clinical purposes, residual BAL specimen, when available, was tested.
The volume and frequency of blood draws was limited by the weight of the child. Total blood draw volume could not exceed 3 mL/kg in an 8-week period and a single blood draw could not exceed 2.5% of the child's total blood volume. Specifically, blood was drawn weekly in children weighing less than 10 kg, and for those between 10 and 16 kg, blood draws were limited to a maximum of 10 over an 8-week period.
All specimens (blood, urine, and BAL) were frozen at –70°C in the clinical laboratories of the participating study sites and were batch-shipped to the Fred Hutchinson Cancer Research Center. Results did not factor into the care of the patients. At the time of testing, specimens were thawed at room temperature and GM EIA was performed according to manufacturer-specified directions [13]. If a sample resulted with an OD index ≥ 0.5, the same sample was retested for confirmation.
Definitions
Cases of IA were defined by criteria as established by the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) 2002 criteria [14]. Clinical, radiographic, and laboratory data were collected prospectively by clinical research associates. These data were reviewed for all patients with suspected IA by study investigators and labeled as proven, probable, possible, or no IA. Study investigators were blinded to GM EIA test results; hence, study GM EIA results did not contribute to clinical care. For patients receiving chemotherapy for malignancy, the time period during which an IA diagnosis was considered included the entirety of the neutropenic period as well as two weeks after recovery of neutropenia (ANC >1000/µL). For HSCT patients, the time period for evaluation of IA was the entire inpatient admission for the transplant as well as two weeks after discharge.
Data Collection
Patients were followed prospectively after enrollment to record demographic characteristics, treatment characteristics, receipt of antifungal medications, presence of neutropenia, GI tract mucositis (and severity), and diagnostic criteria for IFI. Two follow-up visits were performed at monthly intervals after enrollment.
Statistical Analysis
Summary statistics to describe demographic characteristics were constructed using frequencies and proportions for categorical data elements and medians for continuous variables. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using STATA statistical software version 10.0 (College Station, TX). Initial calculations assumed presence of IA if a patient was given a probable or proven designation. Subsequent calculations included possible IA designation as a true case. Because the GM OD index of 0.5 is established as the threshold for positive in adults, it is not clear that the kinetics for this assay will act in a similar fashion for children. Therefore, in an exploratory analysis, the operating characteristics for both the urine and serum testing were calculated using GM OD index cutoffs of 1.0 and 1.5. Additionally, it was not clear that performing confirmation testing of all specimens with an initial test that was positive resulted in improved operating characteristics. Thus, the sensitivity, specificity, PPV, and NPV were calculated for each of the three GM OD index cutoffs but without the requirement of a confirmatory positive assay result.
Human Subjects Oversight
The conduct of this study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center, Seattle, WA, and by each enrolling study site (Cincinnati Children's Hospital Medical Center, Seattle Children's Hospital, Children's Hospital Boston/Dana-Farber Cancer Institute, and Johns Hopkins Hospital).
RESULTS
From May 2004 through July 2007, 215 patients who met enrollment criteria were approached for study enrollment. Two hundred thirteen were enrolled at 5 different pediatric centers, with 198 contributing at least one urine or serum specimen. Table 1 displays the demographic characteristics of the entire study cohort. The median age of patients was 7.8 years, males accounted for 61% of participants, and most patients were Caucasian (81%). Most patients (85%) had received conditioning chemotherapy for HSCT.
Table 1.
Demographic and Characteristics for 198 Enrolled Patients
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 121 (61) |
| Female | 77 (39) |
| Race | |
| Caucasian | 161 (82) |
| African American | 15 (8) |
| Othera | 21 (10) |
| Ethnicity | |
| Hispanic | 19 (10) |
| Non-Hispanic | 177 (89) |
| Unknown | 2 (1) |
| Age, Median (IQR) | 7.8 (3.7–13.5) |
| HSCT, with underlying diagnosis | |
| Total | 169 (85) |
| HSCT for ALL | 36 (18) |
| HSCT for AML/MDS | 39 (20) |
| HSCT for solid tumor | 30 (15) |
| HSCT, otherb | 64 (32) |
| Chemotherapy, with underlying diagnosis | |
| Total | 29 (15) |
| ALL | 6 (3) |
| AML/MDS | 17 (9) |
| Solid tumor | 6 (3) |
| Duration neutropeniac | |
| Mean | 24.8 |
| Median | 20 |
| Range | 3–343 |
| Antifungal prophylaxisc | |
| Any prophylaxis | 185 |
| Fluconazole | 143 (77) |
| Voriconazole | 15 (8) |
| Caspofungin | 7 (4) |
| Amphotericin product | 20 (11) |
| Chemotherapy contained steroid exposured | 65 (37) |
| TBI exposuree | 83 (47) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML/MDS, acute myeloid leukemia/ myelodysplastic syndrome; HSCT, hematopoietic stem cell transplant; IQR, interquartile range; TBI, traumatic brain injury.
aIncludes Asian (n = 4), Native American (n = 6), Pacific Islander (n = 2), other (n = 7), and unknown (n = 2).
bThe most common other diagnoses included severe aplastic anemia (n = 11), chronic myelogenous leukemia (n = 7), Hodgkin's lymphoma (n = 7), Wiskott-Aldrich syndrome (n = 4), Fanconi anemia (n = 4).
cData available for 185 of the 198 enrolled patients.
dData available for 178 of the 198 enrolled patients.
eData available for 176 of the 198 enrolled patients.
In total, 1865 serum specimens were collected from 198 patients, 886 urine specimens were collected from 183 patients, and 7 BAL specimens were collected from 4 patients. The initial assay was positive on 146 serum specimens, but only 54 of these specimens were positive on confirmatory testing. These 54 positive specimens were isolated from 10 patients. Likewise, the initial urine assay was positive on 139 specimens, but only 84 of these were positive on confirmatory testing. These 84 positive specimens were obtained from 37 patients. Only one BAL specimen was positive. During the follow-up period, no patient was assigned the diagnosis of proven or probable IA, and 24 patients were considered to have possible IA. Retrospectively, 1 patient was identified as having probable IA when study-derived GM EIA results considered in establishing the presence or absence of IA. For subsequent analyses, this patient was consider to have had probable IA.
Table 2 displays the operating characteristics of the serum and urine GM EIA for detecting probable IA only, or probable or possible IA together, using OD index threshold cutoffs of 0.5 and 1.0, respectively. In these data, the samples were considered positive only if a confirmatory test replicated the initial positive result. The serum testing did not correctly identify the single case of probable IA, and thus had 0% sensitivity, but it maintained a high specificity and NPV using both threshold cutoffs. The urine testing successfully identified the single probable IA case, resulting in 100% sensitivity and thus maintained a 100% NPV. Neither serum nor urine testing provided high PPV results, as would be expected given the low incidence of disease. The false-positive rate (calculated as proportion of patients with a positive test result per total number of patients without IA) for serum and urine sample testing when considering an OD index threshold cutoff of 0.5 was 5.2% and 20.2%, respectively. The operating characteristics when using an OD index of 1.5 as the cutoff are not shown, as they were equivalent to those calculated using a cutoff of 1.0 for both serum and urine testing.
Table 2.
Performance Characteristics of Serum and Urine Galactomannan Enzyme Immunoassay Test, Requiring Confirmationa
| Performance Characteristic |
Sensitivity, % | Specificity, % | PPV, % | NPV, % | False-Positive Rate, % | |
|---|---|---|---|---|---|---|
| Serum GM (threshold 0.5) | Probable IAb | 0 | 95 | 0 | 99 | 5.2 |
| Possible and Probable IA | 13 | 96 | 30 | 89 | 4.1 | |
| Serum GM (threshold 1.0) | Probable IAb | 0 | 96 | 0 | 99 | 3.6 |
| Possible and Probable IA | 13 | 98 | 43 | 89 | 2.3 | |
| Urine GM (threshold 0.5) | Probable IAb | 100 | 80 | 2.7 | 100 | 20.2 |
| Possible and Probable IA | 19 | 79 | 11 | 88 | 20.9 | |
| Urine GM (threshold 1.0) | Probable IAb | 100 | 83 | 3.2 | 100 | 16.9 |
| Possible and Probable IA | 14 | 82 | 9.6 | 88 | 17.7 | |
Abbreviations: GM, galactomannan; IA, invasive aspergillosis; NPV, negative predictive value; PPV, positive predictive value.
aA specimen was considered positive if the test result exceeded the indicated threshold, in duplicate.
bCalculated using one case meeting criteria for probable IA.
The operating characteristics were recalculated for each of the OD index thresholds without the restriction that all initially positive specimens required confirmation by repeat testing. This approach effected only a minimal change in the sensitivity, PPV, and NPV. There did appear to be a moderate reduction in specificity with an associated substantial increase in false positivity rate across each of the three OD index thresholds.
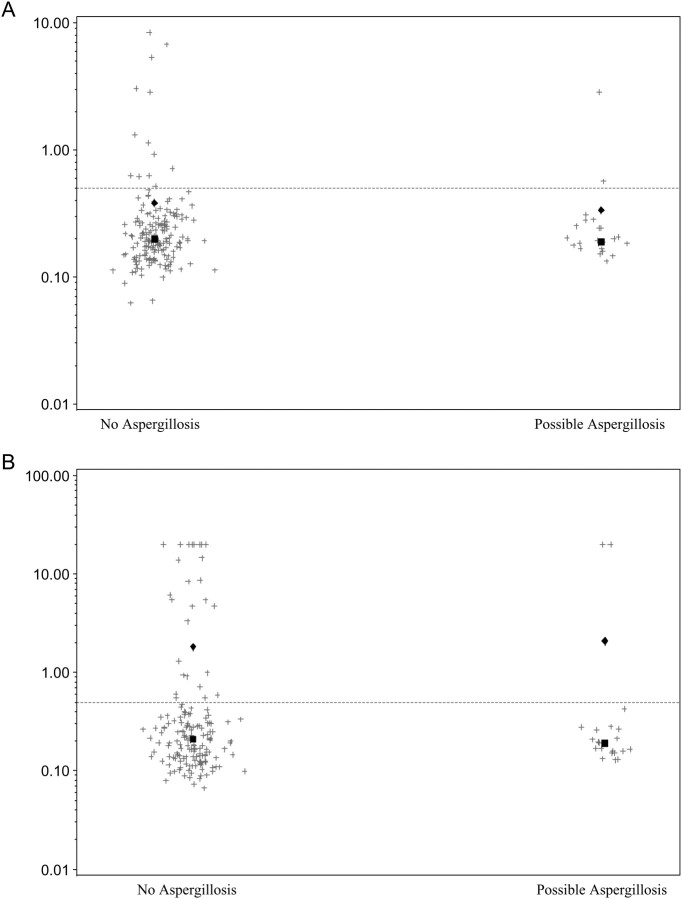
The operating characteristics are less impressive when possible cases are considered as having “true disease,” resulting in a decrease in NPV. As there is considerable diagnostic uncertainty in the category of “possible IA,” we plotted serum and urine GM EIA values among patients with no evidence of disease compared with “possible” IA. Results showed a lack of quantitative differences between the two groups, with results consistently low in samples from patients with no evidence of IA and possible IA (Figure 1).
Figure 1.
Results (optical density [OD] indices) of (A) serum and (B) urine testing in patients with “no” or “possible” invasive aspergillosis. The diamond represents the median for each group and the square represents the mean. Line is drawn at current OD index cutoff to define positivity.
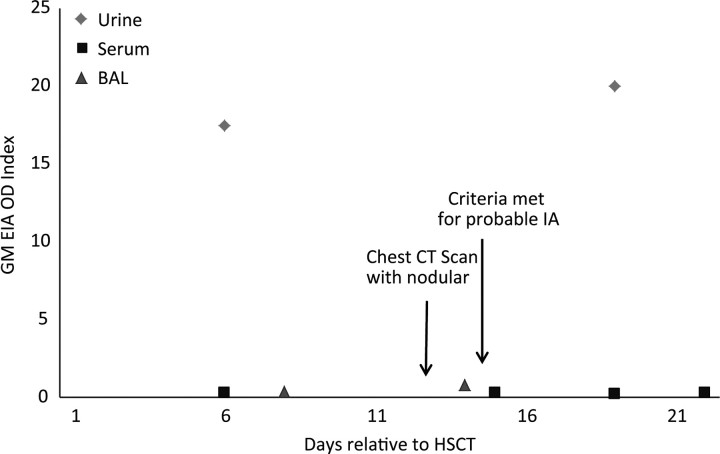
The probable IA diagnosis was illustrative. This patient was a 13-month-old male who had received an autologous transplant for a solid tumor. As noted previously, the designation of probable IA was applied in retrospect and based on the presence of bilateral pulmonary nodules identified on a chest CT scan, in conjunction with a positive study GM EIA result from a stored BAL specimen. Figure 2 illustrates the clinical, radiographic, and laboratory findings for this patient from the time of enrollment until the time of probable IA. Although all serum tests remained negative, two urine samples for this patient had high positive GM EIA values, consistent with the BAL-based designation of probable infection.
Figure 2.
Results of serial testing of urine, serum, and bronchoalveolar lavage specimens in one patient with probable. Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; IA, invasive aspergillosis.
DISCUSSION
This study provides important data to evaluate the operating characteristics of the serum GM EIA in a large prospective observational pediatric cohort at risk for IA, and the only study to date to evaluate the urine GM EIA in this population. Although false-positive results were seen, our data demonstrated lower false-positive rates of serum GM EIA in neutropenic children. Only one subject developed probable/proven IA (on retrospective analysis), limiting our ability to present conclusions on sensitivity of the serum assay. Interestingly, testing GM EIA in urine may offer an alternative specimen source for noninvasive diagnostic aid and/or screening tool for IA.
A prior study evaluating the GM EIA in a combined cohort of adult and pediatric febrile and neutropenic patients suggested that the false positivity rate was much higher in children than in adults [7]. Several pediatric-focused studies have since shown that the false-positive rate for the GM EIA is not as high as once believed. The results of these studies are summarized in Table 3, with our data included for comparison [8–12, 15]. However, small numbers and/or a lack of a standardized definition for IA limited conclusions from these prior publications. The present study was the largest to evaluate specificity and false-positive rates (using the EORTC/MSG criteria) in a pediatric population, and results support the conclusion that false-positive assays occur, but at a lower rate than previously reported, even when applying the low OD index of 0.5. It is also important to note that certain positive results may be misclassified as “false positive,” as the incidence of biologically real antigenemia in the absence of disease has not been determined. Specifically, some of these patients may have had transient antigenemia without development of subsequent clinical disease. Also, other factors may have contributed to development of falsely positive assays, such as receipt of contaminated β-lactam antibiotics. These data were not captured, as this study was initiated prior to knowledge of the reported association.
Table 3.
Operating Characteristics of Galactomannan Testing for Diagnosis of Proven or Probable Aspergillosisa in Pediatric Patients
| Author Year | Total No. Patients/Episodes in Study | Proven or Probable Aspergillosis | Sensitivity, % | Specificity, % | TP | FP | TN | FN | False-Positive Rate, %b |
|---|---|---|---|---|---|---|---|---|---|
| Hayden 2008 [15]c | 56 | 17 | 65 | 87 | 11 | 5 | 34 | 6 | 12.8 |
| Steinbach 2007 [10] | 64 | 1 | 0 | 87 | 0 | 8 | 55 | 1 | 12.7d |
| Hovi 2007 [8] | 89 | 1 | 100 | 93 | 1 | 6 | 82 | 0 | 6.8 |
| Sulahian 2001 [11] | 347 | 9 | 100 | 89.9 | 9 | 34 | 304 | 0 | 10.1 |
| Rohrlich 1996 [9] | 37 | 10 | 100 | 92.6 | 10 | 2 | 25 | 0 | 7.4 |
| Armenian 2009 [12] | 78 | 3e | 100 | 98.7 | 3e | 1 | 74 | 0 | 1.3 |
| Our Study | |||||||||
| Serum (threshold 0.5) | 195 | 1 | 0 | 95 | 0 | 10 | 184 | 1 | 5.2 |
| Urine (threshold 0.5) | 179 | 1 | 100 | 80 | 1 | 36 | 142 | 0 | 20.2 |
Abbreviations: FP, false positive; FN, false negative; TN, true negative; TP, true positive.
aThe definition of proven or probable aspergillosis varied depending on the publication.
bDefined as the number of false-positive results per total number of patients without IA.
cCase-control study design may inflate the calculated false-positive rate for this study.
dAfter accounting for piperacillin-tazobactam exposure, the false-positive rate was 8.5%.
eAll 3 probable cases required inclusion of positive GM EIA results to qualify for this designation.
The incidence of probable or proven IA in this cohort was lower (0.5%) than anticipated. This may in part be because over 20% of enrolled patients were receiving antifungal prophylaxis that included anti-Aspergillus coverage at some point in time during the study period. Additionally, a large proportion (85%) of the study cohort received an HSCT; we evaluated samples predominantly during the pre-engraftment period in order to evaluate specificity during periods of GI tract mucositis. As such, this cohort did not contain the HSCT patients who were at highest risk of IA during periods of graft versus host disease [16].
Although the EORTC/MSG criteria for defining IA include the finding of a positive BAL GM EIA, the uptake of BAL GM EIA testing in clinical application has been variable. Our one case of retrospectively defined probable IA is illustrative, as the BAL performed in this patient did not reveal an etiology of disease, but the study GM EIA was positive. It is notable that the serum GM EIA failed to identify this single probable case, which resulted in 0% sensitivity. The urine GM EIA successfully identified the case and was positive 1 week before a chest CT scan identified pulmonary nodules consistent with pulmonary aspergillosis. As would be expected in the setting of low incidence, both tests had poor PPV and high NPV. Prior published discussions have highlighted the issue that diagnostic tests applied to cohorts with low disease prevalence will have resultant low PPV even if the diagnostic test has reasonable sensitivity and specificity parameters [17].
Our results suggest that further investigation of EIA GM testing of urine specimens is warranted. Galactomannan, or a similar polysaccharide recognized by the EB-A2 antibody, is known to be present in urine from infected animals and humans. Animal studies have shown that the kidneys excrete at least some of the polysaccharide rapidly after IV infusion in rabbits [18]. A small number of samples have been evaluated from adult patients, and results suggest that the test may be useful, especially if the urine is pretreated by dialysis, centrifugation or filtration [19]. However, we do not yet know what specific antigen the antibody is recognizing in the urine nor do we know the optimal approach for processing urine specimens. We did not process our urine samples before testing; nonetheless, our results suggest that the GM EIA may be amenable to serial urine testing. This is especially attractive given the potential for development of a test that can be applied in a point-of-care fashion. Certainly, to be useful, the testing approach will have to be improved to reduce the false-positive rate while preserving the sensitivity.
The primary analysis included repeat testing of a first positive result. The importance of requiring this duplicate testing is controversial. Interestingly, when the operating characteristics of the GM EIA were calculated using the first specimen result only, sensitivity, PPV, and NPV remained stable but there was a moderate reduction in specificity. The increased cost for confirmatory testing needs to be balanced against the intended goals for testing. If the hope is to optimize sensitivity, then duplicate testing may not be appropriate. However, if the goal is to reduce false-positive rates (and thus reduce clinical interventions), then duplicate testing should be considered.
When considering the utility of the GM EIA, one can entertain multiple possible approaches for interpreting the results relative to clinical care decisions. The first is to initiate antifungal therapy with anti-Aspergillus coverage in the setting of 1 GM EIA positive specimen found during serial testing. In our cohort, using this approach with the serum GM EIA would have resulted in 10 (5%) patients being treated unnecessarily (false positives) and 1 patient not receiving appropriate therapy (false negative). Given the severity of the disease, clinicians may be willing to tolerate unnecessary therapy in 5% of patients but would be wary of missing even one case of IA. Interestingly, the urine GM EIA results would not have missed the one patient with probable IA but would have resulted in the unnecessary treatment of 36 patients. When the threshold cutoff for the urine test was increased to 1.0, there were still no false negatives and there was a reduction to 30 false positives. Whether these patients with false-positive results would have developed IA during longer-term follow up is not clear. As urine is an easy sample to obtain for testing, serial monitoring with urine should be further explored.
A second approach would argue for using the GM EIA to guide decisions for de-escalation of therapy, as the test maintains a high NPV for both serum and urine specimens. In this setting, the clinician can be reassured by serially negative results and may elect to de-escalate previously initiated broad-spectrum antifungal coverage. In applying this scenario to our study population, the challenge still exists that one would not be able to identify the 1 false-negative serum result among all the true negative results.
Instead of using the GM EIA test in isolation to diagnose IA, it may be most appropriate to use these results as an adjunct with other clinical and radiographic results to gauge the probability of IA. In an adult population, Cordonnier et al [20] compared the initiation of empiric antifungal therapy after persistent fever with that of a preemptive approach guided by the presence of various clinical, radiographic, and laboratory findings. They found that there was no difference in mortality secondary to IFI, but a reduction in antifungal cost among those treated preemptively was shown. The recently updated Infectious Diseases Society of America's adult fever and neutropenia guidelines proposed a similar preemptive strategy as an alternative to the traditional empiric approach of adding antifungal therapy in the setting of prolonged fever and neutropenia [21]. This preemptive strategy proposes that clinicians do not need to add antifungal therapy if antigen testing, chest and sinus CT imaging, and physical exam do not suggest the presence of fungal infection. A similar approach in children may be useful, but randomized trials should address this question in both children and adults.
It is important to note that when patients with possible IA were included in the analysis as “true disease,” the operating characteristics of both the urine and serum sample EIA were poor. This highlights the difficulty of studying a disease that lacks a good diagnostic gold standard. In comparing the GM EIA serum and urine testing in Figure 1, one can see that the results were similar between those without IA and those with possible IA, raising the likelihood that those patients designated as possible IA did not actually have IA.
In summary, GM EIA testing may be useful in diagnosing and/or excluding IA in at-risk pediatric patients, but the low (0.5%) incidence rate of probable or proven IA in this study precludes the ability to make any definitive conclusions. Future work should focus on defining the operating characteristics of this assay in specific subpopulations as they may vary. Subsequently, there should be a focus on developing predictive models that would include but not be limited to GM EIA results for defining the probability of IA. Finally, further investigation of urine GM EIA testing is warranted, as it may offer a mechanism for noninvasive IA screening in the future.
Acknowledgments
We thank Dr Arunmohzi Balajee for technical assistance.
Financial support. This work was supported by the National Institutes of Health (NIH; grant U01 AI054736), with partial support for test kits provided by Bio-Rad Laboratories.
Potential conflicts of interest. Dr. Marr reports a pending patent involving urine testing for antigens to aid diagnosis of aspergillosis. All other authors report no potential conflicts.
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 2.Groll AH, Kurz M, Schneider W, et al. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999;42:431–42. doi: 10.1046/j.1439-0507.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis. 2001;33:641–7. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 5.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–9. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 6.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004;190:641–9. doi: 10.1086/422009. [DOI] [PubMed] [Google Scholar]
- 7.Herbrecht R, Letscher-Bru V, Oprea C, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J Clin Oncol. 2002;20:1898–906. doi: 10.1200/JCO.2002.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Hovi L, Saxen H, Saarinen-Pihkala UM, Vettenranta K, Meri T, Richardson M. Prevention and monitoring of invasive fungal infections in pediatric patients with cancer and hematologic disorders. Pediatr Blood Cancer. 2007;48:28–34. doi: 10.1002/pbc.20717. [DOI] [PubMed] [Google Scholar]
- 9.Rohrlich P, Sarfati J, Mariani P, et al. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr Infect Dis J. 1996;15:232–7. doi: 10.1097/00006454-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach WJ, Addison RM, McLaughlin L, et al. Prospective Aspergillus galactomannan antigen testing in pediatric hematopoietic stem cell transplant recipients. Pediatr Infect Dis J. 2007;26:558–64. doi: 10.1097/INF.0b013e3180616cbb. [DOI] [PubMed] [Google Scholar]
- 11.Sulahian A, Boutboul F, Ribaud P, Leblanc T, Lacroix C, Derouin F. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer. 2001;91:311–8. doi: 10.1002/1097-0142(20010115)91:2<311::aid-cncr1003>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Armenian SH, Nash KA, Kapoor N, et al. Prospective monitoring for invasive aspergillosis using galactomannan and polymerase chain reaction in high risk pediatric patients. J Pediatr Hematol Oncol. 2009;31:920–6. doi: 10.1097/MPH.0b013e3181b83e77. [DOI] [PubMed] [Google Scholar]
- 13.Package insert. Hercules, CA: Bio-Rad Laboratories; Platelia Aspergillus EIA. [Google Scholar]
- 14.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 15.Hayden R, Pounds S, Knapp K, et al. Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis. Pediatr Infect Dis J. 2008;27:815–9. doi: 10.1097/INF.0b013e31817197ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–66. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 17.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–9. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 18.Bennett JE, Friedman MM, Dupont B. Receptor-mediated clearance of Aspergillus galactomannan. J Infect Dis. 1987;155:1005–10. doi: 10.1093/infdis/155.5.1005. [DOI] [PubMed] [Google Scholar]
- 19.Klont RR, Mennink-Kersten MA, Verweij PE. Utility of Aspergillus antigen detection in specimens other than serum specimens. Clin Infect Dis. 2004;39:1467–74. doi: 10.1086/425317. [DOI] [PubMed] [Google Scholar]
- 20.Cordonnier C, Pautas C, Maury S, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48:1042–51. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 21.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–31. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]