Abstract
A substantial, but uncertain, number of patients with cancer develop brain metastases. Risk of brain metastasis is recognized to vary with type of primary cancer. Within specific types of primary cancer, prognostic factors for development of brain metastases are being recognized. Recent data suggest that molecular biomarkers that relate to cellular function can predict risk of developing brain metastases. Such information could optimize surveillance standards and/or be used to select patients for preventive interventions. Though average survival for patients with brain metastases is typically less than 6 months, it is well-recognized that subgroups of patients have significant probability of longer survival. Multiple prognostic models have been proposed, validated, and compared without clearly demonstrating superiority of one model over another. However, some factors show consistency as predictive variables across models, and performance status is almost universally significant. Application of predictive models to specific treatments has been difficult. Tumor-specific prognostic models are evolving, and combinations of biological and clinical factors may be used to optimize models for particular primary tumor types.
Keywords: Biomarkers, brain metastasis, prognosis model
INTRODUCTION
Early studies of patients with brain metastases revealed poor prognosis with median survival of 1 month reported for patients not treated with either radiation or surgery, and about 3-4 months among treated patients.[27,39] However, the functional status of patients reported in these early series was generally poor compared with those reported today, probably because of limited diagnostic capabilities leading to more advanced symptoms before referral for treatment. For example, one study of 108 patients treated with whole brain radiation therapy (WBRT) for brain metastases from 1958 to 1966 reported 81% of their patients having a functional status that would correspond on the Karnofsky Performance Status (KPS) scale to 30 or less.[39] In studies of WBRT patients treated in the 1950s and 1960s, 17-22% did not complete treatment.[9,11,39] Modern series typically consist of patients of higher functional status than those diagnosed in the era predating computerized axial tomography. It has been recognized that subgroups of patients can be identified with significantly different survival prognosis. Extensive effort has been made over the past two decades to derive and improve prognostic models, which have the potential to aid in patient counseling, guide treatment standards, and optimize clinical trials. Similar efforts have been applied from an epidemiologic perspective to identifying cancer patients who may be at increased risk for developing brain metastases, potentially leading to the ability to most effectively select patients for surveillance and preventive treatments.
EPIDEMIOLOGY
Incidence
All studies on the incidence of brain metastases have methodological limitations. Population, hospital, and autopsy studies all have deficiencies related to insensitivity or inaccuracy of data collection, differences in diagnosis practices, referral patterns, access to health care, and selection of subpopulations for study.[19] In general, population-based studies from the past four decades suggest an incidence rate of approximately 10 per 100,000 population.[13,18,32,44,55] A current estimate has been derived of 21,000 to 43,000 patients diagnosed with brain metastases per year in the United States by applying population-based incident rates to 2010 census data.[19] Application of autopsy and clinical data to the total number of cancer cases diagnosed in the United States suggests that over 100,000 patients develop brain metastases each year.[19] Despite the uncertainty in determining the incidence rate of brain metastases, it is clear that the magnitude of the problem is substantial, affecting patients in numbers that approach the incidence rates of the most common specific primary cancer types.
Risk Factors
There is increasing interest in understanding risk factors for developing brain metastases that are specific to primary cancers. Breast cancer is the most common source of brain metastases in women, and 5-15% of women with breast cancer are estimated to develop brain metastases.[22] Commonly identified factors predictive for brain metastases in breast cancer are human epidermal growth factor receptor 2 (HER2) over expression, negative estrogen receptor (ER) status, high histologic grade, high proliferative rate, extensive extracranial metastases, and younger age.[23,40,52] Graesslin et al. derived a nomogram to predict the risk of subsequent brain metastases among breast cancer patients with nonbrain metastases.[22] Potential prognostic factors for 2136 patients with nonbrain metastatic breast cancer were prospectively entered into a database as a training set to develop the nomogram. Seventeen percent subsequently developed brain metastases with a median time to diagnosis of brain metastases of 8.9 months after diagnosis of nonbrain metastases. Independent prognostic factors for subsequent development of brain metastases were younger age, higher histologic grade, shorter time between initial breast cancer diagnosis and diagnosis of first metastasis, higher number of nonbrain metastatic sites, hormone receptor and HER2 status [HER2 positive worse than ER negative/PR negative/HER2 negative (“triple negative”), worse than HER2 negative with either ER or PR positive]. The nomogram ascribed points to each of these five independent prognostic factors. The points were added together, and the total points were projected onto a scale for predicting the percent probability of developing brain metastases. For example, a 50-year-old patient with a grade 3, HER2 positive tumor, 60-month interval from initial diagnosis to first metastasis, and more than one nonbrain metastasis would be predicted to have about a 25% chance of developing brain metastases. The nomogram was externally validated by application to a population from a different institution, and excellent correlation was observed. The nomogram was applied to a virtual clinical trial of prophylactic brain radiation to prevent brain metastases, demonstrating the nomogram's potential to select a study population to enhance the power of the trial while treating far fewer patients compared with an “all-comers” trial (without the nomogram). Similarly, Berghoff et al. found HER2/ER/PR subtypes and shorter time to develop progression of tumor after diagnosis to be significantly associated with shorter brain metastases free survival.[8] Additionally, presence of pulmonary metastasis was associated with shorter brain metastases-free survival.
Lung cancer is considered the most common source of brain metastases, making up about 30-60% of cases.[19] The high probability of developing brain metastases in small cell lung cancer (SCLC) is well-known, lending this disease to clinical trials of prophylactic brain radiation that have successfully accrued and demonstrated survival benefit.[4,43] Nonsmall cell lung cancer (NSCLC) also has a propensity for brain metastases. Prospective data from locally advanced (stage III) NSCLC patients enrolled on a randomized clinical trial of prophylactic brain radiation demonstrated 18% over 1 year developed brain metastases without prophylactic brain radiation.[34] Risk of brain metastases is less well defined in patients with early stage (stage I-II) NSCLC. Retrospective study of 975 consecutive patients undergoing surgery at a single institution for early stage NSCLC with a median follow-up of 33 months revealed that 60 (6.1%) developed brain metastases, with a 5-year cumulative probability of 10%.[24] Independent risk factors for developing brain metastases in these early stage patients were younger age, larger size of primary tumor, lymphovascular space invasion, and hilar lymph node involvement. Although each of these risk factors was statistically significant, the hazard ratios were small, ranging from 1.03 per year of age to 1.87 for lymphovascular space invasion, leading the authors to question the prognostic reliability of these clinical features. Furthermore, prognostic factors for brain metastases have varied among this and other studies in early stage NSCLC patients with mixed conclusions regarding patient age, histology, and size/stage.[5,16,17,31,53]
The desire to enhance the power of clinical trials by selecting patients most likely to benefit from prophylactic treatment against brain metastases, along with the question regarding reliability of clinical prognostic factors, has generated interest in determining which molecular biomarkers are effective predictors of brain metastasis risk. Arora et al. profiled microRNAs (miRNAs) from resected NSCLC specimens.[3] MiRNAs are noncoding RNAs (18-25 nucleotides) that regulate hundreds of genes. A matched discovery cohort of seven patients with brain metastases and six patients without brain metastases from NSCLC (mostly stage III and IV at diagnosis) had tumor samples analyzed by miRNA microarray chip hybridization. Eight miRNAs were confirmed to be significantly differentially expressed. There was no significant correlation of clinical features in this small sample with development of brain metastases, nor was there a correlation between expression of the eight miRNAs with clinical features. The combination of miR-328 and miR-330-3p were determined to be the best markers for brain metastasis risk in the discovery cohort. A validation cohort of 15 patients (stage I and II) who had primary tumor resection at the same institution showed miR-328 was significantly over-expressed in tumors from patients who developed brain metastases compared with those who did not. miR-330-3p over-expression was nearly significant as a marker for brain metastasis development in the validation cohort. Likewise, resected NSCLC brain metastasis tissue in a third cohort of patients from a different institution confirmed significant over-expression of miR-328 compared with the lung specimens from the validation cohort without brain metastases (miR-330-3p was nearly significant). Signaling pathways were shown to be affected by genes deregulated in miR-328 over-expressing cells including interleukin-1, vascular endothelial growth factor, and platelet derived growth factor. Finally, miR-328 was found to enhance migration of a NSCLC cell line, and results suggested that protein kinase C-alpha, could mediate that process.
Melanoma is another primary tumor type with a propensity for brain metastases. A prospectively accrued cohort of 900 melanoma patients (52% stages I or II, 48% stages III or IV) were studied.[57] With a median follow-up of 2.7 years, 89 developed brain metastases. Primary tumor ulceration and location on head and neck were found to independently correlate with development of brain metastases on multivariate analysis. Another study involving patients with more advanced melanoma (stages III and IV) found that 329 of 740 patients (44%) developed brain metastases.[7] Elevated lactate dehydrogenase (LDH), presence of visceral metastases, central (head, neck, and trunk) location of primary, and increased thickness of the primary were significant independent clinical factors for time to developing brain metastases after primary diagnosis.
PROGNOSIS
Seminal indices
A series of clinical trials by the Radiation Therapy Oncology Group (RTOG) evaluating various dose-fractionation regimens for treating brain metastases failed to reveal an advantage to any regimen. By the 1990s, reports of more aggressive interventions such as surgery and stereotactic radiosurgery (SRS) suggested longer survival than previously reported with WBRT regimens. In the absence of randomized trials comparing the therapies, the question remained as to whether aggressive therapies for brain metastases were truly more effective or if the longer survival could be due to selection of patients with better prognosis. In order to better characterize cohorts of patients with brain metastases, several seminal analyses were conducted of prognostic factors for survival. These analyses include the recursive partitioning analysis (RPA),[20] the Rotterdam Score,[26] the scoring index for radiosurgery (SIR),[56] the basic score for brain metastases (BSBM),[30] a scoring system reported by Rades et al. that was subsequently modified to include an additional prognostic factor,[41,42] the graded prognostic assessment (GPA),[45] and a nomogram tool.[6] There are several prognostic factors that are common to these indices. There are also a few differences as might be anticipated due to differences in the study populations, number of patients, and treatments. For example, study populations for the RPA, GPA, and nomogram tool were from the RTOG prospective database of patients treated on randomized clinical trials. However, even with use of the RTOG database in common, the temporal difference between the three studies resulted in cumulatively more patients and greater variety of therapies (WBRT dose-fractionation, radiation sensitizers, SRS) depending on recentness of the particular analysis.
The RPA analyzed 1276 patients entered on three consecutive RTOG trials.[20] This method built decision trees to form a predictive model with successive splitting into 2 homogenous subgroups based on the most significant predictor of survival in the successive populations being split. Terminal nodes were defined by having fewer than 25 patients or if no additional variables resulted in statistically significant splitting. Terminal nodes were merged if survival was similar. Variables included in this study were age, KPS, neurological function, neurologic signs and symptoms graded by severity, primary pathology, control status of primary lesion, presence or absence of extracranial metastases, number of brain metastases, side and location of the sentinel brain lesion, time from primary diagnosis to brain metastases, prior surgery to the brain, total radiation dose, and tumor response in the brain. The first split was based on KPS, with patients having a KPS <70 defining Class III and having a median survival of only 2.3 months. The most favorable group (Class I, median survival 7.1 months) consisted of those with the combination of KPS ≥70, primary controlled, age <65 years, and metastases to brain only. Three other terminal nodes were combined into Class II (median survival 4.2 months), and these patients were characterized as having KPS ≥70 combined with either primary uncontrolled, or primary controlled plus age ≥65 years, or primary controlled plus age <65 years plus extracranial metastases. The RPA was later validated by applying it to the 445 patients treated on RTOG 91-04, a randomized trial of two WBRT dose fractionation regimens, and comparing the results to those from the RTOG database used in the original RPA report.[21] Because of eligibility requirements on RTOG 91-04, none of the patients on that trial were Class III. Median survival for Class I (6.2 months) and Class II (3.8 months) did not differ significantly from the original RPA study. The RPA was also validated in a retrospective study of 528 patients treated with WBRT+/-surgery.[37] Results were similar to those from the RTOG studies, but only 3% of patients were categorized as Class I.
The Rotterdam scoring system was derived from retrospective analysis of 1292 patients with brain metastases from a single institution.[26] The significant prognostic factors with the largest hazard ratios – performance status, response to steroids, and extracranial tumor activity – were used in a prognostic model within the subgroup of patients treated with WBRT alone. Significant differences in survival were found between three groups based on combinations of these prognostic factors. However, by incorporating a subjective response to a treatment, this scoring system has largely been ignored in subsequent reports validating prognostic models.
The SIR was initially reported using a cohort of only 65 patients treated with SRS at a single institution.[56] Exclusion criteria were >5 brain metastases, any lesion ≥30 cm3, KPS <50, and need for “urgent neurosurgical intervention, or a very poor overall prognosis due to progressive systemic disease.” Most patients (89%) also received WBRT, with dose-fractionation regimen selected based on KPS. The primary endpoint was survival after first episode of SRS. Prognostic factors were analyzed by log-rank test, univariate Cox model, and multivariate Cox model. Based on those tests, five prognostic factors were deemed most significant for inclusion in the SIR – age, KPS, extracranial disease status, number of brain lesions, and volume of largest brain lesion. Each of these prognostic factors was separated into three levels that could be scored 0, 1, or 2 for a combined SIR score of 0 (worst condition) to 10 (best condition). The SIR scores were grouped as low (0-3), intermediate (4-7), and high (8-10), with significantly different median survivals of 2.9 months, 7.0 months, and 31.4 months, respectively. The RPA was also applied to these patients, and median survival for Classes I, II, and III of 20.2 months, 7.8 months, and 3.4 months, respectively, were observed. Only the SIR reached significance as an independent factor when compared with the RPA in a Cox analysis.
Lorenzoni et al. also reported the SIR to be superior to the RPA for predicting survival when applied to a cohort of 110 patients treated with SRS from a single institution.[30] In addition, this group reported a BSBM that was also an independent significant variable to predict survival, pointing out that the BSBM was a simplified method, easier to use than the SIR. This simplified method used only the three most significant binary prognostic factors to classify patients – KPS (50-70 vs. 80-100), primary tumor control (yes vs. no), and the existence of extracranial metastases (yes vs. no). The individual prognostic factors were assigned a score of 0 or 1, resulting in four classes of patients with total score ranging from 0 (worst) to 3 (best). Only three patients had a score of 0, and 86% patients had scores of 2 or 3, reflecting the selection of favorable patients for SRS at this institution. None of the 3 patients with BSBM score of 0 survived more than 4 months, while median survival for classes with scores of 1, 2, and 3 were 3.3 months, 13.1 months, and not reached (55% survival at 32 months), respectively.
In contrast to the goal of simplifying prognostication presented by the BSBM, the scoring system of Rades et al. originally described using four prognostic factors and was refined to use five prognostic factors.[41,42] All 1797 patients in the cohort received radiation to the brain, 75% of which received WBRT without SRS or neurosurgical resection. Patients were randomly assigned either to a test group or a validation group in a 2:1 ratio. Seven potential prognostic factors were evaluated in the test group – age, sex, KPS, primary tumor type, number of brain metastases, extracranial metastases, and time between diagnosis of malignancy and radiotherapy of brain metastases. Significant prognostic factors after multivariate analysis were age (>60 years vs. younger), KPS (<70 vs. 70 vs. >70), extracranial metastases (yes vs. no), number of brain metastases (1 vs. 2 to 3 vs. ≥4), and interval from tumor diagnosis to brain radiation (>6 months vs. shorter). Individual scores were assigned to each factor by dividing the percent 6 month survival rate by 10 and rounding to an integer. This gave 12 possible individual scores ranging from 1 through 7, and a total score ranging from 15 (worst) to 30 (best). Total scores were grouped into three ranges: 15-19, 20-25, and 26-30 with 6 month survival rates of 9%, 41%, and 78%, respectively. Nearly identical survivals were found when the scoring system was applied to the validation group. The authors noted that this scoring system with five prognostic factors had the potential to be more discriminating than other indices.
In 2008, Sperduto et al. reported a new prognostic index termed the GPA based on 1960 patients from five RTOG randomized trials involving radiation therapy for brain metastases.[45] Motivation for this new prognostic index included desire to incorporate number of brain metastases, avoid the uncertainties inherent to determining control of extracranial disease, and avoid prognostic factors that cannot be determined until treatment is administered (e.g., volume of largest lesion at time of SRS). Additionally, emphasis was placed on determining prognostic factors (indicative of outcome for categories of patients irrespective of treatment) as opposed to predictive factors (indicative of outcome after a specific treatment). The analysis was limited by the need to exclude two RTOG trials from the GPA analysis (and its comparison to the SIR) because those trials did not collect adequate data on number of brain metastases. Potential prognostic factors tested for use in the GPA included age, sex, KPS, histologic diagnosis of brain metastases, coexistence of brain and bone-only metastases, and number of brain metastases. Significant prognostic factors were age (>60 years, 50-59 years, <50 years), KPS (<70, 70-80, 90-100), number of brain metastases (>3, 2-3, 1), and extracranial metastases (present vs. absent). Each individual factor was assigned a score of 0, 0.5, or 1 (0 or 1 for the binary extracranial metastases factor). Summed scores were significantly different for median survival as follows: GPA 0-1, 2.6 months; GPA 1.5-2.5, 3.8 months; GPA 3, 6.9 months; and GPA 3.5-4.0, 11.0 months. The GPA was as prognostic for survival as the RPA with the added advantage of incorporating number of brain metastases, and it was more prognostic than the BSBM and SIR while avoiding the uncertainty of coding extracranial tumor control status.
Most recently, a nomogram was derived from the RTOG database inclusive of 2367 patients from seven randomized trials.[6] The goal was to determine predicted survival for an individual patient as opposed to an estimate based on the median for a prognostic group into which a patient fits. Based on a Cox model, the variables included in the nomogram were primary site and histology, control status of primary disease, existence of extracranial metastases, age, KPS, and number of brain metastases. Individual variables were projected to a point scale, and total points were projected to scales predicting 6-month survival, 12-month survival, and median survival. Interestingly KPS <70 vs. ≥70 provided the least incremental accuracy for estimating survival by the nomogram in contrast to that variable's primary significance as the first branch point in the RTOG's RPA.[20]
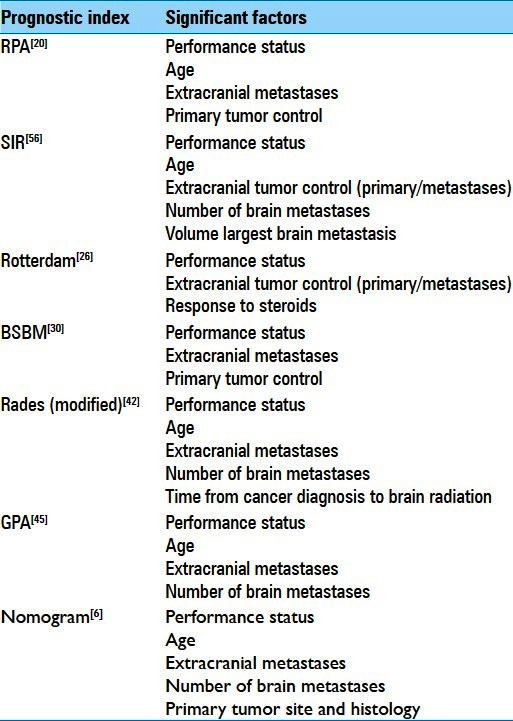
Table 1 shows the significant prognostic factors for each of the indices discussed above. With the variety of analytical methods, patient selection, data collection, and treatments, along with investigator emphasis on the distinguishing features of the particular index being espoused, one can be distracted from the striking consistency across studies in the significant prognostic factors. Clearly performance status (mainly by the KPS scale), age, status of extracranial tumor (primary and/or metastases), and number of brain metastases can be considered as generally important in determining prognosis of patients with brain metastases. The similarities between prognostic indices (RPA, BSBM, and GPA) were noted in a multicenter, multinational, prospective analysis of 285 patients.[54] The prospectively acquired data included the clinician's calculation of each patient's prognostic indices along with the individual components that make up the calculation. As a result each patient's prognostic indices could be recalculated for comparison to assess accuracy of the clinician-user. Minor discrepancies between clinician and recalculated scores (defined as 1 for BSBM/RPA or 0.5 for GPA) were observed in 7.8% for GPA, 20.4% for BSBM, and 9.8% for RPA. Major discrepancies in scores of ≥2 for BSBM/RPA or ≥1 for GPA were observed in 7.1% for GPA, 2.7% for BSBM, and 0% for RPA. These data suggest that clinicians do reasonably well in calculating these indices with trends toward more minor errors with BSBM and major errors for GPA. The latter observation was possibly due to use of a less-familiar noninteger scoring system as this study would have begun accruing a very short time after the first publication of GPA in 2008. Based on the recalculated indices, no superiority of one index over the others was determined, and the investigators cautioned against “developing a zealotry about these indexes, in which we, as physicians, rely too heavily on them.” Interestingly, multivariate analysis with inclusion of the indices found treatment center to be a significant independent variable. This unexpected finding was explained as potentially influenced by selective recruitment of patients to this study. About one-quarter of patients were prospectively enrolled out of routine clinical practice, and most of those were from one radiation oncology center. About three-quarters of patients were accrued from multiple Spanish sites on two consecutive prospective trials, one of which involved WBRT with or without temozolomide and included patients with KPS 50-60, and the other of which monitored neurological outcome in patients who received WBRT and excluded good prognosis patients treated with surgery or SRS. Though significance of treatment center may seem a minor finding, it shows that prognostic indices are unlikely to be robust enough to withstand subtle patient selection or recruitment factors. For example, the seminal studies of the SIR and BSBM apply specifically to patients undergoing SRS, and included assessment of RPA.[30,56] In both reports, the survival for RPA classes (particularly Class I) were longer than those reported from the seminal RPA study.[20] The temptation could be to attribute this longer survival to the therapy (SRS over WBRT in this example) though patient selection/recruitment may be the dominant cause.
Table 1.
Significant prognostic factors for seminal indices of survival of patients with brain metastases
Subgroup application of indices – Treatment
With establishment of multiple prognostic indices for patients with brain metastases, attention has been given to determining their utility among subgroups distinguished by therapy. In particular, there has been interest in applying prognostic indices to patients treated neurosurgically for brain metastases. This has proved to be a challenging endeavor due to common clinical factors that go into selecting patients for surgery of brain metastases, variability in the population being studied (e.g., limiting the study group to those with single brain metastasis vs. no restriction on number), inclusion vs. exclusion of patients in which the index operation is for recurrence after previously treated brain metastasis, use of other adjuvant therapies (particularly WBRT), and small study populations that confound indices that separate patients into more than three groups.
Within the topic of surgery for brain metastases, variable results were reported in studies on the utility of the RPA, with one study finding the RPA to distinguish three distinct classes,[2] and the other showing identical survival between classes II and III despite being the larger of the two studies by a factor of about 2.[51] Nieder et al. compared the five main indices [RPA, SIR, BSBM, Rades (original scoring system based on 4 variables), and GPA] in a retrospective multi-institutional analysis of 74 consecutive patients with a single brain metastasis that was surgically resected over a 15-year interval.[35] Patients accrued in the early phase of the interval received immediate postoperative WBRT, whereas more recently accrued patients were given the choice of immediate WBRT or surveillance after counseling. Eighty percent of patients received WBRT. Multivariate analysis showed significant predictive factors for longer survival were high KPS, absence of extracranial metastases, and controlled primary tumor, with KPS being the strongest variable. Limitations in each of these methods were found. For example, the SIR and GPA include a score for single vs. multiple brain metastases, which cannot be varied when applied to a cohort of patients with single brain metastases. The systems that utilize four patient classes (BSBM, Rades, GPA) all had certain classes that coalesced and did not show differences in survival. The RPA distinguished survival among all three of its classes. The SIR distinguished survival between its intermediate and favorable classes, but no patients fit into the SIR unfavorable group. All five systems defined most favorable subgroups with similar superior median survival (about 20 months). However, the systems could not be shown to define a subgroup with clearly unfavorable prognosis that could be used to exclude poor candidates for surgery. In great part, that was due to the selective nature in determining surgical candidacy that was routine in this patient population.
This concept of predicting which patients are likely to have short survival after surgery for brain metastases has been explored using the GPA.[25] The study population was retrospectively analyzed, and consisted of 141 consecutive patients operated on for brain metastases from a single institution. Most (72%) had a single brain metastasis and 68% had WBRT either before or after surgery. Confounding the study was inclusion of patients (21%) that had previously been treated with surgery for brain metastasis. Significant overall difference in survival between GPA classes was observed, but the two most favorable classes had very similar survival curves. Furthermore, attempts to use the GPA to predict 30-day and 3-month mortality were unsuccessful. All four GPA classes showed very similar mortality at 30 days (5-11%) and 3 months (14-23%). In fact, counter intuitively, the lowest mortality was in the least favorable class at 30 days and second least favorable class at 3 months. To illustrate the confusing nature of this topic, this study showed no significant difference between GPA classes within 3 months of surgery, yet Villa et al. found that the GPA, RPA, and BSBM differentiated survival between classes only within 3 months.[54] Most likely, the explanation for the apparent discrepancy lies in the selection of patients to undergo surgery. Visual inspection of the survival curves in the initial reports of the seminal indices shows that the worst classes consistently have mortality at 3 months of about 50%. This was also true in Villa et al.'s validation study.[54] Jakola et al.'s mortality at 3 months for the worst class was 23%.[25] Their stated goal was to determine if patients with very poor survival could be predicted in order to avoid surgery. However, they applied their test to a patient population that had already undergone evaluation and been determined to be suitable candidates for surgery. Consequently, the analysis simply confirmed that they had an effective screening process already occurring in their neurosurgical consultations.
Another retrospective neurosurgical series involved 309 consecutive patients from a single institution with newly diagnosed brain metastases.[48] Patients were considered eligible for surgery if the primary tumor was under control and metastases were surgically accessible. Seventy-two percent had a single brain metastasis, 24% had 2-3 brain metastases, and 4% had >3 brain metastases (treated for a life-threatening resectable lesion). Ninety-three percent received postoperative WBRT. Patients were classified by RPA and showed a very even distribution across the three classes; however, survival was not reported as a function of RPA class. Instead, the investigators focused on multivariate analysis and attempted to define an “age threshold” for surgery. The multivariate analysis identified significant independent predictive factors for survival after surgery as age, absence of extracranial metastases, postoperative KPS, radiotherapy, and re-craniotomy. Since three of these four factors were determined after surgery, it is difficult to understand how this analysis could add distinctive information to the process of selecting patients for surgery. By examining various thresholds to separate patient into older and younger groups, 65 years was found to be the most significant age threshold for predicting survival (in agreement with the RPA). Multivariate analysis was applied to patients separately for ages >65 years and ≤ 65 years. Some differences in the predictive variables were noted above and below the age threshold, but again many of those variables were determined postoperatively.
Treatment-specific analysis using RPA and GPA has been applied to Gamma Knife radiosurgery in a retrospective study of 56 consecutive patients from a single institution.[10] Seventy-five percent had SRS alone and others had WBRT either before or after SRS. Survival after SRS was the endpoint. RPA Classes I and II had significantly different median survivals of 16.5 and 6.5 months, respectively, and no patients fit Class III. GPA analysis was confounded by the small number of patients. Even after condensing into a 3-group stratification, significant difference in survival by GPA could not be determined. The most significant finding of this study was longer median survival in females (15 months) compared with males (5.5 months) that was maintained even after removal of the nine female patients with breast cancer primary tumors from the analysis. The investigators proposed a modified GPA system that added a score based on gender (0 for male, 1 for female), and collapsed the summed scores into two groups of scores 0-3 and 3.5-5 that had significantly different median survivals of 7 and 15 months, respectively.
Subgroup application of indices – Tumor type
With the goal of determining if prognostic indices could be more reliable within specific tumor types, diagnosis-specific studies were undertaken. Initially, RPA was the prognostic index of focus in these studies, and most attention was given to patients with breast cancer. To illustrate the potential problem of applying a general index like the RPA to a specific diagnosis, only 12% of patients had breast cancer in the original RPA report.[20] Furthermore, while 61% of patients had brain metastases only in the original RPA series,[20] reports of prognostic models specific to breast cancer have had only 2-35% of patients with brain metastases only.[12,28,38,47] As a result, the original RPA model when applied to breast cancer patients has resulted in a very small subset categorized as RPA Class I, forcing segregation into only two groups (Class I+II vs. Class III).[12,28] In doing so, the RPA essentially became the same as a single split of patients into those with KPS ≥70 vs. <70.[28]
Niwinska and Murawska, applying the RPA technique to 441 prospectively studied patients with brain metastases from breast cancer, reported three more distinct prognostic classifications for survival.[38] WBRT was the treatment in 98%, with 2% not treated due to poor functional status. Seventeen percent had surgical resection of 1-2 brain metastases prior to WBRT. The model included eight patient or cancer-related factors as covariates to design an RPA tree. The factors found to be significant for splitting the population in this tree were number of brain metastases, KPS, and control of extracranial disease. Their RPA model defined three classes with median survivals of 29, 9, and 2.4 months that were different from each other with a high degree of statistical significance (P < 0.0001 for all pairs). The class consistencies were as follows: Class I (brain metastases ≤2, extracranial tumor absent or controlled, and KPS = 100), Class III (>2 brain metastases and KPS <60), and Class II (all others). Application of the original RTOG RPA model to this population of breast cancer patients also generated three distinct classes of patients for survival. In fact, the median survivals using the original and breast-specific RPAs, respectively, were 10 and 9 months for Class II, and 2.7 and 2.4 months for Class III. The breast-specific RPA model only distinguished itself from the original RTOG RPA model by defining an ultra-favorable prognostic Class I with median survival of 29 months compared with 10 months with application of the RTOG RPA model. To further complicate interpretation, a separate RPA analysis was done that included treatment factors with patient and cancer factors. In doing so, surgical resection was a significant splitting factor, whereas number of brain metastases was not. In other words, the significance for number of brain metastases ≤2 vs. more, which was the primary splitting factor in the RPA model using only patient and cancer factors, was likely a reflection of the therapy selected for those patients. Difficulty with interpretation of tumor-specific RPAs (and probably other prognostic indices) is illustrated by comparing this RPA by Niwinska and Murawska with that by Sperduto et al. in breast cancer patients.[38,47] The two series were similar in median age, distribution for subtypes of HER2/ER/PR status, and percentage of patients getting surgery. There were somewhat higher percentages of patients in Sperduto et al. with 1-2 brain metastases and absence of extracranial tumor. The most striking difference in the patient populations was almost a 4-fold higher likelihood for patients to have KPS >60 in Sperduto et al. Interestingly, the breast-specific RPA of Sperduto et al. was clearly different from that of Niwinska and Murawska in that number of brain metastases and extracranial tumor control were not significant splitters, while ER/PR status, HER2 status, and age were. KPS was the only significant factor common to these two contemporary breast-specific RPA models. Yet this was the factor that was most different between the two study populations with respect to patient characteristics. The data were prospectively acquired in one population,[38] and retrospectively acquired in the other.[47] It seems logical that the degree of subjectivity and variability in assigning KPS score would be accentuated by retrospective determination from patient records that may often lack statement of KPS. With performance status consistently showing significance in these prognostic indices, perhaps it is essential for performance status to be assigned prospectively in order to minimize inconsistencies between studies and maximize the possibility of discovering universal principles.
The GPA system was used to generate a Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) in a retrospective review of 4259 patients from 11 institutions with newly diagnosed brain metastases treated with various combinations of WBRT, SRS, and/or surgery.[46] Significant factors on multivariate analysis were explored by primary tumor site. For patients with NSCLC and SCLC, the significant prognostic factors were the same as those for the original GPA analysis (age, KPS, extracranial metastases, and number of brain metastases). For patients with melanoma and renal cell cancer, only two variables (KPS and number of brain metastases) were significant. For breast and GI cancers, only one variable (KPS) was significant. The DS-GPA scoring scales for their particular variables were constructed such that the total score would be 0-4.0 as in the original GPA system. For example, in breast or GI the KPS was divided into <70, 70, 80, 90, and 100 with a whole number of 0-4 assigned, respectively. Median survivals based on groupings of the GPA were significantly different within all six specific diagnoses.
The DS-GPA for breast cancer was analyzed further in 400 patients with information on tumor subtype based on ER/PR/HER2 status.[47] This database included 283 breast cancer patients from the first DS-GPA study.[46] As usual, KPS was one of the significant prognostic factors and was used in the scoring system. Additionally, tumor subtypes based on combinations of HER2/ER/PR status were significant factors, and could be divided into four subtype combinations for scoring purposes, with triple negative having the worst prognosis. In an RPA analysis of this database, age with cutoff at 60 years was also found to be significant among patients with KPS 60-80, and was introduced back into the multivariate Cox regression model. Reintroducing age improved the Cox model and was adopted into their final breast DS-GPA scoring system along with KPS and tumor subtype. The resulting DS-GPA for groups 1-4 had significantly different median survivals ranging from 3.4 to 25.3 months, with almost no overlap in 95% confidence intervals, and a level of statistically significant difference between groups greater than what was previously observed.
While breast cancer has received the most attention for deriving diagnosis-specific prognostic indices in patients with brain metastases, there is growing information among NSCLC and melanoma patients. Agarwal et al. retrospectively studied 100 patients with brain metastases from NSCLC treated with palliative WBRT at their institution.[1] None of the patients had complete excision of brain metastases (10% had brain metastasis biopsy to establish diagnosis), and 90% received accelerated WBRT of 20 Gy in five fractions. The original RTOG RPA classification was applied.[20] Only one patient in this obviously selected population was Class I. Consequently, the analysis was essentially a splitting of patients into those with KPS ≥70 vs. <70, similar to results using the original RPA model in breast cancer patients getting WBRT reported by Le Scodan et al.[28] Median survivals were 6 months for RPA Class II and 4 months for RPA Class III,[1] closely resembling the results reported by Le Scodan et al.[28] A common theme may be that application of the generic RTOG RPA to specific tumor diagnoses results in patient factors (particularly KPS) overriding the specific diagnosis itself. It is likely that the RPA needs to be adapted to the specific diagnosis to retain its utility in that context, as has been more thoroughly investigated with the DS-GPA concept. To that end, a DS-GPA analysis on a multi-institutional database of 51 patients with melanoma brain metastases has been reported.[36] Treatments included surgery, SRS, WBRT, and various combinations. The only two significant prognostic factors for better survival on univariate analysis were higher KPS and normal LDH. The DS-GPA groups had higher median survival with higher GPA score, but the differences were not statistically significant with small numbers of patients in the subgroups. This series differed from Sperduto et al.[46] in that the number of patients studied was much smaller, number of brain metastases was not found to be significant, and LDH was analyzed. This last difference between the studies illustrates the importance of focus within a specific diagnosis when deriving diagnosis-specific indices for prognosis. The study of Sperduto et al. was exploratory in that it addressed specific tumor diagnoses, but did so by applying a wide variety of potential prognostic factors to their whole database.[46] Conversely, Nieder et al. were focused only on melanoma and thereby considered LDH as a factor because of its known impact on the overall prognosis for melanoma.[36] It appears there is potential to discover new prognostic factors in focused smaller series that could be applied to larger existing databases to further refine a DS-GPA through an iterative process. Other examples of prognostic factors specific to melanoma include presence of bone metastases,[49] hemorrhage of brain metastasis,[29] ulceration of primary tumor,[57] and elevated serum protein S-100.[15] An additional example of a prognostic factor that could refine an original DS-GPA is epidermal growth factor receptor mutation in NSCLC.[14]
Another important perspective in disease specific analysis is consideration of which patients with brain metastases should be considered for limiting treatment to shorter WBRT courses or comfort measures only. One study reported the results of 275 patients with brain metastases from NSCLC treated with WBRT alone.[50] “Early death” was defined as survival ≤6 weeks from time of recommendation to receive WBRT. On multivariate analysis, only ECOG performance status >2 was a significant prognostic factor for early death with 66% of patients experiencing early death for performance status >2 vs. 18% for performance status ≤2. Eight prognostic factors were assigned a score based on weightings from a multivariate analysis (even though only ECOG performance status was statistically significant), and the total score was used to calculate a prognostic index with a cutoff score that could define a subgroup 8.6 times more likely to experience early death. In a study of survival after WBRT for patients with brain metastases originating from “radioresistant” tumors (melanoma, renal cell carcinoma, and colorectal cancer), more protracted WBRT courses to 37.5-40 Gy (compared with 30Gy in 10 fractions) were independently associated with longer survival in RPA Class I and II patients.[33] In RPA Class 3 patients, 6 month survival was only 8% and more protracted, higher dose WBRT courses were not associated with increased survival.
Conclusions
The incidence rate of brain metastases is difficult to estimate, but its magnitude is likely on the order of 104-105 per year in the United States. Progress has been made in determining predictive factors for occurrence of brain metastases in specific primary cancer types. Early studies on biomarkers with relevance to the cellular process of metastasis are promising for enhancing predictive models. Such predictive models could be used to guide surveillance and preventive therapies individualized to patients with brain metastases. Perhaps variations on such models could be applied to patients treated with SRS or surgery for brain metastases to determine who would most likely benefit from adjuvant WBRT with the goal of preventing subsequent intracranial metastases.
Multiple prognostic models for survival after diagnosis of brain metastases have been proposed, but all seem subject to variability in results depending on the populations of patients to which the models are applied. Consequently, universal conclusions have been elusive. The most consistent finding between various prognostic models has been the significance of performance status (most commonly using the KPS system). The apparent significance of performance status, which is subject to judgment of the clinician, illustrates that data sets used to derive prognosis should be prospectively acquired with careful attention to performance status assessment. Refinements in prognostic models are ongoing, with attention to specific tumor types and biomarkers to optimize predictive tools for guiding treatment decisions and clinical trial design.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/5/192/111296
REFERENCES
- 1.Agarwal JP, Wadasadawala T, Munshi A, Chadda P, Apsani R, Upasani M, et al. Validation of recursive partitioning analysis classification in patients with brain metastases from non-small cell lung cancer treated with short-course accelerated radiotherapy. Clin Oncol. 2010;22:837–43. doi: 10.1016/j.clon.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Agboola O, Benoit B, Cross P, Da Silva V, Esche B, Lesiuk H, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys. 1998;42:155–9. doi: 10.1016/s0360-3016(98)00198-9. [DOI] [PubMed] [Google Scholar]
- 3.Arora S, Ranade AR, Tran HL, Nasser S, Sridhar S, Korn RL. MicroRNA-328 is associated with non-small cell lung cancer (NSCLC) brain metastasis and mediates NSCLC migration. Int J Cancer. 2011;129:2621–31. doi: 10.1002/ijc.25939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission.Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;34:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 5.Bajard A, Westeel V, Dubiez A, Jacoulet P, Pernet D, Dalphin JC, et al. Multivariate analysis of factors predictive of brain metastases in localized non-small cell lung carcinoma. Lung Cancer. 2004;45:317–23. doi: 10.1016/j.lungcan.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14:910–8. doi: 10.1093/neuonc/nos087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedikian AY, Wei C, Detry M, Kim KB, Papadopoulos NE, Hwu WJ, et al. Predictive factors for the development of brain metastasis in advanced unresectable metastatic melanoma. Am J Clin Oncol. 2011;34:603–10. doi: 10.1097/COC.0b013e3181f9456a. [DOI] [PubMed] [Google Scholar]
- 8.Berghoff A, Bago-Horvath Z, De Vries C, Dubsky P, Pluschnig U, Rudas M, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer. 2012;106:440–6. doi: 10.1038/bjc.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady LW, Mancall EL, Lee DK, Neff B, Shockman AT, Faust DS, et al. Radiation therapy for intracranial metastatic neoplasia. Radiol Clin Biol. 1974;43:40–7. [PubMed] [Google Scholar]
- 10.Chiou SM. Validity of the graded prognostic assessment-derived index to predict brain-metastatic patients’ survival after Gamma Knife radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78:1156–62. doi: 10.1016/j.ijrobp.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Chu FCH, Hilaris BB. Value of radiation therapy in the management of intracranial metastases. Cancer. 1961;14:577–81. doi: 10.1002/1097-0142(199005/06)14:3<577::aid-cncr2820140318>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Claude L, Perol D, Ray-Coquard I, Petit T, Blay JY, Carrie C, et al. Lymphopenia: A new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol. 2005;76:334–9. doi: 10.1016/j.radonc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Counsell CE, Collie DA, Grant R. Incidence of intracranial tumors in the Lothian region of Scotland 1989-1990. J Neurol Neurosurg Psychiatr. 1996;61:143–50. doi: 10.1136/jnnp.61.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, et al. EGFR mutation status and survival after diagnosis of brain metastases in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–9. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eigentler TK, Figl A, Krex D, Mohr P, Mauch C, Rass K, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer. 2011;117:1697–703. doi: 10.1002/cncr.25631. [DOI] [PubMed] [Google Scholar]
- 16.Feld R, Rubinstein LV, Weisenberger TH. Sites of recurrence in resected stage I non-small-cell lung cancer: A guide for future studies. J Clin Oncol. 1984;2:1352–8. doi: 10.1200/JCO.1984.2.12.1352. [DOI] [PubMed] [Google Scholar]
- 17.Figlin RA, Piantadosi S, Feld R. Intracranial recurrence of carcinoma after complete surgical resection of stage I, II, and III non-small-cell lung cancer. N Engl J Med. 1988;318:1300–5. doi: 10.1056/NEJM198805193182004. [DOI] [PubMed] [Google Scholar]
- 18.Fogelholm R, Uutela T, Murros K. Epidemiology of central nervous system neoplasms.A regional survey in central Finland. Acta Neurol Scand. 1984;69:129–36. doi: 10.1111/j.1600-0404.1984.tb07791.x. [DOI] [PubMed] [Google Scholar]
- 19.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22:1–6. doi: 10.1016/j.nec.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 21.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–6. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 22.Graesslin O, Abdulkarim BS, Coutant C, Huguet F, Gabos Z, Hsu L, et al. nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010;28:2032–7. doi: 10.1200/JCO.2009.24.6314. [DOI] [PubMed] [Google Scholar]
- 23.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, et al. Breast cancer with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 24.Hubbs JL, Boyd JA, Hollis D, Chino JP, Saynak M, Kelsey CR. Factors associated with the development of brain metastases.Analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer. 2010;116:5038–46. doi: 10.1002/cncr.25254. [DOI] [PubMed] [Google Scholar]
- 25.Jakola AS, Gulati S, Nerland US, Solheim O. Surgical resection of brain metastases: The prognostic value of the graded prognostic assessment score. J Neurooncol. 2011;105:573–81. doi: 10.1007/s11060-011-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 27.Lang EF, Slater J. Metastatic brain tumors: Results of surgical and nonsurgical treatment. S Clin N Am. 1964;44:865–72. doi: 10.1016/s0039-6109(16)37308-x. [DOI] [PubMed] [Google Scholar]
- 28.Le Scodan R, Massard C, Mouret-Fourme E, Guinebretierre JM, Cohen-Solal C, de Lalande B. Brain metastases from breast carcinoma: Validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys. 2007;69:839–45. doi: 10.1016/j.ijrobp.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Liew DN, Kano H, Kondziolka D, Mathieu D, Niranjan A, Flickinger JC, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. J Neurosurg. 2011;114:769–79. doi: 10.3171/2010.5.JNS1014. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzoni J, Devriendt D, Massager N, David P, Ruiz S, Vanderlinden B, et al. Radiosurgery for treatment of brain metastases: Estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–24. doi: 10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–9. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 32.Materljan E, Materljan B, Sepcic J, Tuskan-Mohar L, Zamolo G, Erman-Baldini I. Epidemiology of central nervous system tumors in Labin area, Croatia, 1974-2001. Croat Med J. 2004;45:206–12. [PubMed] [Google Scholar]
- 33.Meyners T, Heisterkamp C, Kueter JD, Veninga T, Stalpers LJ, Schild SE, et al. Prognostic factors for outcomes after whole-brain irradiation of brain metastases from relatively radioresistant tumors: A retrospective analysis. BMC Cancer. 2010;10:582. doi: 10.1186/1471-2407-10-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Movsas B, Bae K, Meyers C, Gore E, Bonner J, Sun A, et al. Phase III study of prophylactic cranial irradiation vs. observation in patients with stage III non-small-cell lung cancer: Neurocognitive and quality of life analysis of RTOG 0214. Proceedings of ASTRO. Int J Radiat Oncol Biol Phys. 2009;75:S1. [Google Scholar]
- 35.Nieder C, Astner ST, Andratschke NH, Marienhagen K. Postoperative treatment and prognosis of patients with resected single brain metastasis: How useful are established prognostic scores? Clin Neurol Neurosurg. 2010;113:98–103. doi: 10.1016/j.clineuro.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Nieder C, Marienhagen K, Geinitz H, Grosu AL. Can current prognostic scores reliably guide treatment decisions in patients with brain metastases from malignant melanoma? J Cancer Res Ther. 2011;7:47–51. doi: 10.4103/0973-1482.80458. [DOI] [PubMed] [Google Scholar]
- 37.Nieder C, Nestle U, Motaref B, Walter K, Niewald M, Schnabel K. Prognostic factors in brain metastases: Should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys. 2000;46:297–302. doi: 10.1016/s0360-3016(99)00416-2. [DOI] [PubMed] [Google Scholar]
- 38.Niwinska A, Murawska M. New breast cancer recursive partitioning analysis prognostic index in patients with newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2065–71. doi: 10.1016/j.ijrobp.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 39.Order SE, Hellman S, Von Essen CF, Kligerman MM. Improvement in quality of survival following whole-brain irradiation for brain metastasis. Radiology. 1968;91:149–53. doi: 10.1148/91.1.149. [DOI] [PubMed] [Google Scholar]
- 40.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–44. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 41.Rades D, Dunst J, Schild SE. A new scoring system to predicting the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2008;84:251–5. doi: 10.1007/s00066-008-1831-5. [DOI] [PubMed] [Google Scholar]
- 42.Rades D, Dziggel L, Haatanen T, Veninga T, Lohynska R, Dunst J, et al. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys. 2011;80:1122–7. doi: 10.1016/j.ijrobp.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 43.Slotman B, Faivre-Finn C, Karmer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 44.Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101:1919–24. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 46.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberg D, et al. Diagnostic-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–7. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark AM, Stohring C, Hedderich J, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: Prognostic factors and survival in 309 patients with regard to patient age. J Clin Neurosci. 2011;18:34–8. doi: 10.1016/j.jocn.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 49.Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer. 2010;102:1213–8. doi: 10.1038/sj.bjc.6605622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sundaresan P, Yeghiaian-Alvandi R, Gebski V. Prognostic index to identify patients who may not benefit from whole brain radiotherapy for multiple brain metastases from lung cancer. J Med Imaging Radiat Oncol. 2010;54:69–75. doi: 10.1111/j.1754-9485.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 51.Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, Jin T, et al. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys. 2006;66:810–7. doi: 10.1016/j.ijrobp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 53.The Ludwig Lung Cancer Study Group. Patterns of failure in patients with resected stage I and II non-small-cell carcinoma of the lung. Ann Surg. 1987;205:67–71. doi: 10.1097/00000658-198701000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villa S, Weber DC, Moretones C, Manes A, Combescure C, Jove J, et al. Validation of the new graded prognostic assessment scale for brain metastases: A multicenter prospective study. Radiat Oncol. 2011;6:23. doi: 10.1186/1748-717X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219–26. doi: 10.1212/wnl.35.2.219. [DOI] [PubMed] [Google Scholar]
- 56.Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, et al. Radiosurgery for brain metastases: A score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–61. doi: 10.1016/s0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 57.Zakrzewski J, Geraghty LN, Rose AE, Christos PJ, Mazumdar M, Polsky D, et al. Clinical variables and primary tumor characteristics predictive of the development of melanoma brain metastasis and post-brain metastasis survival. Cancer. 2011;117:1711–20. doi: 10.1002/cncr.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]


