Abstract
Background.
Infant laboratory abnormalities have been associated with exposure to antiretrovirals and to trimethoprim/sulfamethoxazole (TMP/SMX).
Methods.
We analyzed data from International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Protocol P1025, a prospective cohort study of human immunodeficiency virus type 1 (HIV)–infected women and their infants. Live-born, singleton, HIV-uninfected infants with at least 6 months of follow-up who represented the first pregnancy on study of HIV-infected mothers with at least 1 prenatal visit, CD4 count, and viral load during pregnancy and who used at least 1 antiretroviral during pregnancy were eligible for inclusion in this analysis.
Results.
The study population comprised 1524 infants. During the first 6 months of life, 7.4% of laboratory serious adverse events (SAEs) were related to glucose, 7.2% were related to hemoglobin, 8.7% were related to absolute neutrophil count, and 4.0% were related to total lymphocyte count. The likelihood of laboratory SAEs decreased with increasing age for hemoglobin, absolute neutrophil count, and glucose. Infant preterm birth and current receipt of antiretroviral(s) were the factors with the strongest associations with laboratory SAEs.
Conclusions.
The overall frequency of laboratory SAEs was low and decreased with age. Preterm infants are at higher risk of hemoglobin- and total lymphocyte count–related SAEs.
Use of antiretrovirals during pregnancy is recommended, whether for treatment of the woman's human immunodeficiency virus type 1 (HIV) infection or for prevention of mother-to-child transmission of HIV [1]. Irrespective of an HIV-infected woman's use of antiretrovirals during pregnancy, all infants of HIV-infected women should receive antiretroviral prophylaxis during the first few weeks of life, beginning at birth [1]. In addition, trimethoprim/sulfamethoxazole (TMP/SMX) for opportunistic infection prophylaxis has been recommended for infants of unknown HIV infection status from 6 weeks of age until HIV infection in the infant is ruled out [2]. However, infant laboratory abnormalities have been associated with in utero exposure to antiretrovirals. Other potential adverse events related to in utero exposure to antiretrovirals include congenital anomalies [3–7], malignancies [8–10], mitochondrial toxicity [11–19], and preterm birth [20–32]. In addition, infant laboratory abnormalities have been associated with infant receipt of TMP/SMX [33]. Laboratory abnormalities associated with in utero or early postnatal exposure to antiretrovirals include anemia, and newborns with in utero or early postnatal exposure to zidovudine should have a hematological evaluation [1]. Based on studies suggesting other laboratory abnormalities among infants with in utero or early postnatal exposure to antiretrovirals [11, 34–40], some experts recommend more extensive laboratory assessments, such as a complete blood count with differential and hepatic transaminase assays. Use of TMP/SMX is associated with bone marrow suppression, especially neutropenia, and severe liver damage [33]. The objectives of this analysis are to describe laboratory abnormalities among eligible HIV-exposed but uninfected infants born to HIV-infected women enrolled in International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Protocol P1025 and to identify factors associated with such laboratory abnormalities, including in utero/early postnatal exposure to antiretrovirals and infant exposure to TMP/SMX.
METHODS
IMPAACT Protocol P1025
IMPAACT Protocol P1025 is a prospective cohort study of HIV-infected women and their infants at multiple clinical sites in the United States and Puerto Rico. Enrollment began in 2002 and is ongoing. Mothers were enrolled during pregnancy (≥8 weeks gestation) or within 2 weeks after delivery. Follow-up on study continued for at least 6 months after delivery/birth. Some infants enrolled in P1025 continued to be followed in the Pediatric AIDS Clinical Trials protocol 219C after completion of their participation in protocol P1025, and HIV diagnostic testing data from 219C were included for these infants when they were available. The primary objectives of P1025 are to assess the effectiveness and safety of interventions for the prevention of mother-to-child transmission of HIV and of adherence to antiretrovirals by enrolled women and infants. All women enrolled in the study provided written informed consent for themselves and their infants.
Study visits for infants are scheduled at birth, at 2 and 6 weeks of age, and at 4, 6, 9, and 12 months of age. During study visits, physical examinations were performed (except at the 2-week visit), and medical histories were obtained. The results of laboratory tests obtained for routine clinical care (including HIV diagnostic assays) were abstracted from the infant's medical record whenever possible. Because these assays were obtained for routine clinical care, generally the cost of these assays was not reimbursed by the study. Thus, it was anticipated before this analysis was begun that several laboratory assays for which results were abstracted from the medical record would have large proportions of missing values.
Inclusion Criteria and Definitions for This Analysis
The study population for this analysis comprised infants who were born to women enrolled in P1025 by September 7, 2010, who had at least 1 prenatal visit and at least 1 CD4 and 1 plasma HIV RNA concentration (viral load) assay during pregnancy, and who used at least 1 antiretroviral during pregnancy; were live born; were singleton; had at least 6 months of follow-up; were HIV uninfected; and represented the product of their mothers' first eligible pregnancy.
The outcomes of interest were laboratory serious adverse events (SAEs) during the first 6 months of life, defined as grade 3 or 4 adverse events [41, 42] (or, for total lymphocyte counts, values less than the fifth percentile for age and race [43]) (Tables 1 and 2), among those laboratory assays with <20% missing values and with at least 5% of assay results representing SAEs. As part of the protocol, newborns had blood glucose measurements performed on blood obtained by heelstick prior to the first feeding. Any infants with abnormal results had blood obtained for analysis in the hospital's clinical laboratory. For this analysis, only results from the latest blood glucose measurement were analyzed.
Table 1.
Laboratory Serious Adverse Event Grading: Hemoglobin, Absolute Neutrophil Count, Glucose
| Laboratory Assay (units) | Age (days) | Grade 3 | Grade 4 |
|---|---|---|---|
| Hemoglobin (g/dL) | 1–7 | <12 | Cardiac failure secondary to anemia |
| 8–21 | <10 | Cardiac failure secondary to anemia | |
| 22–35 | <8 | Cardiac failure secondary to anemia | |
| ≥36 | <7 | Cardiac failure secondary to anemia | |
| Absolute neutrophil count | 1 | 1500–2999 | <1500 |
| 2–7 | 750–1249 | <750 | |
| 8–56 | 500–899 | <500 | |
| ≥57 | 250–399 | <250 | |
| Glucose (mg/dL) | 30–39 | <30 or mental status changes |
Table 2.
Laboratory Serious Adverse Event Grading: Total Lymphocyte Count (109 cells/L)
| Age | Race | Fifth Percentilea |
|---|---|---|
| 1 day | White | 2.16 |
| Black | 1.89 | |
| 1 month | White | 3.50 |
| Black | 3.25 | |
| 3 months | White | 3.50 |
| Black | 3.06 | |
| 6 months | White | 3.59 |
| Black | 3.17 |
aEuropean Collaborative Study [43].
The factors considered as potential predictors of infant laboratory abnormalities included maternal characteristics (ie, age at delivery, education, trimester of study enrollment, CD4 count, viral load, Centers for Disease Control and Prevention (CDC) clinical category, and use of antiretrovirals during pregnancy) and infant characteristics (ie, birth weight, gestational age, receipt of antiretrovirals, and TMP/SMX exposure during the first 6 months of life). Maternal use of antiretrovirals during pregnancy and infant receipt of antiretrovirals and TMP/SMX during the first 6 months of life were prescribed according to the decision of the clinicians caring for the HIV-infected woman and the HIV-exposed infant at the clinical site. Three different strategies were employed to define the postnatal antiretroviral and TMP/SMX exposures: (1) cumulative duration of exposure by the time of study visit, (2) exposure ever before the study visit, and (3) current exposure at the time of study visit (if subject had exposure within 7 days prior to the study visit). The drug initiated on the same day as the study visit time was not considered as an exposure. The clinical stage of infants' HIV-infected mothers was classified according to the 1993 CDC revised classification system for HIV infection [44]. Viral load was categorized as ≤400 copies/mL rather than <400 copies/mL due to inconsistencies in the database regarding quantifier codes (< or =) and potential difficulties in abstracting such codes from medical charts. Low birth weight infants were those with a birth weight <2500 grams. Infants born preterm were those with <37 completed weeks of gestation at birth.
Statistical Analysis
Associations between the potential predictors and trends in laboratory SAEs over time were modeled using repeated measures generalized estimating equation models that account for correlations between measures within each subject. Correlations within subjects were modeled using the exchangeable correlation structure. The parameters of the model are interpreted as population-averaged effects on each respective laboratory measure over time. Similar analyses were conducted for all laboratory SAEs selected for multivariable analyses based on prevalence distributions. First, a crude model was fit to look at the association between the laboratory visit time and each of the laboratory SAEs. The other covariates of interest were then added individually to the crude model. Variables with a P value < .1 from these bivariable models were retained in the final multivariable model. Due to colinearity, if >1 of the infant antiretroviral and TMP/SMX exposures (duration, ever, current) were significant in bivariable analyses, the covariate chosen to be included in the final multivariable model was based on the following hierarchy: duration of use > ever use > current use. Adjusted odds ratios (ORs), 95% confidence intervals (CIs), and P values were obtained from the final multivariable model for each laboratory SAE.
RESULTS
Of 2237 women enrolled in P1025 by 7 September 2010, 2198 women had at least 1 prenatal visit. Of these 2198 women, 2097 had at least 1 CD4 and 1 viral load assay during pregnancy, and 2003 women used at least 1 antiretroviral during pregnancy. Of these 2003 women, 1982 delivered live-born infants, of whom 1941 were singletons. Of these live-born, singleton infants, 1684 had at least 6 months of follow-up, of whom 1598 were HIV uninfected (the other children were either HIV infected [n = 8] or had indeterminate HIV infection status [n = 78]). Of these, 1524 were products of their mothers' first eligible pregnancy on study. Therefore, the study population comprised 1524 infants. Characteristics of these infants and their mothers are shown in Tables 2 and 3.
Table 3.
Characteristics of the Study Population: Categorical Variables
| Characteristics | Total (N = 1524), No. (%) | |
|---|---|---|
| Maternal | ||
| Age at delivery (years) | <20 | 97 (6) |
| 20–34 | 1168 (77) | |
| ≥35 | 259 (17) | |
| Education | <High school graduate | 577 (38) |
| High school graduate/GED | 637 (42) | |
| >High school graduate | 308 (20) | |
| Unknown | 2 | |
| Trimester at study enrollment | First | 13 (1) |
| Second | 541 (35) | |
| Third | 728 (48) | |
| Intrapartum/postpartum | 242 (16) | |
| CD4 count at entry (cells/mm3) | ≥500 | 626 (41) |
| 350–499 | 395 (26) | |
| 200–349 | 317 (21) | |
| <200 | 171 (11) | |
| Unknown | 15 | |
| Plasma viral load at entry (copies/mL) | ≤400 | 1131 (75) |
| >400 | 378 (25) | |
| Unknown | 15 | |
| CDC clinical category at entry | A | 1234 (81) |
| B | 113 (7) | |
| C | 177 (12) | |
| Antiretroviral regimen of the longest duration during pregnancy | PI-containing regimen | 1129 (74) |
| NNRTI-containing regimen | 140 (9) | |
| NRTI(s) only | 254 (17) | |
| NRTI + other | 1 (<1) | |
| Infant | ||
| Low birth weight | No | 1300 (86) |
| Yes | 216 (14) | |
| Unknown | 8 | |
| Preterm birth | No | 1259 (83) |
| Yes | 265 (17) | |
| Antiretroviral receipt during the first six months of life | Zidovudine only | 1387 (91) |
| Zidovudine and single dose nevirapine | 46 (3) | |
| Zidovudine and lamivudine | 30 (2) | |
| Zidovudine and other | 61 (4) | |
| TMP/SMX receipt during the first six months of life | No | 766 (50) |
| Yes | 758 (50) | |
Subjects missing measurements of characteristics are excluded from calculations of percentage.
Abbreviations: CDC, Centers for Disease Control and Prevention; GED, general educational development; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; TMP/SMX, trimethoprim/sulfamethoxazole.
Table 4.
Characteristics of the Study Population: Continuous Variables
| Characteristic | No. | Min. | Max. | Median (IQR) |
|---|---|---|---|---|
| Duration of maternal antiretroviral regimen during pregnancy (regimen of longest duration during pregnancy) (weeks) | 1524 | 0.29 | 44.86 | 21.43 (15.86–27.00) |
| Cumulative duration of infant antiretroviral receipt during the first 6 months of life (weeks) | 1477 | 0.10 | 19.00 | 6.10 (6.10–6.40) |
| Cumulative duration of infant TMP/SMX receipt during the first 6 months of life (weeks) among infants who received TMP/SMX | 635 | 0.10 | 26.30 | 12.10 (9.90–16.10) |
Abbreviations: IQR, interquartile range; TMP/SMX, trimethoprim/sulfamethoxazole.
At study entry, most women were asymptomatic or mildly symptomatic (CDC category A) (81%), had low plasma viral loads (75% had ≤400 copies/mL), and had CD4 counts of ≥ 350 cells/mm3 (67%). The antiretroviral regimen used for the longest duration during pregnancy was categorized into mutually exclusive categories in the following hierarchy: protease inhibitor (PI)–containing regimens, nonnucleoside reverse transcriptase inhibitor (NNRTI)–containing regimens, regimens with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) only, and other regimens. Most women used PI-containing regimens (74%), with zidovudine + lamivudine + nelfinavir (n = 327; 29%) and zidovudine + lamivudine + lopinavir/ritonavir (n = 309; 27%) being the most common regimens (data not shown). Of those women using NNRTI-containing regimens, zidovudine + lamivudine + nevirapine represented the most common regimen (n = 94; 57%). Finally, of those women using NRTIs only, zidovudine + lamivudine + abacavir represented the most common regimen (n = 189; 74%). Most women used only 1 (68%) or 2 (23%) regimens during pregnancy, with <10% using ≥3 regimens (data not shown). Fourteen percent of the infants were of low birth weight, and 17% were preterm. All infants received antiretroviral prophylaxis, and 50% received TMP/SMX prophylaxis. There were no deaths among the 1524 infants in the study population.
For the following assays, missing values represented at least 20% of the data collected during the first 6 months of life, and the assays were therefore not considered further: potassium (20% missing), calcium (33%), magnesium (85%), uric acid (84%), serum gamma glutamyl transferase (95%), triglycerides (50%), total bilirubin (20%), pancreatic amylase (99%), cholesterol (50%), total amylase and lipase (47%), creatine phosphokinase (46%), and CD4+ lymphocyte count (44%). Of the remaining assays, the following had grade 3 or 4 values (or, for total lymphocyte count, values less than the fifth percentile) representing <5% of all values obtained during the first 6 months of life: platelet count (0.7%), sodium (1.4%), serum glutamic oxaloacetic transaminase (0.1%), serum glutamic pyruvic transaminase (0.1%), and creatinine (0%). Therefore, only the following laboratory assays were evaluated further: hemoglobin, absolute neutrophil count, total lymphocyte count, and glucose (hypoglycemia).
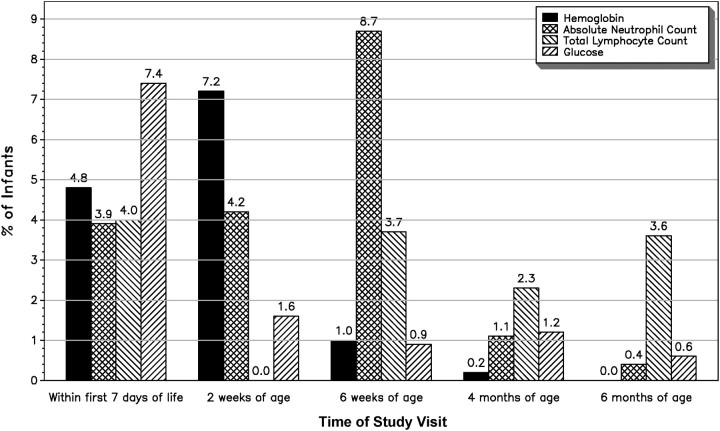
The percentages of subjects with laboratory SAEs at each study visit during the first 6 months of life are shown in Figure 1. Of 1524 infants, 1259 were term and 265 were preterm. A total of 118 infants had grade 3 or 4 hypoglycemia during the first 6 months of life (92 [7%] term infants and 26 [10%] preterm infants). Within the first 7 days of life, grade 3 or 4 hypoglycemia was observed among 7.4% of infants, but this proportion decreased significantly thereafter (1.6% at 2 weeks, 0.9% at 6 weeks, 1.2% at 4 months, and 0.6% at 6 months). A total of 124 infants had grade 3 or 4 anemia during the first six months of life (90 [7%] term infants and 34 [13%] preterm infants). Hemoglobin SAEs were observed in 4.8% of infants within the first 7 days of life but increased in frequency to 7.2% of infants at 2 weeks of life before decreasing significantly thereafter (1.0% at 6 weeks, 0.2% at 4 months, and 0% at 6 months). Sixteen of the 124 infants received blood transfusions. A total of 185 infants had grade 3 or 4 neutropenia during the first 6 months of life (147 [12%] term infants and 38 [15%] preterm infants). Absolute neutrophil count SAEs occurred in 3.9% of infants within the first 7 days of life but increased to 4.2% at 2 weeks and 8.7% at 6 weeks before decreasing to 1.1% at 4 months and 0.4% at 6 months. None of the infants with absolute neutrophil count SAEs received granulocyte colony stimulating factor. Finally, a total of 126 infants had total lymphocyte count SAEs during the first 6 months of life (91 [7%] term infants and 35 [14%] preterm infants). Such events were observed in 4.0% of infants within the first 7 days of life, although no infants had such values at 2 weeks of life. However, some infants did have these low values at 6 weeks (3.7%), 4 months (2.3%), and 6 months (3.6%).
Figure 1.
Laboratory serious adverse events by time of study visit.
Adjusted ORs for laboratory SAEs (hemoglobin, absolute neutrophil count, total lymphocyte count, and glucose) are shown in Table 5. Reinforcing the data shown in Figure 1, the likelihood of SAEs decreased with increasing age of the infant for hemoglobin, absolute neutrophil count, and glucose (overall P value < .001, < .001, and .02, respectively). Infants whose mothers had a plasma viral load of >400 copies/mL at study entry were more likely to have hypoglycemia during the first 6 months of life (adjusted OR, 1.63; 95% CI, 1.10–2.41). Compared with PI-containing regimens, maternal use of regimens consisting of NRTIs only was associated with glucose SAEs (adjusted OR, 1.90; 95% CI, 1.21–2.97). Preterm infants had a greater likelihood of hemoglobin and absolute lymphocyte SAEs (adjusted OR, 1.79; 95% CI, 1.18–2.73; and adjusted OR, 1.90; 95% CI, 1.28–2.83, respectively), and infants currently receiving antiretroviral(s) were at increased risk of hemoglobin SAEs (adjusted OR, 1.99; 95% CI, 1.20–3.31). A greater cumulative duration of infant receipt of antiretroviral(s) was associated with a greater likelihood of absolute neutrophil count SAEs (adjusted OR, 1.16; 95% CI, 1.01–1.32)], but receipt of TMP/SMX was associated with a lower likelihood of such SAEs (adjusted OR, 0.43; 95% CI, .19–.95).
Table 5.
Adjusted Odds Ratios of Laboratory Serious Adverse Events
| Hemoglobina |
Absolute Neutrophil Count |
Total Lymphocyte Count |
Glucose |
|||||
|---|---|---|---|---|---|---|---|---|
| Covariate | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Study visit | <.001 | <.001 | .28 | .02 | ||||
| Within first 7 days after birth | Reference | Reference | Reference | Reference | ||||
| 2 weeks of age | 0.95 (.60–1.50) | 0.80 (.48–1.35) | NAc | 0.20 (.07–.63) | ||||
| 6 weeks of age | 0.09 (.05–.16)b | 1.07 (.45–2.55) | 0.90 (.58–1.40) | 0.11 (.01–1.10) | ||||
| 4 months of age | … | 0.16 (.05–.54) | 0.56 (.31–1.01) | 0.10 (.01–.94) | ||||
| 6 months of age | … | 0.06 (.02–.23) | 0.90 (.57–1.44) | 0.03 (.00–.36) | ||||
| Maternal CD4 count at study entry (cells/mm3) | .05 | .07 | ||||||
| >350 | Reference | Reference | ||||||
| ≤350 | 1.39 (1.00–1.92) | 1.40 (.97–2.01) | ||||||
| Maternal plasma HIV-1 RNA concentration at study entry (copies/mL) | .01 | |||||||
| ≤1400 | Reference | |||||||
| >400 | 1.63 (1.10–2.41) | |||||||
| Maternal clinical class at study entry | .23 | |||||||
| A | Reference | |||||||
| B | 0.93 (.51–1.70) | |||||||
| C | 1.42 (.94–2.15) | |||||||
| Maternal antiretroviral regimen of longest duration during pregnancyd | .02 | |||||||
| PI-containing regimen | Reference | |||||||
| NNRTI-containing regimen | 1.43 (.75–2.71) | |||||||
| NRTI(s) only | 1.90 (1.21–2.97) | |||||||
| Infant preterm birth | .006 | .001 | ||||||
| No | Reference | Reference | ||||||
| Yes | 1.79 (1.18–2.73) | 1.90 (1.28–2.83) | ||||||
| Infant was currently receiving antiretroviral(s) at study visit | .008 | |||||||
| No | Reference | |||||||
| Yes | 1.99 (1.20–3.31) | |||||||
| Cumulative duration of infant receipt of antiretroviral(s) by study visit (weeks) | 1.16 (1.01–1.32) | .04 | 1.01 (.66–1.55) | .97 | ||||
| Infant received TMP/SMX before the date of study visit | ||||||||
| No | Reference | |||||||
| Yes | 0.43 (.19–.95) | .04 | ||||||
| Cumulative duration of TMP/SMX receipt by study visit (weeks) | 1.09 (.99–1.19) | .07 | ||||||
Variables with a P value < .1 from the bivariable model for each laboratory outcome were retained in the final multivariable models and were presented in this table.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus type 1; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor; TMP/SMX, trimethoprim/sulfamethoxazole.
aNone of the infants with hemoglobin severe adverse events received TMP/SMX before the date of hemoglobin assay; therefore, the TMP/SMX exposure variable was excluded during the model fitting procedure.
bHemoglobin assay results at the 6-week, 4-month, and 6-month study visits were collapsed due to the sparseness of event counts (1 at 4 months, 0 at 6 months).
cThere is no standard cut-off value for infants at 2 weeks of age [43]; therefore, total lymphocyte count measurements at this time point were excluded from the analysis.
dThe antiretroviral regimen was classified into 3 mutually exclusive categories following the hierarchy: PI-containing regimen > NNRTI-containing regimen > NRTI-only regimen > NRTI + other regimen. One woman who received NRTI + other regimen was excluded from the analysis.
DISCUSSION
In our analysis of >1500 HIV-exposed but uninfected infants, SAEs related to hemoglobin, absolute neutrophil count, total lymphocyte count, and glucose were experienced by <10% of infants at any study visit during the first 6 months of life. The likelihood of such adverse events decreased significantly over time for absolute neutrophil count, hemoglobin, and glucose (with SAEs related to total lymphocyte count remaining ≤ 4% at any time point). Both maternal and infant factors were associated with SAEs (maternal plasma viral at study entry, maternal antiretroviral regimen of the longest duration during pregnancy, infant preterm birth, and infant antiretroviral and TMP/SMX receipt).
A major strength of this analysis is the set of inclusion criteria utilized to derive the study population. The study population of infants included only those infants whose HIV-infected mothers had at least 1 prenatal visit, 1 CD4 count, and 1 viral load assay during pregnancy and used at least 1 antiretroviral during pregnancy. Of the 1524 mothers, most were asymptomatic or mildly symptomatic, had low plasma viral loads, and had CD4 counts of ≥350 cells/mm3. All women received antiretrovirals during pregnancy for either treatment or prophylaxis, and all infants received antiretroviral prophylaxis. Thus, the results of this analysis are generalizable to populations of HIV-exposed but uninfected infants who, along with their mothers, are receiving at least a minimum of general and HIV-specific clinical care according to current guidelines [1]. The fact that, due to cost constraints, clinical sites were not reimbursed for infant laboratory studies could be considered a limitation of the P1025 prospective cohort study. Clinical sites only reported results of laboratory studies that were obtained as part of routine clinical care and monitoring of children of HIV-infected women.
The results of this analysis are reassuring in terms of the overall low frequency of laboratory SAEs (hemoglobin, absolute neutrophil count, total lymphocyte count, and glucose) and the decreasing frequency of such SAEs with increasing age of the infant. Infant preterm birth and the infant's current receipt of antiretroviral(s) were the factors with the strongest associations with laboratory SAEs during the first 6 months of life. The latter finding is consistent with the overall pattern of decreasing likelihood of laboratory SAEs as the infant ages (with infant antiretroviral prophylaxis being most commonly administered during the first 6 weeks of life and discontinued thereafter). Prevention of preterm birth is an overarching goal of maternal-child health programs. Continuing efforts to achieve this goal are essential. Preterm, HIV-exposed infants are at higher risk of hemoglobin and total lymphocyte count SAEs.
Acknowledgments
Johns Hopkins University Baltimore National Institute of Child Health and Human Development (NICHD) Clinical Research Site (CRS) #5092: Allison Agwu, MD; Jennifer Chang, BS; Todd Noletto, MPH; Joan Bess, BA. St. Jude/University of Tennessee Health Science Center (UTHSC) CRS #6501 and Regional Medical Center at Memphis CRS #6502: Edwin Thorpe Jr, MD; Nina Sublette, RN, PhD; Katherine Knapp, MD; Jill Utech, RN, MSN, CCRC. New York University NICHD CRS #5012: William Borkowsky, MD; Mariam Minter, RN; Nagamah Deygoo, MS; Aditya Kaul, MD. Bronx-Lebanon Hospital IMPAACT CRS #6901: Jenny Guttierez, MD; Rodney Wright, MD; Mavis Dummitt, RN; Murli Purswani, MD. University of Connecticut Health Center, Department of Pediatrics CRS #7303 and Connecticut Children's Medical Center CRS #7304: Juan C Salazar, MD, MPH; Gail Karas, RN. Baystate Medical Center CRS #7302: Barbara W. Stechenberg, MD; Donna J. Fisher, MD; Eileen Theroux, RN, BSN; Maripat Toye, RN, MS. Western New England Maternal Pediatric Adolescent AIDS CRS #7301: Katherine Luzuriaga, MD; Sharon Cormier, RN. Maternal, Child and Adolescent Center for Infectious Diseases and Virology, University of Southern California Keck School of Medicine CRS #5048: LaShonda Spencer, MD; James Homans, MD; Michael Neely, MD; Francoise Kramer, MD. Texas Children's Hospital CRS #3801: Mary E. Paul, MD; Shelley Buschur, RN, CNW; Chivon D. McMullen-Jackson, RN, BSN; Norma Cooper, RN, BSN, MA. Rush University Cook County Hospital Chicago NICHD CRS #5083: James B. McAuley, MD; Kenneth M. Boyer, MD; Maureen Haak, RN, MSN; Elizabeth Jones, RN. Jacobi Medical Center Bronx NICHD CRS #5013: Andrew Wiznia, MD; David Garry, MD; Franciso Reinoso, RN; Karen Kassen, RN. New Jersey Medical School CRS #2802: Charmane Calilap-Bernardo, RN; Linda Bettica, RN. Children's Hospital of Boston NICHD CRS #5009: Ruth Tuomala, MD; Sandra Burchett, MD, MS; Arlene Buck, CNS; Catherine Kneut, CPNP. University of Miami CRS #4201: Amanda Cotter, MD; Charles Mitchell, MD; Erika Lopez, MD; Claudia Florez, MD. Yale University School of Medicine NICHD CRS #5038: Warren A. Andiman, MD; B. Joyce Simpson, RN, MPH; Leslie Hurst, MS. St. Jude/UTHSC CRS #6501: Katherine M. Knapp, MD; Nehali Patel, MD; Jill Utech, RN, MSN; Pam Finnie, RN, MSN. Howard University Washington, DC NICHD CRS #5044: Sohail Rana, MD; Caroline Reed, RN, FNP; Meseret Deressa, MD; Folasade Akereyeni, MD. Tulane University New Orleans NICHD CRS #5095: Yvette Luster, RN; Sheila Bradford, RN; Chi Dola, MD; Robert Maupin, MD. University of California San Diego Maternal, Child, and Adolescent HIV CRS #4601: Andrew Hull, MD; Rolando M. Viani, MD, MTP; Mary Caffery, RN, MSN; Stephen A. Spector, MD. University of California Los Angeles (UCLA) CRS #3601: Yvonne Bryson, MD; Jaime Deville, MD; Karin Nielsen, MD, MPH; Nicole Falgout, RN. State University of New York (SUNY) Upstate Medical University Department of Pediatrics CRS #5039: Leonard B. Weiner, MD; Kathie A. Contello, CPNP; Wendy A. Holz, CPNP; Maureen Butler, RN. University of Colorado Denver NICHD CRS #5052: Carol Salbenblatt, RN, MSN; Kay Kinzie, MSN; Jennifer Dunn, MS, FNP; Carrie Chambers, RNC, BSN. University of Alabama Birmingham NICHD CRS #5096: Marilyn Crain, MD, MPH; Alan Tita, MD; Sharan Robbins, BA; Mickey Parks, CRNP, MSN. Duke University Medical Center Pediatrics CRS #4701: Carole Mathison, MA; John Swetnam, Med; Joan Wilson RN, MPH; Margaret Donnelly, PA-C. SUNY Downstate Medical Center Children's Hospital at Downstate NICHD CRS #5008: H. J. Moallem, MD; E. Handelsman, MD; J. Kaye, RN; D. Swindell; Hutzel Hospital NICHD CRS #5089: Theodore B. Jones, MD; Tameka Watson, RN; Ernestine Brown, RN. Harbor UCLA Medical Center NICHD CRS #5045: Margaret A. Keller, MD; Judy Hayes, RN; Yolanda Gonzalez, RN; Spring Wettgen, RN, PNP. University of California San Francisco Pediatric AIDS CRS #4501: Diane W. Wara, MD; Deborah Cohan, MD; Nicole Tilton, PNP; Mica Muscat, PNP. Long Beach Memorial Medical Center, Miller Children's Hospital CRS #3606: Audra Deveikis, MD; Jagmohan Batra, MD; Tempe Chen, MD; Janielle Jackson-Alvarez, RN. Chicago Children's CRS #4001: Lynn Heald, PNP; Amy Talksy, PNP; Molly Hartrich, MPH; Jessica Shore, BSN. Children's Hospital of Philadelphia CRS #6701: Steven D. Douglas, MD; Jenell Coleman, MD; Richard M. Rutstein, MD; Carol Vincent, CRNP, MSN.
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); and the National Institute of Mental Health (AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under NIAID cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1–infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at: http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf . Accessed November 7, 2011. [Google Scholar]
- 2.Mofenson LM, Brady MT, Danner SP, et al. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 3.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry international interim report for 1 January 1989 through 31 January 2011. Available at: http://www.apregistry.com/forms/interim_report.pdf . Accessed November 7, 2011. [Google Scholar]
- 4.Patel D, Thorne C, Fiore S, et al. Does highly active antiretroviral therapy increase the risk of congenital abnormalities in HIV-infected women? J Acquir Immune Defic Syndr. 2005;40:116–8. doi: 10.1097/01.qai.0000156854.99769.a5. [DOI] [PubMed] [Google Scholar]
- 5.Watts DH, Li D, Handelsman E, et al. Assessment of birth defects according to maternal therapy among infants in the Women and Infants Transmission Study. J Acquir Immune Defic Syndr. 2007;44:299–305. doi: 10.1097/QAI.0b013e31802e2229. [DOI] [PubMed] [Google Scholar]
- 6.Townsend CL, Willey BA, Cortina-Borja M, et al. Antiretroviral therapy and congenital abnormalities in infants born to HIV-infected women in the UK and Ireland, 1990 to 2007. AIDS. 2009;23:519–24. doi: 10.1097/QAD.0b013e328326ca8e. [DOI] [PubMed] [Google Scholar]
- 7.Joao EC, Calvet GA, Krauss MR, et al. Maternal antiretroviral use during pregnancy and infant congenital anomalies: the NISDI Perinatal Study. J Acquir Immune Defic Syndr. 2010;53:176–85. doi: 10.1097/QAI.0b013e3181c5c81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culnane M, Fowler MG, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. JAMA. 1999;13:151–7. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 9.Brogly S, Williams P, Seage GR, et al. In utero nucleoside reverse transcriptase inhibitor exposure and cancer in HIV-uninfected children: an update from the pediatric AIDS clinical trials group 219 and 219C cohorts. J Acquir Immune Defic Syndr. 2006;41:535–6. doi: 10.1097/01.qai.0000194735.66322.d9. [DOI] [PubMed] [Google Scholar]
- 10.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–9. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 11.The Perinatal Safety Review Working Group. Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25:261–8. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hanson IC, Antonelli A, Sperling RS, et al. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:463–7. doi: 10.1097/00042560-199904150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Sperling RS, Shapiro DE, McSherry GD, et al. Safety of the maternal infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS. 1998;12:1805–13. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–85. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Easley KA, Orav EJ, et al. Absence of cardiac toxicity of zidovudine in infants. N Engl J Med. 2000;353:759–66. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- 16.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–7. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Alimenti A, Forbes JC, Oberlander TF, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118:e1139–45. doi: 10.1542/peds.2006-0525. [DOI] [PubMed] [Google Scholar]
- 18.Brogly SB, Ylitalo N, Mofenson LM, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–38. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 19.Hankin C, Lyall H, Peckham C, et al. Monitoring death and cancer in children born to HIV-infected women in England and Wales: use of HIV surveillance and national routine data. AIDS. 2007;21:867–9. doi: 10.1097/QAD.0b013e3280b01822. [DOI] [PubMed] [Google Scholar]
- 20.European Collaborative Study, Swiss Mother and Child HIV Cohort Study. Combination antiretroviral therapy and duration of pregnancy. AIDS. 2000;14:2913–20. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 21.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346:1863–70. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 22.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18:2337–9. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 23.Tuomala RE, Watts DH, Li D, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2005;38:449–73. doi: 10.1097/01.qai.0000139398.38236.4d. [DOI] [PubMed] [Google Scholar]
- 24.Szyld EG, Warley EM, Freimanis L, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS. 2006;20:2345–53. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 25.Cotter AM, Garcia AG, Duthely ML, et al. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193:1195–201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 26.Kourtis AP, Schmid CH, Jamieson DJ, et al. Use of antiretroviral therapy in pregnancy HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. 2007;21:607–15. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 27.Townsend Cl, Cortina-Borja M, Peckham CS, et al. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. 2007;21:1019–26. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 28.Schulte J, Dominguez K, Sukalac T, et al. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric spectrum of HIV disease, 1989–2004. Pediatrics. 2007;119:e900–6. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 29.Ravizza M, Martinelli P, Bucceri A, et al. Treatment with protease inhibitors and coinfection with hepatitis C virus are independent predictors of preterm delivery in HIV-infected pregnant women. J Infect Dis. 2007;195:913–4. doi: 10.1086/507045. [DOI] [PubMed] [Google Scholar]
- 30.Grosch-Woerner I, Puch K, Maier RF, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1–infected women. HIV Med. 2008;9:6–13. doi: 10.1111/j.1468-1293.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 31.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009;85:82–7. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel K, Shapiro DE, Brogly SB, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201:1035–44. doi: 10.1086/651232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes WT, LaFon SW, Scott JD, Masur H. Adverse events associated with trimethoprim-sulfamethoxazole and atovaquone during the treatment of AIDS-related Pneumocystis carinii pneumonia. J Infect Dis. 1995;171:1295–301. doi: 10.1093/infdis/171.5.1295. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzi P, Spicher VM, Laubereau B, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS. 1998;12:F241–7. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sperling RS, Shapiro DE, McSherry GD, et al. Safety of the maternal infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS. 1998;12:1805–13. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, Blanche S. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–61. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 37.Bellon Cano JM, Sanchez-Ramon S, Ciria L, et al. The effects on infants of potent antiretroviral therapy during pregnancy: a report from Spain. Med Sci Monit. 2004;10:CR179–84. [PubMed] [Google Scholar]
- 38.European Collaborative Study. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1–infected mothers. AIDS. 2004;18:2009–17. doi: 10.1097/00002030-200410210-00005. [DOI] [PubMed] [Google Scholar]
- 39.Bunders M, Thorne C, Newell ML. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1–infected mothers. AIDS. 2005;19:1071–9. doi: 10.1097/01.aids.0000174454.63250.22. [DOI] [PubMed] [Google Scholar]
- 40.Mussi-Pinhata MM, Rego MAC, Freimanis L, et al. Maternal antiretrovirals and hepatic enzyme, hematologic abnormalities among human immunodeficiency virus type 1–uninfected infants: the NISDI Perinatal Study. Ped Infect Dis J. 2007;26:1032–7. doi: 10.1097/INF.0b013e31812f56ed. [DOI] [PubMed] [Google Scholar]
- 41.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Table for grading severity of pediatric (≤3 months of age) adverse experiences. Available at: http://rsc.tech-res.com/safetyandpharmacovigilance/ . Accessed November 7, 2011. [Google Scholar]
- 42.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Table for grading severity of pediatric (>3 months of age) adverse experiences. April 1994. Available at: http://rsc.tech-res.com/safetyandpharmacovigilance/ . Accessed November 7, 2011. [Google Scholar]
- 43.Bunders M, Cortina-Borja M Newell M-L; European Collaborative Study. Age-related standards for total lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe. Pediatr Infect Dis J. 2005;24:595–600. doi: 10.1097/01.inf.0000168835.01233.64. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Diseases Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]