Abstract
Many patients with refractory epilepsy are treated with polytherapy, and nearly 15% of epilepsy patients receive two or more anti-convulsant agents. The anti-convulsant stiripentol is used as an add-on treatment for the childhood epilepsy syndrome known as severe myoclonic epilepsy in infancy (Dravet Syndrome). Stiripentol has multiple mechanisms of action, both enhancing GABAA receptors and reducing activity of metabolic enzymes that break down other drugs. Stiripentol is typically co-administered with other anti-convulsants such as benzodiazepines which also act through GABAA receptor modulation. Stiripentol slows the metabolism of some of these drugs through inhibition of a variety of cytochrome P450 enzymes, but could also influence their effects on GABAergic neurotransmission. Is it rational to co-administer drugs which can act through the same target? To examine the potential interaction between these modulators, we transiently transfected HEK-293T cells to produce α3β3γ2L or α3β3δ recombinant GABAA receptors. Using whole-cell patch clamp recordings, we measured the response to each benzodiazepine alone and in combination with a maximally effective concentration of stiripentol. We compared the responses to four different benzodiazepines: diazepam, clonazepam, clobazam and norclobazam. In all cases we found that these modulators were equally effective in the presence and absence of stiripentol. The δ-containing receptors were insensitive to modulation by the benzodiazepines, which did not affect potentiation by stiripentol. These data suggest that stiripentol and the benzodiazepines act independently at GABAA receptors and that polytherapy could be expected to increase the maximum effect beyond either drug alone, even without consideration of changes in metabolism.
Index words: anti-convulsant, electrophysiology, diazepam, clobazam, norclobazam, clonazepam, recombinant, patch-clamp, Dravet Syndrome
1. Introduction
The anti-convulsant stiripentol (Diacomit®) has been investigated for clinical effectiveness in epilepsy for several decades (Trojnar et al., 2005; Chiron, 2007). Although stiripentol did not show greater activity than other common anti-epileptic drugs in clinical trials with adult patients, studies in pediatric populations provided more promising results. The addition of stiripentol to polytherapy reduced the frequency and severity of seizures and status epilepticus in infants and children with a variety of epilepsy syndromes (Perez et al., 1999; Rey et al., 1999; Chiron et al., 2000; Kassai et al., 2008; Inoue et al., 2009). Stiripentol has since been approved by the European Medicines Agency for the treatment of pharmacoresistant patients with severe myoclonic epilepsy in infancy (Dravet syndrome).
Stiripentol has both direct and indirect anticonvulsant actions. It inhibits a variety of hepatic cytochrome P450 enzymes which metabolize other anti-epileptic drugs (Tran et al, 1997), increasing their duration of action. In addition, stiripentol alone is effective in animal models of acute and chronic seizures (Shen et al., 1992; Trojnar et al, 2005; Luszczki et al., 2010). Recent studies suggest that the mechanism underlying this direct activity is positive allosteric modulation of GABAA receptors (Quilichini et al., 2006; Fisher, 2009).
The GABAA receptors are ligand-gated ion channels responsible for fast, inhibitory neurotransmission. Stiripentol acted both pre- and post-synaptically to increase the frequency and slow the decay of GABAergic mIPSCs in hippocampal brain slices (Quilichini et al., 2006). In studies with recombinant GABAA receptors, stiripentol increased the response in a subunit-dependent manner, with greatest effectiveness at receptors containing an α3 subunit (Fisher, 2009). This subunit is one of the predominant α subtypes in the developing brain (Laurie et al., 1992), which may explain stiripentol’s greater clinical efficacy in childhood epilepsy syndromes. Stiripentol was also highly active at δ-containing receptors. These benzodiazepine-insensitive receptors are located extrasynaptically where they produce a tonic current in response to ambient GABA (Belelli et al, 2009).
Stiripentol is approved only for use as add-on therapy, and as such will always be co-administered with another anti-epileptic drug. In many cases, the co-therapy includes a drug targeting the GABAA receptors, such as the 1,4- or 1,5-benzodiazepines. While a number of 1,4-benzodiazepines, including diazepam and clonazepam, are widely used, the only 1,5-benozodiazepine used clinically for epilepsy is clobazam (Ng and Collins, 2007). Interestingly, much of the anti-convulsant activity of clobazam may be mediated through its active metabolite, norclobazam (N-desmethyl-clobazam) (Kinoshita et al., 2007). Stiripentol greatly increases the plasma levels of norclobazam by slowing hydroxylation through CYP2C19 (Giraud et al., 2006).
Stiripentol was initially considered for polytherapy because of its action on metabolic enzymes. With the new understanding of its activity at GABAA receptors, the potential for interactions between stiripentol and other modulators at this target becomes a concern. Therefore, we examined the effect of co-application of stiripentol with benzodiazepines on the activity of recombinant GABAA receptors to determine if these modulators interacted at the level of the receptor or if their actions were independent.
2. Materials and Methods
2.1. Transfection of HEK-293T cells
Full-length cDNAs for rat GABAA receptor subunits in pCMV expression vectors were transiently transfected into the human HEK-293T cell line. HEK-T cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/ml penicillin and 100 µg/ml streptomycin. Cells were passaged by a 2 min. incubation with 0.25% trypsin/0.1% EDTA solution in phosphate-buffered saline (10 mM Na2HPO4, 150 mM NaCl, pH = 7.3).
The cells were transfected using calcium phosphate precipitation. Plasmids encoding GABAA receptor subunit cDNAs were added to the cells in 1:1:1 ratios of 2 µg each(α:β:γ or α:β:δ). To identify positively transfected cells, 1 µg of the plasmid pHook™-1 (Invitrogen, San Diego, CA) was also included. Following a 4–6 hr. incubation at 3% CO2, the cells were treated with a 15% glycerol solution in BBS buffer (50 mM BES(N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid), 280 mM NaCl, 1.5 mM Na2HPO4) for 30 sec. The selection procedure for pHook expression was performed 44–52 h. later. The cells were passaged and mixed with 3–5 µl of magnetic beads coated with antigen for the pHook antibody (approximately 6 × 105 beads) (Chesnut et al., 1996). Following a 30–60 min. incubation to allow the beads to bind to positively transfected cells, the beads and beadcoated cells were isolated using a magnetic stand. The selected cells were resuspended into DMEM, plated onto glass coverslips treated with poly L-lysine and coated with collagen and used for recordings 18–28 h. later.
2.2. Electrophysiological recording solutions and techniques
For whole-cell recording the external solution consisted of (in mM); 142 NaCl, 8.1 KCl, 6 MgCl2, 1 CaCl2, and 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) with pH = 7.4 and osmolarity adjusted to 295–305 mOsm. Recording electrodes were filled with an internal solution of (in mM); 153 KCl, 1 MgCl2, 5 K-EGTA (ethylene glycolbis (β-aminoethyl ether N,N,N′N′-tetraacetate) and 10 HEPES with pH = 7.4 and osmolarity adjusted to 295–305 mOsm. These solutions provided a chloride equilibrium potential near 0 mV. Patch pipettes were pulled from borosilicate glass with an internal filament (World Precision Instruments, Sarasota FL) on a two-stage puller (Narishige, Japan) to a resistance of 5–10 MΩ. Drugs were applied to cells using a stepper solution exchanger with a complete exchange time of <50 msec (SF-77B, Warner Instruments, Hamden CT). There was continuous flow of external solution through the chamber. Currents were recorded with an Axon 200B (Foster City, CA) patch clamp amplifier and stored on a computer hard drive for off-line analysis. All experiments were performed at room temperature (near 25° C).
Drugs were diluted from frozen stocks in water (GABA) or made fresh in DMSO (stiripentol, benzodiazepines) on the day of the experiment. Stiripentol was provided by Biocodex (Beauvais, France) and norclobazam was synthesized by Chemtos (Austin, TX). Diazepam, clonazepam and clobazam were purchased from Sigma-Aldrich (St. Louis, MO)
2.3. Analysis of whole-cell currents
Whole-cell currents were analyzed off-line using the programs Clampfit (pClamp8 suite, Axon Instruments, Foster City CA) and Prism (Graphpad, San Diego, CA). Normalized concentration-response data were fit with a four-parameter logistic equation (Current = (Minimum current + (Maximum current-Minimum current))/(1+(10^(log EC50- log [drug])*n) where n represents the Hill number. All fits were made to normalized data with the current expressed as a percentage of the peak current elicited by the response to GABA alone or GABA + 100 µM stiripentol. Statistical tests were performed using the Instat program (Graphpad). Differences between treatments were determined with a Student’s t- test with a minimum P value for significance of 0.05.
3. Results
3.1. 1,4- and 1,5-benzodiazepines modulate recombinant α3β3γ2L GABAA receptors with differing efficacy and potency
Because stiripentol is most effective at recombinant GABAA receptors that contain the α3 subunit (Fisher, 2009), all experiments were conducted with α3β3γ2L or α3β3δ receptor isoforms. We first compared the ability of the benzodiazepines to modulate the response of recombinant α3β3γ2L receptors to a sub-maximal concentration(EC10–20) of GABA.
3.1.1 - Diazepam and clonazepam
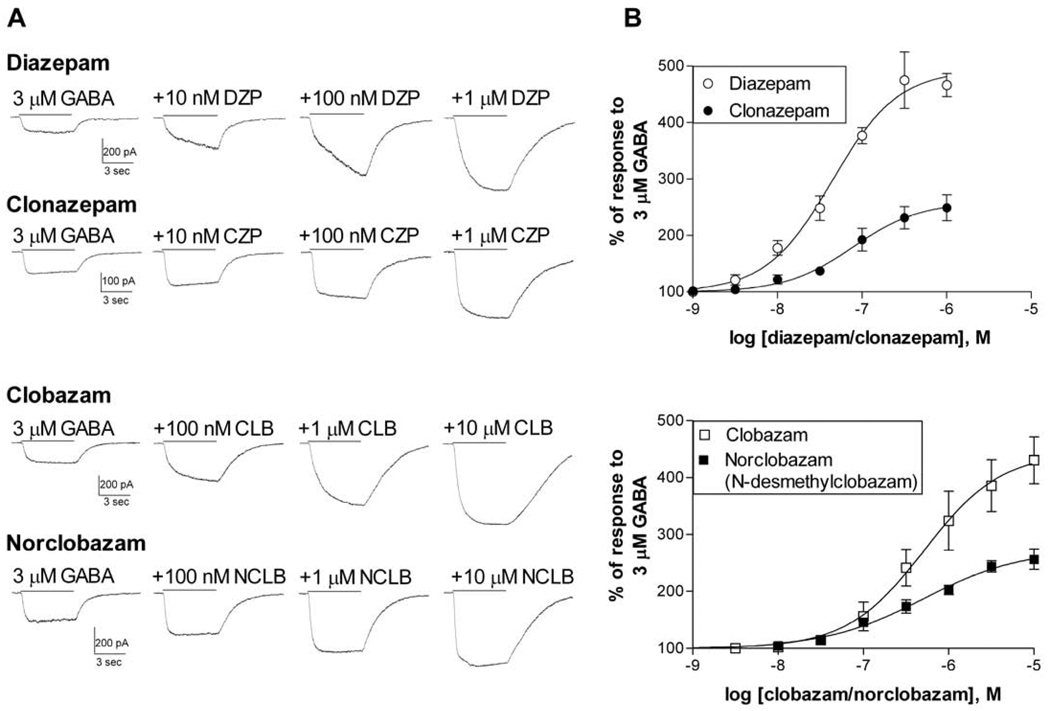
Diazepam strongly enhanced the response of α3β3γ2L receptors to 3 µM GABA, with an average EC50 of 59.4 ± 19.0 nM and maximum potentiation of 508.4 ± 28.4% (N=5) (Figure 1). Clonazepam was also a very potent modulator of these receptors, with an average EC50 of 89.8 ± 22.5 nM (N=4). However, clonazepam had lower efficacy than diazepam, with an average maximum potentiation of 262.7 ± 22.3% (N=4).
Figure 1. 1,4- and 1,5-benzodiazepines show varying potency and efficacy at recombinant GABAA receptors.
HEK-293T cells were transfected with α3, β3, and γ2L subunits. Cells were voltage-clamped at -50 mV and the peak current was measured in response to 3 µM GABA alone or with the indicated modulator.
A. Representative traces in response to GABA and GABA + the modulator at varying concentrations. Drugs were applied together for 5 sec as indicated by the bar. The amplitude of the current was increased in a concentration-dependent manner by all modulators.
B. Concentration-response relationships were constructed by measuring the peak current with co-application of the benzodiazepine as a percentage of the response to GABA alone for each cell. Symbols and bars represent the mean ± S.E.M.. Averaged data were fit with a four-parameter logistic equation. The EC50 (and maximum enhancement) from the fit shown was 44.3 nM (493.4%, N=5) for diazepam, 78.0 nM (258.9%, N=4) for clonazepam, 514.1 nM (444.0%, N=4) for clobazam and 508.4 nM (274.4%, N=4) for norclobazam.
3.1.2 - Clobazam and norclobazam
Few studies have examined the action of clobazam or norclobazam on recombinant GABAA receptors. We found that at α3β3γ2L receptors clobazam and norclobazam had lower potency than diazepam, but were similar to one another, with average EC50 values of 493.0 ± 63.2 nM (N=4) and, 554.7 ± 209.2 nM (N=4) respectively (Figure 1). The maximum potentiation in response to norclobazam was substantially lower than that of clobazam, with an average of 270.5 ± 24.8% (N=4), compared to 487.3 ± 38.7% (N=4) for clobazam. This is consistent with the lower in vivo activity associated with the metabolite compared to the parent drug (Brogden et al., 1980).
3.2. Co-application with stiripentol
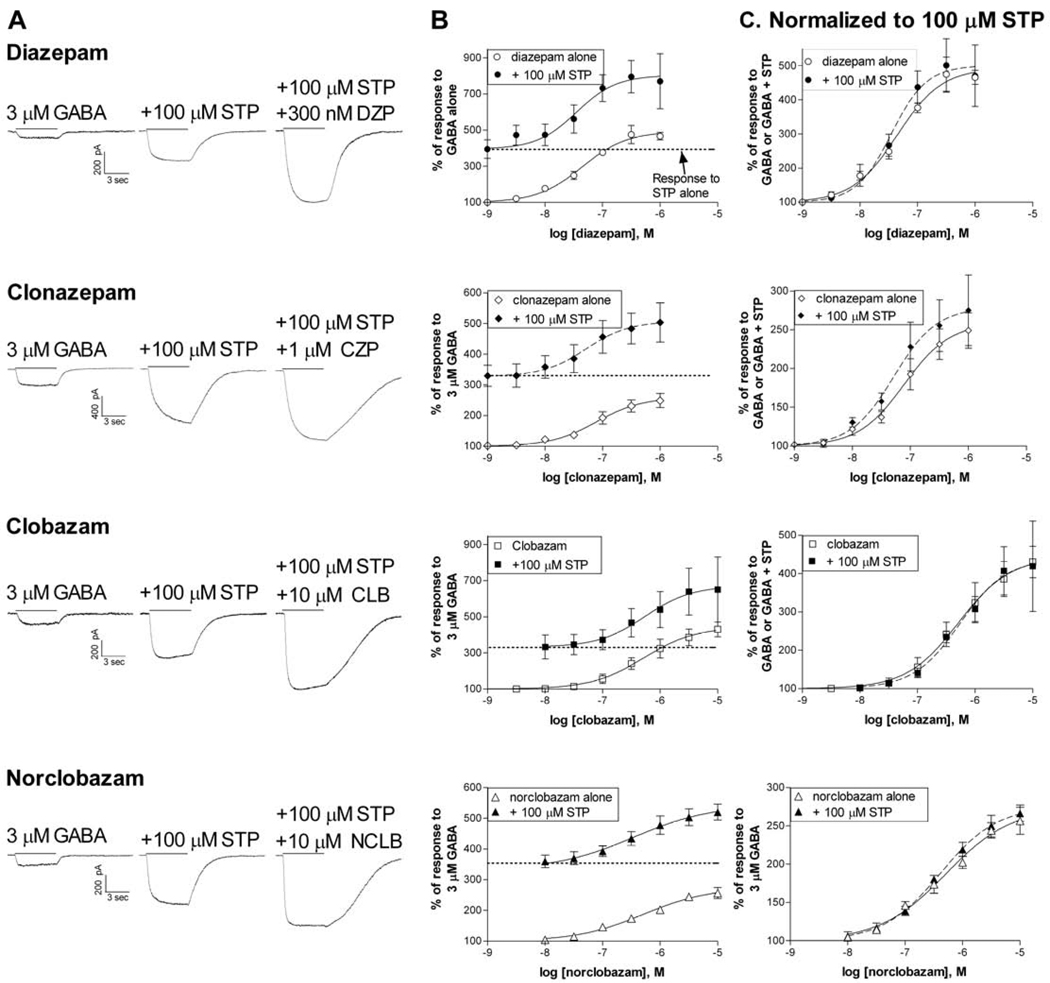
Since stiripentol is always used clinically in combination with other anti-epileptic drugs, it is important to characterize potential interactions at the GABAA receptors. Therefore, we examined the ability of diazepam, clonazepam, clobazam or norclobazam to increase the GABA-activated current amplitude of α3β3γ2L receptors in the presence of a maximally effective concentration of stiripentol (100 µM) (Figure 2).
Figure 2. Benzodiazepines and stiripentol show additive effects at recombinant Receptors.
A. HEK-293T cells expressing α3β3γ2L receptors were voltage-clamped at -50 mV. Whole-cell current traces are shown in response to GABA alone, + 100 µM stiripentol, or + stiripentol and + benzodiazepine. Drugs were co-applied for 5 sec as indicated by the bar.
B. Concentration-response relationships in the presence (filled symbols) and absence (open symbols) of 100 µM stiripentol. The enhancement of the current produced by 100 µM stiripentol alone is indicated by the dotted line.
C. The concentration-response relationships were normalized to the new baseline in the presence of stiripentol to clarify the potency and efficacy of the BZ modulator under both conditions. The EC50 (and maximum potentiation) from the fits shown + stiripentol (dashed lines) were 35.0 nM (500.8%, N=4) for diazepam, 51.8 nM (279.0%, N=4) for clonazepam, 544.1 nM (439.1%, N=3) for clobazam and 408.3 nM (275.7%, N=4) for norclobazam. Data in the absence of stiripentol is from Figure 1.
3.2.1 - Diazepam and Clonazepam
The EC50 for stiripentol modulation of α3β3γ2L receptors was reported to be ~25 µM with a peak potentiation at concentrations near 100 µM. Higher concentrations can produce an inhibitory block, reducing the impact of the positive modulation (Fisher, 2009). Similar to our previous study, we found that 100 µM stiripentol alone increased the response of α3β3γ2L receptors to GABA (Figure 2A,2B). When co-applied with stiripentol, both diazepam and clonazepam further enhanced this response (Figure 2). In the presence of stiripentol, the average EC50 for diazepam was 42.5 ± 2.5 nM and the maximum additional increase in current was 533.2 ± 76.9% (N=4) (Figure 2). These values were not significantly different from those found in the absence of stiripentol (P>0.05, unpaired t-test). Clonazepam also showed similar activity in the presence of stiripentol, with an average EC50 of 49.6 ± 10.7 nM (N=4) and maximum increase of 276.7 ± 47.3% (N=4) (P>0.05 compared to clonazepam alone, unpaired t-test).
3.2.2 - Clobazam and Norclobazam
Stiripentol is most commonly used clinically in combination with clobazam, a combination which will also produce high plasma levels of norclobazam. Just as we found for the 1,4-benzodiazepines, the presence of a maximally effective concentration of stiripentol had no impact on the ability of either clobazam or norclobazam to increase the response of the receptor to GABA (Figure 2). In the presence of stiripentol, the average EC50 for clobazam was 631.2 ± 362.6 nM and the maximum additional increase in current was 461.2 ± 132.5% (N=3) (Figure 2). Neither of these values was significantly different from clobazam alone (P>0.05, unpaired t-test). Norclobazam also showed the same characteristics in the presence of stiripentol, with an average EC50 of 483.9 ± 118.5 nM (N=4) and maximum increase of 280.3 ± 17.5% (N=4) (both P>0.05 compared to norclobazam alone, unpaired t-test).
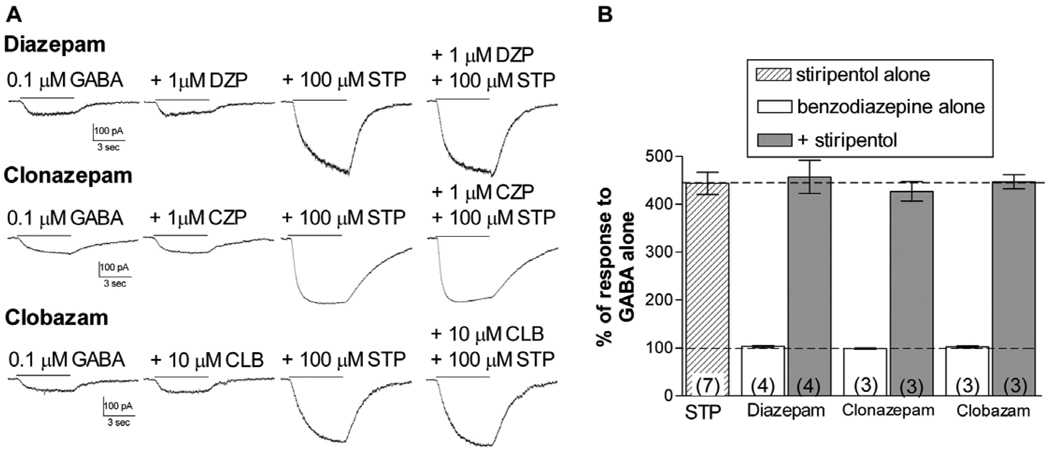
3.3. Effect of co-application at benzodiazepine-insensitive GABAA receptors
Positive modulation by benzodiazepines requires the presence of a γ subunit, while modulation by stiripentol does not (Fisher, 2009). We examined the effect of co-application of diazepam, clonazepam and clobazam with 100 µM stiripentol to α3β3δ receptors to determine if the presence of any of these compounds altered the response to stiripentol. As expected, none of these benzodiazepines alone affected the response to GABA (Figure 3). stiripentol strongly potentiated the GABA-activated current, and this response was not altered by co-application with the benzodiazepine. These results suggest that there is no interaction between these modulators at δ-containing receptors, and that stiripentol’s predicted ability to enhance extrasynaptic GABAA receptor populations should not be impacted by co-administration with benzodiazepines.
Figure 3. δ-containing receptors.
A. Representative whole-cell traces from cells transfected with α3, β3, and δ subunits, showing the current response to GABA alone or GABA + the modulator(s) indicated. Drugs were applied for 5 sec as indicated by the solid line to transfected cells voltage-clamped at -50 mV.
B. Bars represent the mean potentiation (± S.E.M.). Dashed lines indicate the response to GABA alone (100%) or the response to stiripentol alone. Addition of the benzodiazepine did not increase the response to GABA or influence the ability of stiripentol to modulate these receptors
4. Discussion
The anti-convulsant stiripentol has been approved as co-therapy to treat pharmacoresistant forms of severe childhood epilepsy syndromes. Stiripentol is often co-administered with benzodiazepines, particularly clobazam. Because both stiripentol and benzodiazepines modulate the activity of GABAA receptors, they have the potential to interact, although most evidence suggests that they act through separate sites on the receptor. They show different subunit dependence (Fisher, 2009) and the action of stiripentol is not blocked by benzodiazepine-site antagonists (Quilichini et al. 2006). However, other positive modulators of GABAA receptors acting at distinct sites have been shown to interact to enhance (Reynolds and Maitra, 1996) or to inhibit (Zhong and Simmonds, 1997) one another. We co-applied a maximally effective concentration of stiripentol with the benzodiazepines diazepam, clonazepam, clobazam and norclobazam to recombinant α3β3γ2L or α3β3δ receptors. In all cases, we found that the modulators appeared to act through independent and additive mechanisms at the GABAA receptors, and that therefore co-therapy could produce increased effects on neuronal activity.
We examined the response of α3-containing GABAA receptors because they are the most responsive to modulation by stiripentol (Fisher, 2009). Activity of benzodiazepines also depends upon the identity of the α subunit (Puia et al., 1991). In particular, the α3 subunit confers higher efficacy to potentiation by diazepam, compared to α1-containing receptors (Puia et al., 1991; Verdoorn, 1994). The responses of α3-containing receptors to clonazepam or to the 1,5-benzodiazepines had not been as well-characterized. We found that both clobazam and norclobazam were positive modulators of the α3β3γ2L receptors, confirming previous reports that norclobazam is an active metabolite of clobazam (Haigh et al., 1987; Nakamura et al., 1996). Benzodiazepines with different subunit selectivity may produce distinct effects on seizure activity, sedation and anxiety(Rudolph et al., 2001; Rudolph and Möhler, 2006), and could also produce different levels of tolerance development and abuse potential (Rowlett et al., 2005). At the α3β3γ2L receptors we found differing activity among the four compounds examined. The 1,4 benzodiazepines had the highest potency, but diazepam had greater efficacy than clonazepam. The 1,5 benzodiazepines had lower potency, but clobazam was as efficacious at these receptors as diazepam. Norclobazam had lower efficacy, similar to that of clonazepam. It has been suggested that benzodiazepine agonists with lower efficacy might show a different side-effect profile than full agonists such as diazepam (Whiting, 2006). In general, however, clinical studies have not supported this proposal. Norclobazam has been reported to produce less tolerance to its anti-convulsant action than clobazam in an animal model (Haigh et al., 1987) an effect that could be due to differences in efficacy. A comparison of the activity of clonazepam, clobazam and norclobazam at recombinant receptors containing different α, β or γ subunit subtypes has not yet been reported, and might provide important clues regarding the potential role of subunit selectivity on their clinical activities.
We also examined the response of δ-containing receptors to stiripentol and benzodiazepine co-administration and found no interaction between them. In neurons, the δ-containing receptors are exclusively found in extra-synaptic locations where they produce a tonic, long-lasting current in response to low levels of ambient GABA (Belelli et al., 2009). While these receptors are insensitive to modulation by benzodiazepines, our data suggest that stiripentol would enhance activity of both synaptic and extrasynaptic populations of GABAA receptors.
In clinical trials, stiripentol has been shown to be most effective with pediatric patients, including those with Dravet Syndrome (severe myoclonic epilepsy in infancy). This syndrome is commonly associated with de novo mutations in the α subunit of the voltage-gated sodium channel (Catterall et al., 2008). It is characterized by the onset of myoclonic epilepsy in the first year of life with progression to include partial and generalized tonic-clonic seizure, absence seizures and frequent status epilepticus (Wolff et al,. 2006; Kassai et al, 2008). Seizures continue into adulthood and are associated with reduced psychomotor and cognitive development. These patients exhibit pharmacoresistance to many anti-epileptic drugs and are typically treated with a combination of valproate, benzodiazepines and ketogenic diet. The combination of clobazam with stiripentol has been shown to have positive effects in clinical trials (Chiron et al., 2000; Kassai et al., 2008) but neither of these drugs is yet approved for use in the United States.
The choice of anti-epileptic drugs for polytherapy of poorly controlled seizures can be guided by a number of considerations, including mechanism of action and metabolic pathways (French and Faught, 2009; St. Louis, 2009). The selection of stiripentol as add-on therapy should consider both its inhibitory effect on cytochrome P450 enzymes and its enhancement of GABAA receptor activity. The optimum effect observed with the combination of stiripentol and clobazam could be due to the multiple levels of interaction between these compounds. Stiripentol slows metabolism of both clobazam and norclobazam and increases norclobazam levels above those achieved through clobazam administration (Giraud et al., 2006; Inoue et al., 2009). In addition, our results suggest that combining stiripentol with clobazam or norclobazam can further enhance GABAergic neurotransmission beyond the effect of either alone. While clobazam and stiripentol might represent the best combination because of these dual effects, our data indicate that stiripentol is also a rational choice for polytherapy with other benzodiazepine agonists.
ACKNOWLEDGMENTS
Thanks to Dr. James Warren, III for technical assistance and Dr. David Mott for helpful discussion regarding experimental design.
This work was supported by a research grant from Biocodex pharmaceuticals and funds from NIH-NINDS (RO1-NS045950).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden RN, Heel RC, Speight TM, Avery GS. Clobazam: a review of its pharmacological properties and therapeutic use in anxiety. Drugs. 1980;20:161–178. doi: 10.2165/00003495-198020030-00001. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J. Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut JD, Baytan AR, Russell M, Chang MP, Bernard A, Maxwell IH, Hoeffler JP. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J. Immunol. Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J, Dulac O, Pons G. Stiripentol in severe myoclonic epilepsy in infancy: a randomized placebo-controlled syndrome-dedicated trial, STICLO study group. Lancet. 2000;356:1638–1642. doi: 10.1016/s0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- Chiron C. Stiripentol. Neurother. 2007;4:123–125. doi: 10.1016/j.nurt.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL. The anti-convulsant stiripentol acts directly on the GABAA receptor as a positive allosteric modulator. Neuropharm. 2009;56:190–197. doi: 10.1016/j.neuropharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50:63–68. doi: 10.1111/j.1528-1167.2009.02238.x. [DOI] [PubMed] [Google Scholar]
- Giraud C, Treluyer J-M, Rey E, Chiron C, Vincent J, Pons G, Tran A. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab. and Dispos. 2006;34:608–611. doi: 10.1124/dmd.105.007237. [DOI] [PubMed] [Google Scholar]
- Haigh JR, Pullar T, Gent JP, Dailley C, Feely M. N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy. Br. J. Clin. Pharmacol. 1987;23:213–218. doi: 10.1111/j.1365-2125.1987.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Ohtsuka Y, Oguni H, Tohyama J, Baba H, Fukushima K, Ohtani H, Takahashi Y, Ikeda S. Stiripentol open study in Japanese patients with Dravet syndrome. Epilepsia. 2009;50:2362–2368. doi: 10.1111/j.1528-1167.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- Kassaï B, Chiron C, Augier S, Cucherat M, Rey E, Gueyffier F, Guerrini R, Vincent J, Dulac O, Pons G. Severe myoclonic epilepsy in infancy: a systematic review and meta-analysis of individual patient data. Epilepsia. 2008;49:343–348. doi: 10.1111/j.1528-1167.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Ikeda A, Begum T, Terada K, Shibasaki H. Efficacy of low-dose, add-on therapy of clobazam (CLB) is produced by its major metabolite N-desmethyl- CLB. J. Neurol. Sci. 2007;263:44–48. doi: 10.1016/j.jns.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczki JJ, Trojnar MK, Ratnaraj N, Patsalos PN, Czuczwar SJ. Interactions of stiripentol with clobazam and valproate in the mouse maximal electroshock-induced seizure model. Epilepsy Res. 2010;90:188–198. doi: 10.1016/j.eplepsyres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Suzuki S, Nishimura S, Yagi K, Seino M. Effects of clobazam and its active metabolite on GABA-activated currents in rat cerebral neurons culture. Epilepsia. 1996;37:728–735. doi: 10.1111/j.1528-1157.1996.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Ng Y, Collins SD. Clobazam. Neurother. 2007;4:138–144. doi: 10.1016/j.nurt.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P, Vincent J, Dulac O. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40:1618–1626. doi: 10.1111/j.1528-1157.1999.tb02048.x. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant γ- aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of γ-aminobutyric acid-gated Cl− currents. Mol. Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABAA-receptor channels. Epilepsia. 2006;47:704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- Rey E, Tran A, d’Athis P, Chiron C, Dulac O, Vincent J, Pons G. Stiripentol potentiates clobazam in childhood epilepsy: a pharmacological study. Epilepsia. 1999;40:112–113. [Google Scholar]
- Reynolds JN, Maitra R. Propofol and flurazepam act synergistically to potentiate GABAA receptor activation in human recombinant receptors. Eur. J. Pharm. 1996;314:151–156. doi: 10.1016/s0014-2999(96)00527-4. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharm. Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Shen DD, Levy RH, Savitch JL, Boddy AV, Tombret F, Lepage F. Comparative anticonvulsant potency and pharmacokinetics of (+)-and (−)- entantiomers of stiripentol. Epilepsy Res. 1992;12:29–36. doi: 10.1016/0920-1211(92)90088-b. [DOI] [PubMed] [Google Scholar]
- St. Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr. Neuropharm. 2009;7:96–105. doi: 10.2174/157015909788848929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A, Rey E, Pons G, Rouseau M, d’Athis P, Olive G, Mahter GG, Bishop FE, Wurden CJ, Labroo R, Trager WF, Kunze KL, Thummel KE, Vincent JC, Gillardin J-M, Lepage F, Levy RH. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: In vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin. Pharmacol. Ther. 1997;62:490–504. doi: 10.1016/S0009-9236(97)90044-8. [DOI] [PubMed] [Google Scholar]
- Trojnar MK, Wojtal K, Trojnar MP, Czuczwar SJ. Stiripentol. A novel antiepileptic drug. Pharm. Reports. 2005;57:154–160. [PubMed] [Google Scholar]
- Verdoorn TA. Formation of heteromeric γ-aminobutyric acid type A receptors containing two different α subunits. Mol. Pharm. 1994;45:475–480. [PubMed] [Google Scholar]
- Wolff M, Cassé-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet Syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47:45–48. doi: 10.1111/j.1528-1167.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Simmonds MA. Interactions between loreclezole, chormethiazole and pentobarbitone at GABAA receptors: functional and binding studies. Br. J. Pharmacol. 1997;121:1392–1396. doi: 10.1038/sj.bjp.0701269. [DOI] [PMC free article] [PubMed] [Google Scholar]