Abstract
In soft-tissue sarcoma patients, enhanced expression of NG2/CSPG4 proteoglycan in pre-surgical primary tumours predicts post-surgical metastasis formation and thereby stratifies patients into disease-free survivors and patients destined to succumb to the disease. Both primary and secondary sarcoma lesions also up-regulate collagen type VI, a putative extracellular matrix ligand of NG2, and this matrix alteration potentiates the prognostic impact of NG2. Enhanced constitutive levels of the proteoglycan in isolated sarcoma cells closely correlate with a superior engraftment capability and local growth in xenogenic settings. This apparent NG2-associated malignancy was also corroborated by the diverse tumorigenic behaviour in vitro and in vivo of immunoselected NG2-expressing and NG2-deficient cell subsets, by RNAi-mediated knock down of endogenous NG2, and by ectopic transduction of full-length or deletion constructs of NG2. Cells with modified expression of NG2 diverged in their interaction with purified Col VI, matrices supplemented with Col VI, and cell-free matrices isolated from wild-type and Col VI null fibroblasts. The combined use of dominant-negative NG2 mutant cells and purified domain fragments of the collagen allowed us to pinpoint the reciprocal binding sites within the two molecules and to assert the importance of this molecular interaction in the control of sarcoma cell adhesion and motility. The NG2-mediated binding to Col VI triggered activation of convergent cell survival- and cell adhesion/migration-promoting signal transduction pathways, implicating PI-3K as a common denominator. Thus, the findings point to an NG2–Col VI interplay as putatively involved in the regulation of the cancer cell–host microenvironment interactions sustaining sarcoma progression.
Keywords: proteoglycans, sarcoma, collagen type VI, tumour–stroma interaction, cell migration, prognostic biomarker
Introduction
Gene expression data available for patients affected by various cancer types suggest that altered expression/distribution of chondroitin sulphate proteoglycan 4 (CSPG4)/NG2 may serve as a prognostic factor for both solid and haematological tumours (Kageshita et al., 1992; Smith et al., 1996; Benassi et al., 2009; Petrovici et al., 2010; Wang et al., 2010a, b; Svendsen et al., 2011). The clinical significance of the aberrant tumour expression of NG2 is thoroughly substantiated by experimental data accrued over the years and proposing a role for the proteoglycan (PG) in the control of tumour growth and spreading in a variety of cellular and animal models (Burg et al., 1998; Al-Mayhani et al., 2011; Wang et al., 2011a). Through exploitation of xenograft models involving transplantation of cells constitutively expressing NG2, or cells engineered to ectopically express the PG, and the use of conjugated or non-conjugated anti-NG2 antibodies, it has been possible to demonstrate an effective eradication of the implanted tumours in haematological malignancies, melanoma, glioblastoma, breast carcinoma and mesothelioma (Wagner et al., 2008; Wang et al., 2010a, 2011a, b; Rivera et al., 2012). The clinical potential of NG2 as a target for passive, adoptive immunotherapy (Mittelman et al., 1995; Murray et al., 2004) has been further emphasized by the successful outcome of a vaccination Phase I/II trial entailing an idiotypic anti-NG2 antibody (a trial recently completed at our Institute on >100 advanced melanoma patients). Thus, NG2 has a consolidated role in the process of tumorigenesis, including angiogenesis, and the modes through which it exerts this effect deserve to be explored in more detail. It remains particularly challenging to establish whether the tumour-promoting activity of NG2 is entirely cell-autonomous, or NG2 acts as a promoter of the cancer cells' interaction with the microenvironment, which secondarily activates signalling pathways governing cell survival (Chekenya et al., 2008).
It could be assumed that NG2 may affect tumour progression via its documented multitude of functions. These include, among others, a docking receptor activity in growth factor signalling, a modulatory activity of membrane-bound metalloproteinases (Iida et al., 2001), sequestration of angiostatin, surface binding of galectin-3, modulation of integrin activity, and facilitation of pericyte recruitment and sprouting during angiogenesis (Iida et al., 1992, 1995; Fukushi et al., 2004; Virgintino et al., 2007; Tigges et al., 2008). The indirect linkage of the NG2 cytoplasmic tail with the actin cytoskeleton (Lin et al., 1996; Fang et al., 1999; Chatterjee et al., 2008) may provide a means through which NG2 contributes to the stabilization of the contacts between cancer cells and their neighbouring host (including stromal) cells and/or with the surrounding intra- and extra-lesional extracellular matrix (ECM). This latter function could plausibly be exerted through cooperation of the PG with integrins α3β1 and α4β1, or by direct binding to discrete ECM constituents. Accordingly, specific interactions of ‘non-tumour’ NG2 with collagen types V and VI (Col V and VI) have previously been reported in some experimental models in vitro and confirmed by binding studies with the isolated molecules (Stallcup et al., 1990; Nishiyama and Stallcup, 1993; Burg et al., 1996, 1997; Midwood and Salter, 2001).
In the tumour context, of particular relevance may be the NG2 interaction with Col VI. Noteworthily, secretion of this collagen is strongly increased in the stromal compartment of breast carcinoma, ovarian carcinoma, melanoma, and glioblastoma lesions (Han et al., 1995; Daniels et al., 1996; Sherman-Baust et al., 2003; Iyengar et al., 2005) and, hence, particular emphasis has been given to the potential of Col VI in the control of tumour development. Whether Col VI can promote the loco-regional growth of tumours through an interaction with NG2 remains to be more firmly established by experimental means. In fact, in a simulated ‘melanoma brain metastasis’ syngenic model, loss of Col VI reduces lesion growth by interfering with intra-lesional neovessel maturation (You et al., 2012) and this observation underscores a generalized importance of Col VI in structuring tumour-permissive microenvironments. Cumulatively, these previous observations have led us to hypothesize that a direct NG2–Col VI interaction may indeed play a key role in the control of the local propagation of primary and secondary lesions. To address this possibility, we have approached the clinical significance of NG2 and Col VI expression in lesions of soft-tissue sarcoma (STS) patients and have explored how the interplay between these molecules governs tumour cell behaviour.
Results
Combined up-regulation of NG2 and Col VI predicts metastasis formation and a dismal clinical course
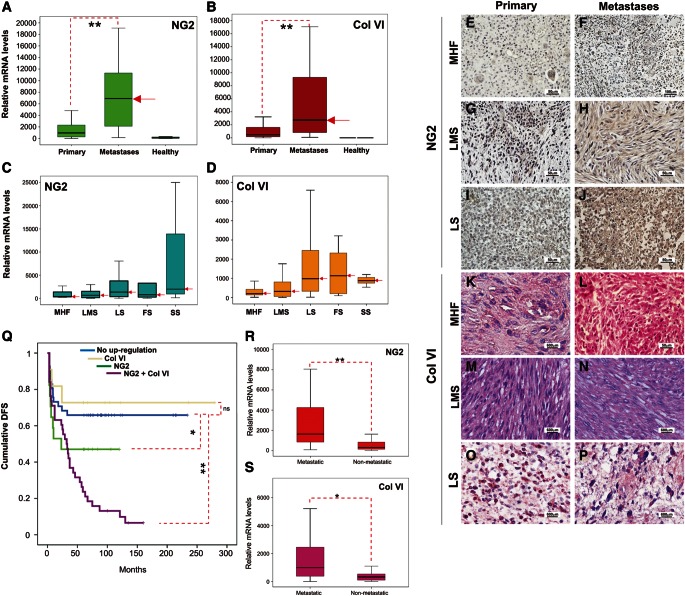
In a previous study we have shown that NG2 is strongly up-regulated in primary and metastatic STS lesions and that its relative levels of expression may serve as a prognostic indicator of disease evolution and post-surgical metastasis formation (Benassi et al., 2009). To further substantiate this finding, we have re-examined the prognostic significance of NG2 in a larger and more comprehensive cohort of patients from whom both primary and secondary STS lesions were accessible and for whom the full clinical history could be evaluated (Supplementary Table S1). To this end, we selected homogeneously treated patients, considering both pre- and post-surgical treatments, who remained free from other ‘non-sarcoma’ tumours (see Supplementary material). Analysis of mRNA expression in this cohort of patients confirmed the enhancement of NG2 (up to 100-fold) in metastatic lesions (primarily pulmonary ones) and additionally demonstrated consistently higher levels of the α3(VI) chain mRNA in metastases, compared with primary lesions and the adjacent healthy tissue of the surgical resection margins (Figure 1A and B). A direct comparison of the metastasis-associated expression patterns of NG2 with those displayed by healthy lung tissue surrounding the metastatic formations was not technically possible because of the lack of accessibility to such healthy material from these patients. Nevertheless, despite the higher NG2 expression levels in lung tissues compared with dermis, visceral and urogenital tissues surrounding primary lesions, neoplastic STS lesions within the lungs had nearly 50-fold higher quantities of NG2 mRNA than that detected in commercially obtained healthy lung tissue. Thus, although some contaminating lung tissue may have contributed to the overall NG2 mRNA signal measured in pulmonary metastatic specimens, we conclude that the predominant NG2 transcript was, to a large extent, associated with the metastatic cells composing such lesions.
Figure 1.
NG2 and Col VI are up-regulated in STS lesions and their expression levels predict the clinical disease course and metastasis formation. (A and B) Levels of NG2 and α3(VI) chain mRNAs in primary lesions (Primary), pulmonary metastases (Metastases) and surgical resection margins (Healthy) of the primary lesions removed from patients affected by various STS histological subtypes (red arrows point to median values). (C and D) Patterns of expression of NG2 and Col VI mRNAs in the different STS histotypes. MHF, malignant fibroshistocytoma-like pleomorphic sarcoma; LMS, leiomyosarcomas; LS, various variants of liposarcoma; FS, fibrosarcoma; and SS, synovial sarcoma (red arrows point to median values). Representative immunohistochemical staining for NG2 (E–J) and Col VI [α3(VI) chain; K–P] proteins in primary and metastatic lesions derived from patients with the indicated STS variants. (Q) Probability rates of disease-free survival (DFS) in patients presenting enhanced NG2 and Col VI mRNAs levels in their metastatic lesions. (R and S) Relative levels of NG2 and α3(VI) chain mRNA expression in primary lesions of STS patients who developed metastases (Metastatic), when compared with individuals who did not develop such secondary lesions (Non-metastatic), and as evaluated during a (post-surgical) follow-up period of up to 9 years. *P < 0.05, **P < 0.01 by the Kruskal–Wallis one-way analysis of variance.
Interestingly, when we considered the individual STS histotypes, the maximal detectable levels of NG2 versus Col VI mRNA expression showed a substantial diversity and did not perfectly coincide; synovial sarcomas showed the highest overall NG2 expression, whereas fibrosarcomas and leiomyosarcomas showed equal or superior levels of Col VI (Figure 1C and D). The enrichment of NG2 on the surface of neoplastic cells and neovascular structures of primary and secondary tumour masses was independently confirmed by immunolabelling with the anti-NG2 polyclonal antiserum D2 (Virgintino et al., 2009) and with selected anti-NG2 mAbs recently generated in our laboratory (see Supplementary material). More than 60% of the cells in primary lesions and >90% in metastatic lesions stained positively for NG2, detected either within the cytoplasm or on the cell surface, and occasionally within the interstitial spaces (Figure 1E–J) for secreted forms of the PG (i.e. as a cell surface shedded molecule). In contrast to NG2, Col VI was found to be prevalently associated with the intra-lesional stroma and to a lower degree with the neoplastic cells themselves (Figure 1K–P). Collectively, these findings indicate a precise spatial relationship in the distribution of the two molecules: NG2 was predominantly associated with the tumour cells (as well as decorating pericytes of the neovasculature), whereas Col VI was tied to the tumour stroma.
Comparing the disease course of patients with NG2 levels in their metastatic lesions above median values with that of patients whose NG2 levels below median disclosed a group of individuals with a significantly more unfavourable clinical course (Figure 1Q). Accordingly, a multivariate Cox regression analysis, which took into account the prognostic factors recognized to date to be of clinical relevance in STS (beyond those upon which patient selection was based), substantiated that the high expression of NG2 (i.e. above median values) was an independent prognostic parameter (Supplementary Figure S1). Enhanced expression of Col VI alone did not significantly impact on disease course, but high expression of both Col VI and NG2 correlated with the worst disease course (Figure 1Q). An analogous multivariate analysis of Col VI expression did not highlight a significant impact of the collagen up-regulation (not shown).
We next re-evaluated whether relative expression levels of NG2 and Col VI mRNAs in primary lesions could provide information on the possible occurrence of future metastasis in patients free of metastatic disease at the time of diagnosis. To this end, we assessed the relative levels of the two transcripts in primary lesions of patients who developed distant metastases and compared these values with those found in analogous lesions of patients who remained free of metastases during a 7-year follow-up period (Supplementary Table S1). We noted that both NG2 and Col VI were more abundant in primary lesions of patients who manifested post-surgical metastatic disease (Figure 1R and S), thus implying a close correlation between augmented NG2 mRNA levels in primary tumours and the development of post-surgery metastases. The relative probability of the event of metastatic disease in patients harbouring NG2 overexpressing primary lesions (i.e. lesions with NG2 mRNA levels above the median value) was determined to be higher than that in those harbouring Col VI overexpressing primary lesions and to reach 60%. The absolute NG2 mRNA levels defining the cluster of patients at a high risk of developing metastases are currently being assessed and will be reported elsewhere.
A closer examination of the clinical course of patients with augmented levels of NG2 in their primary lesions and the timing of development of metastatic disease revealed a further patient stratification. Individuals with lower levels of NG2 developed metastases after preferentially five or more years after removal of their primary tumour masses. By contrast, patients with the highest levels of NG2 were more likely to develop metastases within 12 months of surgical intervention (Supplementary Figure S1). Thus, expression levels of NG2 mRNA in primary pre-surgical lesions also determined a temporal parameter of metastases formation and discriminated patient subsets with longer and shorter metastasis-free lag periods. At this stage, we cannot establish whether the NG2 up-regulation seen in cells constituting metastatic lesions is a primary event (i.e. occurring prior to or concurrently with the tissue dissemination of the pro-metastatic cells and defining a pre-committed metastatic phenotype) or whether NG2 is up-regulated in metastatic lesions after their formation (i.e. as a secondary event).
NG2 surface expression, but not Col VI secretion, correlates with malignancy
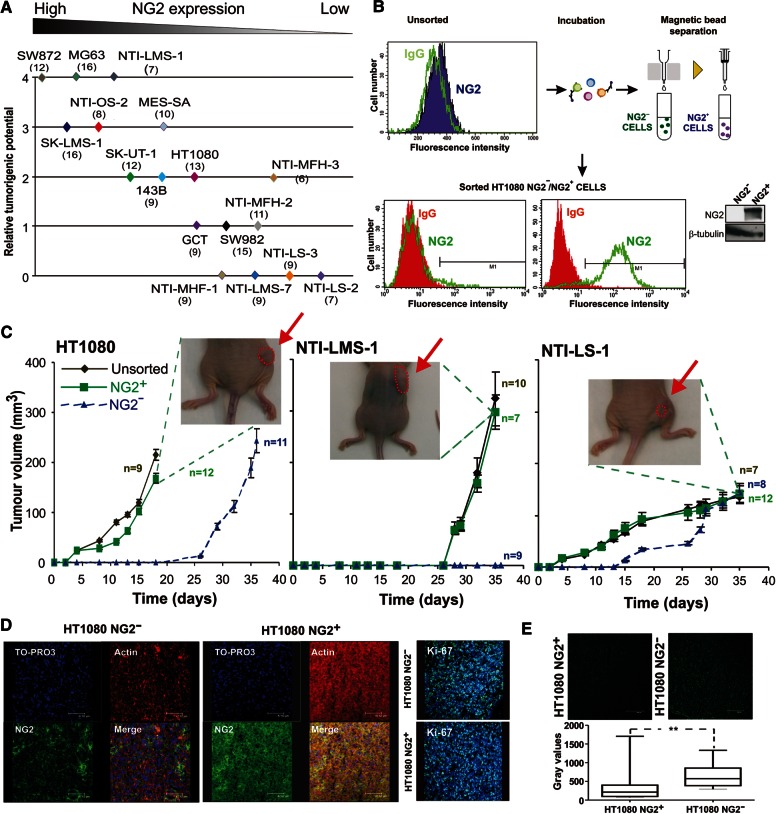
To verify whether surface NG2 or secreted Col VI could be directly responsible for the aggressiveness of cells overproducing the molecules, we assayed by xenografting the tumorigenic capacity of sarcoma cells with different NG2 surface levels and diverse Col VI secretion patterns (Supplementary Figure S2). Independent of their histological derivation, growth rates and overall capability of sarcoma cells to engraft and form subcutaneous tumour masses were found to closely correlate with the cell surface abundance of NG2 (Figure 2A), but not relate to the ability of the cells to secrete Col VI. These finding suggested that NG2-positive and NG2-negative cells of STS lesions may have represented subsets with distinct tumorigenic potential (e.g. NG2-expressing cells being a putative cancer initiating cell subset). Since a heterogeneous expression of NG2 was similarly noted in several sarcoma cell lines, we separated by immunosorting the NG2+ and NG2− subpopulations of these cell lines (Figure 2B) and comparatively assayed the tumorigenic potential of the resulting subpopulations in vitro or by transplantation into nude mice. Antibody-assisted enrichment of the NG2+ cell subsets was effectively accomplished in cell lines in which the NG2-expressing subpopulation represented >15% of the entire cell population. We investigated subsets separated in these cell lines only when the two subpopulations maintained their phenotype for up to 40 population doublings. There was no evidence of methylation being the factor responsible for the lack of NG2 expression in the NG2− subset.
Figure 2.
Surface levels of NG2 dictate the tumorigenic potential of sarcoma cells. (A) Graphical representation of the relative degree of tumour engraftment and local growth (following subcutaneous implantation of 3 × 106 cells/mouse) as a function of the constitutive surface levels of NG2 (Supplementary Figure S2). Relative tumorigenic potential of the implanted sarcoma cell lines was arbitrary scored 0–4 by adopting the algorithm reported in Supplementary material. (B) Typical outcome of the immunosorting strategy used to isolate minority NG2+ cell subsets in established sarcoma cell lines via one of our recently generated anti-NG2 mAb (coded 2161D3). Flow cytometry (lower graphs) on the immunosorted cells were performed using the anti-NG2 mAb 7.1 and yielded a close to 90% enrichment of weakly NG2-expressing cells. Inset to the right shows the corresponding immunoblotting with anti-NG2 mAb 9.2.27 on lysates of NG2− and NG2+ cell subsets resolved by SDS–PAGE. (C) Representative growth kinetics of locally growing tumours induced by subcutaneous implantation (1.5×106 cells/flank/animal) of the indicated NG2− (0–5% NG2 expression) and NG2+ (>90% NG2 expression) cell subsets into nude mice. Behaviour of the ‘unsorted’ cell population presenting surface levels of NG2 in the range of 30%–35% (Supplementary Figure S2) is indicated for reference. (D) Representative confocal laser microscopy images of sections taken from tumour masses formed by NG2− and NG2+ cells and double stained with antibodies against NG2 (mAb 9.2.27) and F-actin. The patchy staining in masses formed by NG2− cells corresponds to intra-lesional neovascular pericytes. Right panel shows representative distributions of Ki-67-positive cells in the two types of tumour lesions. Nuclear counterstaining was performed with TO-PRO-3. (E) Representative staining (upper panel) and quantification of TUNEL assay by In Situ Apoptosis Detection Kit - Fluorescein TUNEL-based Apoptosis Detection Assay. The graph reports the median value of three independent experiments. **P < 0.005 by Student's t-test.
Both unilateral and paired bilateral flank transplantations (i.e. each of the cell subsets was implanted on either flank of the same animal) were performed, yielding two distinct xenograft scenarios: (i) the NG2+ cell subset developed conspicuous tumours, whereas the NG2− subset largely failed to engraft and (ii) the NG2− cell subset was significantly (P < 0.001) delayed in its engraftment and subsequent growth, but eventually formed masses analogous to those seen with the NG2+ subset (Figure 2C). Both scenarios were observed when testing five different immunosorted cell lines (SK-UT-1 NG2+/NG2− cells behaved as in scenario i, whereas MG63 NG2+/NG2− cells behaved as in scenario ii). Accentuated malignant potential of the NG2+ subpopulations was clearly asserted in the HT1080 cell line in which as low as 30% of NG2-positive cells in the unsorted population produced masses similar to the ones seen with sorted populations containing <90% of the cells expressing NG2 (Figure 2C). Again, differences in tumorigenic behaviour of STS cells in vivo did not seem to relate to the histological subtype or to correlate with the kinetics/latency with which each of the parental cell lines (i.e. the unsorted cells) developed discernible lesions (Figure 2C). However, a 3–5-fold lower amount of NG2+ cells was normally needed to obtain optimal engraftment and formation of palpable tumour masses, further emphasizing the malignant potency of this latter phenotype.
Histological and immunohistochemical analyses of explanted tumour lesions formed by NG2+ and NG2−cells show that they had indistinguishable morphologies and neovascularisation degrees and that neovessel density was largely proportional to the size of the masses. These analyses also indicated that both NG2 cell subsets preserved their phenotype after transplantation and expansion in vivo. In fact, lesions formed by the NG2+ subset contained roughly 46% of the cells exhibiting a detectable NG2 expression, whereas the smaller lesions formed by NG2− cells were composed nearly exclusively of NG2-negative cells (i.e. only 7% of cells of the entire lesion including microvascular pericytes and stromal cells were positive for NG2; Figure 2D). Although this point was not systematically addressed hitherto, re-implantation of ‘unsorted’ cells derived from SK-UT-1 NG2+ masses seemed to effectively regenerate lesions in a second host. Conversely, cells derived from NG2− masses were strongly impaired in bringing about a second engraftment and a pronounced tumour formation. Ki-67 staining of lesions explanted at experimental end-points showed equally high cycling frequencies in the two types of tumour masses; virtually all cells were positive for the cell-cycle marker (Figure 2D). TUNNEL staining did not indicate gross differences in the apoptotic rates between the two types of tumours (Figure 2E). These observations suggested that size differences in the NG2+ and NG2− tumour masses were accounted by differences in the engraftment capacities and initial growth rates of the two cell phenotypes.
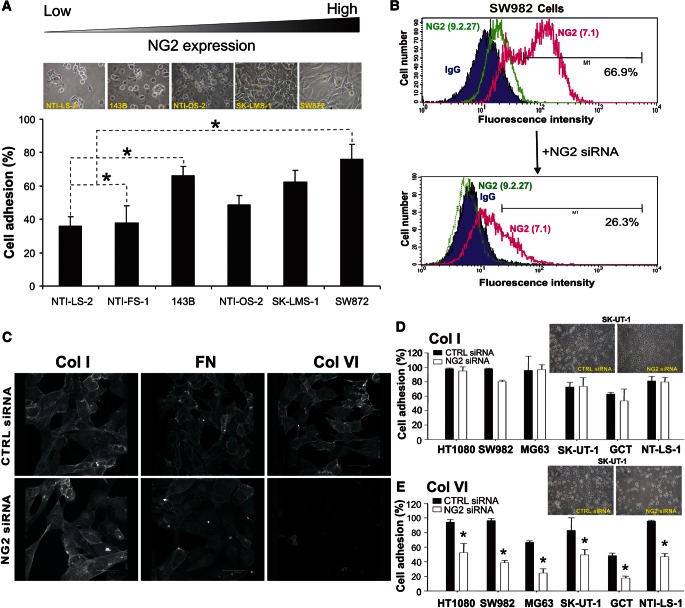
NG2 mediates cell adhesion and spreading on Col VI by cooperating with integrinα2β1
It has been proposed that NG2 is capable of binding to Col VI (Stallcup et al., 1990; Nishiyama and Stallcup, 1993; Burg et al., 1997; Tillet et al., 1997, 2002; Midwood and Salter, 2001), and it has been suggested that this collagen, in turn, is an ECM component affecting progression of several tumour types (Daniels et al., 1996; Sherman-Baust et al., 2003; Iyengar et al., 2005; You et al., 2012). We, therefore, hypothesized that the interaction between sarcoma NG2 and stromal Col VI could be a determining factor in the control of the growth and dissemination of these tumours. Our panel of sarcoma cells attached and spread on Col VI substrates with an efficacy that was largely proportional to their constitutive NG2 surface levels (Figure 3A). Accordingly, siRNA-mediated NG2 abrogation significantly reduced both adhesion and spreading of the cells onto these substrates, but did not affect binding to other ubiquitous ECM components (Figure 3B–E). Integrins of the β1 class were identified as the predominant NG2-cooperating ECM receptors and the use of specific function-blocking antibodies disclosed the α2β1 as the one directly implicated (Supplementary Figure S3), a finding that was supported by the discrete Mg2+/Mn2+ dependency for optimal cell binding. In line with these observations, combined knockdown of NG2 and addition of function-blocking anti-β1 integrin antibodies produced additive inhibitory effects and virtually abolished cell attachment to intact Col VI tetramers. Furthermore, the cells' interaction with Col VI was marginally inhibited by exogenously added heparin or heparatinase/chondroitinase ABC-treatment of the cells (not shown). This finding precluded any ancillary involvement of the NG2 GAG chains in the interaction with collagen.
Figure 3.
NG2 promotes tumour cell adhesion to Col VI. (A) Extents of cell adhesion to intact Col VI tetramers in relation to the surface levels of NG2 displayed by the cells. (B and C) Representative transient knockdown of NG2 using a previously validated siRNA probe (B) and the corresponding effect on cell binding and spreading onto the indicated ECM substrates (C). Fluorescence micrographs show phalloidin-Alexa staining of the actin microfilaments in SW982 cells after transfection with a control siRNA (CTRL siRNA), or an NG2-specific siRNA (NG2 siRNA). (D and E) Selective perturbation of cell adhesion to Col VI (E), but not Col I (D), by siRNA-mediated knockdown of NG2 in a panel of different human sarcoma cell lines with different constitutive surface levels of the PG. Upper insets show exemplifying phase contrast views of SK-UT-1 cells adhering to each of the collagens following transfection with the control or NG2-directed siRNAs. *P < 0.05 by Student's t-test.
To finally gain more detailed information about the modes through which NG2 contributed to Col VI binding, as well as attempt to pinpoint its putative binding sites within the collagen, we assayed the NG2-dependent cell adhesion to disassembled and/or tryptic forms of the collagen (Perris et al., 1993). It was found that cells bound more tenaciously to intact Col VI tetramers encompassing the C5 segment of the α3(VI) chain, previously demonstrated to be crucial for proper macromolecular assembly of the tetrameric units into microfilaments (Lamande et al., 2006), than to any other form of the collagen (Supplementary Figure S3). This observation strongly suggests that the NG2-mediated cellular interaction with Col VI requires a native configuration of the collagen and that the NG2 binding site on the collagen has a conformational nature. The partial inhibitory effect seen with antibodies to β1-class integrins and the lack of inhibitory effects by anti-β3/β5/β6 antibodies further suggest that NG2 may directly bind to the N- and/or C-terminal globular domains of the α3(VI) chain.
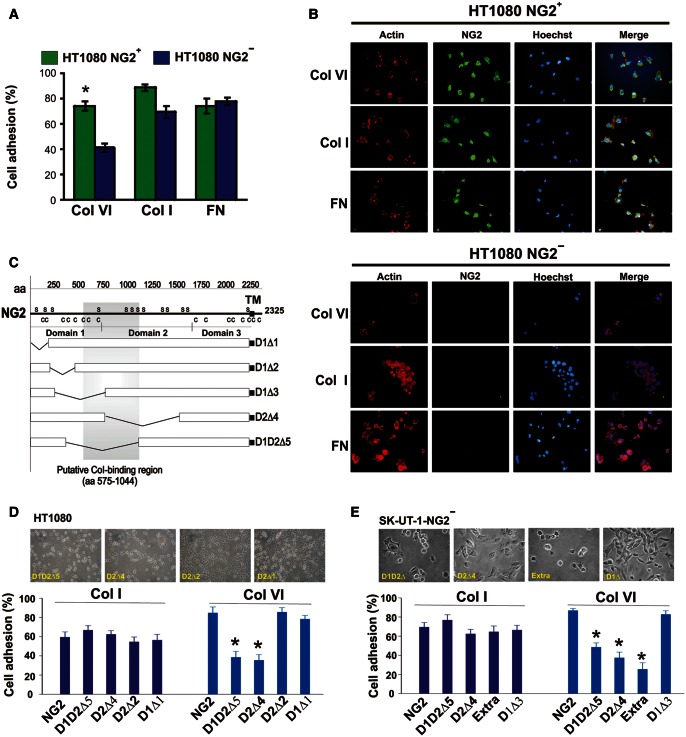
Incontrovertible evidence for a role of NG2 in the cells' interaction with Col VI was ultimately provided by the superior binding of immunosorted NG2+ cell subsets to Col VI, whereas adhesion to Col I and fibronectin (FN) was indistinguishable from that of NG2− cells (Figure 4A and B). We also wondered whether the NG2-mediated cell attachment to Col VI could be exploited by NG2-expressing cells to form ECM-promoted cellular aggregates. Hints for this being the case were provided by culturing cells on poly-HEMA substrates (to force anchorage-dependent cells to interact with each other rather than with the culture substrate) in the presence of soluble Col VI. In this condition non-treated cells formed spheroids to a significantly higher extent than cells in which NG2 had been knocked down (Supplementary Figure S4). Col VI/NG2-promoted anchorage-independent cell growth was also demonstrated in colony formation assays (Supplementary Figure S4). In these assays and independently performed cell proliferation experiments, there was, however, no evidence that the NG2–Col VI interaction directly influenced cell mitosis.
Figure 4.
Sarcoma cell adhesion to Col VI is mediated by the N-terminal mid-portion of the ectodomain of NG2. (A) Quantification of immunosorted HT1080 NG2+ and NG2− spreading to different ECM molecules (Col VI, Col I and FN) by cell counting within arbitrarily chosen representative fields. *P < 0.05 by Student's t-test. (B) Representative cell adhesion and spreading of NG2+ and NG2− subsets on the indicated purified ECM molecules as highlighted by immunofluorescence using antibodies to F-actin and NG2. Nuclear counterstaining was performed with the Hoechst dye. (C) Schematic view of the rodent NG2 deletion constructs used to engineer cells to overexpress, or express ectopically, distinct truncated forms of the PG. (D and E) Non-manipulated HT1080 cells (D) and immunosorted NG2− SK-UT-1 cells (E) were transduced to express full-length NG2 (‘NG2’), the NG2 deletion constructs described in C, or NG2 molecules lacking the cytoplasmic tail (‘Extra’). Cells were then assayed for their ability to adhere and spread on Col I or Col VI substrates. Upper panels show representative phase contrast views of the engineered cells interacting with the two substrates. *P = 0.0033 in D, *P = 0.0087 in E by the Mann–Whittney U-test.
The putative collagen-binding domain of NG2 is essential for cell anchorage to Col VI
When SK-UT-1 cells were forced to overexpress rodent NG2 constructs lacking the cytoskeleton-interacting C-terminal domain (a construct named NG2extra; Cattaruzza et al., 2013), or constructs in which we had partially or entirely deleted the central ‘collagen-binding’ segments of the ectodomain (Burg et al., 1997) (i.e. constructs denoted D1D2Δ5 and D2Δ4; Figure 4C), the cells bound significantly less well to Col VI (Figure 4D). In contrast, cell adhesion to Col I remained largely unaffected. Identical results were obtained with immunosorted NG2-negative cells transduced with the same deletion constructs (Figure 4E).
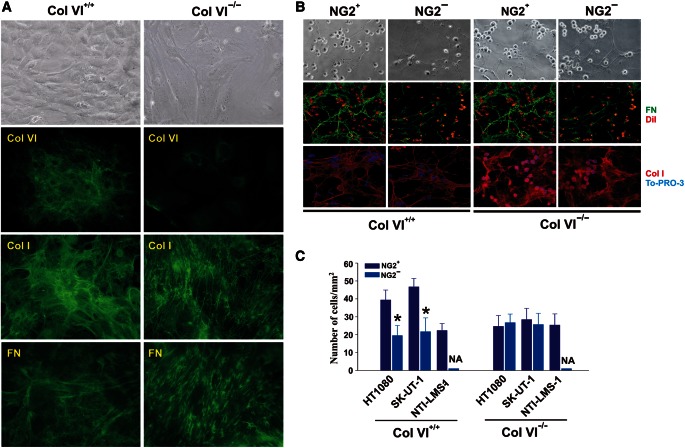
The impaired adhesion of NG2-deficient cells to purified Col VI was indicative of an involvement of the PG in cell binding to this mono-molecular substrate, but did not fully clarify whether the same interplay was essential for cells interacting with matrix-assembled Col VI. To approach this question, we isolated native, cell-free ECM from human transformed fibroblasts, or murine embryonic fibroblasts derived from wild-type or Col VI knockout mice (Figure 5A). Immunosorted NG2+ sarcoma cells adhered more tenaciously to Col VI-containing native matrices than NG2− and siRNA-treated cells, and again, higher surface levels of NG2 favoured a more pronounced binding to these ECMs (Figure 5B and C). Intriguingly, native ECMs isolated from Col VI knockout fibroblasts were overall less supportive of adhesion of both NG2+ and NG2− cell subsets, albeit matrices from the two genotypes exhibited apparently equivalent topographical arrangements (Figure 5A).
Figure 5.
NG2 is essential for cell binding to Col VI-rich cell-free matrices. (A) In vitro matrix deposition of wild-type (Col VI+/+) and Col VI knockout (Col VI−/−) MEFs as highlighted by immunolabelling with antibodies to murine Col VI, Col I, or FN. Since Col VI null mice were created by deletion of the gene encoding the α1(VI) chain, the anti-Col VI polyclonal antiserum raised against the intact heterotrimeric collagen molecule reveals some retention of the newly synthesized α2(VI) and α3(VI) chains within the cytoplasm (presumably in part within the endoplasmatic reticulum) of the null fibroblasts generated by deletion of the COLVIA gene. (B) Immunosorted NG2+ and NG2− HT1080 cells binding to Col VI-containing (left panel) or Col VI-deficient isolated matrices (right panel). The ECM was stained with antibodies to the indicated ECM molecules and cells either tagged with DiI prior to incubation with the matrices or labelled with TO-PRO-3 after fixation of the specimens. (C) Assessment of cell adhesion to Col VI+/+ and Col VI−/− matrices of the indicated NG2+ and NG2− subsets by cell counting within randomly selected microscopic fields. NA, not assessable (i.e. value close to 0); *P < 0.01 by the Mann–Whittney U-test.
NG2 is a key surface component for cell movement on Col VI
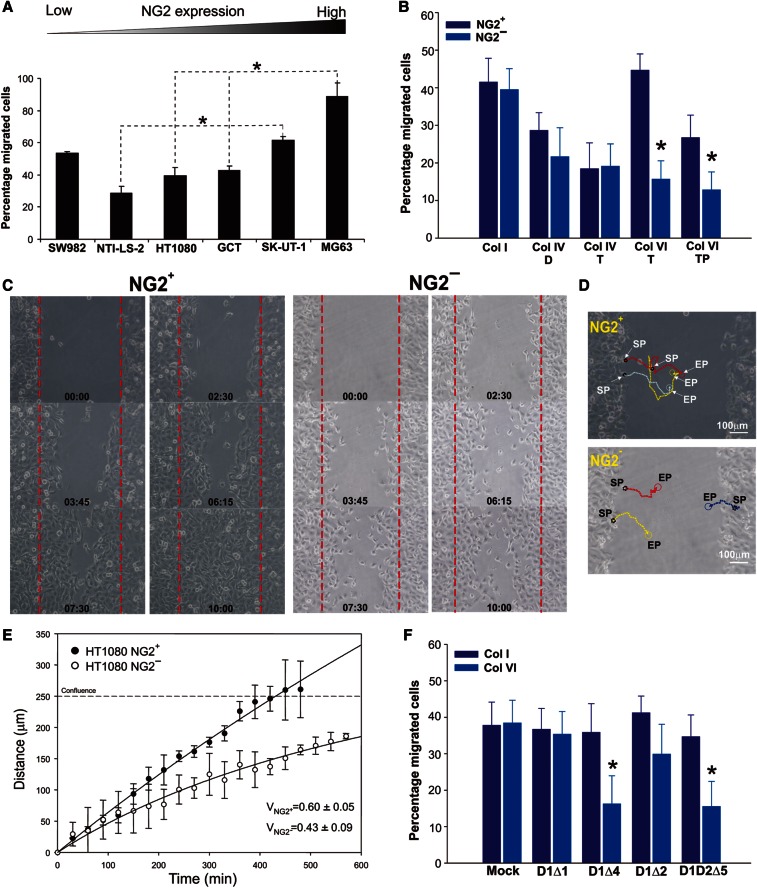
The above findings indicated that cell-matrix interplays promoted by the NG2–Col VI association were crucial for the control of cell adhesion, survival and aggregation. To determine whether such interactions could also be important for cell motility in response to Col VI, we set up a number of dedicated in vitro assays using the cellular models and matrix substrates described above. Similar to what was observed for cell-substratum adhesion, the extent of haptotactic movement on isolated Col VI tetramers closely correlated with the diverse NG2 surface levels displayed by the cells (Figure 6A). Loss-of-function of the PG strongly compromised this migratory behaviour, but did not perturb the lower levels of motility seen on Col I and other widespread ECM components (Supplementary Figure S5). Immunosorted NG2+ and NG2− cell subsets and siRNA-treated cells also showed different levels of migration when confronted with collagen substrates: NG2-deficient cells were substantially less motile on Col VI than on Col I or Col IV substrates and, consistent with the cell adhesive behaviour, moved significantly better on more intact forms of Col VI (Figure 6B). Furthermore, siRNA-treated cells and immunosorted NG2− cells migrated significantly slower and less directionally than NG2-expressing cells (Figure 6C–E; Supplementary movies S1, S2).
Figure 6.
NG2 enhances cell motility in response to 2D Col VI substrates. (A) Extents of haptotactic movement (FATIMA system) of different sarcoma cell lines on Col VI tetramers, relative to their measured NG2 surface levels. *P < 0.01 by the Mann–Whittney U-test. (B) Extents of migration of NG2-deficient SK-UT-1 cells on Col I, dimeric (Col IV-D) or tetrameric (Col IV-T) forms of collagen type IV, intact (Col VI-T) or pepsin-digested (Col VI-TP) tetramers of Col VI (same experimental setting as in A). *P < 0.001 by the Mann–Whittney U-test. (C) Representative migratory behaviour of HT1080 NG2+ and NG2− cell subsets in response to tetrameric Col VI (scratch assay). (D) A typical reconstruction of the migratory trajectories of the cells. SP, starting point; EP, end point. (E) NG2+ migrate significantly faster and with a higher persistence of directionality than NG2− cells (see also Supplementary movies S1 and S2). ‘Confluence’ refers to the distance within the scratch at which there was complete coverage of the scratch area and cells were progressively arrested in their movement by contact inhibition. (F) Extents of migration (FATIMA system) of immunosorted SK-UT-1-NG2− cells transduced with truncated forms of the PG (Figure 4C) and allowed to migrate on Col I or tetrameric Col VI substrates. *P < 0.001 by two-sided ANOVA.
Cells overexpressing NG2 variants lacking the putative collagen-binding domain (i.e. variants D1Δ2 and D1D2Δ5) showed a significantly reduced motility on Col VI substrates (Figure 6F). SiRNA-mediated abrogation of NG2 also affected the capability of the cells to penetrate complex, Col VI-containing matrices, as shown by experiments involving inclusion of cells into Matrigel droplets supplemented with Col VI. In this experimental setting, cells that had retained NG2 on their surface migrated out from the droplets more effectively than NG2 deprived cells (Figure 7A and B). By exploiting our FATIMA system (Spessotto et al., 2009) and polymeric Col I or Matrigel supplemented with Col VI, it was also possible to appreciate the higher invasive capability of NG2-expressing cells (Figure 7C). Inclusion of Col VI favoured sarcoma cell movement through these 3D matrices and, consistent with the observed adhesive and motile behaviour, the invasive capabilities of the cells were intimately related to their NG2 surface levels.
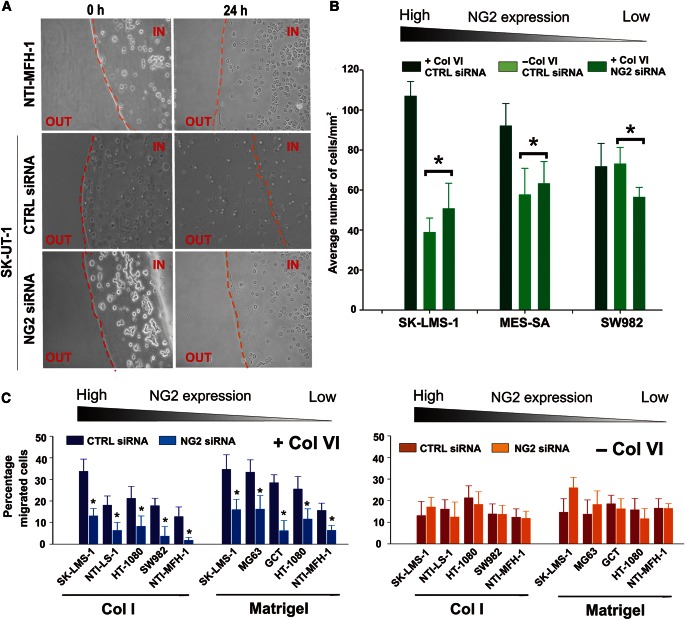
Figure 7.
NG2 affects the ability of sarcoma cells to move through in vitro reconstituted Col VI-containing basement membrane (Matrigel)-type matrices. (A) Representative phase-contrast views of the capability of NTI-MFH-1 (sarcoma cells with poor surface expression of NG2; Supplementary Figure S2), SK-LMS-1 treated with a control (CTRL siRNA) or NG2-directed siRNA (NG2 siRNA) to move through Matrigel droplets supplemented with tetrameric Col VI. (B) Assessment (by cell counting of randomly selected microscopic fields) of the ability of cell lines displaying diverse surface levels of NG2 (SK-LMS-1, MES-SA, SW982) to evade Col VI-supplemented (+ Col VI) or non-supplemented (− Col VI) Matrigel after treatment with a control siRNA (CTRL siRNA), or an NG2-directed siRNA (NG2 siRNA). *P < 0.01 by Student's t-test. (C) Relative capabilities of different sarcoma cell lines to invade polymeric Col I substrates supplemented (+ Col VI) or not (− Col VI) with tetrameric Col VI, after treatment with the CTRL or NG2-directed siRNAs. In the presence of Col VI, NG2 knockdown consistently and significantly (*P < 0.01–0.001 by the Mann–Whittney U-test) reduced the invasive capabilities of the cells.
NG2–Col VI interaction triggers cell adhesion- and migration-associated PI-3K activations
To establish whether the observed NG2/Col VI-mediated promotion of cellular interactions involved the activation of defined intracellular signalling cascades, we carried out a series of antibody array- and immunoblotting-based phospho-proteomic screenings. For these screens, we used NG2-expressing and NG2-deficient cells exposed to either purified Col VI or Col I. To circumvent a number of technical caveats (see Supplementary material), we adopted an experimental strategy entailing the plating of siRNA-treated cells onto poly-L-lysine-coated substrates and the addition of molar equivalents of soluble Col VI or Col I. Effectiveness of an experimental design involving soluble ECM ligands was partly supported by previous observations showing that both Col I and Col VI are capable of eliciting signal transduction events when added in solution (Ruhl et al., 1999a, b). To ensure the optimal neutralization of signalling phenomena evoked by α2β1 integrin binding to the collagens, we simultaneously treated cells with anti-α2 integrin function-blocking antibodies and β1 integrin-directed siRNA probes.
The appropriateness of this experimental strategy was initially verified by examining the phosphorylation patterns of the cells by semi-quantitative immunoblotting, using a platform in which about 70 phosphorylation sites in 63 selected signal transduction components were simultaneously probed (Supplementary Figure S6). The phosphorylation screening was performed as a four-way comparative analysis of the signal transduction cascades triggered in ‘integrin-deprived’ SK-UT-1 cells, exposed to either Col VI or Col I in the presence or absence of cell surface NG2, according to the following scheme: NG2-expressing versus NG2-deficient cells exposed to Col I; NG2-expressing versus NG2-deficient cells exposed to Col VI; NG2-expressing cells exposed to Col I versus Col VI; and NG2-deficient cells exposed to Col I versus Col VI (Supplementary Figure S6). The outcome of these comparisons indicated that when either NG2-positive or NG2-negative cells were confronted with Col I, 39 phosphorylation sites were either equally modulated in the two cell types or were not identifiable in any of them (either because the molecule was not expressed or because the screened site was not phosphorylated). These sites were ‘depleted’ from the list leaving 33 phosphosites as the ones specifically modulated in cells interacting with Col VI in the presence or absence of NG2. The 12 sites exhibiting enhanced phosphorylation (two sites were de novo phosphorylated) in NG2-expressing cells encompassed sites in components associated with cell survival, apoptosis, stress-related responses, and cytokine-typical signal transduction cascades. Conversely, 14 sites pertaining to cell-cycle regulators, growth factor/cytokine response mediators, and controllers of cytoskeleton dynamics were found to be less phosphorylated in cells deficient in NG2, compared with NG2-positive ones (Supplementary Figure S6).
In a parallel approach, we pursued a direct comparison of the signal transduction patterns elicited by Col I or Col VI between murine melanoma cells ectopically expressing NG2 and wild-type counterpart cells lacking the PG. Through this alternative approach, we could corroborate the NG2/Col VI-induced up-regulation of 19 phosphosites harboured by the same classes of signalling components that were unveiled with the simpler immunoblotting-based screening approach. By contrast, 11 phosphosites were found to be strongly down-regulated or completely abrogated (Supplementary Figure S6). Eighteen of the phosphosites that were not found to be modulated upon the cells' interaction with Col I also remained unvaried when cells interacted with Col VI. Comprehensively, through two independent experimental paradigms we could assert the usefulness and reproducibility of the adopted phospho-proteomic strategy and could gain important information about the signalling pathways elicited by the NG2–Col VI binding.
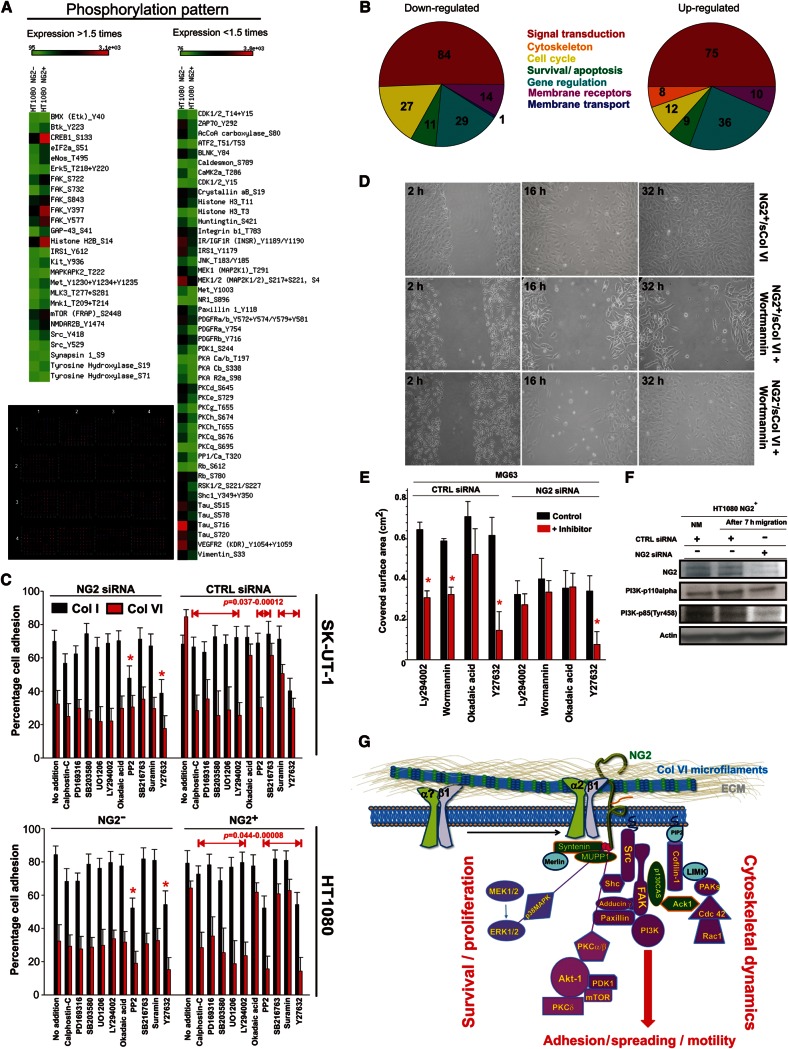
We then sought to generate a more comprehensive portrait of the NG2/Col VI-induced signal transduction by relying upon a wider and more quantitative phospho-proteomic screening. For this purpose, we applied the four-way comparative Col I/Col VI-based paradigm to immunosorted HT1080 NG2+ and NG2− cells. The screening was performed using a 627-spot (spotted in duplicate plus control spots) fluorescent antibody-array platform, followed up by verification of the 36 primary lead phosphosites by semi-quantitative immunoblotting (most of these sites are reported in Supplementary Figure S7). In this case, we adopted an arbitrary threshold setting of the system at 1.5-fold difference (95% confidence interval) for the assertion of the divergent phosphorylation status of the examined molecules. Delineation of the modulated phosphorylation pattern was based upon normalization of the actual expression levels of the molecules in the two cell types, as established by the parallel reactivity patterns of phosphorylation-independent antibodies against the individual components. Through this approach we found that phosphorylation of 84 sites in 76 components was higher in NG2+ versus NG2− cells interacting with Col VI (i.e. the molecules were more de-phosphorylated in NG2-deficient cells; 16–48-fold difference). Vice versa, 75 sites in 58 components were found to be more phosphorylated in the latter cells: 11 phosphosites differed in the range of 81–148-folds and sites within PKN/PKR1, MST3, and RSK2 were virtually de novo phosphorylated (202–529-fold difference) (Figure 8A and B).
Figure 8.
NG2-mediated interaction of sarcoma cells with Col VI activates multiple signal transduction pathways. (A) Heat map of the outcome of a representative phospho-proteomic comparative screening performed according to the four-way, pair-wise comparisons depicted in Supplementary Figure S6 and using a fluorescent 627-spot antibody array platform (lower image). The map was generated by arbitrarily adopting a cut-off of 1.5-fold difference (within a 95% confidence interval) of the degree of phosphorylation of single molecules, after normalization for their relative, constitutive expression levels. (B) Summary of the ontological clustering of the molecules with modulated phosphorylation. (C) Effect of signalling antagonists on adhesion to Col I and Col VI of SK-UT-1 cells treated with the NG2-directed or CTRL siRNA. The lower graph shows the results obtained, using the same experimental paradigm, with immunosorted NG2− and NG2+ HT1080 cells. (D) Representative migration rates of NG2+ and NG2− cells in the presence of soluble Col VI (sCol VI) and 5 µM/ml of Wortmannin. (E) Extent of cell migration of MG63 cells treated with a CTRL or NG2-directed siRNA probe in the simultaneous presence of Col VI and the indicated signalling inhibitors. (F) Western blot analysis of PI-3K activation by detection of p110α and p85 (Tyr458) regulatory subunit phosphorylation in HT1080 NG2+ cells treated with a CTRL or NG2-specific siRNA probe and allowed to migrate in the presence of soluble Col VI. (G) Proposed scheme of the signal transduction pathways activated by the NG2–Col VI interaction and believed to converge and/or agonize with the ones activated by growth factor receptors and integrins. Signal transduction components highlighted in purple are the ones confirmed and/or newly discovered in this study, components in green are molecules previously discovered to directly interact with and/or be activated by NG2, and molecules in light blue are inferred from previous studies.
A total of 82 phosphosites in 76 molecules, including sites within FAK, ERK1, PAK5, PP4C, p38aMAPK, MEK4/6, PCKβ2/PCKh, β-catenin, PDGFRα, Fos, ErbB2, PTEN, Pyk, p53, S6Kα, ZAP70, PP1/Cα, Lck, MAPKAPK2, Btk, and several STATs, were found to be unchanged (i.e. <1.5-fold difference). The remaining phosphosites detected by the platform were not detectable in either cell type. In NG2-deficient cells, lower phosphorylation degree and/or complete de-phosphorylation was observed for a number of molecules controlling cell survival and promoting apoptosis, negative regulators of the cell-cycle, and components of the cytostatic IFN signalling pathway, as previously documented in these cells (Gazziola et al., 2005). Conversely, molecules displaying de novo and/or enhanced phosphorylation were prevalently associated with actin microfilaments and microtubule dynamics, formation of focal adhesions, positive regulation of the cell-cycle, and propagation of PCKs and MAPK/ERK pathways (Figure 8A). Notably, while it was possible to assert the increased phosphorylation of cofilin-1, phosphorylation of cofilin-2 was not found to be modulated. Another notable finding was the diversified phosphorylation of sites within ERK and PCK family members (Supplementary Figure S7). We further observed activation of a number of down-stream effectors of various receptor systems, consistent with the idea that upon interaction with extracellular ligands, NG2 may directly, or indirectly, modulate these systems.
To approach the functional significance of the observed NG2/Col VI-dictated phosphorylation profiles, we set up short-term cell adhesion assays with siRNA-treated and immunosorted cell subsets, using cells transduced to overexpress the NG2extra deletion construct (i.e. NG2 mutants lacking cytoskeletal linkage) as reference. Cells were allowed to adhere to Col VI or Col I substrates in the presence or absence of drugs selectively antagonizing individual signal transduction components. Thus, for these experiments, we largely reproduced the paradigm originally used for the phospho-proteomic screenings, with the exception that β1-integrins were maintained operational to avoid impairment of cell-substrate binding. Addition of signalling antagonists to NG2-deficient cells did not significantly affect their poor adhesion to Col VI, whereas both the ROCK1 inhibitor Y27632 and the Src inhibitor PP2 impaired adhesion to both Col I and Col VI in either cell phenotype (Figure 8C).
ATP-competitive and non-competitive antagonists of p38MAPK (PD169316 and SB203580, but not the control drug SB202474), an antagonist of MEK1/MEK2 (UO126, but not UO124), and two potent and rather selective antagonists of PI-3K (LY294002 and Wortmannin) produced selective blockades of NG2+ cell binding to Col VI (Figure 8C). Preliminary observations using more specific inhibitors (i.e. GDC-0941, TGX-221, and AS252424) also suggested that the PI-3K isoforms p110α/δ could be the one primarily associated with signalling events modulated by the NG2–Col VI interaction. NG2-dependent and NG2-independent cell adhesion to either collagen was not affected by SB216763, Suramin, or okadaic acid indicating that it did not require the activity of GSK3α, CAMK, PP1, PP2A, or other MAPKs, including JNK and MK2a (the latter blocked by SP600125 and SP475863).
To finally investigate the potential involvement of PI-3K in cell motility regulated by NG2–Col VI interactions, we performed migration assays with NG2+ and NG2− cells in which, along with soluble Col VI, LY294002 or Wortmannin was added. In the presence of either inhibitor, NG2+ cells were largely impeded in their movement, whereas the poorer migration rate of NG2− cells seemed to be unaffected (Figure 8D and E). PI-3K activation was confirmed by the enhanced phosphorylation of the p85 regulatory subunit of the enzyme (Figure 8F). On the basis of these results, the cumulative findings of our phospho-proteomic screening, and the previously published information about the NG2 connection with defined signalling cascades, we propose a scheme of how NG2 binding to Col VI microfilaments may, through a putative modulation of cytoskeletal dynamics, contribute to the convergence of integrin- and growth factor-elicited signal transduction pathways (Figure 8G).
Discussion
Molecular analyses on a cohort of >100 STS patients confirm that NG2 is associated with neoplastic transformation, being de novo expressed in primary lesions and being strongly up-regulated in metastases developed in these individuals. A first unsupervised clustering of the patients according to their metastatic NG2 expression profile unveiled a subgroup with a more dismal clinical course. A second clustering exercise that took into consideration both up-regulation of NG2 and its putative ligand Col VI identified a subgroup exhibiting a dramatically unfavourable prognosis, suggesting that co-enhancement of the two molecules drives tumorigenesis. The application of multivariate regression analyses established that up-regulation of NG2, but not Col VI, was an independent prognostic factor, thus paralleling the previously proposed clinical importance of NG2 in glioblastoma multiforme, neuroblastoma, breast carcinoma, melanoma, and mesothelioma (Kageshita et al., 1992; Al-Mayhani et al., 2011; Svendsen et al., 2011; Rivera et al., 2012). The putative clinical impact of NG2 in STS adds to the previously discovered value of the PG in the prognostication of rare infant and adult leukaemia (Smith et al., 1996; Petrovici et al., 2010).
Rather than simply adding another post to the infinite ‘list’ of tumour biomarkers correlating with overall patient survival, the present findings on STS suggest that evaluation of NG2 expression may have a more direct utility in the daily clinical management of cancer patients. In fact, relative abundance of the PG in primary lesions of metastasis-free individuals affords a unique potential in predicting with ≥60% probability the future occurrence of post-surgical metastases. Relative levels of NG2 expression in primary tumours also defined the tentative timing of appearance of such secondary lesions. Therefore, assessment of the transcriptional rates of NG2 in surgically removed (or sampled through biopsy) primary tumour masses may represent an unprecedented molecular tool for the disclosure of occult metastatic lesions emerging after removal of loco-regional ones, this parameter being independent of the type of adjuvant or neoadjuvant treatment, tumour histotype, or clinical history of the patient. To our knowledge altered expression of NG2 in primary lesions is the first tangible metastasis-predicting factor ever to be identified in STS and, as such, it may be a more potent biomarker than what was previously proposed in other solid tumours. Larger case studies than the present one should be able to decipher the clinical significance of this finding and more precisely define its probability scoring.
Comparative assaying of the tumorigenic potential of NG2-expressing cells by xenografting in athymic mice highlighted a marked correlation between surface abundance of the PG and tumour growth. This observation provided a biological rationale for the observed pathological impact of NG2 expression in cancer patients. In situ analyses of NG2 distribution in STS lesions showed a certain inter- and intra-tumour variability with respect to the percentage of neoplastic cells expressing NG2. A similar heterogeneity was observed in sarcoma cells isolated from various metastatic and relapsing tumour specimens. It would be technically impossible to tell whether cells overexpressing NG2 in the primary tumours of STS patients were actually the same cells that later on contributed to the metastatic process in these individuals. However, apart from the observed correlation between NG2 cell surface abundance and tumour engraftment/growth in animal models, another experimental finding sustained a link between elevated NG2 expression and enhanced tumour growth.
When we separated by immunosorting NG2+ and NG2− cell subsets, and comparatively assayed their tumorigenic behaviour in nude mice, we found that the former subset gave rise to local tumour masses more rapidly and more extensively than the latter one. This observation was consistent with corollary in vitro findings showing sustained anchorage-independent growth of NG2-expressing (but not NG2-deficient) cells and called upon a possible, more direct implication of the PG in the control of tumour cell engraftment and survival. However, since the ability of NG2+ cells to engraft and form tumours in nude mice did not seem to correlate with their intrinsic proliferation index, the pro-tumorigenic role of NG2 was unlikely to be restricted to a mere control of cell division. Conversely, our current belief is that high surface levels of NG2 may confer to cancer cells a more malignant behaviour by controlling multiple facets of their host microenvironmental interactions.
Up-regulation of NG2 in STS lesions was accompanied by a corresponding augmented stromal deposition of one of its presumptive matrix ligands, Col VI. The elective stromal association of the collagen, i.e. lack of significant expression in the tumour cells themselves, was consistent with previous observations indicating that mesenchymal tumours lose their ability to produce Col VI upon neoplastic transformation (Schenker and Trueb, 1998). Thus, within STS lesions, the origin of NG2 and Col VI appear to be spatially separated in two primary components of the lesion, i.e. tumour cells and stroma. The preferential association of Col VI with the latter compartment of the lesions suggested that the collagen exerted a critical role in the host-contributed support of the lesion.
NG2 effectively mediated the interaction of cells with both naturally assembled forms (i.e. microfilaments) of Col VI and with its isolated tetrameric units. By contrast, it was ineffective when cells were confronted with fragmented forms of the collagen. While the N-terminal portion of the PG core protein appeared to bind the trimeric globular domains of the collagen, other parts of NG2 synergized with integrin α2β1 (which in turn recognizes the triple-helical region of the collagen) to stabilize the PG–collagen interaction. Cooperation of NG2 with integrins has previously been documented for both α3β1 (Fukushi et al., 2004) and α4β1 (Iida et al., 1992, 1995), but the precise modes of these interplays have remained veiled. Our findings are somewhat at variance with a previous publication suggesting a role for NG2 in mediating β1 integrin-independent binding to Col VI (Tillet et al., 2002). We also noted that the NG2–Col VI interaction induced cell–cell cohesion and promoted anchorage-independent growth. These observations suggested that NG2-driven retention of the collagen on the cell surface could be exploited by mesenchymal tumour cells to form ECM-mediated homotypic cellular aggregates.
Cells with reduced levels of NG2 showed a strongly attenuated ability to adhere and migrate on both purified Col VI and native Col VI-containing matrices, and markedly failed to invade reconstituted basement membrane matrices supplemented with Col VI. Similar observations were done with cells engineered to overproduce, or express ectopically, NG2 molecules deprived of their N-terminal collagen-binding region or cytoplasmic domain. These cells were found to be defective in their interaction with Col VI, but were not impaired in their binding and migration on other ECM components. Collectively, these findings sustain a cardinal role of the cytoplasmic tail of NG2 in promoting cell–substrate interactions and cell motility (Fang et al., 1999; Makagiansar et al., 2004) and provide novel insights into the importance of the NG2–Col VI interaction in governing ECM-promoted cellular phenomena. To what extent the PG mediates these phenomena by acting beyond a direct binding to Col VI remains to be further asserted.
By binding to extracellular ligands, in a possible cooperation with integrins, NG2 is believed to affect filopodia extension and stabilization and activate lamellopodial signalling cascades propagated via p130CAS, Rac/cdc42, and FAK-ERK phosphorylations (Eisenmann et al., 1999; Majumdar et al., 2003; Yang et al., 2004). The NG2-induced rearrangement of the actin cytoskeleton may occur through ancillary actin-binding intermediates and more recently syntenin-1 has been added to the spectrum of putative NG2-cytoskeletal linker/adaptor molecules in migrating oligodendrocytes (Chatterjee et al., 2008). In accordance with some of these previous findings, NG2-expressing sarcoma cells showed a defined spreading behaviour on Col VI substrates that involved a canonical reorganization of the cytoskeleton and an accompanying paxillin phosphorylation. The augmented adhesive, motile, and invasive properties of NG2-expressing cells in response to Col VI substrates prompted us to investigate the signal transduction cascades triggered through NG2 in cells confronted with the collagen. To this end, we carried out a comparative profiling of the intracellular phosphorylation patterns triggered by alternative exposure to Col I or Col VI in three distinct, β1/α2 integrin subunit-depleted cellular models, which were matched by their counterpart non-manipulated cells. These included siRNA-treated cells, cells ectopically expressing NG2, and cells enriched for their constitutive NG2 surface levels by immunosorting. Experiments with these cellular models consistently delineated the differential activation of multiple signal transduction pathways in NG2-expressing versus NG2-deficient cells upon selective confrontation with Col VI.
We identified diverse phosphorylation patterns in both components linked to cell survival pathways of the PCK–PI-3K–Akt-1–mTOR-type and molecules associated with growth-promoting pathways of the ERK–MAPK-type; the latter already known to be associated with the phosphorylation of the cytoplasmic NG2 threonine residues (Makagiansar et al., 2004, 2007). Modulation of the phosphorylation state of molecules affecting the actomyosin-regulated cell contractility and focal adhesion formation was in partial agreement with previous Col VI-unrelated findings and provided additional evidence for a tight NG2 exerted control of the actin microfilament dynamics in critical cytoplasmic domains of motile cells. The NG2-induced cofilin-1 and PCK-dependent adducin γ phosphorylations were particularly intriguing in the light of our preliminary evidence of a physical NG2–cofilin-1 intracellular linkage and the previously documented bilateral NG2–PCKα inter-relationship. Furthermore, engagement of NG2 in Col VI binding induced enhanced phosphorylation of the FAK-controlled tyrosine residue Y118 of paxillin, but not residue Y31, suggesting that upon specific interactions with certain ECM components, NG2 may also influence focal adhesion disassembly and the dynamics of the retracting end apparatus of locomotory cells.
The putative significance of the signalling pathways triggered by the NG2–Col VI interplay was, therefore, further addressed in the context of cell adhesion and movement through the use of drug antagonists. These experiments unravelled a NG2–Col VI induction of PI-3K isoform-specific phosphorylation and functionally confirmed the activation of pathways dependent upon and/or involving p38MAPK and MEK1/2/ERK1/2 enzymatic activities. Conversely, NG2 binding to Col VI did not specifically influence the Rho–ROCK-1, Crk, or Csk participation in cell–substrate interactions. Since PI-3K has recently been implicated in the control of cell spreading and random cell movement, through a bypass of the canonical integrin-elicited signal transduction machinery (Weiger et al., 2009), we specifically assayed whether a Col VI–NG2–PI-3K axis was established during sarcoma cell adhesion and motility. Such connection was suggested by the adhesion/migration-inhibitory effects exerted by antagonists of this kinase in NG2-expressing cells, but not NG2-deficient ones, or in cells overexpressing cytoplasmic deletion constructs of the PG.
Conclusively, the present findings consolidate the unprecedented role of NG2 as a metastasis-predicting biomarker in STS and as a growth- and survival-promoting factor in discrete cell subsets of these tumours. The PG appears to control tumour progression by mediating the interaction of neoplastic cells with the host ECM, in particular with the Col VI that accumulates in the peri- and intra-lesional stroma. The importance of this interaction in cancer is thus underscored and proposed to be central for governing aggressiveness of sarcomas. It is proposed that sustained NG2–Col VI interplays, alongside other molecular interactions that ‘sarcoma NG2’ may mediate with discrete cues in the tumour microenvironment, and be pivotal in predisposing for enhanced local growth and tissue infiltration. Hence, these interactions are believed to contribute to the predisposition of the STS cells' ability to disseminate to distant sites.
Materials and methods
Tumour specimens
Informed consent for collection of sarcoma specimens was given to Rizzoli Orthopaedic Research Institutes and the study was carried out with the full approval of the institutional Ethical Committee. See Supplementary material for details.
Cell lines and RNAi
Human sarcoma and melanoma cell lines SW872, SW982, HT1080, MES-SA, 143B, MG63, A375, RD/KD, SK-UT-1, and SK-LMS-1 (Supplementary Figure S2) were obtained from ATCC and grown in DMEM (Lonza Walkersville) with 10% FBS (Sigma-Aldrich). The sarcoma cell line GCT was provided by Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (Brescia, Italy) and maintained in McCoy's 5A medium (GibcoTM, Invitrogen) with 10% FBS. A number of human lines were produced from cells isolated from STS specimens surgically removed from patients undergoing treatment at the National Cancer Institute in Aviano (Supplementary Figure S2). Wild-type and Col VI knockout embryonic fibroblasts were isolated as previously described (Sabatelli et al., 2001) and maintained in DMEM (4.5 g/L glucose) containing 15% of FBS. Non-manipulated, mock-transfected, and NG2 stably transduced murine B16-F10 melanoma cells (Burg et al., 1998) were grown in DMEM with 10% FBS. Twenty-one-mer siRNA probes against human NG2 and scrambled versions of these probes were synthesized with the Dicer siRNA Generation kit (Gene Therapy Systems Inc.). Additional siRNA probes against NG2 and integrin β1 were obtained through Ambion.
RNA extraction and qPCR
See Supplementary material for details. Immunohistochemistry and TUNEL assay
See Supplementary material for details.
ECM substrates
The various forms of Col VI and the intact tetramers were purified as previously described by Perris et al. (1993). Other purified ECM molecules were obtained as follows: human fibronectin, collagen type III, tetrameric collagen type IV, and vitronectin from Sigma-Aldrich; rat tail Col I and Matrigel from BD Biosciences; dimeric mouse collagen type IV from Merck Laboratories-Collaborative Research. Native Col VI-containing and Col VI-negative matrices were isolated from passage 3 to 7 embryonic fibroblasts derived from wild-type (Col VI+/+) and Col VI knockout (Col VI−/−) mice and grown on glass coverslips for 4–5 days in the presence of 0.25 M ascorbic acid until they reach confluence. For more details see Supplementary material.
Immunocytochemistry and FACS
See Supplementary material for details. Transfection constructs and gene transduction
Sarcoma cell lines were transiently or stably transfected (using Lipofectamine Plus as a delivery vehicle; Life Technologies, Inc.) with a plasmid containing either the cytoplasmic tail and membrane-spanning domain of the PG, or the membrane-spanning domain plus the entire ectodomain. For more details, see Supplementary material.
Cell adhesion and migration assays
See Supplementary material for details. Phospho-proteomic profiling and functional follow-up screening
Relative expression levels and phosphorylation patterns of signal transduction components elicited by engagement of NG2 in Col VI interactions were defined by relying upon the Kinetworks™ Immunoblot KPSS 1.3 Phospho-Site Screen, entailing detection of >70 phospho-sites in 64 molecules, and Kinex™ (KAM 1.1) antibody microarray services (Kinexus Bioinformatic Corporation). For more details, see Supplementary material.
In vivo tumorigenesis
All experiments in mice were approved by the Review Board on animal experimentation of the National Cancer Institute of Aviano and were performed in accordance with the international guidelines for tumorigenesis by xenografting in nude mice. Experiments aimed at assaying tumour formation in relation to NG2 surface expression involved the evaluation of 170 nude mice out of 208 animals that were totally manipulated. See Supplementary material for details.
Statistical analyses
Data on human tumour specimens are shown as median (m) and 25–75th percentile for their strong non-Gaussian distribution. The non-parametric Mann–Whitney U-test and the Kruskal–Wallis one-way analysis of variance were performed to compare gene expression in unpaired and paired samples, respectively; P-values <0.05 were consistently considered to be statistically significant. Metastasis-free survival was calculated by the Kaplan–Meier analysis and comparison of curves was performed through Breslow's test. Multivariate meta-analyses were performed according to Cox proportional hazard regression and significance levels in cell adhesion and migration assays in vitro were established by Student's two-tailed t-test and one- and two-way ANOVA of variance.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
The work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC to R.P. and A.C.), the Italian Ministry of Health (grants ‘Rare Diseases’ and ‘Alleanza Contro il Cancro to R.P and M.S.B.), and intramural research funds from the University of Parma, the National Cancer Institute of Aviano and Rizzoli Orthopeadic Research Institutes. During the course of the study S.C., P.A.N., and E.R. were supported by post-doctoral fellowships from the Associazione ‘Via di Natale’, Pordenone, Italy.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We are indebted to Dr Francesca Rossi (The National Tumour Institute Aviano – CRO-IRCCS, Aviano, Italy) for assistance with FACS analyses and Drs Daniela Segat (University of Padova, Padova, Italy) and Zhinan Yin (University of Padova, Padova, Italy) for assistance with some of the cell adhesion assays. Dr Douglas Keene (Shriners Hospital for Children, Portland, USA) is thanked for providing the TEM rotary shadowing images of various forms of Col VI.
References
- Al-Mayhani M.T., Grenfell R., Narita M., et al. NG2 expression in glioblastoma identifies an actively proliferating population with an aggressive molecular signature. Neuro. Oncol. 2011;13:830–845. doi: 10.1093/neuonc/nor088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benassi M.S., Pazzaglia L., Chiechi A., et al. NG2 expression predicts the metastasis formation in soft-tissue sarcoma patients. J. Orthop. Res. 2009;27:135–140. doi: 10.1002/jor.20694. [DOI] [PubMed] [Google Scholar]
- Burg M.A., Tillet E., Timpl R., et al. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J. Biol. Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- Burg M.A., Nishiyama A., Stallcup W.B. A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp. Cell Res. 1997;235:254–264. doi: 10.1006/excr.1997.3674. [DOI] [PubMed] [Google Scholar]
- Burg M.A., Grako K.A., Stallcup W.B. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J. Cell. Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Cattaruzza S., Ozerdem U., Denzel M., et al. Multivalent proteoglycan modulation of FGF mitogenic responses in perivascular cells. Angiogenesis. 2013;16:309–327. doi: 10.1007/s10456-012-9316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N., Stegmuller J., Schatzle P., et al. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J. Biol. Chem. 2008;283:8310–8317. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- Chekenya M., Krakstad C., Svendsen A., et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels K.J., Boldt H.C., Martin J.A., et al. Expression of type VI collagen in uveal melanoma: its role in pattern formation and tumor progression. Lab. Invest. 1996;75:55–66. [PubMed] [Google Scholar]
- Eisenmann K.M., McCarthy J.B., Simpson M.A., et al. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat. Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- Fang X., Burg M.A., Barritt D., et al. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol. Biol. Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi J., Makagiansar I.T., Stallcup W.B. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazziola C., Cordani N., Carta S., et al. The relative endogenous expression levels of the IFNAR2 isoforms influence the cytostatic and pro-apoptotic effect of IFNalpha on pleomorphic sarcoma cells. Int. J. Oncol. 2005;26:129–140. [PubMed] [Google Scholar]
- Han J., Daniel J.C., Pappas G.D. Expression of type VI collagen during glioblastoma cell invasion in brain tissue cultures. Cancer Lett. 1995;88:127–132. doi: 10.1016/0304-3835(94)03627-u. [DOI] [PubMed] [Google Scholar]
- Iida J., Skubitz A.P., Furcht L.T., et al. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J. Cell Biol. 1992;118:431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J., Meijne A.M., Spiro R.C., et al. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995;55:2177–2185. [PubMed] [Google Scholar]
- Iida J., Pei D., Kang T., et al. Melanoma chondroitin sulfate proteoglycan regulates matrix metalloproteinase-dependent human melanoma invasion into type I collagen. J. Biol. Chem. 2001;276:18786–18794. doi: 10.1074/jbc.M010053200. [DOI] [PubMed] [Google Scholar]
- Iyengar P., Espina V., Williams T.W., et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageshita T., Yamada M., Arao T., et al. Expression of high molecular weight-melanoma associated antigen (HMW-MAA) in primary ALM lesions is associated with a poor prognosis. Pigment Cell Res. 1992:132–135. doi: 10.1111/j.1600-0749.1990.tb00362.x. Suppl 2. [DOI] [PubMed] [Google Scholar]
- Lamande S.R., Morgelin M., Adams N.E., et al. The C5 domain of the collagen VI alpha3(VI) chain is critical for extracellular microfibril formation and is present in the extracellular matrix of cultured cells. J. Biol. Chem. 2006;281:16607–16614. doi: 10.1074/jbc.M510192200. [DOI] [PubMed] [Google Scholar]
- Lin X.H., Dahlin-Huppe K., Stallcup W.B. Interaction of the NG2 proteoglycan with the actin cytoskeleton. J. Cell. Biochem. 1996;63:463–477. doi: 10.1002/(sici)1097-4644(19961215)63:4<463::aid-jcb8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Majumdar M., Vuori K., Stallcup W.B. Engagement of the NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell. Signal. 2003;15:79–84. doi: 10.1016/s0898-6568(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Makagiansar I.T., Williams S., Dahlin-Huppe K., et al. Phosphorylation of NG2 proteoglycan by protein kinase C-alpha regulates polarized membrane distribution and cell motility. J. Biol. Chem. 2004;279:55262–55270. doi: 10.1074/jbc.M411045200. [DOI] [PubMed] [Google Scholar]
- Makagiansar I.T., Williams S., Mustelin T., et al. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. J. Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K.S., Salter D.M. NG2/HMPG modulation of human articular chondrocyte adhesion to type VI collagen is lost in osteoarthritis. J. Pathol. 2001;195:631–635. doi: 10.1002/path.985. [DOI] [PubMed] [Google Scholar]
- Mittelman A., Chen G.Z., Wong G.Y., et al. Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2–23: modulation of the immunogenicity in patients with malignant melanoma. Clin. Cancer Res. 1995;1:705–713. [PubMed] [Google Scholar]
- Murray J.L., Gillogly M., Kawano K., et al. Fine specificity of high molecular weight-melanoma-associated antigen-specific cytotoxic T lymphocytes elicited by anti-idiotypic monoclonal antibodies in patients with melanoma. Cancer Res. 2004;64:5481–5488. doi: 10.1158/0008-5472.CAN-04-0517. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Stallcup W.B. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol. Biol. Cell. 1993;4:1097–1108. doi: 10.1091/mbc.4.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris R., Kuo H.J., Glanville R.W., et al. Neural crest cell interaction with type VI collagen is mediated by multiple cooperative binding sites within triple-helix and globular domains. Exp. Cell Res. 1993;209:103–117. doi: 10.1006/excr.1993.1290. [DOI] [PubMed] [Google Scholar]
- Petrovici K., Graf M., Hecht K., et al. Use of NG2 (7.1) in AML as a tumor marker and its association with a poor prognosis. Cancer Genomics Proteomics. 2010;7:173–180. [PubMed] [Google Scholar]
- Rivera Z., Ferrone S., Wang X., et al. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin. Cancer Res. 2012;18:5352–63. doi: 10.1158/1078-0432.CCR-12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl M., Johannsen M., Atkinson J., et al. Soluble collagen VI induces tyrosine phosphorylation of paxillin and focal adhesion kinase and activates the MAP kinase erk2 in fibroblasts. Exp. Cell Res. 1999a;250:548–557. doi: 10.1006/excr.1999.4540. [DOI] [PubMed] [Google Scholar]
- Ruhl M., Sahin E., Johannsen M., et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J. Biol. Chem. 1999b;274:34361–34368. doi: 10.1074/jbc.274.48.34361. [DOI] [PubMed] [Google Scholar]
- Sabatelli P., Bonaldo P., Lattanzi G., et al. Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 2001;20:475–486. doi: 10.1016/s0945-053x(01)00160-3. [DOI] [PubMed] [Google Scholar]
- Schenker T., Trueb B. Down-regulated proteins of mesenchymal tumor cells. Exp. Cell Res. 1998;239:161–168. doi: 10.1006/excr.1997.3896. [DOI] [PubMed] [Google Scholar]
- Sherman-Baust C.A., Weeraratna A.T., Rangel L.B., et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3:377–386. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Smith F.O., Rauch C., Williams D.E., et al. The human homologue of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor-prognosis patients with abnormalities of chromosome band 11q23. Blood. 1996;87:1123–1133. [PubMed] [Google Scholar]
- Spessotto P., Lacrima K., Nicolosi P.A., et al. Fluorescence-based assays for in vitro analysis of cell adhesion and migration. Methods Mol. Biol. 2009;522:221–250. doi: 10.1007/978-1-59745-413-1_16. [DOI] [PubMed] [Google Scholar]
- Stallcup W.B., Dahlin K., Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J. Cell Biol. 1990;111:3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen A., Verhoeff J.J., Immervoll H., et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011;122:495–510. doi: 10.1007/s00401-011-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges U., Hyer E.G., Scharf J., et al. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- Tillet E., Ruggiero F., Nishiyama A., et al. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J. Biol. Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- Tillet E., Gential B., Garrone R., et al. NG2 proteoglycan mediates beta1 integrin-independent cell adhesion and spreading on collagen VI. J. Cell. Biochem. 2002;86:726–736. doi: 10.1002/jcb.10268. [DOI] [PubMed] [Google Scholar]
- Virgintino D., Girolamo F., Errede M., et al. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Virgintino D., Perissinotto D., Girolamo F., et al. Differential distribution of aggrecan isoforms in perineuronal nets of the human cerebral cortex. J. Cell. Mol. Med. 2009;13:3151–3173. doi: 10.1111/j.1582-4934.2009.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Krepler C., Allwardt D., et al. Reduction of human melanoma tumor growth in severe combined immunodeficient mice by passive transfer of antibodies induced by a high molecular weight melanoma-associated antigen mimotope vaccine. Clin. Cancer Res. 2008;14:8178–8183. doi: 10.1158/1078-0432.CCR-08-0371. [DOI] [PubMed] [Google Scholar]
- Wang X., Osada T., Wang Y., et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J. Natl Cancer Inst. 2010a;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang Y., Yu L., et al. CSPG4 in cancer: multiple roles. Curr. Mol. Med. 2010b;10:419–429. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- Wang J., Svendsen A., Kmiecik J., et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One. 2011a;6:e23062. doi: 10.1371/journal.pone.0023062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Katayama A., Wang Y., et al. Functional characterization of an scFv-Fc antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Res. 2011b;71:7410–7422. doi: 10.1158/0008-5472.CAN-10-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger M.C., Wang C.C., Krajcovic M., et al. Spontaneous phosphoinositide 3-kinase signaling dynamics drive spreading and random migration of fibroblasts. J. Cell Sci. 2009;122:313–323. doi: 10.1242/jcs.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Price M.A., Neudauer C.L., et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J. Cell Biol. 2004;165:881–891. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You W.K., Bonaldo P., Stallcup W.B. Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. Am. J. Pathol. 2012;180:1145–1158. doi: 10.1016/j.ajpath.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.