Abstract
Sprouting of angiogenic perivascular cells is thought to be highly dependent upon autocrine and paracrine growth factor stimulation. Accordingly, we report that corneal angiogenesis induced by ectopic FGF implantation is strongly impaired in NG2/CSPG4 proteoglycan (PG) null mice known to harbour a putative deficit in pericyte proliferation/mobilization. Conversely, no significant differences were seen between wild type and knockout corneas when VEGF was used as an angiocrine factor. Perturbed responsiveness of NG2-deficient pericytes to paracrine and autocrine stimulation by several FGFs could be confirmed in cells isolated from NG2 null mice, while proliferation induced by other growth factors was equivalent in wild type and knockout cells. Identical results were obtained after siRNA-mediated knock-down of NG2 in human smooth muscle-like cell lines, as also demonstrated by the decreased levels of FGF receptor phosphorylation detected in these NG2 deprived cells. Binding assays with recombinant proteins and molecular interactions examined on live cells asserted that FGF-2 bound to NG2 in a glycosaminoglycan-independent, core protein-mediated manner and that the PG was alone capable of retaining FGF-2 on the cell membrane for subsequent receptor presentation. The use of dominant-negative mutant cells, engineered by combined transduction of NG2 deletion constructs and siRNA knock-down of the endogenous PG, allowed us to establish that the FGF co-receptor activity of NG2 is entirely mediated by its extracellular portion. In fact, forced overexpression of the NG2 ectodomain in human smooth muscle-like cells increased their FGF-2-induced mitosis and compensated for low levels of FGF receptor surface expression, in a manner equivalent to that produced by overexpression of the full-length NG2. Upon FGF binding, the cytoplasmic domain of NG2 is phosphorylated, but there is no evidence that this event elicits signal transductions that could bypass the FGFR-mediated ones. Pull-down experiments, protein-protein binding assays and flow cytometry FRET coherently revealed an elective ligand-independent association of NG2 with FGFR1 and FGFR3. The NG2 cooperation with these receptors was also corroborated functionally by the outcome of FGF-2 treatments of cells engineered to express diverse NG2/FGFR combinations. Comprehensively, the findings suggest that perivascular NG2 may serve as a dual modulator of the availability/accessibility of FGF at the cell membrane, as well as the resulting FGFR transducing activity.
Keywords: Proteoglycan, angiogenesis, FGF signalling, NG2/CSPG4, pericytes
INTRODUCTION
FGFs are key factors in the regulation of angiogenesis where one of their main contribution is to convert quiescent-stationary vascular and non-vascular cells to mitotic and motile phenotypes. We have previously shown that microvascular pericytes – a cell type known to be highly responsive to FGF [1] - may serve as pathfinding pioneer cells during tubular sprouting in normal, pathological and experimental growth factor-induced angiogenesis [2–5]. Reorganization of pericytes within the perivascular zone allows for the subsequent recruitment of endothelial cells to line the inner side of the tubular structures formed by the activated pericytes. To accomplish their rearrangement immature pericytes and their bone marrow-derived precursors are highly dependent upon the transmembrane NG2/CSPG4 proteoglycan (PG). This dependence has been documented in numerous experimental paradigms by the overt vasculogenic defects caused by targeting the PG in pro-angiogenic conditions [5–9]. In these previous investigations a primary link has been drawn between the ability of NG2 to regulate the pericytes’ responses to PDGF and the need of pericytes to receive PDGF stimulation. Such bivalent relationship has been emphasized in a wealth of studies confirming the pivotal role of PDGF for the survival, migration and maturation of pericytes [8, 10–15]. NG2 has therefore being proposed to be a critical adapter molecule in the growth factor-activation of perivascular, non-endothelial cells and this has led to the identification of a growth factor-binding site within the central portion of its core protein [16].
Several cell surface-associated components may intervene in the control of FGF responses exhibited by different cell types [17–22]. FGF signalling is known to be initiated by the conversion of the growth factor from a latent to an active form and this conversion is currently believed to require a heparan sulfate (HS)-induced dimerization of the factor. Ternary complexes comprised of HS, FGF and FGFR are formed on the cell surface to drive the intracellular signal transduction [23– 32]. The dynamics of this interaction is convincingly established for FGFR1 and FGFR2, whereas it is less documented for the other FGFRs.
Although substantial information has accrued regarding the nature of the GAG moieties capable of engaging in growth factor-encompassing ternary complexes [29, 33–39], the precise stoichiometry of these molecular assemblies is still somewhat debated [40–43]. It also remains to be asserted to what extent other FGF-binding molecules can affect the HSPG dependence, complement the function of these PGs, and possibly supplant the GAG-mediated mechanisms of FGF signalling. Coinciding, or alternating, activities of multiple FGF co-receptor molecules could provide a means to fine tune the cells’ mitogenic responses to these growth factors [44–45]. Such a finer regulation of growth factor-induced cell proliferation/activation might be particularly relevant in complex biological processes such as angiogenesis, where distinct cell types need to be stimulated in a diversified manner by FGFs and other angiocrine factors. We have explored here the precise modes by which NG2 expressed by immature, sprouting pericytes [2–4, 46] may contribute to the modulation of their spatio-temporal regulated autocrine and paracrine mitogenic responses.
MATERIALS AND METHODS
Recombinant proteins and other reagents
Recombinant FGFR1-4-Fc fusion proteins were produced in HEK293 human kidney cells by transfection of the corresponding CMV-constructs inserted into pRK5Tkneo vectors (Genentech, Inc, San Francisco, CA, USA). Full-length rat NG2 ectodomain (residues 1–2223), inserted into the CMV plasmid, pcDNA3.1 (Invitrogen, Carlsbad, CA, USA), and the membrane-proximal NG2/D3 fragment (residues 1587–2218) inserted into the PCEP4/γ2III vector, were similarly produced in HEK293 cells. Recombinant GAG-containing and GAG-free NG2 ectodomains were separated by ion-exchange chromatography on DEAE resins as previously described [16]. PDGFRα inhibitor imatinib mesylate/Glivec and FGFR inhibitors 5′ deoxy- 5′ methyl-thioadenosine (MTA) and PD173074 were from Novartis (Origgio, Italy) and Sigma-Aldrich (Saint Louis, MI, USA). Recombinant human growth factors were obtained as follows: FGF-16-18 from Cell Sciences Inc. (Canton, MA, USA); FGF-1–10 from Sigma; PDGF-AA from Oncogene Research Products (Cambridge, MA, USA); HGF and IGF from Millipore Corp (Billerica, MA, USA). HS oligosaccharides of 6–16 disaccharides units in length (HS6–16) were a kind gift of John Gallagher (Department of Medical Oncology, Christie CRC Research Centre, Manchester UK). Heparatinase III, chondroitinase ABC, mixed chondroitin isomers, high Mr heparin and tracheal keratan sulfate were purchased from Sigma-Aldrich. Antibodies against syndecans 1–4 and glypicans 1–6 were purchased from R&D Systems, Inc, Millipore Corp., Santa Cruz Biotechnology, Inc., ABCAM and AbD Serotec Ltd. Rabbit polyclonal antibodies against Ki67 and PCNA were purchased from ABCAM.
Real-time qPCR and RNAi
Conventional and real-time qPCR was performed as reported in Supplemental Materials and Methods. For RNAi experiments 19-mer siRNA probes against human NG2 and scrambled versions of these probes were synthesized with the Dicer siRNA Generation kit (Gene Therapy Systems Inc., San Diego, CA, USA). Probes were directed against seven well-separated stretches of the mRNA encoding the extracellular portion of the PG and were comparatively assayed for their protein knock-down efficacy by FACS, immunocytochemistry and Western blotting (Supplemental Fig. 1). Two of these probes were found to be particularly effective in ablating surface NG2, i.e. probes siRNA3289 and siRNA4402 (superscript numbers refer to the first “5′ end-localized” nucleotide of the targeted sequence within the NG2 transcript), yielding on average 70–80% knock-down levels when tested on different cell types. An additional 3′-end anti-NG2 probe (siRNA6189) was obtained through Ambion (Austin, TX, USA). RNAi probes against FGFR1 and FGFR3 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and Thermo Scientifics-Dharmacon (Lafayette, CO, USA). Cells were transfected with up to 500ng of the siRNA oligos/2.5×104 cells using Gene Silencer™ siRNA Transfection Reagent (Gene Therapy Systems Inc.). siRNAs against GAPDH were produced using the corresponding Silencer™ GAPDH Template Set (Ambion). In some cases, knock-down efficiency was validated functionally by assaying the levels of adhesion to collagen type VI and the anchorage-independent growth in the presence PDGF-AA of the siRNA-treated cells. Levels of siRNA-mediated abrogation of FGFR1 and FGFR3 were similarly established first by FACS and Western blotting and then verified functionally by cell proliferation assays in the presence of FGF. Knock-down efficiency of FGFR1/FGFR3 varied in the different cell types from 53% to 65%.
Engineering of NG2 expression patterns
Human transformed smooth muscle cell lines were stably lipo-transfected (Lipofectamine Plus) with a plasmid containing either the cytoplasmic tail and membrane-spanning domain of NG2 (cyto), or its membrane-spanning domain plus the entire extracellular domain (extra). For this purpose, human NG2 cDNA clones B, C and D (kindly provided by Gerd Pluschke) [47] were cut with XhoI and SacI, SacI and HindIII, HindIII and BamHI, respectively. These fragments were ligated and inserted into the pEGFP-N1 vector (Clontech Laboratories Inc., Mountain View, CA), and the sequence of the entire insert comprising bases 4030–7216 of the NG2/CSPG4 sequence reported with the NCBI accession number X96753 was verified by automated DNA sequencing. A variant of dominant-negative mutant SK-LMS-1 and SK-UT-1 cells (coded NG2cyto), displaying NG2 molecules retaining cytoskeletal linkage and putative intracellular signalling abilities but lacking extracellular activities, were generated by treating cells overexpressing the construct NG2cyto with probe siRNA3289. This probe was designed to specifically knock down the endogenous NG2 while sparing the transduced NG2 deletion construct. Cell mutants named NG2extra (dominant-negative with respect to the ability of the overexpressed truncated NG2 molecules to engage extracellular interactions while lacking cytoskeletal associations and signalling potential) were generated by transfection of the same cell lines with a GFP-plasmid containing the entire extracellular portion and the transmembrane domain of human NG2. For this purpose, NG2 cDNA clones H, G and F [47] were cut with XhoI and ApaI, ApaI and BamHI, BamHI and HindIII, respectively, and inserted between the XhoI and HindIII sites in pEGFP-N1 vector (BD Biosciences, Bedford, MA). A cDNA from A375 melanoma cells was used as template to amplify the sequence corresponding to nucleotides 2230–5025, the fragment was inserted into a pGEM-T vector (Promega), and its sequence verified by automated DNA sequencing. The construct was then subcloned into the pEGFP-N1 expression vector containing fragments H, G and F as described above (BD Biosciences). Cells stably expressing the NG2 ectodomain were treated with the 3′-end-directed NG2 probe siRNA6189 to specifically knock-down the endogenous NG2 without affecting the transduced deletion construct. In both types of cell mutants the relative levels of expression of the full-length endogenous NG2 versus the truncated NG2 variant were determined at the mRNA level by real-time qPCR and at the protein level by flow cytometry and immunoblotting (Supplemental Fig. S2). Transient overexpression of NG2 and phenotype rescuing in NG2-deficient cells were performed by liposome transfer of full-length rat NG2 inserted into a pcDNA3.1 plasmid. The murine BaF3 lymphocytic cell line was transiently transfected with full-length rodent NG2 inserted into the same plasmid using the Amaxa Nucleofect technology. For rescuing experiments, aortic smooth muscle cells from NG2 null mice and siRNA-treated SK-LMS-1 cells that were transduced with the above NG2-containing plasmid, or a control empty plasmid, were enriched by panning on the D3 anti-NG2 polyclonal antiserum. In other cases, immunosorting and enrichment of cells highly expressing NG2 was performed using the MACS technology and the anti-NG2 mAb 9.2.27 (Millipore Corp.).
Cellular assays
SK-UT-1, SK-LMS-1, MES-SA, SW982, HT1080, SW872, A375, U87, U373 and HeLa cells were grown in DMEM with 10% FCS and antibiotics. Cell lines established in the laboratory and coded NTI-OS-1 (osteosarcoma), NTI-LS-1 (liposarcoma), NTI-LMS-1 (leiomyosarcoma), NTI-MFH-1 (pleomorphic sarcoma), and NTI-FS-1 (fibrosarcoma) were cultivated under similar conditions. Aortic smooth muscle cells from wild type and NG2 null mice [12] were grown in DMEM supplemented with MITO+ Serum Extender (Becton-Dickinson, Bedford, MA). The murine pro-B cell line BaF3 cells stably expressing the FGFR1-IIIc isoform was kindly received from David Ornitz and maintained in RPMI-1640 with 10% FCS and 2 ng/ml IL-3 as previously described [48]. For proliferation assays, cells were seeded onto uncoated, or poly-L-lysine-coated (0.1%, Sigma), 48 wells plates at an initial density of 5×103 cells/well and grown for up to 5 days in DMEM with and without the following components: 10% FCS; 0–20 ng/ml of the different FGFs, HGF, IGF-1 or PDGF-AA; 1–100 μg/ml of mixed chondroitin sulfates (CS) or keratan sulfate (KS); 1–15 μg/ml of HS6–16; 1–15 μg/ml of soluble recombinant NG2; and 0.1–10 μg/ml of heparin. For heparitinase III and chondroitinase ABC digestions, cells were incubated with 0.02–2.0 U/ml of the enzymes diluted in PBS 0.1% BSA for 2 hours at 37°C. Growth factors were replenished at 12 hours intervals. Rates of cell proliferation were determined in separate wells for each condition starting at 4 hours after cell plating and ending at 4–5 days. For these measurement we used interchangeably the CyQUANT Cell Proliferation Assay (Invitrogen) and the CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI), in accordance to the manufacturers’ instructions. The Live/Dead Cytotoxicity kit was occasionally used to verify the potential occurrence of programmed cell death. Fluorescent cellular assays were evaluated with a Genius Plus UV microplate reader (Tecan Group) or with the Acunen eX3 scanning laser cytometer (TTP Labtech Ltd, Melbourn, UK). Transformed cells used for proliferation experiments were starved for 24–48 hours by culturing in DMEM with 0.5% FCS. Primary murine fibroblasts were “synchronized” by incubation in serum-free conditions for 4 hours prior to be exposed to growth factors. An estimated amount of 2,000–10,000 synchronized cells was inoculated into each well of 24 well-plates, yielding an initial plating density of about 2,550–12,740 cells/cm2. Cells were allowed to attach to the substrate for 2–4 hours. Prior to addition of growth factors, relative amount of cells bound to the bottom of the wells was assessed by random field counting which confirmed that >90% of the cells initially inoculated were bound to the substrate. In one set of experiments, partial desulfation of cell surface PGs of the cells was carried out as previously described [36] by incubating cells in sulfate-free DMEM supplemented with 1% FCS, 15 mM NaClO3 and 250 μg/ml BSA for up to 24 hours prior addition of growth factors. For antibody blocking experiments, 5×103 cells were preincubated for 45 min with 10 μg/ml of the function-blocking antibodies anti-FGFR1, anti-FGFR3 or anti-CD44 (Millipore Corp.) and then grown in the presence or absence of FGF-2 for up to 4 days. For flow cytometric cell-cycle analyses, cells were detached, washed and fixed/permeabilized with cold 70% ethanol and incubated with 12.5 μg of propidium iodide/106 cells in the presence of 0.1% NP40 and 125 μg/ml RNAse. For FGF “pulse-capture” assays cells were pre-cooled on ice for 15–30 min, washed with ice-cold 1% BSA in MCDB to remove any FGF bound to the cell surface, incubated at 4°C for another 15–30 min with 10 μg/ml of the antibodies to FGFR1 and FGFR3 (or control antibodies), and then pulsed with 20 μg/ml FGF-2 at 37°C.
Immunofluorescence and FRET analyses
For immunofluorescent assays we used the following antibodies: mouse mAbs to FGFR1 (VBS6; VBS7; 19B2, #3472; Life Technologies, Inc., EMD-Calbiochem, Millipore and Cell Signaling Technologies Inc., Danvers, MA, USA); rabbit polyclonal anti-FGFR2 antiserum (Abnova Corp., Taipei, Taiwan); mouse mAb and rabbit polyclonal anti-FGFR3 (B-9, Santa Cruz Biotechnology, Inc.; and C51F2, Cell Signaling Technologies, Inc.); rabbit polyclonal anti-FGFR4 antiserum (Santa Cruz Biotechnology, Inc.); goat anti-PDGFRα antiserum (R&D Systems Inc., Minneapolis, MN, USA), mAb (clone FB-8) against FGF-2, (Sigma-Aldrich), rabbit anti-syndecan-4 antisera, (Santa Cruz Biotechnology Inc.; ABCAM), anti-NG2 mAbs 9.2.27, (Millipore Corp.) and B5/M28, (ATCC), and our proper anti-NG2 polyclonal antisera EC, D2 and D3. Flow cytometry was carried out primarily with the anti-NG2 PE-conjugated mAb 7.1 (Immunotech, Marseille, France), anti-syndecan antibody BB4 (anti-CD138; IQP Lagitre srl, Milan, Italy), anti-syndecan-2, anti-syndecan-3 and anti-syndecan-4 antisera (BD Biosciences Inc.; R&D Systems), anti-glypican-1, -glypican-2, glypican-3, glypican-5 and glypican-6 mAbs and polyclonal antibodies were from R&D Systems Inc. and the corresponding isotype matched control antibodies were from Sigma-Aldrich, BD Biosciences and Life Technologies. FITC and PE-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA, USA). For FRET analyses, SK-LMS-1 cells were double-labelled with antibody 7.1 and anti-FGFR1 or anti-FGFR3 antibodies that had been pre-tagged with Alexa Fluor 647 using the corresponding labelling kit (Life Technologies). For reference we used PE-conjugated anti-α4 subunit and an anti-β1 integrin subunit antibodies (BD Biosciences) tagged in a similar manner as above with Alexa Fluor 647. The same protein tagging kit was utilized to label FGF-2 for flow cytometric analyses of its binding to the cell surface in the presence or absence of NG2 (i.e. following siRNA-mediated knock-down of the PG).
Immunochemistry, proteomics and protein binding assays
For immunoprecipitation, SK-LMS-1 cells, treated or not with FGF-2, were lysed with CHAPS buffer containing 500 mM NaCl, 0.25% sodium deoxycholate, 10 mM β-glycerolphosphate, 1 mM EDTA, 1 mM sodium vanadate and the Complete Protease Inhibitor Cocktail from Roche Biosciences Inc.. Cell lysates were immunoprecipitated with either the D2 anti-NG2 or the anti-FGFR1/anti-FGFR3 antisera followed by extensive washing and enrichment on Protein A-Sepharose (Sigma). In some cases, cells were pre-treated with 4 mM of the membrane-impermeable cross-linker BS3 (Pierce Protein Research Products-Thermo Scientifics, Rockford, IL, USA) on ice for 15–30 min, followed by incubation in 50 mM Tris-HCl, pH 7.8, for 15 min at room temperature. Immunoprecipitated material was resolved by SDS-PAGE on 4–12% gradient gels and either stained with Commassie blue or electro-transferred onto nitrocellulose membranes and immunoblotted (following saturation of the membrane with 5% dry milk in TBST for 1 hr at room temperature) with D2 or D3 anti-NG2 antisera (1:200 dilution in PBS), or anti-FGFR1–4 antibodies. In parallel, immunoprecipitated material was resolved by SDS-PAGE, extensively stained with Commassie brilliant blue, and the bands cut out from the gel for digestion with trypsin and the resulting fragments injected into a MALDI-TOF mass spectrometer (Applera, Inc.) for molecular mass determination. For detection of phosphorylated threonine residues in the NG2 cytoplasmic domain, cells were starved for 72 hours and treated for 15 min with optimal concentrations of FGF- 2, FGF-8 or HGF. Growth factor-treated cells were solubilized in SDS-containing sample buffer and pellets resolved by PAGE on 4–12% gradient gels, transferred to nitrocellulose membranes and probed with pan-antibodies to phosphotyrosine (clone, 4G10), PhosphoFGF receptor (Tyr653/654; clone 55H2), PhosphoFGF receptor 1 (Tyr766; clone 1E5) or PhosphoEGF receptor (Tyr1068; Cell Signalling Technologies, Inc.). For phosphosite immuno-proteomic profiling of the signal transduction components activated following NG2-mediated and NG2-independent FGF signalling, an amount of 106 cells treated or not with the probe siRNA3289 and stimulated with 20 ng/ml of FGF-2 for 30 min was extensively washed, lysed with 1% CHAPS buffer solution and the cocktail of protease inhibitors described above. The lysate sonicated twice for 15 seconds. Solubilized material was subjected to ultracentrifugation for 30 min at 95,000×g and the supernatant collected for subsequent determination of the total protein concentration. This was adjusted to 1 mg/ml for each sample using the PAGE buffer, containing 31.25 mM Tris-HCl (pH 6.8), 1% SDS (w/v), 12.5% glycerol (v/v), 0.02% bromophenol blue (w/v) and 1.25% β-mercaptoethanol and samples further denatured by boiling for 4 min at 100°C. Samples were resolved by one-dimensional SDS-PAGE, electro-blotted onto nitrocellulose and the membrane subdivided in 20 lanes (plus one for the MW standards). Each lane was separately blotted with given combinations of 3 antibodies directed against a total of 82 phosphosites in 69 signal transduction components specifically selected out from the panels of components contained by phospho-screens KPSS1.3 and KPSS3.1 (Kinexus Bioinformatic Corp., Canada). Protein binding assays were performed as described in Supplemental Materials and Methods.
Corneal neovascularization assays
To induce local neovascularisation in the mouse cornea we used implantation of slow-release polyhydroxyethyl methacrylate (hydron; Hydro Med Sciences, Cranbury, NJ, USA) pellets (0.4×0.4×0.2 mm) formulated to contain 45μg sucrose aluminum sulfate (sucralfate; Sigma-Aldrich) and 75 ng FGF-2 or 180 ng of VEGF (previously established to be optimal quantities of the growth factors). Mice were anesthetized with Avertin (0.015–0.017 ml/g body weight), and under an operating microscope a hydron pellet was surgically implanted into the corneal stroma at a distance of 0.7 mm from the corneo-scleral limbus. A total of 22 NG2 wild type eyes and 20 NG2 knockout eyes received pellets containing either FGF-2 or VEGF. Mice were monitored by stereomicroscopy over a period of 7–10 days post-surgery to evaluate the progress of corneal angiogenesis in the operated eyes. In some cases, on days 3 and 7 mice received intra-peritoneal injections of BrdU (Sigma.Aldrich) at a dose of 240 μg/g body weight. Between days 7–10, angiogenesis was quantified by determining the area of vascularisation according to a previously described method [50]. Briefly, clock hours of neovascularization (CN) and maximal vessel length (VL) were measured and the vascularised area was calculated according to: area (mm2) = 0.2 × π × VL (mm) × CN (mm). Following fixation of tissues in 4% PFA, cryopreservation in 20% sucrose in PBS at 4°C, embedding in OCT compound and snap-freezing, collected cryostat sections were processed for immunohistochemistry. Pericytes were immunolocalized by labelling with anti-αSMA and anti- PDGFRβ antibodies and proliferating cells incorporating BrdU were visualized through an anti- BrdU antibody [6]. For this purpose, frozen sections were digested with 0.005% pepsin (Sigma-Aldrich) in 0.01M HCl for 30 min at 37°C followed by treatment with 4M HCl for 30 min at room temperature. Sections were then blocked by incubation in 5% goat serum in PBS for 30 min prior to incubation with antibody. Slides were mounted with DAPI (Vectashield 1200, Burlingame, CA, USA). Sections were viewed with a Fluoview 1000 confocal microscope (Olympus USA, Melville, NY) and serial optical sections (1μm each) across the entire thickness (40μm) of the histological specimens were overlaid (Z-stack) to provide reconstructions of entire vessels. This allowed unambiguous identification of the immunolabelled pericytes exhibiting nuclear BrdU labelling. The Prism 4.0 software (GraphPad, San Diego, CA, USA) was used for statistical analyses. Systematic random sampling of serial histological sections was carried out according to previously described methods [6].
Statistical analyses
In most cases, significance levels were established using the non-parametric Mann-Whittney U test, Student’ T test, Kruskal Wallis one-way analysis of variance, or the two-way ANOVA test. Confidence intervals were mostly set at 95% and statistical significance at p<0.05.
RESULTS
Deletion of NG2/CSPG4 impairs FGF-induced corneal angiogenesis
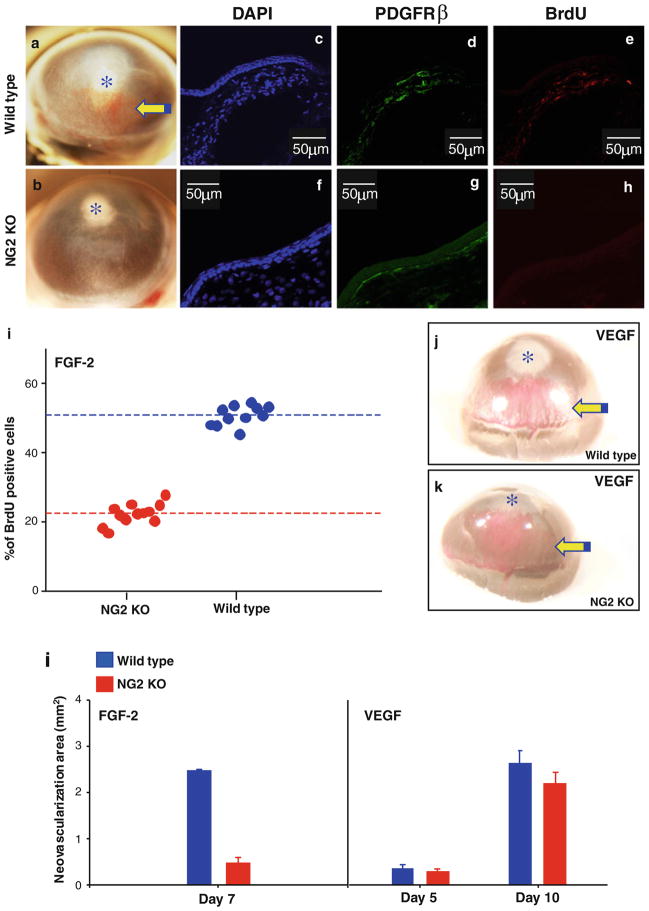
To explore the possible involvement of NG2 expressed by pericyte/smooth muscle cells in FGF signalling in vivo we implanted FGF-2-containing hydron pellets into corneas of wild type and NG2/CSPG4 null mice and evaluated the extent of FGF-2-induced neovascularization in the two genotypes. In the absence of NG2/CSPG4, a significantly reduced size of the neovessel network growing toward the implanted FGF-2-containing pellet could be noted in the cornea of these mutant mice (Fig. 1a, b, l). BrdU incorporation indicated that the reduced vascularization in NG2 knockout mice was accompanied by a decrease in the cycling rate of PDGFRβ-expressing pericytes associated with the newly formed vessels (Fig. 1c–i). Accordingly, the mitotic index for pericytes in NG2 null mice was lower than in wild type animals by a factor of 2.5 (p<0.0001). Although angiogenic sprouting induced by a similar local implantation of VEGF-microbeads had an apparently slower kinetics than that induced by FGF-2, a distinct neovascular network formed in both wild type and NG2 null mice in response to this growth factor (Fig. 1j–l). Thus, ablation of NG2 caused a selective loss of FGF-promoted pericyte involvement in the angiogenic process and did so in a manner closely resembling that previously observed in other experimental models [4–8].
Figure 1.
Experimentally induced corneal neovascularization in wild type and NG2 knockout (KO) mice by local implantation of microbeads releasing FGF-2 or VEGF. (a, b) Macrographs of the neovessel network formed in wild type (a; n=11) and NG2 KO mice (b; n=15) receiving FGF-2-containing beads. Confocal laser microscopy images (c–h) and corresponding assessment of the relative amount of cycling PDGFRβ-expressing pericytes (i) by incorporation BrdU in the two genotypes. (j, k) Outcome of a similar implantation of VEGF-soaked microbeads in wild type (j; n=9) and NG2 KO (k; n=13) mice. (l) Comparative assessment of the neovascularization induced by FGF-2 and VEGF as determined by morphometry (arrows; p<0.001).
NG2 is essential for optimal FGF mitogenic responses in the absence of HSPGs
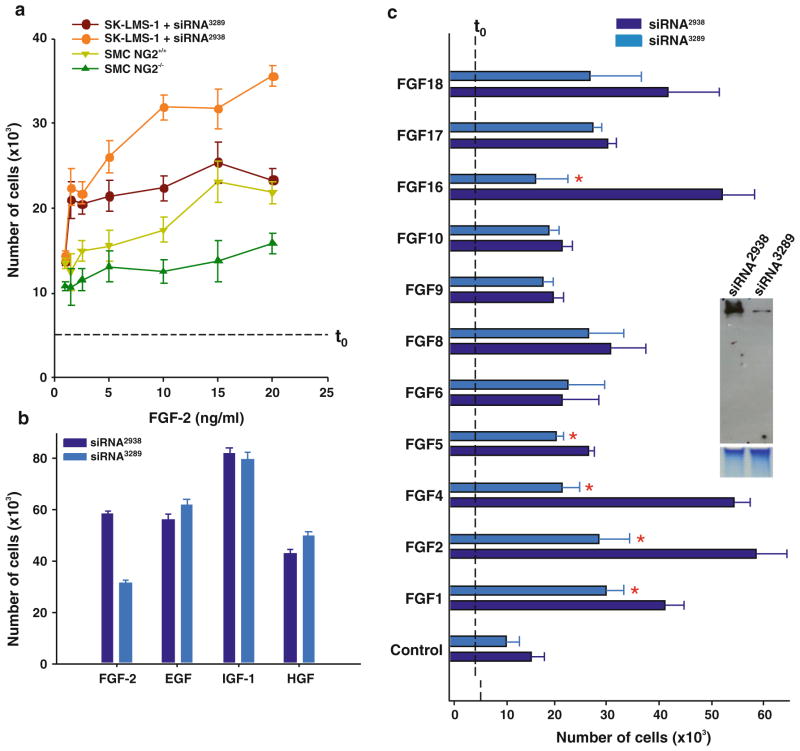
The altered FGF-induced angiogenesis seen in NG2/CSPG4 null mice prompted us to examine more in detail the mechanisms underlying the involvement of the PG in FGF-related signalling phenomena. For this purpose, we comparatively assayed the mitogenic responses of isolated wild type and NG2/CSPG4 null aortic smooth muscle cells and untreated or siRNA-treated (Supplemental Fig. 1a, b) human smooth muscle-like cell lines (expressing some syndecan-4 and glypican-1 and minor amounts of glypican-4 and -6; Supplemental Fig. 2) to a panel of growth factors (using PDGF-AA as reference; not shown). In parallel, we verified that NG2/CSPG4 gene ablation/mRNA knock-down did not alter the overall PG surface profile (Supplemental Fig. 2). Although the magnitude of response to the different growth factors that were tested varied, both murine NG2/CSPG4 null and human siRNA-treated cells proliferated significantly less upon FGF-2 (and PDGF) stimulation than their counterpart wild type and untreated cells (Fig. 2a). However, both NG2-deficient cell types exhibited a minor NG2-independent FGF response. This left open the possibility that the NG2’s effect on FGF-induced cell proliferation required a certain cooperation with HSPGs (or other FGF co-receptor molecules), even if such were present in scantier amounts than NG2. Thus, to unequivocally ascertain a direct involvement of NG2 in the regulation of FGF- 2-induced mitosis, we utilized the BaF3 cell line as a well-established cellular HS-deficient model. Upon transduction with full-length rodent NG2, a robust mitogenic response of the HSPG-negative/FGFR1-IIIc-expressing BaF3 cells was observed (Supplemental Fig. 3a). This finding corroborated that NG2 could alone mediate the cells’ response to FGF stimulation, as was also asserted by a cell-cycle analysis and further confirmed by semi-quantitative assessment of Ki67 and PCNA immunolabelling (Supplemental Fig. 3b, c). We finally sought to exclude the possibility that the reduced levels of FGF-induced proliferation were attributed to an irreversible loss of the cells’ ability to respond to FGF stimulation caused by off-target effects of the NG2 knock-down. This was accomplished by first confirming the ability of the siRNA-treated cells to resume their FGF-responsiveness upon surface recovery of NG2 (Supplemental Figs 1c and 4a). Then, we performed an additional control experiment entailing exposure of siRNA-treated cells to FGF-2 in the presence of soluble high molecular weight heparin, or structurally defined HS oligosaccharides known to act as potent FGF activators [36, 42]. Both intact heparin and HS fragments were able to rescue, in a dose-dependent manner, the NG2-deficient phenotype, whereas unrelated GAGs were ineffective (Supplemental Fig. 4b).
Figure 2.
(a) Dose-dependent mitogenic responses to FGF-2 of primary smooth muscle cells isolated from wild type (SMC) and NG2 KO (SMC-NG2−/−) mice and human smooth muscle-like SK-LMS-1 cells, treated with either the effective anti-NG2 probe siRNA3289 or the control probe siRNA2938 (Supplemental Fig. S1a; p<0.005 for SMC and p<0.001 for SK-LMS-1 cells by Mann-Whittney U test). (b) NG2-dependence of cell proliferation induced by the indicated growth factors (10 ng/ml; 4 days treatment) in siRNA-treated SK-UT-1 cells (p<0.001 by Student’s T test). (c) Mitogenic responses of siRNA-treated SK-LMS-1 cells following stimulation with a panel of FGFs (10 ng/ml; 4 days treatment; asterisks; p<0.005 by Mann-Whittney U test). Right inset shows a representative abrogation of NG2 levels after siRNA treatment (mAb B5/M28). Dashed t0 lines indicate the amount of cells/well at the start of the experiment.
To next determine which FGF receptors were implicated in the NG2-dependent mitogenic action of FGF-2, and establish whether our cells produced endogenous FGFs that could contribute to NG2-mediated autocrine signalling loops, we mapped the transcriptional profiles of the currently known FGFRs and their cognate ligands. These analyses revealed that our human smooth muscle-like cells expressed different combinations of FGFR1-IIIb, FGFR1-IIIc, FGFR3-IIIc and FGFR3-IIIs, whereas the murine wild type and NG2/CSPG4 null cells expressed discrete isoforms of all four primary FGFRs (Supplemental Fig. 5a, b). From these observations we could infer that NG2 may have preferentially acted in cooperation with FGFR1 and FGFR3.
Among the recognized FGFR1/FGFR3 ligands [48], we detected FGF-1, FGF-8, FGF-10 and FGF-16-18 as the ones endogenously produced by the cells (Supplemental Fig. 5c). When we next approached the putative involvement of NG2 in the mitosis induced by a spectrum of FGFs, we noted a selective NG2-dependence for FGFs known to bind with highest affinity to FGFR1 and FGFR3. Thus, virtually no involvement of NG2 was seen in proliferation events induced by FGFs predominantly binding to other FGF receptors (Fig. 2c). The assessable efficiency of the FGFs apparently requiring NG2 for their mitogenic action decreased in the order: FGF-16>FGF-4>FGF-2>FGF-1>FGF-5 (Fig. 2c; an observation that was corroborated by parallel dose-dependent assays). Although not more deeply investigated in this study, the above findings suggest that NG2 may display an interactive bias for FGFs with a discrete preference for FGFR1/FGFR3.
NG2 acts as a GAG-independent FGF co-receptor
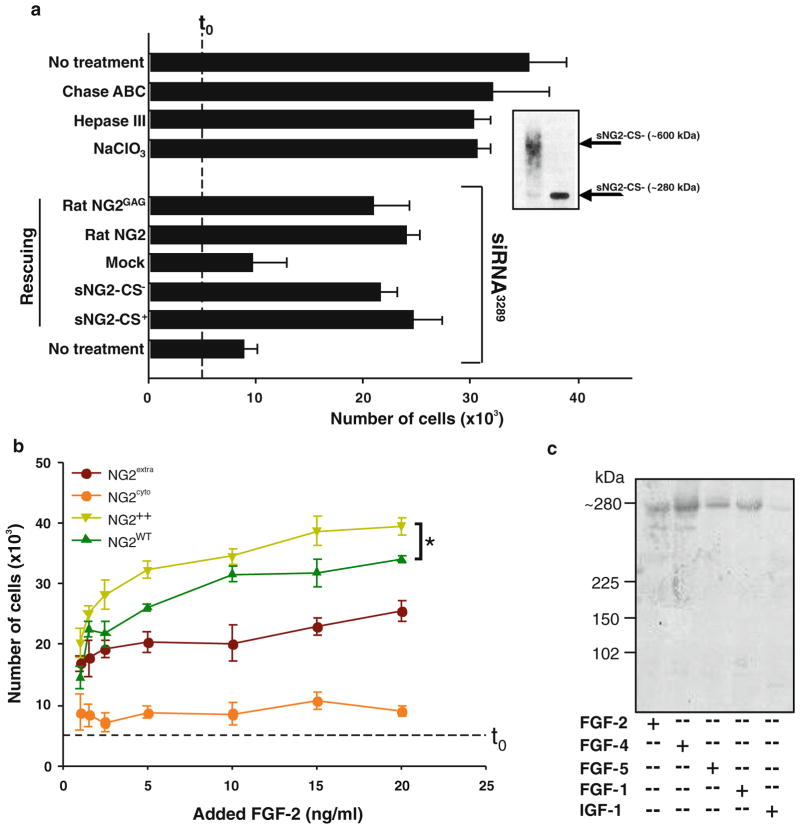
The capability of NG2 to participate in FGF signalling independently of its GAG chain(s) was initially tested by exposing NG2-deficient cells to FGF-2 in the presence of recombinant glycanated and non-glycanated forms of the NG2 ectodomain. In these conditions, up to a 70% recovery of FGF-induced cell proliferation was noted after addition of either form of the PG (Fig. 3a). Furthermore, in NG2-deprived human smooth muscle-like cells, FGF-induced mitosis could be rescued in a similar fashion by transient transduction of the cells with either full-length rodent NG2 (Fig. 3a), or NG2 with a mutated GAG-attachment site [50]. Because of the divergence in the nucleotide sequence between the human and rat NG2 transcripts in the region targeted by probe siRNA3289, ectopic expression of the rodent NG2 was not affected by the co-transduced probe.
Figure 3.
(a) Effect of enzymatic (heparatinase III; Hep III; or chondroitinase ABC; Chase ABC) removal or desulfation (NaClO3 treatment) of cell-surface HSs and CSs on FGF-2-induced proliferation in SK-LMS-1 cells. Anti-HS mAb 10E4 and anti-CS mAb CS56 were used to verify the extent of cell surface removal of the corresponding GAGs. In “Rescuing” experiments, siRNA-treated cells were exposed to the glycanated (sNG2-CS+) or non-glycanated (sNG2/CS−) recombinant ectodomains of rodent NG2 (Inset; p<0.001 by Mann-Whitney U Test), or were transfected with a wild type (rat NG2) or modified rodent NG2 construct in which the primary GAG-attachment site was mutated (rat NG2GAG; p<0.0001 by ANOVA). Dashed t0 line indicates the starting amount of cells/well. (b) Comparative levels of FGF-2-induced mitosis observed in SK-LMS-1 cells overexpressing full-length NG2 (NG2++; asterisk, p<0.001 by ANOVA), the NG2 ectodomain (NG2extra mutants), or a transmembrane-cytoplasmic segment (NG2cyto mutants; see Materials and Methods). (c) Phosphorylation pattern of the ERK-dependent Thr2314 residue of the cytoplasmic domain of NG2 upon stimulation of the cells with the indicated growth factors (IGF-1 was used as a control), as determined by immunoblotting using antibodies to the xTpP phosphorylation motif.
These rescuing experiments confirmed that NG2 acted in a GAG-independent manner to mediate the interaction of FGF-2 with its cognate signalling receptors. However, the observation left unresolved whether NG2 merely contributed to FGF-2-induced signal transduction through modulation of FGFR-ligand binding, or whether it also directly transduced intracellular signals upon interaction with FGF-2. To address this issue we generated two types of “dominant-negative” cell mutants with respect to the potential involvement of NG2 in FGF responses (Supplemental Fig. 6a–c): one ectopically expressing the NG2 ectodomain (NG2extra), while the endogenous NG2 was knock-down, and one ectopically expressing the cytoplasmic tail of NG2 (NG2cyto) in a similar NG2-deficient background.
In the absence of any exogenous growth factor, human smooth muscle-like cells were found to be dependent upon surface NG2 for their survival and proliferation and the ectodomain was the portion of the molecule responsible for this dependence (Supplemental Fig. 6e). Furthermore, in “growth factor-poor” experimental conditions, NG2 seemed to make an equal contribution to autocrine PDGF- and FGF-dependent activities. This since siRNA-treated and non-treated cells behaved in a similar manner in the presence of either PDGFR or FGFR antagonists (Supplemental Fig. 6e). Assessment of the proliferation rates of NG2cyto and NG2extra mutant cells exposed to increasing doses of FGF-2 revealed a strongly attenuated mitotic rate of NG2cyto mutant cells and only a modestly reduced proliferation of NG2extra mutants (Fig. 3b). These results were consistent with the general idea that any contribution made by the NG2 cytoplasmic domain to FGFR-FGF binding, or to the down-stream signalling events, was negligible compared to that exerted by the membrane-proximal ectodomain of the PG. Thus, even though FGF stimulation was found to induce ERK-mediated phosphorylation of the NG2 cytoplasmic threonine residue Thr2314 (Fig. 3c), there was no evidence that this phosphorylation event contributed to the FGF-induced cell proliferation. Finally, increasing the concentrations of exogenous FGF-2 did not rescue the mitotic deficiency of NG2-poor cells, whereas cells transiently overexpressing full-length NG2 proliferated to a higher degree than cells with normal levels of NG2 (Fig. 3b). This latter finding would imply that surface abundance of NG2 may increase a cell’s ability to respond to FGF(s), especially when the growth factor(s) is poorly accessible in the extracellular environment.
Loss of NG2 abrogates FGF canonical signalling pathways
Despite of the positive outcome of the different rescuing experiments performed with NG2-deficient cells, it could still be argued that siRNA-induced loss of NG2 may have caused, as a secondary effect, alterations of the canonical FGF-FGFR signal transduction pathways, i.e. defects in the cell’s signalling machinery such as to render the cell partially unresponsive to FGF stimulation. To rule out this possibilityand ascertain the direct association between loss of NG2 and impaired FGF-triggered signal transduction we treated SK-LMS-1 cells with the effective siRNA3289 probe NG2, or with the non-effective probe NG22938, stimulated both types of cells with FGF-2 (10 ng/ml for 30 min) and comparatively assayed by semi-quantitative immunoblotting the phosphorylation status of 53 primary mediators (62 phosphosites) of growth factor signalling, cell survival and stress response. Non-stimulated NG2-expressing and NG2-deficient cells differed only marginally in their constitutive baseline phosphorylation pattern. Upon FGF-2 treatment, however, a marked difference could be noted between siRNA-treated and non-treated cells; the latter cells showed a clearly reduced phosphorylation, or complete de-phosphorylation, of primary FGF-activated signalling effectors and cell survival mediators, including ERK1/2/5, Shc, MSK1, MEK1/2/3/6, Jun, Src, p38αMAPK, MAPKs, Raf1, JNK and Akt-1 (Supplemental Table 1). Phosphorylation of Cdk-1/cdc2 and serine phosphorylation of Rb protein (i.e. of residues S612 and S807) were also found to be strongly reduced. These findings confirmed that, upon FGF stimulation, loss of NG2 impaired activation of canonical FGF signal transduction components and did, apparently, not elicit activation of anti-proliferative stress-related signal transduction events.
NG2 captures and retains FGF-2 on the cell surface
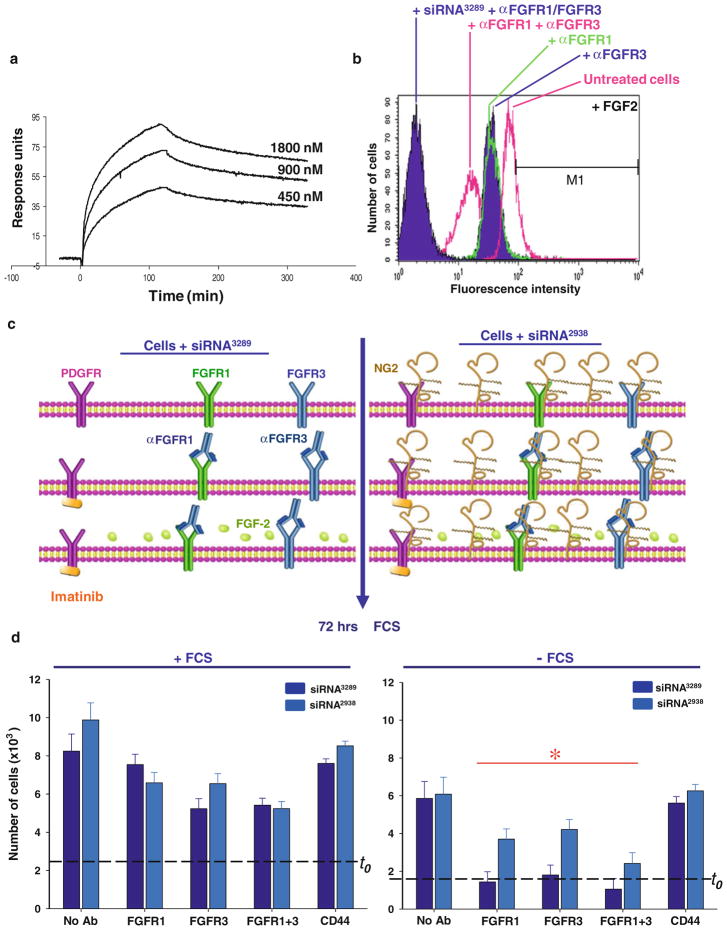
To establish whether the putative FGF co-receptor activity of NG2 was truly associated with its ability of the PG to reversibly sequester FGF molecules, we first determined whether FGF-2 could directly bind to the NG2 core protein. Through surface plasmon resonance we could measure binding strengths between FGF-2 and the NG2 ectodomain (putative binding site residing within the D3 membrane-proximal module) revealed a Kd in the range of 732–809 nM (Fig. 4a). These binding values appear substantially lower than those previously determined with a different approach between FGF-1/-2 and HSPGs isolated from cancer cells [51].
Figure 4.
(a) Surface plasmon resonance binding profiles of an NG2 ectodomain fragment to chip-immobilized FGF-2. (b) Alexa-647-tagged FGF-2 binding to the surface of SK-LMS-1 cells, prior to or following knock-down of NG2, when assayed by flow cytometry in the concomitant presence of anti-FGFR1 and/or anti-FGFR3 antibodies. Antibodies to CD44 were used as a control. (c) Schematic overview of the experimental design adopted to address functionally the FGF capturing and receptor presentation ability of NG2. Dashed t0 line indicates the amount of cells/well at the start of the experiment. (d) Assessment of cell proliferation in the two experimental conditions (“*”, p<0.01–0.001 by Mann-Whittney U Test). In conditions in which no significant increase in cell number was detected, apoptotic cells accounted for <15%.
We next used fluorescently-tagged FGF-2 to verify the capacity of the growth factor to bind membrane-bound NG2 on live cells. These experiments were performed in the presence of anti-FGFR1 and -FGFR3 antibodies, to delimit as much as possible a direct interaction of soluble FGF-2 to their cognate receptors, i.e. bypassing interactions with surface-bound co-receptors. Untreated cells bound a significant amount of FGF-2 (Fig. 4b), whereas pre-incubation of the cells with either anti-FGFR1 or anti-FGFR3 antibodies substantially reduced surface binding. In this latter experimental condition, addition of heparin did not significantly modify the surface fluorescence signal (not shown). Combined siRNA-mediated knock-down of NG2 and pre-incubation of cells with antibodies against both FGFRs virtually eliminated the FGF-2 association with the cell surface.
The functional significance of the receptor-independent FGF-capturing ability of NG2 observed above was then assayed by exposing cells for a short time period (“pulse”) to an optimal mitogenic dose of FGF-2 in the presence of anti-FGFR1 and -FGFR3 antibodies (Fig. 4c). Unbound FGF-2 was subsequently removed and cells were grown for additional 72 hours in the absence of serum. A parallel set of cells were grown in the presence of serum to ascertain that the experimental manipulation had not affected their overall capability to respond to mitogenic stimuli. As could be expected, cells pre-incubated with antibodies to either FGFR showed a reduced proliferation when compared to control untreated cells. However, NG2-expressing cells were able to proliferate significantly better than NG2-deficient ones, demonstrating that, during the pulse period, the PG had captured a sufficient amount of FGF-2 to stimulate cell replication in the absence of other exogenous growth factors (Fig. 4d). The findings did not highlight any specific functional bias of NG2 for either FGFR.
NG2 associates with FGFR1 and FGFR3 in a GAG-independent manner
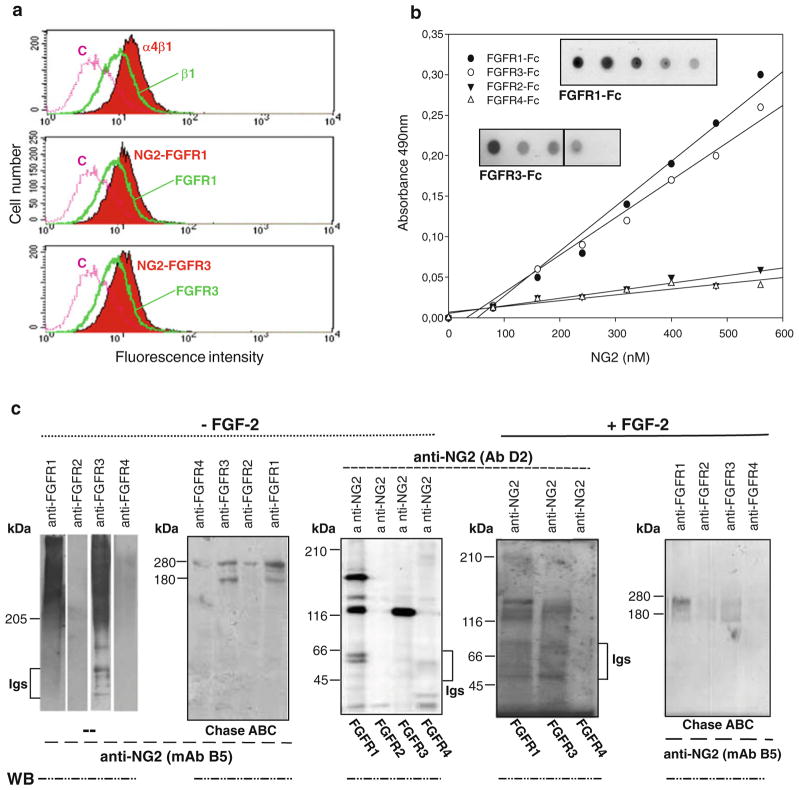
In addition to capturing and retaining FGFs on the cell surface for subsequent receptor presentation, NG2 might also physically associate with FGFR1 and FGFR3 through its core protein or GAG chain(s) and by engaging exogenous ligands in the interaction. To determine if this was the case we first performed a FRET analysis by flow cytomtery in the presence or absence of exogenous FGF-2. For this purpose we used cells incubated with combinations of anti-NG2 and anti-FGFR1/FGFR3 antibodies. These assays revealed a close apposition of NG2 to both FGF receptors (Fig. 5a), but not to CD44 (used as a control surface PG molecule). Adding FGF-2 to the system did not affect the NG2-receptor interaction. Selective NG2-FGFR1/-FGFR3 linkages in the absence of FGFs were also measured with purified recombinant molecules in two-ways solid-phase and overlay assays. In fact, when we allowed the purified ectodomain NG2 fragment and Fc-tagged FGFR1-FGFR4 molecules to interact with each other (using alternatively NG2 or the receptors as immobilized ligands), a dose-dependent binding of NG2 to FGFR1 and FGFR3 was observed both in the presence and absence of FGF-2. In contrast, no binding of the PG was detected to the recombinant FGFR2 or FGFR4 (Fig. 5b).
Figure 5.
(a) Flow cytometric FRET analyses of NG2-FGFR1/FGFR3 ligand-independent complex formation on the cell surface using pair-wise combinations of fluorescently-tagged anti-NG2 and anti-FGFR1 or anti-FGFR3 antibodies. Interaction between the individual subunits of the integrin α4β1 heterodimer is shown as positive control and the combination of tagged anti-NG2 and anti- CD44 antibodies was used as a negative control. (b) Scatchard plots of NG2-FGFR binding as determined by dose-dependent protein-binding assays performed with biotinylated recombinant NG2 and immobilized Fc-tagged human FGFR1–4. Purified human kappa light chain and recombinant human glypican-6 were used as negative controls. Assays performed in the presence of FGF-2 yielded equivalent results. (c) Immunochemical identification of NG2-FGFR surface complexes. SK-LMS-1 membrane preparations were immunoprecipitated with the anti-NG2 antiserum D3 or with anti-FGFR1-FGFR4 antibodies. Immunoprecipitates were resolved in their intact or chondroitinase ABC digested (Chase ABC) forms and reciprocally immunoblotted (WB) with antibodies against the individual FGFRs or against NG2 (mAb B5/M28).
To ultimately confirm by a third independent means that NG2 could assemble with FGFRs in an apparent ligand- and GAG-independent manner we performed pull-down assays based upon the constitutively expressed molecules. Thus, we carried out reciprocal immunoprecipitations of untreated or chondroitinase ABC-treated cell membranes with either anti-NG2 or anti-FGFR1-FGFR4 antibodies. Following separation by SDS-PAGE, the precipitated material was immunoblotted with heterologous (with respect to the antibody used for immunoprecipitation) anti-NG2 or anti-FGFR1-FGFR4 antibodies. Even with this experimental approach we could confirm NG2-FGFR1/-FGFR3 associations, but were not able to detect NG2-FGFR2/-FGFR4 complexes. This neither in the absence nor presence of exogenous FGF-2 and independently of prior removal of the CS chain(s) of NG2 or addition of heparin to the system (not shown). These observations further corroborated the propensity of NG2 to form ligand-unrelated interactions with FGFR1/FGFR3 and confirmed the lack of involvement of its GAG chains in these interactions (Fig. 5c). The possibility that endogenously produced FGFs were responsible for triggering the formation of NG2-FGFR complexes in the absence of exogenous growth factors was also effectively ruled out by complementary MALDI-TOF analyses that asserted the presence of peptides deriving from NG2, FGFR1 and FGFR3 and the lack of peptides derived from any of the known FGFs (not shown).
NG2 dictates the magnitude of FGFR1/FGFR3-mediated mitosis, but does not influence FGFR2-/FGFR4-induced signal transduction
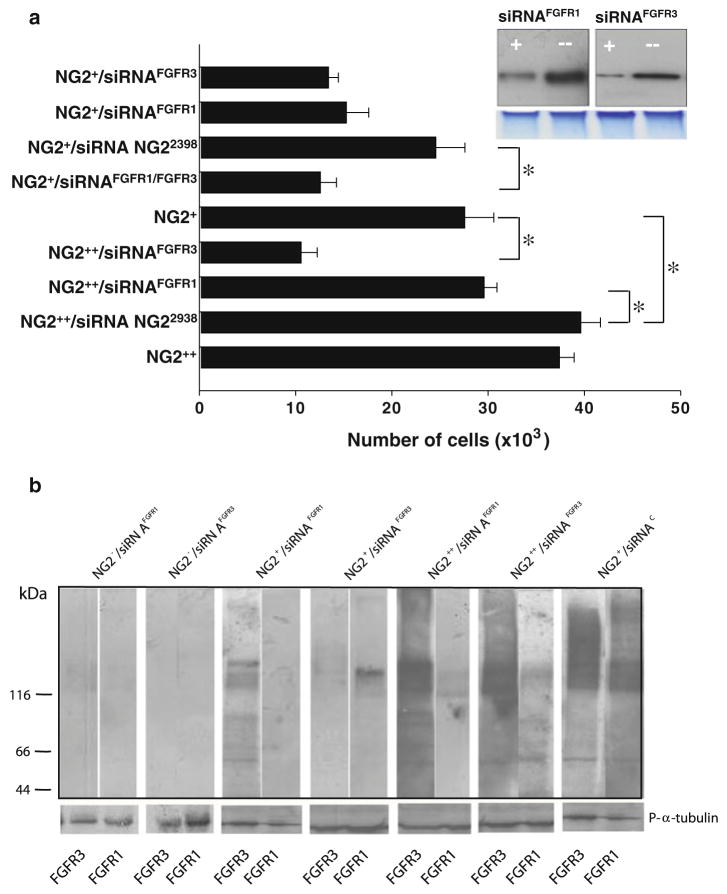
Taken together the above described findings provide evidence for an FGF co-receptor function of NG2, but do not preclude that NG2 may modulate receptor activity at the intracellular level. To test the hypothesis of a direct effect of NG2 on the overall functionality of FGFR1/FGFR3 we experimentally modified the relative surface ratios of NG2 versus FGFR1/FGFR3 in our cells by selective knock-down of the FGFRs, enrichment by immunoselection of cell populations homogenously expressing high constitutive levels of NG2 (cells denoted NG2+), and overexpression of full-length NG2 by in vitro gene transduction (cells denoted NG2++). In NG2+ cells, knock-down of either FGFR produced a marked decrease in FGF2-induced mitosis, as also confirmed by reduced receptor phosphorylation (Fig. 6a, b). NG2 overexpression effectively compensated for low levels of FGFR1, but apparently not for low levels of FGFR3 (Fig. 6a). Furthermore, knock-down of FGFR1 strongly abrogated ERK-dependent phosphorylation of the NG2 cytoplasmic tail, while knock-down of FGFR3 was somewhat less effective in de-phosphorylating the PG (not shown).
Figure 6.
(a) FGF-2 responses in cells expressing altered FGFR1/FGFR3-NG2 ratios. Cells enriched by immunoselection for their constitutive NG2 expression (NG2+) and cells transduced to overexpress the rodent full-length NG2 (NG2++) were exposed to optimal stimulatory concentrations of FGF-2, while their FGFR1 (siRNAFGFR1) and/or FGFR3 (siRNAFGFR3) were selectively knocked down. Probe NG2 siRNA2938 was used as a control probe. Inset shows an immunoblotting of FGFR1 and FGFR3 prior (−) and after (+) siRNA-mediated knock-down (lower panel, protein loading normalization as viewed by Commassie Brilliant Blue staining of the of the top of two gel lanes run parallel to those that were blotted). (b) Phosphorylation patterns of FGFR1 and FGFR3 in the same experimental conditions as depicted in (a). Calibrating immunoblots were performed using antibodies against phosphorylated α-tubulin (P-α-tubulin). “*”, p<0.001 by Mann- Whittney U Test.
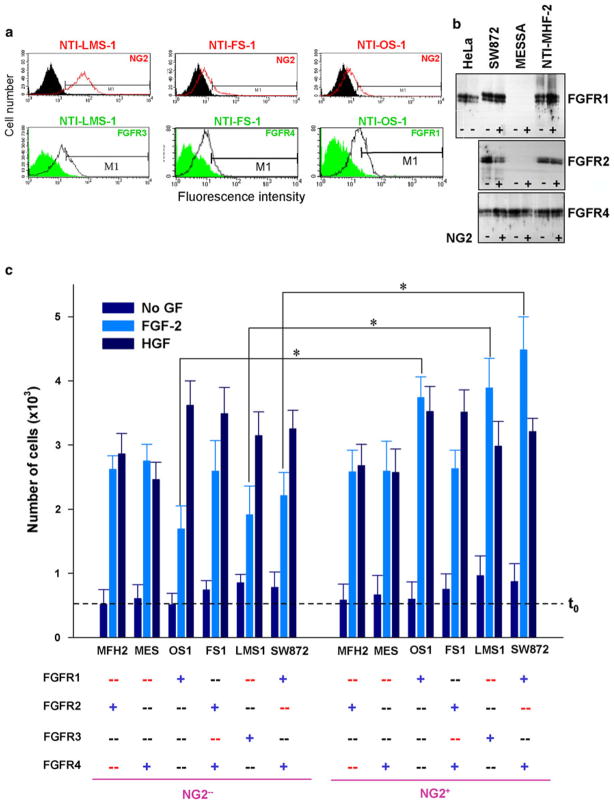
Although NG2 did not appear to interact with FGFR2 and FGFR4 it could not be firmly excluded that NG2 might influence FGF signalling in cells expressing solely these receptors by a mechanism not involving a direct association. Therefore, to address this issue we assayed the FGF-induced mitogenic responses of a panel of mesenchymal cell lines that were selected according to their constitutive levels of NG2 and their diverse profiles of FGFR1-FGFR4 (Supplemental Fig. S5a). In some of these cell lines we additionally separated the NG2+ subset from NG2− one and in the former subset knocked down one or two of the constitutively expressed FGFRs (Fig. 7a, b). In parallel, we verified that knock-down of FGFRs did not affect NG2 surface expression (not shown).
Figure 7.
Selectivity of the functional cooperation of NG2 with FGFR1 and FGFR3. FGF-2-induced mitogenic responses were compared in different fibroblastic cell lines selected on the basis of their diverse FGFR expression patterns (a, b; Supplemental Fig. S3) and their consistently high NG2 surface expression. Some cell lines were additionally immunoselected to yield homogeneously enriched NG2-positive (NG2+) and NG2-deficient (NG2-) cell populations. Constitutive and engineered FGFR expression patterns are highlighted by colour coding: blue “+” = constitutively expressed receptor; black “−“ = receptor not normally expressed by that cell; and red “−“ = receptor knocked down by siRNA probes. (c) Relative levels of FGF-2-induced cell proliferation in cells expressing the indicated FGFR(s) (*p<0.001 by ANOVA). Dashed line t0 indicates the amount of cells per well at the start of the experiment.
In baseline serum-free conditions, the NG2+ and NG2− cell subsets divided in a comparable manner, irrespective of their FGFR expression profile (Fig. 7c). In the presence of FGF-2, NG2+ and NG2− cells presenting FGFR2 and/or FGFR4 were equally stimulated, whereas NG2+ cells expressing either FGFR1 or FGFR3 proliferated more robustly than their counterpart NG2− cells (Fig. 7c). These observations thereby provide a functional support to the model of selective cooperation of NG2 with FGFR1 and FGFR3.
DISCUSSION
Proliferation and sprouting of NG2/CSPG4-expressing perivascular cells is an obligatory early step in normal and pathological angiogenesis. The growth factor-binding capability of NG2 [16] has led us to hypothesize that the PG may contribute to the optimization of the responsiveness of perivascular cells to angiocrine factors. In support of this idea we find that corneal angiogenesis, experimentally induced by ectopic implantation of the angiocrine factor FGF-2, is strongly compromised in NG2 knockout mice due to a marked reduction in pericyte replication and mobilization. Under the same experimental conditions, ectopic VEGF implantation is capable of promoting angiogenesis in the NG2 null background, albeit with lower efficiency than in wild type animals. This observation corroborates the lack of a cell-autonomous endothelial cell function in the NG2 knock-out mice, but leaves to reconcile the apparent integrity of neovessel network assembled under the influence of VEGF with the angiogenic deficits observed in these animals under a variety of experimental conditions [2–8, 52]. The present findings, nonetheless, emphasize a central role of NG2 in FGF signalling and the importance of FGF stimulation of angiogenic pericytes during tubular sprouting.
FGF-mediated signal transduction represents a complex phenomenon, not only due to the wealth of FGF molecules and their interaction patterns with the cognate FGF receptors, but also due to the increasing number of ancillary molecules proposed to dictate the strength of cellular responses in relation to spatio-temporal changes in mitogen availability [53]. The most well-studied FGF co-receptor entities are the HS chains of membrane-associated PGs. Within these GAG structures it has been possible to identify sub- or microdomains displaying high-affinity for FGF and, in some cases, it has even been proposed that different FGFs may have different HS subdomain specificities [32, 33, 35, 36, 42, 43]. A further complexity of the system is given by the fact that cations may modify the HS structure, such as to create FGF signalling-promoting or -inhibitory moieties [54].
The cumulative results of our study depict a novel FGF co-receptor paradigm that, at least when restricted to angiogenic pericytes, may act in an alternative or complementary fashion to that of HSPGs and cell adhesion molecules. Intriguingly, we find that FGF-2 binds to a discrete portion of the NG2 core protein with an apparently lower avidity than that previously determined for structurally defined HS oligosaccharides [31, 42, 51]. This raises the possibility that NG2 and HSPGs may iteratively retain FGF molecules on the cell surface, but since soluble heparin did not appear to affect FGF-2 binding to surface NG2, the two types of proteoglycans may not act in direct competition. While a model of “alternative function” would imply that NG2 could substitute for HSPGs in promoting extracellular capture and receptor presentation of the ligand, a model of “complementary function” would envision that HSPGs may primarily serve the purpose of dimerizing the single FGF molecules while NG2 could in concert favour the subsequent displacement of the dimerized ligands to the receptors. This latter scenario does not preclude a dual participation of HSPGs and NG2 in FGF signalling and, hence, it is fully compatible with the observations that smaller HS fragments may engage in ternary FGF-FGFR-GAG complexes in the concomitant presence of NG2. Accordingly, our findings demonstrate that even when NG2 is optimally accessible on the cell surface, addition of HS fragments/heparin does not further enhance FGF stimulation. This observation indicates that NG2 alone can saturate the cells’ FGF responses, i.e. satisfy the cells’ growth factor needs, without involvement of HSPGs.
In seeking detailed information about the mechanism of NG2 involvement in FGF signalling we adopted wild type and NG2 null perivascular cells and human smooth muscle-like cell lines. These latter cells were selected for their relatively low constitutive levels of HSPGs and lack of other FGFR1/FGFR4-interacting co-receptor molecules, such as N-CAM or N-cadherin. In these cellular models we found that NG2 exerted a multivalent extracellular influence on the cell-autonomous and paracrine signal transduction elicited by certain FGF family members. However, at variance with what is known for syndecans and glypicans, NG2 was not found to influence the cells’ mitogenic responses to a number of other heparin-binding growth factors requiring cell membrane-associated co-receptors for eliciting optimal signal transduction. This finding asserts that NG2 has a higher growth factor specificity than that currently documented for HSPGs and selectively regulates two distinct signalling systems, i.e. the FGF and PDGF ones. The reciprocal involvement of NG2 in these two signalling systems, and their inter-relationship in the context of pericyte proliferation, was not deemed to be a subject of this study. It deserves, however, attention because of potential agonistic or antagonistic effects that these systems may exert depending upon the relative extent with which NG2 may participate in the control of one or the other system.
Through its ability to bind to FGF-2, NG2 captured and retained the growth factor on the cell surface. NG2-mediated sequestration of FGF-2 was entirely independent of binding of the growth factor to its receptors and did not involve the CS chain(s) of the PG. Although not specifically investigated hitherto, it is likely that binding of latent FGFs to NG2 directly or indirectly impinged upon their activation, while we deduce from our findings that the primary role of the PG was to facilitate the interaction of these activated ligands with their cognate receptors. Of note in this context is that the PG was effective in bringing about cell mitosis in the absence of HS-mediated “ligand-activation”, as substantiated by the fact that in NG2-deprived cells, experimentally reintroduced NG2 (ectopic transduction), or NG2 administered to the cells as a soluble ectodomain fragment, rescued the partial FGF signalling deficiency of the cells. The rescuing effect seen with the isolated ectodomain also suggests that, similarly to syndecans, NG2 might influence the FGFs mitogenic actions both in its surface-bound and cell surface released form.
Based upon our results NG2 emerges as a putative key surface molecule for the elaboration and perception of FGF morphogen/mitogen gradients and may additionally contribute to the modulation of the receptor-growth factor binding kinetics. In fact, our findings suggest that elevated surface levels of NG2 may compensate for low surface levels of FGF receptors, as also recently shown for certain HSPGs [55]. Most likely this could be accomplished by augmenting the number of FGF molecules available for receptor binding on the cell surface, or by enhancing FGFR clustering when the receptor molecules are more sparsely distributed on the cell membrane. Since FGFRs and NG2 may both cluster in lipid rafts, they have the potential to synergize at the level of these cell membrane domains. The idea that a putative NG2-FGFR clustering may be critical for optimal regulation of FGF signalling was hinted by the observation that overexpression of truncated NG2 molecules, failing to properly accumulate in lipid rafts [50], were less effective in supporting FGF signalling than intact NG2 molecules.
Crystallographic studies have provided two tentative models of HS-FGF-FGFR assembly based upon two different stoichiometric ratios of the involved components. Thus, trimeric configurations with ratios of 2:2:1 or 2:2:2 between ligand, receptor and GAG moiety have been proposed to be equally effective in triggering down-stream signal transductions. However, more recent biochemical data generated with isolated molecules suggest that a 2:2:1 FGF-FGFR-HS ratio may be the most probable assemblies for optimal signal transducing effects [42]. It remains unresolved to what extent surface HSPGs are capable of “pre-cluster” with FGFRs in a ligand-independent manner and to what degree ternary complexes between HS moiety, ligand and receptor actually form on the cell surface (all studies reported thus far have been based upon interactions between the isolated components). Although we did not attempt to demonstrate here the formation of ternary complexes composed of NG2, FGF and FGFR, the ability of NG2 to physically associate with FGFRs in a ligand-independent fashion, and the ability of the PG to effectively trap FGF on the cell surface, strongly suggest that NG2 has the potential to mediate the assembly of such higher order macromolecular complexes.
NG2 association with FGFR1 and FGFR3 (but not with FGFR2 or FGFR4) in the apparent absence of FGFs further indicates that the PG may be capable of affecting FGF signalling at multiple levels, i.e. at both ligand and receptor level. However, since cells expressing NG2 molecules lacking the intracellular domain still effectively responded to FGF-2, association of the NG2 cytoplastic tail with the kinase domain of FGFRs did not seem to be required for NG2-mediated enhancement of the FGF mitogenic action. In contrast, signal transduction triggered by engagement of FGFR1/FGFR3 resulted in phosphorylation of the cytoplasmic tail of NG2, a phenomenon previously shown to be linked to integrin-dependent cell proliferation and motility [56, 57]. It is not yet clear whether, independently of this phosphorylation, or following such event, NG2 may make a direct contribution to ligand-unrelated FGFR pre-clustering. It is similarly, uncertain to what extent the PG may remain associated with the ligand-receptor complex to influence a possible subsequent cellular internalization of a heterodimeric or heterotrimeric complex. As NG2 seems to promote activation of distinct FGF receptor sets and, simultaneously, may sequester with high avidity more than one type of FGF, the PG may sustain different spatio-temporal “capture-release” FGF-FGFR binding dynamics on the cell surface. It could be envisioned that this NG2 ability may favour a diversification of the pericyte responses to the growth factors when such differentiated responses are needed to bring about complex multistep processes such as angiogenesis.
Supplementary Material
Supplemental Figure 1. Relative knock-down efficacy and surface recovery of NG2 following transient siRNA-mediated abrogation in SK-LMS-1 cells (analogous results were obtained in SK-UT-1 cells). (a) Determination of the relative knock-down efficacy of siRNA probes directed against different regions of the NG2 transcript (a scrambled sequence probe was used as a control) based upon flow cytometry using mAb 7.1. (b, c) Kinetics of NG2 surface knock-down and subsequent cell surface recovery, as determined by flow cytometry using the same anti-NG2 mAb at the indicated days following siRNA transfection, or on day one to six starting from the fourth day after transfection (i.e. Day 1 of recovery = day 4 after transfection). Immunofluorescence (anti-NG2 mAb B5/M28) images show representative cases of maximal knock-down and following surface recovery (i.e. at Day 3 of knock-down and Day 6 of recovery; nuclei were counterstained with TO-PRO-3). When surveying the panel of mesenchymal cell lines used in this study we could estimate by flow cytometry (and corroborate by parallel semi-quantitative Western blotting) that an average of 75–82% of the cells expressed detectable surface levels of NG2. Following transient siRNA-mediated knock-down, the abrogation efficiency that was achieved was in the range of 53–67% reduction of cell surface expression of the PG when compared to untreated cells.
Supplemental Figure 2. Patterns of constitutive syndecan and glypican expression in aortic smooth muscle cells from wild type NG2+/+ and knockout NG2−/− mice, and the two primary human smooth muscle cell-like lines used in the study. Expression levels were determined semi-quantitatively at the (a) mRNA (qPCR) and (b) protein level (flow cytometry; representative analyses of SK-LMS-1 cells are shown in the lower panel). Primers and antibodies were as detailed in Materials and Methods. Flow cytometric scoring of relative surface expression of the detected antigens was as follows: “−“, 1–15%; “+”, 16–40%; “++”, 41–65%; and “+++”, 66–95%. Scoring for relative levels of transcript expression were based upon direct comparisons with calibrating human bone marrow-derived mesenchymal progenitor cells, HUVEC, primary human dermal fibroblasts, A375 human melanoma cells, HT1080 fibrosarcoma, MCF-7 breast carcinoma, HepG2 hepatocellular carcinoma, NIH3T3 cells, B16 murine melanoma cells and mouse embryonic fibroblasts (MEF). The cumulative expression scoring was as follows: “−“, weak mRNA expression and no detectable surface levels of the protein; “+”, moderate transcript levels and weak surface levels of the protein; “++”, high transcript levels and moderate surface levels of the protein; and “+++”, high transcript levels and high surface expression of the protein.
Supplemental Figure S3. (a) FGF-2 mitogenic responses of FGFR1-expressing murine lymphocytic BaF3 cells after transient transduction with rodent NG2 (BaF3NG2). Inset to the right shows the relative levels of ectopic NG2 expression as determined by flow cytometry using the D3 anti-NG2 antiserum. Left inset shows detection of ectopically expressed rodent NG2 in whole cell lysates subjected (BaF3NG2/Chase) or not (BaF3NG2) chondroitinase ABC digestion. (b) Representative immunostaining of untreated and anti-NG2 siRNA-treated SK-LMS-1 cells exposed for 72 hours to the indicated FGFs (10 ng/ml) in the absence of serum. (c) One out of four cell-cycle analyses performed by flow cytometry by propidium iodide incorporation on untreated and anti-NG2 siRNA-treated SK-UT-1 cells exposed to FGF-2.
Supplemental Figure S4. (a) Recovery of FGF-responsiveness in SK-UT-1 cells upon surface recovery of transiently knocked down NG2 (Supplemental Fig. S2). Cells were either treated with an effective anti-NG2 siRNA probe (siRNA3289), or with an ineffective one (siRNA2938), and stimulated with 10 ng/ml of FGF-2. Assessment of cell proliferation was as described for other growth factor stimulation experiments. (b) Verification of the preserved ability of NG2-deficient cells to respond to GAG-activated exogenous FGF-2. SiRNA-treated cells were exposed to 10 ng/ml FGF-2 in the presence of soluble heparin (high molecular weight; 10 μg/ml), HS oligosaccharides of different sizes and compositions (HS6-HS12; 1 μg/ml), or the unrelated GAGs chondroitin sulfate (CS; 10–50 μg/ml) and keratan sulfates (KS; 10–50 μg/ml). Adding heparin or HS oligosaccharides to cells not treated with siRNA probes did not augment their FGF response level.
Supplemental Figure S5. Patterns of FGFR expression and endogenously produced FGFs in the cell types used in the study, including smooth muscle cells from wild type (NG2+/+) and NG2 null (NG2−/−) mice. Determination of the patterns of FGFR expression was based upon combined qPCR (a) and flow cytometry. (b) shows representative flow cytometry analyses of FGFR expression in wild type (red line) and NG2 null (green line) smooth muscle cells. Endogenous FGFs production was determined by qPCR for the corresponding transcripts (c). Arbitrary scoring of the levels of expression of FGFRs was similar to that detailed for PGs in Supplemental Fig. S2. Analyses of FGFs were restricted to those currently known to be preferred ligands of FGFR1 and FGFR3.
Supplemental Figure S6. (a) Schematic overview of the strategy adopted for generating NG2 mutant cells by combined siRNA-mediated mRNA knock-down and gene transduction. SK-LMS-1 cells were stably transduced with a human NG2 construct encoding the entire extracellular domain plus the membrane-spanning portion (NG2extra), or with a human NG2 construct encoding the transmembrane and cytoplasmic domains and a minor portion of the extracellular domain (NG2cyto). Transduced cells were then treated with either a C-terminus-directed siRNA (in the case of NG2extra), or an N-terminus-directed siRNA (in the case of NG2cyto). This approach resulted in an effective abrogation of the expression of the endogenous NG2 (see also Supplemental Fig. S2), while the transduced deletion constructs were not targeted. (b) qPCR-based assessment of the expression levels of the NG2extra and NG2cyto constructs when compared to the endogenous NG2 mRNA. (c, d) Flow cytometry and immunoblotting documenting the overall NG2 surface expression in NG2extra and NG2cyto cells as detected by mAb 7.1 (flow cytometry) and mAb B5/M28 (immunoblotting). The somewhat reduced surface levels of NG2 in NG2extra mutant cells, when compared to wild type cells, are presumably due to a previously observed ineffective membrane localization of NG2 molecules deprived of their cytoplasmic tail. (e) Summarizes the evidence for a putative involvement of NG2 in autocrine FGF and PDGF signallings through activity of its ectodomain. NG2extra, NG2cyto mutant SK-LMS-1 cells or SK-LMS-1 cells in which NG2 was knocked down were grow in low serum content (0.5%) for the indicated time in the presence or absence of the FGFR antagonist PD173074, or the PDGF receptor antagonist Imatinib, and their proliferation rates were scored. Untreated cells and cells treated with a non-effective siRNA were consistently used in a comparative manner as controls.
Acknowledgments
We are indebted to Gerd Pluschke for providing human NG2 cDNAs, David Ornitz for the engineered BaF3 cells and Marco Presta, John Gallagher and Sally Stringer for helpful comments on early versions of the manuscript. Iduron Ltd is thanked for providing HS fragments. April Esquibel is acknowledged for her assistance with histological procedures. The work was primarily supported by research funds from AIRC (Associazione Italiana per la Ricerca sul Cancro, to RP), the Italian Ministry of Health (ACC and Oncology Projects, Research Line 3 to RP), the Italian Ministry of the University and Research (PRIN 2008 to RP), intramural funds from the University of Parma, NIH grants RO1 CA95287 and PO1 HD25938 to WBS and R21HD052126 to UO. SC was supported by a post-doctoral fellowship from the Associazione “Via di Natale”, Pordenone, Italy.
Contributor Information
Sabrina Cattaruzza, Email: sabrina.cattaruzza@libero.it.
Ugur Ozerdem, Email: uozerdem@ljbi.org.
Martin Denzel, Email: mdenzel@burnham.org.
Barbara Ranscht, Email: ranscht@burnham.org.
Pietro Bulian, Email: pbulian@cro.it.
Ugo Cavallaro, Email: ugo.cavallaro@ifom-ieo-campus.it.
William B. Stallcup, Email: stallcup@burnham.org.
Roberto Perris, Email: rperris@cro.it.
References
- 1.Chen C-W, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Péault B. Perivascular multi-lineage progenitor cells in human organs: Regenerative units, cytokine sources or both ? Cytok Growth Fact Rev. 2009;20:429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgintino D, Girolamo F, Erede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between migrating pericytes and endothelial cells governs human foetal brain angiogenesis. Angiogenesis. 2007a;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 4.Virgintino D, Ozerdem U, Girolamo F, Roncali L, Stallcup WB, Perris R. Reversal of the cellular roles in angiogenesis: implications for anti-angiogenic therapy. J Vasc Res. 2007b;45:129–131. doi: 10.1159/000109965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 6.Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:307–311. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozerdem U. Targeting of pericytes diminishes neovascularization and lymphangiogenesis in prostate cancer. Prostate. 2006;66:294–304. doi: 10.1002/pros.20346. [DOI] [PubMed] [Google Scholar]
- 8.Song S, Ewald AJ, Stallcup WB, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumors regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995;221:231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- 11.Lindahl P, Johansson B, Leveen P, Betzholtz C. Pericyte loss and microaneurysm formation in PDGF-B deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 12.Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112:905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- 13.Hirschi K, Rohovski S, Beck L, Smith S, D’Amore P. Endothelial cells modulate the proliferation of mural cell precursors via PDGF-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Sennino B, Falcòn BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 16.Goretzki L, Burg M, Grako KA, Stallcup WB. High-affinity of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- 17.Suyama K, Shapiro I, Guttman M, Hazon RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 18.Böttcher RT, Pollet N, Delius H, Niehrs C. The transmembrane protein XFLRT3 forms a complex with FGF receptors and promotes FGF signalling. Nat Cell Biol. 2004;6:38–44. doi: 10.1038/ncb1082. [DOI] [PubMed] [Google Scholar]
- 19.Gotoh N, Manova K, Tanaka S, Murohashi M, Hadari Y, Lee A, Hamada Y, Hiroe T, Ito M, Kurihara T, Nakazato H, Shibuya M, Lax I, Lacy E, Schlessinger J. The docking protein FRS2α is an essential component of multiple fibroblast growth factor responses during early mouse development. Mol Cell Biol. 2005;25:4105–4116. doi: 10.1128/MCB.25.10.4105-4116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Heras E, Howell FV, Williams GP, Doherty PT. The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural adhesion molecule. J Biol Chem. 2006;281:35208–35216. doi: 10.1074/jbc.M608655200. [DOI] [PubMed] [Google Scholar]
- 21.Francavilla C, Cattaneo P, Berezin V, Bock E, Ami D, De Marco A, Christofori G, Cavallaro U. The binding of NCAM to FGFR1 induces a specific cellular response mediated by receptor trafficking. J Cell Biol. 2009;187:1101–1116. doi: 10.1083/jcb.200903030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 23.Allen BL, Filla MS, Rapraeger AC. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J Cell Biol. 2001;155:845–857. doi: 10.1083/jcb.200106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loo B-M, Kreuger J, Jalkanen M, Lindahl U, Salmivirta M. Binding of heparin/heparan sulfate to fibroblast growth factor. J Biol Chem. 2001;276:18668–18876. doi: 10.1074/jbc.M011226200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Coomans C, David G. Membrane heparan sulfate proteoglycan-supported FGF-2-FGFR1 signaling. J Biol Chem. 2001;276:41921–41929. doi: 10.1074/jbc.M106608200. [DOI] [PubMed] [Google Scholar]
- 26.Wu ZL, Zhang L, Yabe T, Kuberan B, Beeler DL, Love A, Rosenberg RD. The involvement of heparan sulfate (HS) in FGF1/HS/FGFR1 signaling complex. J Biol Chem. 2003;278:17121–17129. doi: 10.1074/jbc.M212590200. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahimi OA, Zhang F, Lang Hrstka SC, Mohammadi M, Linhardt RJ. Kinetic model for FGF, FGFR and proteoglycan signal transduction complex assembly. Biochemistry. 2004;43:4724–4730. doi: 10.1021/bi0352320. [DOI] [PubMed] [Google Scholar]
- 28.Chu CL, Buczek-Thomas JA, Nugent MA. Heparan sulfate proteoglycans modulate fibroblast growth factor-2 binding through a lipid raft-mediated mechanism. Biochem J. 2004;379:331–341. doi: 10.1042/BJ20031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu CL, Goerges AL, Nugent MA. Identification of common and specific growth factor binding sites in heparan sulfate proteoglycans. Biochemistry. 2005;44:12203–12213. doi: 10.1021/bi050241p. [DOI] [PubMed] [Google Scholar]
- 30.Gopalakrishnan M, Forsten-Williams K, Nugent MA, Tauber UC. Effects of receptor clustering on ligand dissociation kinetics: theory and simulations. Biophys J. 2005;89:3686–3700. doi: 10.1529/biophysj.105.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsten-Williams K, Chua CC, Nugent MA. The kinetics of FGF-2 binding to heparin sulfate proteoglycans and MAP kinase signaling. J Theor Biol. 2005;233:483–489. doi: 10.1016/j.jtbi.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Patel VN, Likar KM, Zisman-Rozen S, Cowherd SN, Lassiter KS, Sher I, Yates EA, Turnbull JE, Ron D, Hoffman MP. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 2008;283:9308–9317. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maccarana M, Casu B, Lindahl U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J Biol Chem. 1993;268:23898–23905. [PubMed] [Google Scholar]
- 34.Guimond S, Maccarana M, Olwin BB, Lindahl U, Rapraeger AC. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2 and FGF-4. J Biol Chem. 1993;268:23906–23914. [PubMed] [Google Scholar]
- 35.Kreuger J, Salmivirta M, Sturiale L, Giménez-Gallego G, Lindahl U. Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J Biol Chem. 2001;276:30744–30752. doi: 10.1074/jbc.M102628200. [DOI] [PubMed] [Google Scholar]
- 36.Delehedde M, Lyon M, Gallagher JT, Rudland PS, Fernig DG. Fibroblast growth factor-2 binds to small heparin-derived oligosaccharides and stimulates a sustained phosphorylation of p42/44 mitogen-activated protein kinase and proliferation of rat mammary fibroblasts. Biochem J. 2002;366:235–244. doi: 10.1042/BJ20011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrovsky O, Berman B, Gallagher J, Mulloy B, Fernig DG, Delehedde M, Ron D. Differential effects of heparin saccharides on the formation of specific fibroblast growth factor (FGF) and FGF receptor complexes. J Biol Chem. 2002;277:2444–2453. doi: 10.1074/jbc.M108540200. [DOI] [PubMed] [Google Scholar]
- 38.Powell AK, Fernig DG, Turnbull JE. Fibroblast growth factor receptors 1 and 2 interact differently with heparin/heparan sulfate. Implications for dynamic assembly of a ternary signaling complex. J Biol Chem. 2002;277:28554–28563. doi: 10.1074/jbc.M111754200. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Ye S, Kan M, McKeehan KW. Structural specificity in a FGF7-affinity purified heparin octasaccharide required formation of a complex with FGF7 and FGFRIIIb. J Cell Biochem. 2006;97:1241–1256. doi: 10.1002/jcb.20724. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 41.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 42.Robinson CJ, Harmer NJ, Goodger SJ, Blundell TL, Gallagher JT. Cooperative dimerization of fibroblast growth factor 1 (FGF1) upon single heparin saccharide may drive the formation of 2:2:1 FGF1.FGFR2c.heparin ternary complexes. J Biol Chem. 2005;280:42274–42282. doi: 10.1074/jbc.M505720200. [DOI] [PubMed] [Google Scholar]
- 43.Harmer NJ, Robinson CJ, Adam LE, Ilag LL, Robinson CV, Gallagher JT, Blundell TL. Multimers of the fibroblast growth factor (FGF)-FGF receptor-saccharide complex are formed on long oligomers of heparin. Biochem J. 2006;393:741–748. doi: 10.1042/BJ20050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stringer SE. The role of heparan sulphate proteoglycans in angiogenesis. Biochem Soc Trans. 2006;34:351–353. doi: 10.1042/BST0340451. [DOI] [PubMed] [Google Scholar]
- 45.Fuster MM, Wang L, Castagnola J, Sikara L, Reddi K, Lee PHA, Radek KA, Schuksz M, Bishop JR, Gallo RL, Sriramarao P, Esko JD. Genetic alteration of endothelial heparan sulphate selectively inhibits tumor angiogenesis. J Cell Biol. 2007;177:1–11. doi: 10.1083/jcb.200610086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozerdem U, Grako K, Dahlin-Huppe K, Monosov E, Stallcup WB. The NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 47.Pluschke G, Vanek M, Evans A, Dittmar T, Schmid P, Itin P, Filardo EJ, Reisfeld RA. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 50.Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- 51.Rahmoune H, Chen H-L, Gallagher JT, Rudland PS, Fernig DG. Interaction of heparin sulfate from mammary cells with acidic fibroblast growth factor (FGF and basic FGF). Regulation of the activity of basic FGF by high and low affinity binding sites in heparan sulfate. J Biol Chem. 1998;273:7303–7310. doi: 10.1074/jbc.273.13.7303. [DOI] [PubMed] [Google Scholar]
- 52.Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 53.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:110–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 54.Guimond SE, Rudd TR, Skidmore MA, Ori A, Gaudesi D, Cosentino C, Guerrini M, Edge R, Collison D, McInnes E, Torri G, Turnbull JE, Fernig DG, Yates EA. Cations modulate polysaccharide structure to determine FGF-FGFR signaling: a comparison of signaling and inhibitory polysaccharide interactions with FGF-1 in solution. Biochemistry. 2009;48:4772–4779. doi: 10.1021/bi802318z. [DOI] [PubMed] [Google Scholar]
- 55.Zhu H, Duchesne L, Rudland PS, Fernig DG. The heparan sulfate co-receptor and the concentration of fibroblast growth factor-2 independently elicit different signalling patterns from the fibroblast growth factor receptor. Cell Commun Signal. 2010;8:14. doi: 10.1186/1478-811X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makagiansar IT, Williams S, Dahlin-Huppe K, Fukushi J, Mustelin T, Stallcup WB. Phosphorylation of NG2 proteoglycan by protein kinase C-alpha regulates polarized membrane distribution and cell motility. J Biol Chem. 2004;279:52262–52270. doi: 10.1074/jbc.M411045200. [DOI] [PubMed] [Google Scholar]
- 57.Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. J Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Relative knock-down efficacy and surface recovery of NG2 following transient siRNA-mediated abrogation in SK-LMS-1 cells (analogous results were obtained in SK-UT-1 cells). (a) Determination of the relative knock-down efficacy of siRNA probes directed against different regions of the NG2 transcript (a scrambled sequence probe was used as a control) based upon flow cytometry using mAb 7.1. (b, c) Kinetics of NG2 surface knock-down and subsequent cell surface recovery, as determined by flow cytometry using the same anti-NG2 mAb at the indicated days following siRNA transfection, or on day one to six starting from the fourth day after transfection (i.e. Day 1 of recovery = day 4 after transfection). Immunofluorescence (anti-NG2 mAb B5/M28) images show representative cases of maximal knock-down and following surface recovery (i.e. at Day 3 of knock-down and Day 6 of recovery; nuclei were counterstained with TO-PRO-3). When surveying the panel of mesenchymal cell lines used in this study we could estimate by flow cytometry (and corroborate by parallel semi-quantitative Western blotting) that an average of 75–82% of the cells expressed detectable surface levels of NG2. Following transient siRNA-mediated knock-down, the abrogation efficiency that was achieved was in the range of 53–67% reduction of cell surface expression of the PG when compared to untreated cells.
Supplemental Figure 2. Patterns of constitutive syndecan and glypican expression in aortic smooth muscle cells from wild type NG2+/+ and knockout NG2−/− mice, and the two primary human smooth muscle cell-like lines used in the study. Expression levels were determined semi-quantitatively at the (a) mRNA (qPCR) and (b) protein level (flow cytometry; representative analyses of SK-LMS-1 cells are shown in the lower panel). Primers and antibodies were as detailed in Materials and Methods. Flow cytometric scoring of relative surface expression of the detected antigens was as follows: “−“, 1–15%; “+”, 16–40%; “++”, 41–65%; and “+++”, 66–95%. Scoring for relative levels of transcript expression were based upon direct comparisons with calibrating human bone marrow-derived mesenchymal progenitor cells, HUVEC, primary human dermal fibroblasts, A375 human melanoma cells, HT1080 fibrosarcoma, MCF-7 breast carcinoma, HepG2 hepatocellular carcinoma, NIH3T3 cells, B16 murine melanoma cells and mouse embryonic fibroblasts (MEF). The cumulative expression scoring was as follows: “−“, weak mRNA expression and no detectable surface levels of the protein; “+”, moderate transcript levels and weak surface levels of the protein; “++”, high transcript levels and moderate surface levels of the protein; and “+++”, high transcript levels and high surface expression of the protein.
Supplemental Figure S3. (a) FGF-2 mitogenic responses of FGFR1-expressing murine lymphocytic BaF3 cells after transient transduction with rodent NG2 (BaF3NG2). Inset to the right shows the relative levels of ectopic NG2 expression as determined by flow cytometry using the D3 anti-NG2 antiserum. Left inset shows detection of ectopically expressed rodent NG2 in whole cell lysates subjected (BaF3NG2/Chase) or not (BaF3NG2) chondroitinase ABC digestion. (b) Representative immunostaining of untreated and anti-NG2 siRNA-treated SK-LMS-1 cells exposed for 72 hours to the indicated FGFs (10 ng/ml) in the absence of serum. (c) One out of four cell-cycle analyses performed by flow cytometry by propidium iodide incorporation on untreated and anti-NG2 siRNA-treated SK-UT-1 cells exposed to FGF-2.
Supplemental Figure S4. (a) Recovery of FGF-responsiveness in SK-UT-1 cells upon surface recovery of transiently knocked down NG2 (Supplemental Fig. S2). Cells were either treated with an effective anti-NG2 siRNA probe (siRNA3289), or with an ineffective one (siRNA2938), and stimulated with 10 ng/ml of FGF-2. Assessment of cell proliferation was as described for other growth factor stimulation experiments. (b) Verification of the preserved ability of NG2-deficient cells to respond to GAG-activated exogenous FGF-2. SiRNA-treated cells were exposed to 10 ng/ml FGF-2 in the presence of soluble heparin (high molecular weight; 10 μg/ml), HS oligosaccharides of different sizes and compositions (HS6-HS12; 1 μg/ml), or the unrelated GAGs chondroitin sulfate (CS; 10–50 μg/ml) and keratan sulfates (KS; 10–50 μg/ml). Adding heparin or HS oligosaccharides to cells not treated with siRNA probes did not augment their FGF response level.
Supplemental Figure S5. Patterns of FGFR expression and endogenously produced FGFs in the cell types used in the study, including smooth muscle cells from wild type (NG2+/+) and NG2 null (NG2−/−) mice. Determination of the patterns of FGFR expression was based upon combined qPCR (a) and flow cytometry. (b) shows representative flow cytometry analyses of FGFR expression in wild type (red line) and NG2 null (green line) smooth muscle cells. Endogenous FGFs production was determined by qPCR for the corresponding transcripts (c). Arbitrary scoring of the levels of expression of FGFRs was similar to that detailed for PGs in Supplemental Fig. S2. Analyses of FGFs were restricted to those currently known to be preferred ligands of FGFR1 and FGFR3.
Supplemental Figure S6. (a) Schematic overview of the strategy adopted for generating NG2 mutant cells by combined siRNA-mediated mRNA knock-down and gene transduction. SK-LMS-1 cells were stably transduced with a human NG2 construct encoding the entire extracellular domain plus the membrane-spanning portion (NG2extra), or with a human NG2 construct encoding the transmembrane and cytoplasmic domains and a minor portion of the extracellular domain (NG2cyto). Transduced cells were then treated with either a C-terminus-directed siRNA (in the case of NG2extra), or an N-terminus-directed siRNA (in the case of NG2cyto). This approach resulted in an effective abrogation of the expression of the endogenous NG2 (see also Supplemental Fig. S2), while the transduced deletion constructs were not targeted. (b) qPCR-based assessment of the expression levels of the NG2extra and NG2cyto constructs when compared to the endogenous NG2 mRNA. (c, d) Flow cytometry and immunoblotting documenting the overall NG2 surface expression in NG2extra and NG2cyto cells as detected by mAb 7.1 (flow cytometry) and mAb B5/M28 (immunoblotting). The somewhat reduced surface levels of NG2 in NG2extra mutant cells, when compared to wild type cells, are presumably due to a previously observed ineffective membrane localization of NG2 molecules deprived of their cytoplasmic tail. (e) Summarizes the evidence for a putative involvement of NG2 in autocrine FGF and PDGF signallings through activity of its ectodomain. NG2extra, NG2cyto mutant SK-LMS-1 cells or SK-LMS-1 cells in which NG2 was knocked down were grow in low serum content (0.5%) for the indicated time in the presence or absence of the FGFR antagonist PD173074, or the PDGF receptor antagonist Imatinib, and their proliferation rates were scored. Untreated cells and cells treated with a non-effective siRNA were consistently used in a comparative manner as controls.