Abstract
Bacterial responses to phosphorus limitation, commonly inorganic phosphate (Pi), are important survival mechanisms in a variety of environments. The two-component sensor kinase PhoR and its cognate response regulator PhoB are central to the Pi limitation response of many bacteria and control the large Pho regulon. Limitation for Pi significantly increased attachment and biofilm formation by the plant pathogen Agrobacterium tumefaciens, and this was driven by PhoB. Surprisingly, it was also found that both phoR and phoB were essential in A. tumefaciens. Expression of a plasmid-borne copy of the low affinity Pi transporter (pit) from Sinorhizobium meliloti in A. tumefaciens abolished the phoB and phoR essentiality in A. tumefaciens and allowed direct demonstration of the requirement for this regulatory system in the biofilm response. Increased attachment under Pi limitation required a unipolar polysaccharide (UPP) adhesin. Mutation of a polyisoprenylphosphate hexose-1-phosphate transferase (PHPT) called uppE abolished UPP production and prevented surface attachment under Pi-replete conditions, but this was rescued under Pi limitation, and this rescue required phoB. In low Pi conditions, either uppE or a paralogous gene Atu0102 is functionally redundant, but only uppE functions in UPP synthesis and attachment when Pi is replete. This conditional functional redundancy illustrates the influence of phosphorus availability on A. tumefaciens surface colonization.
Keywords: Biofilm, Phosphate limitation, PhoR-PhoB, Adhesin
1. Introduction
The element phosphorus is an important compound for all life forms and is often a limiting nutrient in natural environments, usually in the form of inorganic phosphate (Pi). Bacteria have evolved a wide range of mechanisms by which they can adapt to Pi limitation, including enhanced uptake of phosphorus-containing compounds from the environment, release of phosphatase enzymes that can release Pi from macromolecules and activation of phosphorus-sparing systems (Benning et al., 1995; Wanner, 1993). The response to limiting phosphate has been well studied in several model bacterial systems. In Escherichia coli, a very large regulon of genes is controlled in response to Pi limitation by the PhoR-PhoB two-component system (Hsieh and Wanner, 2010; Van Bogelen et al., 1996). For this regulatory system, PhoR is kept inactive in conditions with replete Pi. If the levels of Pi are diminished, PhoR senses this change (directly, or indirectly through interaction with the PstSCAB phosphate transporter through the PhoU coupling protein) and its histidine kinase activity is stimulated. Phospho-PhoR donates phosphate to the PhoB response regulator, which is a transcription factor that controls many of the genes influenced by the Pi limitation response. PhoR and PhoB are well conserved and have been studied in many different proteobacteria.
The phosphate limitation response has also been examined in plant-associated members of the Rhizobiaceae, most extensively in Sinorhizobium meliloti, and also relies upon homologues of PhoR and PhoB (McDermott, 2000). The uptake of phosphorus from the environment in S. meliloti can be mediated by at least three different major pathways: (i) Pit low affinity Pi permease; (ii) high affinity ABC-type Pi transporter PstSCAB; and (iii) the similar high affinity PhoCDET ABC transporter, that likely preferentially transports phosphonates, but will also transport Pi (Voegele et al., 1997; Yuan et al., 2006a). In S. meliloti, phospho-PhoB has been shown to activate pstSCAB and phoCDET expression and to repress pit expression under Pi limitation (Yuan et al., 2006a). Conversely, when Pi is replete, expression of neither pstSCAB nor phoCDET is activated and pit expression is derepressed. This regulatory pattern makes sense, as Pit does not require ATP to drive Pi transport when it is abundant, but when conditions are limiting, the cell utilizes energy to scavenge Pi and similar phosphorus-containing compounds from the environment.
In previous studies of the plant pathogen Agrobacterium tumefaciens, a member of the Rhizobiaceae related to S. meliloti, we had found that the Pi limitation response stimulated both adherence to surfaces and biofilm formation (Danhorn et al., 2004). A. tumefaciens is a plant pathogen that causes crown gall and is well known for its ability to transfer DNA into plant cells (Tzfira and Citovsky, 2008). To determine whether A. tumefaciens PhoR-PhoB orthologues were required for enhancement of biofilm formation at low Pi, we had attempted to create independent null mutations in phoR and phoB, but found that neither gene could be disrupted (Danhorn et al., 2004). Provision of plasmid-borne copies of either phoR or phoB allowed disruption of the corresponding genomic copy, suggesting that these genes were essential in A. tumefaciens. This was surprising, as these regulators have been disrupted in many other bacteria, and although the mutations typically debilitate the Pi-limitation response, the genes are clearly non-essential in other species. Using a controlled expression system, we were able to demonstrate the elevation of biofilm formation in static conditions by high-level expression of phoB even under Pi replete conditions, suggesting that the enhanced biofilm levels were a component of the Pi limitation response.
In this study, we report that expression of the S. meliloti pit gene from a plasmid suppresses the observed essentiality of phoR and phoB in A. tumefaciens. Using this information, the requirement of this regulatory system for enhanced biofilm formation is directly tested. Stimulation of attachment under Pi limitation requires a recently discovered polar adhesin called the unipolar polysaccharide (UPP), and mutants which cannot synthesize the UPP do not attach. However, we report that the effect of a presumptive UPP synthesis gene is ameliorated under Pi limitation, and that this is due to a functional redundancy in A. tumefaciens that is only effective at low Pi.
2. Materials and methods
2.1. Strains, plasmids, reagents and growth conditions
All of the strains and plasmids used in this study are described in Table 1. Buffers, antibiotics and microbiological media were obtained from Fisher Scientific (Pittsburgh, PA, USA) and Sigma Chemical Co. (St. Louis, MO, USA). DNA manipulations were performed in accordance with standard protocols (Sambrook et al., 1989). Plasmids were electroporated into A. tumefaciens by a standard method (Mersereau et al., 1990). DNA sequencing was performed with ABI BigDye Terminator version 3.1 on an ABI 3700 sequencer operated by the Indiana Molecular Biology Institute. Oligonucleotides were obtained from Integrated DNA Technologies, Coralville, IA, USA (primer information is listed in Table S1). Alexa Fluor 594 conjugate of wheat germ agglutinin (af-WGA) was obtained from Invitrogen (Grand Island, NY, USA). Fluorescein-labeled Dolichos biflorus agglutinin (fl-DBA) from a fluorescein lectin kit was obtained from Vector Laboratories, (Burlingame, CA, USA). The E. coli strains used for plasmid DNA transformation or conjugation of plasmids were grown in LB broth (Difco Bacto tryptone at 10 g liter−1, Difco yeast extract at 10 g liter−1 and NaCl at 5 g liter−1, pH 7.2) with or without 1.5% (w v−1) agar. A. tumefaciens derivatives were grown on AT minimal salt medium (Tempé et al., 1977) supplemented with 0.5% (wt vol−1) glucose and 15 mM ammonium sulfate (ATGN). To prevent the accumulation of iron oxide precipitate, the FeS04 prescribed in the original AT recipe was omitted, with no adverse growth effect. However, for biofilm cultures, 22uM FeSO4·7H2O was added to ATGN medium immediately before use. For phosphorus limitation experiments, the phosphate buffer of the AT medium was replaced with 5 mM imidazole buffer, pH 7, and 50 μM phosphate was added ((Danhorn and Fuqua, 2007). For sacB counterselection, 5% sucrose (Suc) replaced glucose as the sole carbon source (ATSN). Antibiotics were used at the following concentrations (mg liter−1): for E. coli, ampicillin (Ap), 50; gentamicin (Gm), 25; kanamycin (Km), 50; streptomycin (Sm), 25; and for A. tumefaciens, Gm, 300; Km, 150; Sm, 2,000.
Table 1.
Strains and plasmids
| Strain/plasmid | Relevant features | Reference |
|---|---|---|
| E. coli | ||
| DH5α/λpir | λpir; cloning strain | (Chiang and Rubin, 2002) |
| TOP10 F’ | Cloning strain | Invitrogen |
| S17-1/λpir | λpir; Tra+, cloning host | (Kalogeraki and Winans, 1997) |
| SY327/λpir | λpir; cloning host | (Miller and Mekalanos, 1988) |
| A. tumefaciens 1 | ||
| C58 | Nopaline type strain; pTiC58; pAtC58 | (Watson et al., 1975) |
| JX100 | ΔcrdS (ΔAtu3055-3057) | This study |
| JX101 | ΔchvAB (ΔAtu2728-2730) | This study |
| JX102 | Δcel cluster (ΔAtu3302-8187) | This study |
| PMM26 | Δupp cluster (ΔAtu1235-1240) | Merritt et al. in preparation |
| MLL2 | ΔexoA (ΔAtu4053) | (Tomlinson et al., 2010) |
| JX103 | Δ crdS Δ exoA | This study |
| JX108 | Δ crdS Δ cel Δ exoA | This study |
| JX110 | ΔcrdSΔcelΔexoAΔchvAB, | This study |
| JW6 | phoR::Ω-Km, carrying pPM194 (Plac-pitSm) | This study |
| PMM34 | ΔuppE carrying pPM194, with phoB:: pTD105 |
This study |
| PMM13 | Δ uppE | Merritt et al. in preparation |
| JX112 | ΔAtu0102 | This study |
| JX113 | ΔuppE ΔAtu0102 | This study |
| JX114 | ΔuppE ΔAtu3327 | This study |
| TD5 | NTL4 derivative; pTi−
phoB::pTD105, PtraI-phoB |
(Danhorn et al., 2004) |
| Plasmids | ||
| pGEM-T easy | PCR cloning vector: ApR | Promega |
| pNPTS138 | ColE1 suicide plasmid, sacB, KmR | (Hibbing and Fuqua, 2011) |
| pKNG101 | R6K ori; Sucs SmR | (Kaniga et al., 1991) |
| pHP45 ΩKm | Ω -KmR cassette | (Fellay et al., 1984) |
| pTD114 | pBBR1MCS-5 derivative, GmR; Plac::traR, PtraI |
(Danhorn et al., 2004) |
| pTD115 | pTD114 carrying phoB | (Danhorn et al., 2004) |
| pVIK112 | R6K ori; lacZY for transcription fusions; KmR | (Kalogeraki and Winans, 1997) |
| pBBR1-MCS5 | Broad-host-range Plac expression vector; GmR |
(Kovach et al., 1995) |
| pJX103 | pNPTS138 carrying ΔAtu0102, KmR | This study |
| pJX104 | pNPTS138 carrying ΔAtu3327, KmR | This study |
| pTD105 | pVIK112 carrying phoB internal fragment, KmR |
(Danhorn et al., 2004) |
| pPM194 | pBBR1-MCS5 Plac-pitSm, GmR | This study |
| pTD102 | pKNG101 carrying phoR::Ω-Km, KmR | (Danhorn et al., 2004) |
All A. tumefaciens strains are derivatives of C58, except TD5, which is derived from the Ti plasmidless derivative A. tumefaciens NTL4, originally from C58 (Luo et al., 2001)
2.2. Recombinational mutagenesis and in-frame markerless deletion mutants
Campbell insertion in phoB was performed as reported previously (Danhorn et al., 2004). The pTD105 suicide plasmid (pVIK112 carrying the phoB gene Atu0425 truncated at both ends), was transformed into E. coli S17-1/λpir and then conjugated into A. tumefaciens C58 derivatives either with a plasmid-borne copy of the S. meliloti pit gene expressed from the lac promoter (Plac-pitSm) or a vector control (Fig. S1). The pTD105 plasmid cannot replicate in A. tumefaciens, and transconjugants carrying the plasmid-interrupted genomic copy of phoB were selected on ATGN plates with Km. Presumptive KmR recombinants were analyzed by PCR using a plasmid primer (lacZ) and a primer (phoB-1) located upstream of the recombined fragment of phoB (Fig. S1).
Allelic replacement of A. tumefaciens genes interrupted with antibiotic resistance cassettes and creation of markerless in-frame deletions was performed as reported previously (Danhorn et al., 2004; Merritt et al., 2007). For allelic replacement of phoR (Atu0419), the pTD102 suicide plasmid carrying the phoR gene interrupted with a KmR cassette was transformed into E. coli S17-1/λpir and then conjugated into A. tumefaciens C58 harboring either the pPM194 (Plac-pitSm) or a vector control (Fig. S2). Transconjugants with the integrated plasmid were selected on ATGN plates plus Sm and Km (the plasmid and cassette resistance markers, respectively) and allelic replacement recombinants were subsequently selected for using the pTD102 sacB marker (Kaniga et al., 1991) and sucrose sensitivity (SucS) while also maintaining selection for the KmR cassette. Presumptive SucRKmRSmS recombinants were evaluated by PCR with external primers (phoR-1 and phoR-2) for replacement of the genomic phoR gene with phoR:: Km (Fig. S2).
For allelic replacement of genes with in-frame deletions, approximately 500 to 1,000 bp of flanking sequence upstream (primers P1 and P2) and downstream (primers P3 and P4) of the reading frame targeted for deletion were amplified by PCR. Primers were designed to remove as much of the coding sequence as possible without disrupting any possible translational coupling. Primers P2 and P3 were designed with 18-bp complementary sequences at their 5′ ends (lower case italicized nucleotides in Table S1) to facilitate splicing by overlapping extension (SOE), essentially as described previously (Warrens et al., 1997). Briefly, both flanking sequences were amplified using the high-fidelity Phusion DNA polymerase (NEB) and were agarose-gel-purified. Purified PCR products were used as both templates and primers for a five-cycle PCR. A final PCR step used primers 1 and 4, with 2 μl of the second-step reaction mix as the template, generating the full-length spliced product. The final PCR products were cloned into pGEM-T Easy (Promega), confirmed by sequencing, excised by cleavage with the appropriate restriction enzyme and ligated with suicide vector pNPTS138 cleaved at compatible restriction sites. The pNPTS138 suicide plasmid derivatives, conferring KmRSucS, were introduced into A. tumefaciens C58 by conjugal transfer, and transconjugants with the intergrated plasmid were selected on ATGN plus Km. As described above, allelic replacement recombinants were subsequently selected using the pNPTS138 sacB marker and sucrose sensitivity (SucS) by plating on ATSN. Plasmid excision was verified by patching SucR clones onto ATGN plus Km to identify derivatives that had lost the plasmid KmR marker. Appropriate deletion of the target genes was confirmed by diagnostic PCR and DNA sequencing of the products (with primers P5 and P6, which flank the target region and primer P7, which is within the target region).
2.3. Expression plasmid for S. meliloti pit
Construction of a plasmid to ectopically express the S. meliloti pit gene (pitSm, SMc02861) was performed as reported previously (Merritt et al., 2007). Coding sequences for pitSm were PCR-amplified from S. meliloti 1021 genomic DNA using the corresponding primers Sm pit 5′ and Sm pit 3′ (Table S1) and the Phusion polymerase, ligated into pGEM-T Easy, confirmed by sequencing, excised by restriction enzyme cleavage and ligated with appropriately cleaved pBBR1MCS-5 plasmid. (Kovach et al., 1995). 5′ primers were designed with stop codons in all three reading frames downstream of the pBBR lacZα to prevent translational readthrough and followed by the E.coli lacZ ribosome binding site to optimize expression of pitSm. Plasmid derivatives harboring the correct inserts were verified by restriction digestion and sequencing prior to electroporation into competent A. tumefaciens cells and this Plac-pitSm plasmid was designated pPM194.
2.4. Cultivation and analysis of static culture biofilms
Static culture biofilms were grown essentially as described previously (Ramey et al., 2004). Briefly, sterile polyvinyl chloride (PVC) cover-slips were placed vertically in 12-well polystyrene cell culture plates (Corning Inc., Corning, NY, USA), inoculated with cells in ATGN at an OD600 of 0.05, and incubated at room temperature for 72 h. For crystal violet (CV) staining of biofilms, coverslips were rinsed in double-distilled H2O, stained with 0.1% (wt vol−1) CV for 10 min, and rinsed again in double-distilled H2O. CV-stained biomass adhering to the coverslip was quantified by soaking stained coverslips in 1 ml of 33% acetic acid to solubilize the CV, followed by absorbance measurement of the soluble stain at 600 nm (A600) in a Bio-Tek Synergy HT microplate reader. Absorbance values were normalized to culture growth by dividing the A600 value for solubilized CV by the OD600 of the culture.
2.5. Flow cell configuration and analyses
The flow cell biofilm assay was performed essentially as described previously (Danhorn et al., 2004). Briefly, once-through flow cells (Christensen et al., 1999) with a 200 μl chamber volume were inoculated with A. tumefaciens carrying pJZ383 (Ptac::gfpmut3) for GFP expression. All tubing and bubble traps were autoclaved prior to assembly of the flow cell system. The system was filled with 0.5% sodium hypochlorite and left overnight without flow. A minimum of 2 l of sterile water was flushed through the system prior to treatment with 0.6% hydrogen peroxide at a flow rate of ~30 ml h−1 for at least 3 h. Flow cells were flushed with 2-3 l of sterile water and then equilibrated with AT minimal medium with 0.5% mannitol (ATMN) as a carbon source (flow rate of 3 ml h−1) for at least 12 h prior to inoculation. For each strain tested, three individual flow channels were inoculated. Each chamber was inoculated with 200 μl of cells suspended in ATMN at an OD600 of 0.4. After 30 min, flow was resumed at a rate of 3 ml h−1 and continued uninterrupted for the duration of the experiment. A crude culture extract with high levels of 3-oxooctanoyl-l-homoserine lactone (3-oxo-C8-HSL) was added to the ATMN to a final concentration of 0.1% to induce expression of the PtraI-phoB gene carried on pTD115. Surface colonization of the glass slide was monitored for several days using a Perkin Elmer spinning disk confocal microscope (SDCM). Five stacks of z-sections (0.5 or 0.8 μm spacing) were taken for each of three flow cell chambers per treatment, and the results shown are the average of 15 sample image stacks, corresponding to a surface total area of approx. 236,500 μm2, sufficient to yield representative quantitative biofilm data. Each field of view had an area of approximately 1.58 × 104 μm2. Images were acquired with the Perkin Elmer UltraView software package and analyzed with the autoCOMSTAT program based on the COMSTAT package by Heydorn et al. (Heydorn et al., 2000; Merritt et al., 2007), running in MatLab R2006a.
2.6. Short-term binding assays and lectin labeling
A short-term binding assay was performed essentially as described previously (Merritt et al., 2007). Assays were conducted by growing the appropriate strains in ATGN or limited Pi medium to an OD600~0.6. Glass coverslips were floated on 5 ml of culture in six-well tissue culture plates for 2 h. Coverslips were removed from the plates and rinsed thoroughly with 1X AT buffer (79 mM KH2PO4, pH 7.0). Those coverslips were placed on top of 100 μl 10 μg ml−1 af-WGA or fl-DBA. After 20 min, coverslips were rinsed gently 3 times with 1X AT buffer and mounted on slides for microscopy. Each strain was tested in triplicate, with 10 view fields captured for each coverslip.
3. Results
3.1. The PhoR-PhoB system stimulates
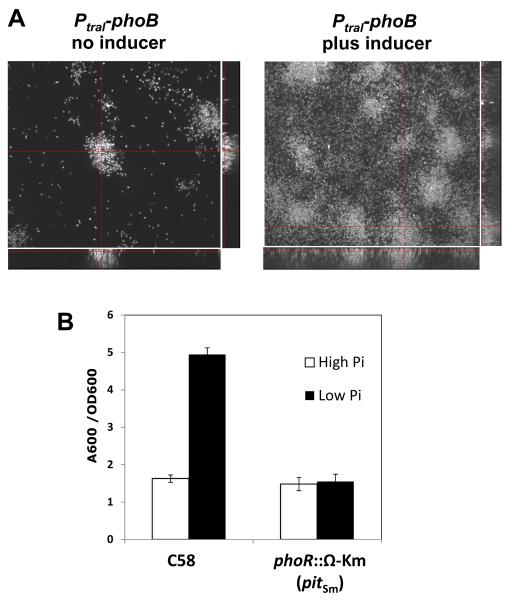
A. tumefaciens biofilm formation in flow cells. Our previous findings clearly indicated that the phoR (Atu0419) and the phoB (Atu0425) genes of A. tumefaciens C58 are essential under a variety of Pi levels and several alternative sources of phosphorus (Danhorn et al., 2004). This observation was surprising and precluded addressing the simple question of whether the enhanced biofilm formation observed under Pi limitation required the PhoR-PhoB response pathway. We circumvented the essentiality of the phoR and phoB genes by constructing a strain in which the resident copy of phoB was disrupted and an intact plasmid-borne copy was expressed from the strong but tightly controlled traI promoter (PtraI) of A. tumefaciens (Danhorn et al., 2004). The PtraI promoter is activated by the TraR transcriptional activator in response to N-3-oxo-octanoyl-L-homoserine lactone (3-oxo-C8-HSL), the quorum sensing signal produced by wild type A. tumefaciens harboring the Ti plasmid (Zhang et al., 1993). The A. tumefaciens strain in which this was used lacks the Ti plasmid and thus does not synthesize this inducing signal (Fuqua and Winans, 1994). Expression of phoB from this plasmid simulated the Pi-limitation response even under Pi-replete conditions, as determined from alkaline phosphatase induction (Danhorn et al., 2004). Under the same conditions, elevated phoB expression also stimulated biofilm formation in static cultures. We have now examined biofilm formation by this phoB controlled expression strain (also harboring a plasmid-borne constitutive gfp gene) by spinning disk confocal microscopy (SDCM) in once-through flow cells. Induction of the PtraI-phoB plasmid with a crude preparation of 3-oxo-C8-HSL (0.1% v/v) resulted in a striking increase in attachment and biofilm formation relative to the same strain in the absence of inducer (Fig. 1A), with far greater biofilm height, percent coverage and biovolume (as quantitated using autoCOMSTAT analysis). The same strain lacking the phoB plasmid does not respond to the crude 3-oxo-C8-HSL preparation at all (Danhorn et al., 2004)(data not shown). The weak biofilm formation by this strain in the absence of inducer may reflect the impact of limited uninduced phoB expression from the plasmid, although this is sufficient to allow growth.
Fig. 1. Biofilm formation in flow cells is stimulated by phoB expression under Pi-replete conditions, and Pi-limitation-enhanced biofilm formation requires phoR.
(A) SDCM images of A. tumefaciens TD5 (phoB::pTD105; pTD115, Plac-traR, PtraI-phoB) mutant derivatives expressing GFP and cultivated in once-through flow cells for 96 h in ATMN medium. Side and bottom panels are orthogonal views of the biofilms. (B) Static biofilm assays of A. tumefaciens WT and JW6 (Plac-pitSm, phoR::Ω Km) with 72 h cultures in ATGN medium (79 mM Pi) and Pi-limited medium (50 μM). The A600 of solubilized CV stain from adherent biomass was normalized by planktonic growth (OD600) of the culture. Values are averages of triplicate assays and error bars are standard deviation.
3.2. Provision of a plasmid-borne copy of the S. meliloti pit gene allows a direct demonstration that PhoR-PhoB stimulates biofilm formation in A. tumefaciens
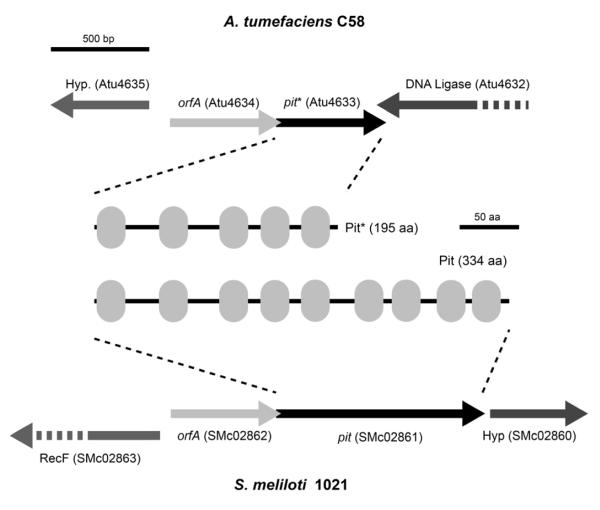
In S. meliloti the low affinity Pit transporter (encoded by S. meliloti 1021 pit, designated here as pitSm) is largely responsible for transporting Pi when it is replete (Voegele et al., 1997). Inspection of the A. tumefaciens C58 genome revealed that the pit gene (Atu4633) product is 139 amino acids shorter at its C-terminal end than the S. meliloti Pit protein, as well as other rhizobial Pit homologues, and thus may have incurred a truncation (Fig. 2). The gene immediately upstream of pitSm in S. meliloti is designated orfA and overlaps by 1 bp with the 5′ coding sequence of pitSm. Although the function of orfA is not understood, this tandem arrangement is exactly conserved in A. tumefaciens and the Atu4634 gene product is 79% identical to OrfA (Fig. 2). Sequence analysis of the oldest obtainable isolate of C58 from the culture collection at Cornell University (C58 was isolated in Geneva, NY from a cherry tree gall in 1958) revealed this pit truncation to also be present (data not shown). The C-terminal truncation of the pit gene in A. tumefaciens C58 removes four predicted transmembrane domains (Fig. 2). We therefore reasoned that provision of the S. meliloti pit gene might abolish the observed essentiality of phoR and phoB and allow us to mutate these genes to directly examine their role in biofilm formation
Fig. 2. A. tumefaciens C58 and S. meliloti orfA-pit loci and gene products.
Diagram shows the genetic context of the orfA-pit genes in A. tumefaciens C58 and S. meliloti 1021. Pit and Pit* gene products are also shown, with gray ovals indicating transmembrane domains.
In the A. tumefaciens strains harboring the Plac-pitSm plasmid, we were readily able to obtain phoB and phoR null mutants, whereas no mutants were obtained in strains harboring a vector control (Fig. S1 and S2). With these mutants in hand, we could thus examine the influence of PhoR and PhoB on biofilm formation under Pi limitation. Consistent with our prediction that the PhoR-PhoB two-component system is required for the Pi response, we observed that biofilm enhancement under Pi limiting conditions is abolished for mutants in which phoR is interrupted (Fig. 1B). Disruption of phoB results in the same lack of response to limiting Pi (data not shown).
3.3. Evaluation of the role for A. tumefaciens exopolysaccharides in biofilms under Pi limitation
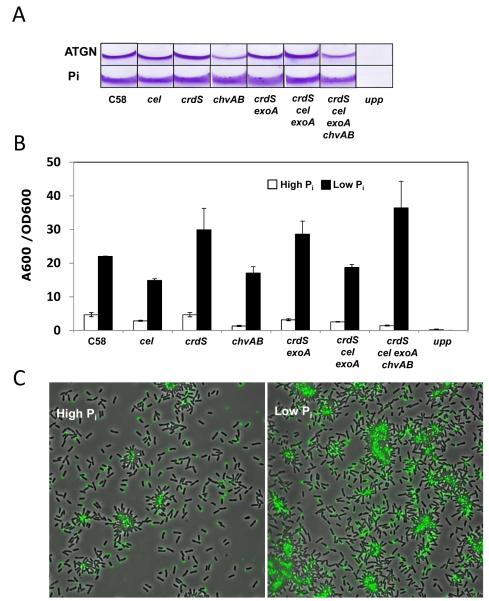
Environmental levels of Pi are known to influence the synthesis of several different exopolysaccharides in S. meliloti, including succinoglycan (EPS I) and galactoglucan (EPS II) (Mendrygal and Gonzalez, 2000). PhoB is required to activate expression of exp genes that direct production of galactoglucan under low Pi conditions. Given the recognized role of EPS in biofilm formation, it seemed possible that one of the A. tumefaciens EPSs might be responsible for the enhancement of biofilm formation under limiting Pi (although A. tumefaciens does not produce galactoglucan). We utilized a series of unmarked deletion mutations (Xu et al., submitted) that were created in biosynthetic genes for several of the known A. tumefaciens EPSs including succinoglycan (ΔexoA), cellulose (Δcel), β-1,2 glucans (ΔchvAB), β-1,3 glucans (also known as curdlan, ΔcrdS), and the unipolar polysaccharide (designated UPP ΔuppABCDEF, a cluster of genes all homologous to polysaccharide biosynthetic functions and required for UPP production). Biofilm formation of the mutants was evaluated on PVC coverslips at both high (79 mM) and low (50 μM) Pi levels. We observed that single deletion mutants disrupted in genes required for succinoglycan, cellulose, curdlan or β-1,2 glucans still maintained the Pi response (Fig. 3A and 3B). Moreover, the Pi response was still maintained in double mutants (such as ΔcrdS ΔexoA deficient in curdlan and succinoglycan), in triple mutants (such as ΔcrdS Δcel ΔexoA deficient in curdlan, cellulose and succinoglycan), and even a quadruple deletion mutant deficient in these polysaccharides plus the cyclic β-1,2 glucan mutation (ΔcrdS Δcel ΔexoA ΔchvAB). It is noteworthy that β-1,2-glucan mutants in which chvAB is deleted exhibit decreased biofilm formation under Pi replete conditions relative to the wild type, but this decrease is abolished under Pi limitation (Fig. 3A and 3B).
Fig. 3. Biofilm formation and the Pi limitation effect require the UPP but not other polysaccharides.
(A) CV-stained coverslip biofilms (48 h) of A. tumefaciens polysaccharide mutant derivatives grown in ATGN (high Pi, 79 mM) and Pi-limiting medium (low Pi, 50 μM). (B) Measurement of A600/OD600 values for acetic-acid-solubilized CV-stained 72-h biofilms under high and low Pi. Values are averages of triplicate assays and error bars are standard deviation. (C) Lectin labeling of A. tumefaciens C58 in short-term binding assays on PVC coverslip inoculated from planktonic cultures grown in ATGN and Pi-limiting medium. Cells were mixed with fl-DBA just prior to inoculation and, after 1 h, viewed at 100X magnification on a Nikon E800 epifluorescence microscope (excitation, 460-500 nm; emission, 510-560 nm) with an overlay of phase contrast and fluorescence images.
A deletion of the entire uppABCDEF gene cluster (Atu1235-1240) completely abolishes detectable UPP for A. tumefaciens (Merritt et al. in preparation). These genes are conserved in other rhizobia and, in Rhizobium leguminosarum bv. viciae, mutations in these homologues block synthesis of a unipolar glucomannan (Williams et al., 2008). Biofilm formation under Pi-replete conditions is completely blocked in the Δupp cluster deletion and Pi limitation does not rescue this phenotype at all (Fig. 3A and 3B). The UPP is only detected in wild type cultures upon surface contact (Li et al., 2012). We often evaluate UPP production using short-term binding assays on coverslips, in which the bacteria have only 1-2 hs to attach, after which they are incubated in the presence of fluorescently labeled lectin (af-WGA specific for N-acetylglucosamine or fl-DBA, specific for N-acetylgalactosamine) and subsequently examined microscopically for attachment and UPP unipolar labeling. Low Pi levels can greatly stimulate attachment and UPP production for wild type A. tumefaciens (3-5X greater attached cells from an equivalent inoculum), particularly within cell clusters, which are much more prevalent in these cultures (Fig. 3C). This effect parallels the strong increase in biofilm formation under Pi limitation. However, even under Pi limitation, planktonic single cells do not produce significant amounts of detectable UPP, but require contact with surfaces or with other cells (within aggregates) to elaborate the structure (data not shown).
3.4. A uppE mutant is rescued in Pi-limiting conditions through PhoR-PhoB
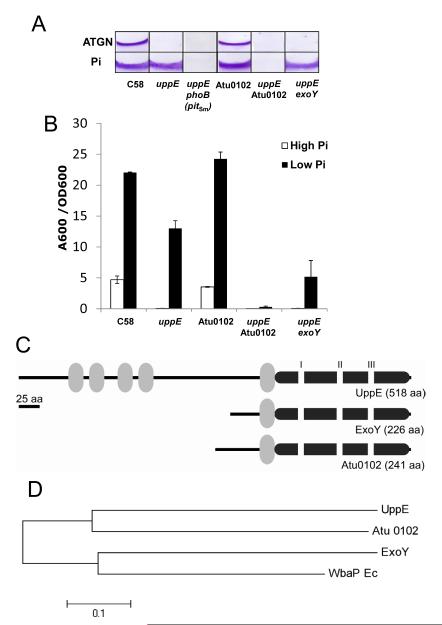
Within the uppABCDEF gene cluster, we first identified uppE (Atu1236) as the disrupted gene in a transposon mutant which could not attach to surfaces and is completely deficient for biofilm formation under Pi-replete conditions (Merritt et al. in preparation). The genes in this cluster all are homologous to polysaccharide biosynthetic proteins and they all influence UPP production. UppE is a polyisoprenylphosphate hexose-1-phosphate transferase (PHPT), defined as the WbaP component in the generalized Group I capsule synthesis pathway (Whitfield, 2006). UppE is homologous to hfsE in Caulobacter crescentus, which is involved in synthesis of the unipolar holdfast at the end of stalks in this bacterium (Toh et al., 2008). By analogy to HfsE, UppE presumably adds the initiating sugar residues, perhaps N-acetyl glucosamines, during UPP synthesis. In-frame deletion of uppE prevents biofilm formation under Pi replete conditions (Fig. 4A), as with the original transposon mutant, and also prevents normal synthesis of UPP as detected by af-WGA labeling of cells in a short-term surface binding assay as compared to the wild type (Fig. 5). Surprisingly, the biofilm deficiency of the uppE mutant was to a great extent corrected in Pi-limiting conditions (Fig. 4A and B) and production of the UPP in the short-term binding assays was also rescued (Fig. 5). No other individual mutations in the uppABCDEF cluster were rescued by low Pi (Merritt et al., in preparation).
Fig. 4. Paralogues of UppE- and PhoB-dependent functional redundancy under Pi limitation.
(A) CV-stained coverslip biofilms (72 h) of A. tumefaciens mutant derivatives grown in ATGN (high Pi, 79 mM) and Pi-limiting medium (low Pi, 50 μM). (B) Measurement of A600/OD600 values for acetic-acid-solubilized CV-stained 72 h biofilms under high and low Pi. Values are averages of triplicate assays and error bars are standard deviation. (C) Diagram of domain structure for UppE and Atu0102 and ExoY paralogues. Gray ovals indicate transmembrane domains, the large dark gray bar is the WbaP domain and the white rectangles indicate the PHPT I, II and III motifs. (D) Neighbor-joining tree of UppE paralogues. The optimal tree with the sum of branch length = 1.22142857 is shown. The tree is drawn to scale, with branch lengths reflecting evolutionary distances. The evolutionary distances were computed using the p-distance method (Nei and Kumar, 2000) and are in the units of the number of amino acid differences per site. All positions containing gaps and missing data were eliminated, and a total of 210 positions were in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011).
Fig. 5. Lectin labeling of UPP in the phoB mutant and PHPT paralogue mutants under high and low Pi.
Short-term binding assays were performed with A. tumefaciens derivatives grown in high Pi (79 mM) and low Pi (50 μM) cultures and incubated in suspension with PVC coverslips after 2 h incubation with af-WGA at room temperature. After washing, coverslips were viewed at 100X magnification on a Nikon E800 epifluorescence microscope (excitation, 510-560 nm; emission, >610 nm) with an overlay of phase contrast and fluorescence images.
Given the observation that enhancement of biofilm formation under Pi-limitation involves the PhoR-PhoB system, the rescue of uppE with Pi limitation was tested in the phoB null mutant (harboring the plasmid-expressed pitSm). Biofilm assays clearly demonstrate that phoB is required for Pi-limitation rescue of the uppE mutant (Fig. 4A).
3.5. Functional redundancy of uppE and one of its paralogues under Pi limitation
The observation that low Pi rescued the ΔuppE mutant but not the ΔuppABCDEF cluster deletion suggested that there might be a functionally redundant gene(s) in A. tumefaciens that can specifically compensate for the loss of uppE under Pi limitation. Analysis of the A. tumefaciens genome revealed the presence of two genes that are paralogous to uppE (Fig. 4C and 4D): exoY (Atu3327), which is involved in the first step of succinoglycan biosynthesis, and Atu0102, another initiating PHPT-type protein most similar to pssY from R. leguminosarum (Janczarek et al., 2009). Non-polar deletion of each gene and double deletion mutants with uppE were constructed and assayed for attachment and biofilm formation in Pi-replete and Pi-limiting medium (Fig. 4A and 4B). The ΔexoY ΔuppE double deletion mutant retained its ability to form biofilms and produce UPP under Pi-limiting conditions, albeit at lower levels than the uppE single mutant. Although the single ΔAtu0102 deletion exhibited essentially wild type levels of biofilm formation in both high and low Pi, introducing the ΔAtu0102 deletion into the ΔuppE mutant abolished the Pi limitation rescue of the uppE mutant phenotype, indicating that Atu0102 could compensate for the absence of uppE. The short-term surface binding assay further confirmed that the ΔuppE ΔAtu0102 mutant is deficient in UPP elaboration in Pi-limiting conditions (Fig. 5). The enhanced biofilm formation of the ΔAtu0102 mutant in low Pi also suggests that it is not exclusively the activity of Atu0102 that leads to this Pi stimulation, despite its ability to compensate for uppE when Pi is limiting.
4. Discussion
In this study, we have circumvented the phoR and phoB essentiality in A. tumefaciens C58 by providing a copy of the pit gene from S. meliloti, and with this construct, have demonstrated the requirement for this regulatory system in enhanced biofilm formation under Pi limitation, further revealing that it requires the UPP adhesin. We have also uncovered a functional redundancy in UPP synthesis that is conditional, only manifested under low Pi.
Our results indicate that the previously observed essentiality of phoR and phoB can be obviated by provision of a full-length pit gene from S. meliloti. The plasmid-borne pitSm likely compensates for the A. tumefaciens pit gene, which is significantly shorter (~139 codons) than pit genes in other rhizobia, suggesting a C-terminal truncation (Fig. 2). The use of the Plac-pitSm plasmid has allowed us to examine the role of the PhoR-PhoB response pathway in enhancement of A. tumefaciens biofilm formation under Pi limitation. This effect of the Plac-pitSm plasmid also suggests the possibility that in A. tumefaciens, the Pit gene product is defective, and hence phosphorus is transported into the cell via the activity of transport systems that are PhoR-PhoB-dependent. In this situation, mutants for either regulator uptake insufficient phosphorus for growth. However, provision of the plasmid-borne Plac-pitSm alters two aspects of pit function, providing a normal-length Pit protein, but also removing the pitSm gene from any endogenous regulation. Although it seems likely that the normal length Pit protein compensates for a defective A. tumefaciens pit gene, our findings do not exclude the possibility that the regulation of pit gene expression in A. tumefaciens also plays an important role in the observed phoR and phoB essentiality. In S. meliloti, the PhoR-PhoB system activates expression of the pstSCAB and phoCDET transporters, and represses the pit gene under Pi limitation (Yuan et al., 2006a). In A. tumefaciens, both of these operons have recognizable upstream pho box sequences (TTGACATTTCCCATTCAT upstream of phoC; TTGTCACAAATCTTTCGT upstream of pstC) that are good matches to the rhizobial pho box (Yuan et al., 2006b), suggesting a similar control pattern as that in S. meliloti. A similar pho box type sequence is also upstream of orfA-pit in A. tumefaciens (TTGTCATAAAACTGTCAT), consistent with the observed repression of orfA-pit in S. meliloti.
Prior to discovering that plasmid-borne pitSm expression could suppress phoR and phoB essentiality in A. tumefaciens, we provided evidence for the involvement of PhoB in the biofilm response under Pi limitation by ectopically expressing the transcription factor from a strongly activated promoter in a derivative in which the resident phoB was inactivated (Danhorn et al., 2004). In this report we expand on this result and demonstrate that phoR and phoB are necessary for enhanced biofilm formation, and thus increased attachment and biofilm formation represent components of the general Pi limitation response. It remains unclear which specific PhoB-regulated functions are responsible for the enhanced adhesion, although our data clearly indicate that the UPP polysaccharide is required for the stimulated attachment observed under Pi limitation. In P. fluorescens, Pi limitation also exerts a significant influence on attachment and biofilm formation through the PhoR-PhoB system, but in this case it inhibits these processes (Monds et al., 2001). The P. fluorescens regulatory pathway has been extensively studied, and a detailed model has developed. In P. fluorescens the transition from reversible to irreversible attachment requires the large surface protein adhesin called LapA (Hinsa et al., 2003). Phospho-PhoB activates expression of rapA, a phosphodiesterase that degrades the intracellular adhesion-stimulating second messenger cyclic diguanosine monophosphate (c-di-GMP). Elevated RapA levels resulting in low c-di-GMP promote proteolytic cleavage of the proteinacious LapA adhesin in its periplasmic domain, leading to release of the cleaved protein into the environment, and decreasing its ability to drive irreversible attachment (Newell et al., 2009). Our findings clearly show that, in contrast to the P. fluorescens system, low Pi stimulates attachment in A. tumefaciens, and that this involves the polysaccharide-based UPP adhesin. Interestingly, preliminary findings suggest that this effect is also mediated through PhoB-dependent modulation of c-di-GMP levels and that this second messenger plays a major role in driving irreversible attachment and biofilm formation through the UPP (Xu et al, in preparation). It is striking how both A. tumefaciens and P. fluorescens utilize similar tool kits to drive the response to low Pi in opposite directions. Why the two bacteria respond so differently is not clear, but it appears that P. fluorescens avoids attachment in environments low in Pi, whereas A. tumefaciens associates more avidly with surfaces under these conditions. Low Pi is recognized to stimulate virulence in A. tumefaciens during association with host plants (Winans, 1990), as plant sequestration is known to deplete local phosphorus levels in rhizosphere soils proximal to plant tissues (Hinsinger, 2001). Phosphorus is a limiting compound in the soil in general (Holford, 1997), but a variety of phosphorus-containing compounds are known to concentrate at surfaces and thus colonization of surfaces in general, both biotic and abiotic, may also enhance access to this nutrient.
An intriguing aspect of the A. tumefaciens Pi limitation response that we discovered in this study was the ability for low Pi to mask the attachment requirement for the UppE protein, a PHPT family initiating-glycosyl transferase homologue, that is needed for UPP production under Pi replete conditions. Examination of two paralogues, ExoY (Atu3327) and the PssY homologue Atu0102, led to the finding that UppE and Atu0102 are functionally redundant, but only under Pi limitation. This was surprising since, although Atu0102 is homologous to UppE and in the same general family of proteins (Fig. 4D), it is approximately 50% the size of UppE (Fig. 4C). Functional redundancy among these PHPT-type proteins in polysaccharide biosynthesis has been previously observed (Patel et al., 2012b). In fact, genetic analysis of the uppE homologue hfsE from C. crescentus identified two additional paralogues to hfsE in the C. crescentus genome (Cc0166, pssY and Cc2384, pssZ), both of which could function in the place of the others (Toh et al., 2008). A C. crescentus hfsE mutant does not manifest a deficiency in holdfast production or attachment, and in fact a triple mutant in all three paralogues is required to observe strong holdfast deficiency. In contrast to these findings in C. crescentus, we do observe a very clear attachment-deficient phenotype for the uppE mutant in high Pi, and only under Pi limitation is the redundancy between UppE and Atu0102 revealed. In further contrast to the C. crescentus results, the third paralogue homologous to ExoY (Atu3327) cannot replace UppE function under high or low Pi levels. The ability of Atu0102 to function in the place of UppE is not unprecedented as, among other PHPT proteins, it has been shown that the C-terminal region with the catalytic PHPT motifs (Fig. 4C), is sufficient to drive the linkage of hexose-phosphates to the undeprenyl carrier during polysaccharide synthesis (Patel et al., 2012a). The same basic observation holds in C. crescentus, where the N terminus of HfsE contains several predicted transmembrane segments, as does UppE, whereas the PssY and PssZ proteins that can function in its absence only contain single transmembrane segments and the catalytic motifs, similar to A. tumefaciens Atu0102 (Fig. 4C). The function of the amino terminal half of UppE and HfsE proteins, with its multiple transmembrane domains, is not yet understood.
Our studies have more deeply investigated the role of Pi limitation in attachment and biofilm formation for A. tumefaciens. These findings provide clear evidence that this represents a programmed response to low Pi, and that this response is mediated at least in part through synthesis or deployment of the UPP adhesin. The mechanism by which PhoR-PhoB mediates this control does not appear to be direct transcriptional regulation of known UPP biosynthetic genes, and is possibly through allosteric control of the attachment process by c-di-GMP.
Supplementary Material
Table S1.Oligonucleotides
Figure S1. Plasmid-borne pitSm enables phoB disruption by Campbell insertion Results from Campbell insertion mutagenesis of phoB with a non-replicating suicide plasmid carrying an internal fragment of phoB. Table indicates numbers of recombinants. The plasmid insertion process is diagrammed, and the positions of diagnostic PCR primers (phoB-4 and lacZ) are indicated. Jagged ends on gene arrows indicate truncations in the coding sequences. Agarose gel (1%) analysis of PCR products from wild type and selected recombinants using ethidium bromide and short wave UV excitation.
Figure S2. Plasmid-borne pitSm enables phoR disruption by allelic replacement. Results from allelic replacement mutagenesis of phoR with a phoR::Ω Km gene. Table indicates numbers of recombinants. The allelic replacement process is diagrammed, and the positions of diagnostic PCR primers (phoR-1 and phoR-2) are indicated. Agarose gel (1%) analysis of PCR products from wild type and selected recombinants using ethidium bromide and short wave UV light excitation.
Acknowledgements
This project was supported by National Institutes of Health grant GM080546 and through a grant from the Indiana University METACyt program, funded in part by a major endowment from the Lilly Foundation. The IU Light Microscopy Imaging Center facilitated our microscopy experiments. We thank Z.-C. Yuan et al. for pointing out the truncation in the A. tumefaciens pit gene, and the lab of Y.V. Brun for helpful input and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benning C, Huang Z-H, Gage DA. Accumulation of a novel glycolipid and a betaine lipid in the cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995;317:103–111. doi: 10.1006/abbi.1995.1141. [DOI] [PubMed] [Google Scholar]
- Chiang SL, Rubin EJ. Construction of a mariner-based transposon for epitope-tagging and genomic targeting. Gene. 2002;296:179–85. doi: 10.1016/s0378-1119(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Christensen BB, Sternberg C, Andersen JB, Palmer RJJ, Nielsen AT, Givskov M, Molin S. Molecular tools for study of biofilm physiology. Methods Enzymol. 1999;310:20–42. doi: 10.1016/s0076-6879(99)10004-1. [DOI] [PubMed] [Google Scholar]
- Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007;61:401–22. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- Danhorn T, Hentzer M, Givskov M, Parsek M, Fuqua C. Phosphorous limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J. Bacteriol. 2004;186:4492–4501. doi: 10.1128/JB.186.14.4492-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R, Frey J, Kirsch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1984;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Toftgaard Nielsen A, Hentzer M, Sternberg C, Givskov M, Kjaer Ersboll B, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Hibbing ME, Fuqua C. Antiparallel and interlinked control of cellular iron levels by the Irr and RirA regulators of Agrobacterium tumefaciens. J. Bacteriol. 2011;193:3461–72. doi: 10.1128/JB.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsa SM, Espinosa-Urgel M, Ramos JL, O’Toole GA. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003;49:905–18. doi: 10.1046/j.1365-2958.2003.03615.x. [DOI] [PubMed] [Google Scholar]
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes. Plant Soil. 2001;237:173–195. [Google Scholar]
- Holford ICR. Soil phosphorous: its measurement and its uptake by plants. Aust. J. Soil Res. 1997;35:227–239. [Google Scholar]
- Hsieh YJ, Wanner BL. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010;13:198–203. doi: 10.1016/j.mib.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczarek M, Kalita M, Skorupska AM. New taxonomic markers for identification of Rhizobium leguminosarum and discrimination between closely related species. Arch. Microbiol. 2009;191:207–19. doi: 10.1007/s00203-008-0447-6. [DOI] [PubMed] [Google Scholar]
- Kalogeraki VS, Winans SC. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene. 1997;188:69–75. doi: 10.1016/s0378-1119(96)00778-0. [DOI] [PubMed] [Google Scholar]
- Kaniga K, Delor I, Cornelis GR. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RMI, Peterson KM. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z-Q, Clemente TE, Farrand SK. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol. Plant-Microbe Interact. 2001;14:98–103. doi: 10.1094/MPMI.2001.14.1.98. [DOI] [PubMed] [Google Scholar]
- McDermott TR. Phosphorous assimilation and regulation in the rhizobia. In: Triplett EW, editor. Prokaryotic Nitrogen Fixation: A Model System for the Analysis of a Biological Process. Horizon Scientific Press; Norfolk, England: 2000. pp. 529–548. [Google Scholar]
- Mendrygal KE, Gonzalez JE. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 2000;182:599–606. doi: 10.1128/jb.182.3.599-606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt PM, Danhorn T, Fuqua C. Motility and chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J. Bacteriol. 2007;189:8005–14. doi: 10.1128/JB.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersereau M, Pazour GJ, Das A. Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene. 1990;90:149–151. doi: 10.1016/0378-1119(90)90452-w. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations:osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae. J. Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monds RD, Silby MW, Mahanty HK. Expression of the Pho regulon negatively regulates biofilm formation by Pseudomonas aureofaciens PA147-2. Mol. Microbiol. 2001;42:415–26. doi: 10.1046/j.1365-2958.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press; New York: 2000. [Google Scholar]
- Newell PD, Monds RD, O’Toole GA. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. U S A. 2009;106:3461–6. doi: 10.1073/pnas.0808933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KB, Ciepichal E, Swiezewska E, Valvano MA. The C-terminal domain of the Salmonella enterica WbaP (UDP-galactose:Und-P galactose-1-phosphate transferase) is sufficient for catalytic activity and specificity for undecaprenyl monophosphate. Glycobiology. 2012a;22:116–22. doi: 10.1093/glycob/cwr114. [DOI] [PubMed] [Google Scholar]
- Patel KB, Toh E, Fernandez XB, Hanuszkiewicz A, Hardy GG, Brun YV, Bernards MA, Valvano MA. Functional characterization of UDP-glucose:undecaprenyl-phosphate glucose-1-phosphate transferases of Escherichia coli and Caulobacter crescentus. J. Bacteriol. 2012b;194:2646–57. doi: 10.1128/JB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey BE, Matthysse AG, Fuqua C. The FNR-type transcriptional regulator SinR controls maturation of Agrobacterium tumefaciens biofilms. Mol. Microbiol. 2004;52:1495–1511. doi: 10.1111/j.1365-2958.2004.04079.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Lab. Press; CSH, NY: 1989. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempé J, Petit A, Holsters M, Van Montagu M, Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. USA. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh E, Kurtz HD, Jr., Brun YV. Characterization of the Caulobacter crescentus holdfast polysaccharide biosynthesis pathway reveals significant redundancy in the initiating glycosyltransferase and polymerase steps. J. Bacteriol. 2008;190:7219–31. doi: 10.1128/JB.01003-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson AD, Ramey-Hartung B, Day TW, Merritt PM, Fuqua C. Agrobacterium tumefaciens ExoR represses succinoglycan biosynthesis and is required for biofilm formation and motility. Microbiology. 2010;156:2670–81. doi: 10.1099/mic.0.039032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T, Citovsky V. Agrobacterium, From Biology to Biotechnology. Springer Science+Business Media; New York, NY: 2008. [Google Scholar]
- Van Bogelen RA, Olson ER, Wanner BL, Neidhardt FC. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 1996;178:4344–66. doi: 10.1128/jb.178.15.4344-4366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegele RT, Bardin S, Finan TM. Characterization of the Rhizobium (Sinorhizobium) meliloti high- and low-affinity phosphate uptake systems. J. Bacteriol. 1997;179:7226–7232. doi: 10.1128/jb.179.23.7226-7232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner BL. Gene regulation by phosphate in enteric bacteria. J Cell Biochem. 1993;51:47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29–35. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- Watson B, Currier TC, Gordon MP, Chilton MD, Nester EW. Plasmid required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- Williams A, Wilkinson A, Krehenbrink M, Russo DM, Zorreguieta A, Downie JA. Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J. Bacteriol. 2008;190:4706–15. doi: 10.1128/JB.01694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans SC. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J. Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZC, Zaheer R, Finan TM. Regulation and properties of PstSCAB, a high-affinity, high-velocity phosphate transport system of Sinorhizobium meliloti. J. Bacteriol. 2006a;188:1089–102. doi: 10.1128/JB.188.3.1089-1102.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZC, Zaheer R, Morton R, Finan TM. Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Res. 2006b;34:2686–97. doi: 10.1093/nar/gkl365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Murphy PJ, Kerr A, Tate ME. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Oligonucleotides
Figure S1. Plasmid-borne pitSm enables phoB disruption by Campbell insertion Results from Campbell insertion mutagenesis of phoB with a non-replicating suicide plasmid carrying an internal fragment of phoB. Table indicates numbers of recombinants. The plasmid insertion process is diagrammed, and the positions of diagnostic PCR primers (phoB-4 and lacZ) are indicated. Jagged ends on gene arrows indicate truncations in the coding sequences. Agarose gel (1%) analysis of PCR products from wild type and selected recombinants using ethidium bromide and short wave UV excitation.
Figure S2. Plasmid-borne pitSm enables phoR disruption by allelic replacement. Results from allelic replacement mutagenesis of phoR with a phoR::Ω Km gene. Table indicates numbers of recombinants. The allelic replacement process is diagrammed, and the positions of diagnostic PCR primers (phoR-1 and phoR-2) are indicated. Agarose gel (1%) analysis of PCR products from wild type and selected recombinants using ethidium bromide and short wave UV light excitation.