Abstract
Stereotactic radiosurgery (SRS) and hypofractionated stereotactic radiotherapy (HFSRT) have become important treatment modalities for brain metastases. While effective, there are still areas of extensive debate on its appropriate use in patients with life-limiting diseases. This review provides an overview of the indications and challenges of SRS and HFSRT in the management of brain metastases.
Keywords: Brain metastases, brainlab, cyberknife, gamma knife, hypofractionated, radiosurgery, stereotactic
INTRODUCTION
Brain metastases cause significant morbidity and mortality for patients with cancer. In the past, the median survival of patients with brain metastases without treatment was generally a few months. This was largely due to the presence of significant symptoms and larger lesions at presentation. It was not routine to scan asymptomatic patients. Treatment with whole brain radiation therapy (WBRT) improved survival over best supportive care and was given with palliative intent to improve symptoms temporarily. Response rates for WBRT were 40-50%.[12] With improved and widespread imaging as well as improved systemic treatments, many patients are now presenting with brain metastases but no clinical symptoms. This has clearly impacted our approach to managing brain metastases. With limited survival, management of symptoms was the dominant goal. However, now that patients are presenting with minimal to no symptoms and have better systemic therapy options, the management of brain metastases should focus not only on symptom management but also on potential long-term complications. It is these changes in presentation and prognosis that have made stereotactic radiosurgery (SRS) critical in the management of brain metastases.
WHAT IS STEREOTACTIC RADIOSURGERY/STEREOTACTIC RADIOTHERAPY
The key component of SRS and hypofractionated stereotactic radiotherapy (HFSRT) is the precise delivery of a high dose of radiation to a target with rapid dose drop off to the surrounding normal tissues. There are a variety of devices that can be used, including Gamma Knife (Elekta AB, Stockholm, Sweden), Cyberknife (Accuray, Sunnyvale, CA, USA), gantry–based linear accelerator (LINAC) systems (e.g., Novalis TX, BrainLab) and less commonly proton beam-based systems.
Gamma Knife uses a fixed immobilization frame and imaging obtained with the frame in place to create a stereotactic grid “space” for treatment planning. Multiple imaging modalities can be used for treatment planning with all scans co-localized into the same space. The most common modality for treatment of brain metastases is fine cut postcontrast magnetic resonance imaging (MRI). Alternatively, computed tomography (CT) with contrast can be used when patients have contraindications to MRI, such as a defibrillator or pacemaker. Positron emission tomography (PET) scans can also be used to incorporate biological relevant information into the treatment planning process.
Using the latest version of Gamma Knife Perfexion, the delivery of radiation is carried out by 192 different radiation beams or ports all focused on a single isocenter. The size of the isocenter can be varied with a 4, 8, or 16 mm collimator. This effectively creates a dose cloud with the same size. Multiple isocenters or shots can be combined to create custom shapes. The collimators can be mixed and matched or blocked to further customize the dose delivery. During treatment, the patient is immobilized by fixing the head frame to the treatment couch, which precisely positions the patient to the correct coordinates for each isocenter or shot. When the treatment is complete, the patient is removed from the treatment machine and the head frame is removed. Generally the prescription doses for brain metastases vary between 12 and 24 Gy to the 50% isodose line. Thus the middle of the target receives twice that dose (24-48 Gy). Generally these doses are based on findings from the Radiation Therapy Oncology Group (RTOG) 90-05, a dose escalation study undertaken to determine the highest dose with acceptable toxicity based on tumor size.[14,17]
Cyberknife utilizes a linear accelerator attached to the end of a robotic arm. Planning is somewhat similar in that CT, MRI, and PET can all be used when co-localized with a treatment planning CT. Since the head frame is not attached to the skull, the scans can be obtained prior to the day of treatment and treatment planning can take place without the patient being present. Treatment planning is achieved by utilizing different sized collimators from thousands of possible beam directions or multiple pencil beams. Doses are generally similar to those used for Gamma Knife but usually are prescribed to the 70-80% isodose line. Cyberknife also allows treatment of larger lesions with multiple treatments (HFSRT) over several days. Patients are treated with an immobilization mask and on-board orthogonal X-rays to assure positioning. The orthogonal imaging is repeated multiple times throughout treatment to assure delivery accuracy.
Gantry-based LINAC systems use either fixed circular collimators or multileaf collimators. As with other systems, treatment planning imaging is based on CT but other images including MRI and PET can be fused to the treatment CT. Once again, on-board imaging is used to assure patient alignment. The treatment can be delivered as either multiple arcs or as one continuous arc. The isocenter is generally in the middle of the target lesions; however, newer systems with Volumetric Modulated Arc Therapy (VMAT) allow for treatment of multiple lesions in a single arc. Doses again are in the 12-24 Gy range for single fraction treatments generally prescribed to the 60-80% isodose line.
There are pluses and minuses to each system. Gamma Knife may have some benefit in treating multiple lesions in terms of shorter treatment times and efficiency. Furthermore the composite brain dose may be less. The down side is the frame-based system, which patients may find uncomfortable, and the need to reload the radiation delivery sources roughly every 5 years. Cyberknife, BrainLab, and LINAC systems can treat solitary lesions the fastest due to the higher radiation output when compared with Gamma Knife. However, most systems are only able to treat one lesion at a time, and multiple lesions take longer. This does not hold true for systems that can use VMAT – an arcing treatment technique where the beam is delivered continuously as the gantry moves around the patient. In addition, LINAC-based systems may be used for anatomic sites other than brain and thus may be more practical for centers with smaller radiosurgery volume.
Proton stereotactic systems are quite rare. Either a frame based or bite block immobilization system can be used. The lesion is generally treated with two to three ports such that the dose drops off rapidly on the distal side of the lesion and the isodose lines can be shaped with compensators. The advantage of protons is that there is no exit dose, decreasing integral dose to the brain.
In general, SRS describes dose delivered in a single treatment, whereas HFSRT is delivered in 2-5 fractions. However, the dose per fraction for HFSRT is generally larger (5-9 Gy) than conventionally fractionated radiation therapy (1.8-2 Gy). Some standard hypofractionated schemes include 18-30 Gy in 3-5 fractions. The limitation to five fractions is likely most prevalent in the US due to reimbursement structure. Stereotactic treatments are not covered after five fractions. There have been no trials that have clearly identified that HFSRT is best performed in 2-5 fractions.
Given the initial prevalence of frame-based immobilization devices (Gamma Knife and some LINAC-based systems), many of the initial studies employed single fraction treatments due to the discomfort of either multiple frame placements or multiple days that patients would wear the frame. Many lesions respond quite well to single fraction treatments particularly smaller lesions. However, larger lesions, generally greater than 3 cm, treated in a single fraction have been associated with increased rates of acute side effects. Furthermore as on-board imaging has become more prevalent, it is easier to administer multiple sessions. There are no randomized trials comparing single fraction treatment with multiple hypofractionated treatments. For larger lesions hypofractionation possibly reduces the risk of toxicity, and there may also be a radiobiological benefit of multiple treatments.
WHY RADIOSURGERY?
SRS and HFSRT have been increasingly used as treatment for brain metastases for several reasons. For few metastases, advantages compared with neurosurgical resection include its noninvasive approach, suitability for outpatient treatment, ability to treat surgically unresectable areas such as the brainstem, and ability to treat multiple lesions. For multiple metastases, advantages compared with WBRT include improved local control, fewer neurocognitive side effects, and a shorter treatment course. In addition, retrospective series have shown that radioresistant histologies including renal cell carcinoma and melanoma have control rates after SRS that are similar to radiosensitive tumor types.
Efficacy
Initially, SRS was used and tested in clinical trials as a way of improving outcomes for patients with few metastases. Multiple trials showed improved intracranial control with adding SRS to WBRT.[1,9] In the RTOG 95-08 trial of 333 patients with one to three brain metastases, survival was similar for patients receiving WBRT or WBRT with SRS (5.7 and 6.5 months, respectively). However, a subset analysis demonstrated improved survival from 4.9 to 6.5 months with the addition of SRS to WBRT in patients with a single brain metastasis and from 9.6 to 11.6 months in patients younger than 65 with good performance status, controlled primary tumor, and no extracranial metastases compared with WBRT alone. Overall, patients receiving SRS were significantly more likely to have a stable or improved performance status at 6 months (43% versus 27%). Given that significant findings were based on a post hoc analysis with a survival advantage of only 1-2 months, this trial did not serve as a ringing endorsement for adding SRS to WBRT.
Therefore, subsequent randomized trials tested SRS alone as a new treatment paradigm. Indeed, multiple randomized trials have shown that withholding WBRT for one to four brain metastases does not compromise overall survival.[2,8,4] The European Organisation for Research and Treatment of Cancer (EORTC) 22952-260001 trial randomized 359 patients with one to three metastases treated with SRS or surgery alone to either WBRT or observation with brain MRI every 3 months.[8] Patients treated with SRS alone and surgery alone had a 2 year relapse rate at the initial site of 31% and 59%, respectively. The rate of relapse at new sites was 42% and 48% for SRS alone and surgery alone, respectively. WBRT improved the rate of relapse at the initial and new sites, but did not change overall survival (median 10.9 and 10.7 months with WBRT and observation, respectively). The Japanese Radiation Oncology Study Group (JROSG) 99-1 trial also randomized 132 patients with one to four brain metastases to SRS alone or SRS and WBRT.[2] The 12 month brain tumor recurrence rate was 46.8% in the WBRT and SRS group and 76.4% for the SRS alone group. However, median survival and death from neurological causes was not significantly different.
There has never been a head to head trial comparing SRS and surgery. However, retrospective series and results from EORTC 22952 suggest that SRS control rates are not inferior to surgical resection. Results from both of these sources are biased by how patients were assigned to surgery or SRS, and so direct comparison is not possible. In reality, SRS and surgery have different strengths and weaknesses as treatment for patients with brain metastases. Whereas surgery may be the optimal treatment for patients with symptoms due to mass effect, radiosurgery allows us to treat surgically inaccessible lesions. Rapid dose drop off provided by radiosurgery allows us to treat lesions that are adjacent to critical structures and still preserve neurological function. Perhaps, this is most clearly seen in treating brain metastases in the brainstem [Figure 1]. Previously, these lesions have been quite difficult to manage; however, SRS has provided good local control with acceptable toxicity.
Figure 1.
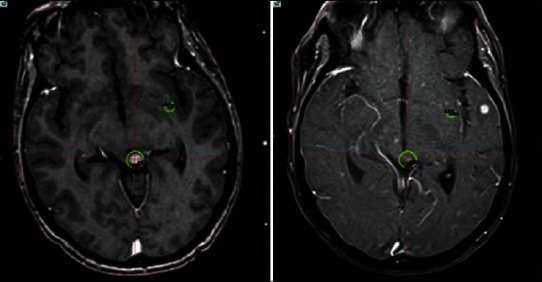
Woman with metastatic breast cancer with metastasis to the upper brain stem. She was treated with stereotactic radiosurgery (left panel) and follow up one month later (right panel). Unfortunately she developed multiple new lesions
Neurocognitive outcomes
Since no overall survival benefit has been shown in adding WBRT to SRS, attention has shifted from using SRS to improve overall survival to using SRS to improve quality of life and neurocognitive outcomes. The neurocognitive decline associated with WBRT, described many years ago by DeAngelis et al., have become increasingly important as patients with metastatic cancer are living longer with improvements in detection, systemic therapies, and supportive care. Though the median survival of patients with brain metastases remain poor overall, certain subgroups with good prognostic factors have median survival of 15-25 months.[16] In the DeAngelis study, 11% of the patients living more than 12 months after WBRT developed clinical dementia, however, all of the affected patients received more than 300 cGy per fraction. A more recent randomized trial at MD Anderson Cancer Center of SRS alone compared with WBRT and SRS, examined the primary endpoint of neurocognitive decline as defined by a 5-point drop compared with baseline in the Hopkins Verbal Learning Test-Revised. The trial was stopped early by the data monitoring committee with only 58 patients. At 4 months, patients randomized to SRS and WBRT were significantly more likely to show decline (52%) in learning and memory function compared with patients receiving WBRT, which had a significant decline in learning and memory function at 4 months than patients treated with SRS alone (24%).[4] 58 patients before it was closed early due to stopping rules based on neurocognitive outcomes.
The EORTC 22952 also assessed quality of life and cognitive function with EORTC QLQ-C30 and BR20 brain cancer specific questionnaires, however, only 45% completed the analysis at one year. Patients who underwent WBRT had lower physical functioning at 8 weeks and cognitive function scores at one year, however, there were no significant differences in global Health Related Quality of Life.[15] Currently we are awaiting results of a larger randomized trial conducted by the North Central Cancer Treatment Group (NCT00377156) was originally slated to accrue 500 patients to look at overall survival, however, with poor accrual, the trial was amended to include approximately 200 patients to detect neurocognitive outcomes.
Patient/provider preference
Though the interpretation of published trials results may be controversial, the use of SRS for brain metastases has been steadily increasing in the US.[6] It is important to consider that one of the important drivers of increasing SRS use in the treatment of brain metastases is patient preference. Patients have become better educated regarding treatment options and have learned about the systems used for SRS through support groups and direct to consumer advertising. Whether true or not, many patients consider SRS as more aggressive than WBRT. In addition, many patients have formed opinions after seeing fellow patients experience side effects of treatment. For instance, many patients refuse to undergo WBRT given the fatigue and neurocognitive side effects they associate with the treatment. Referring physicians, generally medical oncologists have also started requesting SRS alone after seeing late effects of WBRT in their patients and because of the shortened treatment course.
Timing of treatment
In addition to avoiding or delaying WBRT to prevent associated side effects, the one day course of SRS may have quality of life implications. WBRT generally takes 2-3 weeks of daily treatment. For patients with poor prognosis, this may represent a significant percentage of the time they have left. For all patients, this may delay starting systemic treatment since chemotherapy is usually held during WBRT.
CHALLENGES IN RADIOSURGERY
Treatment effect
One of the major challenges after SRS is determining whether imaging results and clinical decline represent treatment effect or true tumor progression [Figure 2]. It has been widely reported that when a large number of tumor cells are killed rapidly during SRS, contrast enhancement and vasogenic edema can increase significantly. These changes may be related to treatment effect as opposed to true tumor progression. Since both tumor progression and treatment effect often appear similarly on imaging and can cause the same symptoms, one often needs to depend on the overall clinical picture to decide which is more likely. This includes the timing of the “progression,” status of systemic disease, response to steroids, and evolution over time. However, treatment effect changes on imaging can be dynamic in nature much like tumor progression. It is been observed that lesions can get larger and smaller over time, just as a result of treatment effect. Several imaging modalities have been investigated for improving the diagnosis of treatment effect versus tumor progression, including fluorine-18 fluorodeoxyglucose positron emission tomography scanning and dynamic susceptibility-weighted contrast-enhanced MRI.[7,5] Though these images show promise, none has yet proven effective in solving this dilemma.
Figure 2.
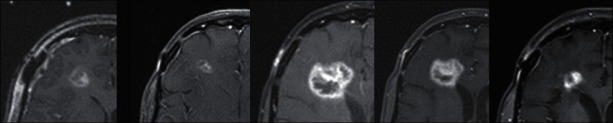
Woman with metastatic breast cancer with multiple brain metastases that had a right frontal resection cavity treated with stereotactic radiosurgery 4 weeks after resection of the lesion. She developed significant radiation necrosis possibly caused by radiation recall after a Vinorelbine infusion. The area of contrast enhancement changed dramatically over a year and a half with the only treatment being steroids (no further radiation or surgery to this lesion). Further resection was not an option due to her overall clinical situation
If treatment effect is suspected, most patients will respond to steroids with improvement in symptoms and can be followed conservatively. However, some patients will become quite symptomatic or intolerant of steroids and require surgical intervention. This treatment decision is often difficult in a patient with progressive systemic disease and/or poor performance status. While surgical resection of an enlarging contrast enhancing lesion may improve neurological symptoms, the recovery required after neurosurgical resection may be difficult for a patient with deteriorating condition. However, increasing doses of steroids often lead to significant side effects including insomnia, anxiety, weight gain, adrenal insufficiency, and hyperglycemia. Bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF) receptor is well known for decreasing contrast enhancement and vasogenic edema in recurrent high-grade gliomas and multiple reports have suggested that bevacizumab may have the same effect on radiation induced necrosis.[5] However, bevacizumab may increase the risk of intracranial or gastrointestinal hemorrhage, and furthermore prohibits any surgical intervention for at least 4-6 weeks after the last infusion due to its effect on wound healing. In addition, insurance coverage may be an issue given that treatment for radiation induced necrosis is currently an off label use.
Tumor/CNS recurrence
If the clinical picture is consistent with recurrence either within or outside of the radiosurgery target, there are multiple options for salvage treatment. However, deciding the best approach is challenging. One of the most important factors in deciding salvage treatment is the overall clinical picture at the time of recurrence. For an isolated tumor recurrence after prior SRS in a patient with control systemic disease then repeat SRS to the same lesion is possible, however, it carries an increased risk of radionecrosis.[11] For tumor recurrence in the context of multiple new brain metastases, then WBRT is most often used for salvage if the patient has not already undergone WBRT. For patients with stable systemic disease and more slowly progressing brain disease, it may be difficult to determine when SRS or WBRT is the appropriate salvage. Additionally, in certain situations such as poor performance status and/or progressive systemic disease or small metastases that are unlikely to be symptomatic in the near future, observation may be the appropriate approach. There are few prospective studies to help guide us in these clinical scenarios.
Size of lesions
The dose escalation RTOG trial 90-05 established that the size of the lesion treated with radiosurgery directly correlates with the risk of side effects due to increased vasogenic edema and radionecrosis [Figure 3]. However, the maximum size of a metastasis that can be safely treated with SRS is unclear. In general, the size cut-off for treating brain metastases with SRS is 3 cm. However, some advocate that larger lesions can be safely treated if the dose is lowered, which may be effective especially for radiation sensitive histologies. Overall, clinical practice varies regarding dose selection for various sized targets. The Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) expert panel reviewed the literature on the dose-volume effects on the risk of toxicity after SRS. Though it seemed that toxicity increased once the volume of brain exposed to >12 Gy was more than 5-10 cm3, they concluded that they could not make toxicity-risk predictions given substantial variation among different reported outcomes.[10] The location of the target, histology, conformality of the plan, and patient's life expectancy all are important factors to take into account when assessing the risk of toxicity.
Figure 3.
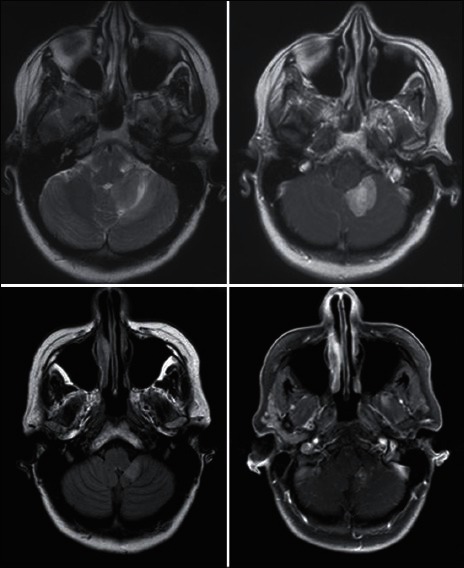
Woman with metastatic breast cancer treated with stereotactic radiosurgery to a large (>3.5 cm) left cerebellum metastasis. Upper panel prior to treatment and the lower is one year later
For larger lesions, many have also taken the approach of HFSRT, which capitalizes on the stereotactic precision of radiosurgical devices but delivers dose over multiple fractions. Many tumors respond quite well to these courses, however, further investigation into the optimal treatment schedules, doses, and their relationship to the size of targets needs to be pursued.
Number of lesions
One of the biggest misconceptions regarding SRS is that it is only helpful for patients with fewer than four lesions [Figure 4]. Initial trials of SRS for brain metastases were limited to patients with 1-4 brain metastases. This was driven by the fact that early trials concentrated on overall survival as the primary endpoint and since SRS is associated with significant costs, it was not felt to be justified in patients with poor prognosis. However, SRS has become increasingly used for treating patients with multiple lesions in a single session, especially among Gamma Knife users.[3] Given the sharp dose drop-off and conformality afforded by stereotactic systems, multiple lesions can be treated with acceptable toxicity. It should be noted that there has never been a comparison of WBRT versus SRS alone for patients with more than four lesions, so we do not know whether overall survival or quality of life differs with either approach. Number of metastases is an adverse prognostic factor no matter what treatment a patient undergoes, but there is also substantial data that volume of disease may be more important than number of metastases.[3]
Figure 4.
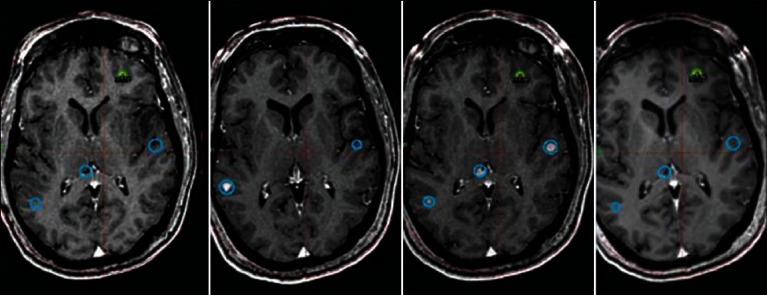
Sixty-six-year-old male with metastatic renal cell cancer. Thirty-three lesions have been treated over two and one-half years. MRI shows representative images of the same anatomical location over time with the oldest scans on the left and newest on the right. The blue circles represent previously treated lesions and the yellow/green represent lesions most recently treated
Cost
Much of the controversy surrounding the efficacy of radiosurgery compared with WBRT is driven by the cost differential between the two treatment approaches. Throughout the US, the radiation therapy costs for patients with brain metastases are increased in those who receive SRS and the more frequent imaging surveillance associated with a SRS alone approach may also add to the overall costs.[6] Given that most patients with brain metastases have limited survival, it is especially difficult for us as a society to determine how much money should be spent for improvement in ones quality of life. Yet, we do think it is important when looking at allocation of resources to consider outcomes of quality of life in addition to overall survival.
Role of radiosurgery in treating resection cavities
When patient do have metastases resected, there is an approximately 50% chance of a local recurrence within the resection cavity. Previously patients underwent whole brain irradiation, which included the resection cavity to improve local control. There have now been retrospective studies showing improved local control when the resection cavity has been treated with SRS.[13] Once again, appropriate criteria for patient selection has yet to be completely defined in terms of size of the resection cavity and timing after surgery.
CONCLUSION
SRS and HFSRT have become increasingly important treatment techniques in the management of brain metastases. An approach of SRS alone as initial treatment of brain metastases has allowed patients to delay or avoid WBRT and its associated side effects. Further studies are necessary to determine which patients may benefit from this approach. One of the most critical questions is how benefit is defined and from who's perspective – patient, provider, payer, or society. Many centers with high volume practices feel comfortable treating multiple lesions at multiple time points in patients with an excellent performance status. However, whether the cost of this approach is justified has yet to be defined.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/5/185/111295
Contributor Information
Lia M. Halasz, Email: lhalasz@uw.edu.
Jason K. Rockhill, Email: jkrock@uw.edu.
REFERENCES
- 1.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV. Assessment of brain metastases with dynamic susceptibility-weighted contrast-enhanced MR imaging: Initial results. Radiology. 2003;228:193–9. doi: 10.1148/radiol.2281020298. [DOI] [PubMed] [Google Scholar]
- 6.Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85:e109–16. doi: 10.1016/j.ijrobp.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horky LL, Hsiao EM, Weiss SE, Drappatz J, Gerbaudo VH. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol. 2011;103:137–46. doi: 10.1007/s11060-010-0365-8. [DOI] [PubMed] [Google Scholar]
- 8.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–34. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–7. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matuschek C, Bölke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187:135–9. doi: 10.1007/s00066-010-2184-4. [DOI] [PubMed] [Google Scholar]
- 12.Mehta MP, Shapiro WR, Glantz MJ, Patchell RA, Weitzner MA, Meyers CA, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: Centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. J Clin Oncol. 2002;20:3445–53. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 13.Robbins JR, Ryu S, Kalkanis S, Cogan C, Rock J, Movsas BS, et al. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71:937–43. doi: 10.1227/NEU.0b013e31826909f2. [DOI] [PubMed] [Google Scholar]
- 14.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–8. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 15.Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients With One to Three Brain Metastases From Solid Tumors After Surgical Resection or Radiosurgery: Quality-of-Life Results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–61. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance Epidemiology and End Results. [Last accessed on 2010]. Available from: http://seer.cancer.gov/index.html .


