Abstract
Aims
Our aim was to identify new microRNAs (miRNAs) implicated in pathological vascular smooth muscle cells (VSMCs) proliferation and characterize their mechanism of action.
Methods and results
MicroRNAs microarray and qRT–PCR results lead us to focus on miR-424 or its rat ortholog miR-322 (miR-424/322). In vitro mir-424/322 level was decreased shortly after the induction of proliferation and increased in a time-dependent manner later on. In vivo its expression increased in the rat carotid artery from Day 4 up to Day 30 after injury. miR-424/322 overexpression in vitro inhibited proliferation and migration without affecting apoptosis and prevented VSMC dedifferentiation. Furthermore, miR-424/322 overexpression resulted in decreased expression of its predicted targets: cyclin D1 and Ca2+-regulating proteins calumenin and stromal-interacting molecule 1 (STIM1). Using reporter luciferase assays, we confirmed that cyclin D1 and calumenin mRNAs were direct targets of miR-322, whereas miR-322 effect on STIM1 was indirect. Nevertheless, consistent with the decreased STIM1 level, the store-operated Ca2+ entry was reduced. We hypothesized that miR-424/322 could be a negative regulator of proliferation overridden in pathological situations. Thus, we overexpressed miR-424/322 in injured rat carotid arteries using an adenovirus, and demonstrated a protective effect against restenosis.
Conclusion
Our results demonstrate that miR-424/322 is up-regulated after vascular injury. This is likely an adaptive response to counteract proliferation, although this mechanism is overwhelmed in pathological situations such as injury-induced restenosis.
Keywords: MicroRNA, Restenosis, Vascular smooth muscle cells, Proliferation, Calcium
1. Introduction
MicroRNAs (miRNAs) are endogenous 18–24 nucleotides long non-coding RNAs with emerging roles in mammals. They are implicated in gene silencing by targeting mRNAs with complementary sequences in their 3′ untranslated regions (UTR). This post-transcriptional regulation of gene expression is conserved among species ranging from plants to mammals and miRNAs appear to be an important way of regulation in many physiological and pathological functions.1
About 1500 different miRNAs are predicted in humans and could regulate >30% of all genes. These miRNAs associate with a complex of proteins called RNA-induced silencing complex (RISC) and with targets mRNAs, which lead to the inhibition of translation either by degradation of mRNAs (if the miRNA and the 3′UTR target site are fully complementary) or by dissociation of the translational complex. It has been shown that nucleotides 2–8 of the miRNA, called ‘seed sequence’, are essential and sufficient to promote translation inhibition of a target mRNA. Based on complementary miRNA-mRNA sequence and its evolutionary conservation, several algorithms have been developed to predict genes regulated by specific miRNAs. Bioinformatics and basic studies have revealed that a single miRNA can regulate many genes and that one gene can be modulated by different miRNAs.2 Despite this complexity of regulation, it appears that many miRNAs are probably responsible for subtle changes of expression allowing for fine regulations of physiological functions. Nonetheless, this can sometimes have major consequences; for instance, when a miRNA targets several mRNAs in a common biological pathway or when many miRNAs act cooperatively and additively on a target gene.3
Recent studies have identified miRNAs that play important roles in vascular pathophysiology.3–5 In particular, miR-143 and miR-145 were shown to be critical modulators of the vascular smooth muscle cells (VSMC) phenotype, since their expression is sufficient to induce differentiation and repress VSMC proliferation.6,7 Other important miRNAs implicated in modulating vascular phenotypes are: (i) anti-proliferative miR-1,8 miR-1339, and miR-19510 and (ii) pro-proliferative miR-21,11 miR-221/miR-222,12 and miR-146a.13
It is likely that many other miRNAs are involved in the regulation of vascular phenotype. Our goal was to identify new miRNAs differentially expressed between proliferative and quiescent VSMC. We focused on miR-424, ortholog of rat miR-322, which was deregulated in the human mammary artery and rat aortic VSMC. We hypothesized that it was an adaptive mechanism to counteract proliferation. We looked at miR-424/322 predicted targets, in particular, at proteins involved in Ca2+ signalling, as alteration of Ca2+ signalling is a mechanism well-known to induce VSMC proliferation (for review, see Marchand et al.14) and on cell-cycle regulator proteins. We investigated the role of this miRNA in VSMC phenotype both in vitro and in vivo in a model of injury of the carotid artery in the rat.
2. Methods
For expanded methods, see supplementary material online.
2.1. Human mammary artery SMC and rat VSMC culture and treatment
Waste fragments of internal mammary arteries were obtained from patients undergoing coronary artery bypass surgery at the Cardiology Institute of Hôpital Pitié-Salpêtrière, Paris, France, after patient consent in accordance with French legislation (L.1211-3-9) and with the principles outlined in the Declaration of Helsinki. Mammary artery segments were dissected and VSMC were isolated using a protocol previously described for rat aortic VSMC.15 Alternatively, rat VSMC were prepared using aortas from 6-week-old rats. Adult male Wistar rats (Janvier, France) were treated in accordance with our institutional guidelines (Ministère de l'Agriculture, France; authorization 75–1090) and with the Directive 2010/63/EU of the European Parliament. At the time of sacrifice, rats were administered with a sodium pentobarbital (Ceva, Santé Animale, France) ip overdose (200 mg/kg). When the animals were completely non-responsive to toe pinching, a thoracotomy was performed, the heart was removed and aortas were retrieved. Cells were used at passages 2–6. To keep VSMC in a quiescent state, cells were maintained at least 2 days in serum-free (rat cells) or 0.1%-serum medium (human cells), which was changed every day.
2.2. Global miRNA expression profile between quiescent and serum-induced proliferative VSMCs
MicroRNAs microarrays (version 9.2 of Ambion® mirVana™ miRNA Bioarrays including 471 human probes and 238 rat probes) were used to compare the miRNAs expression profile between proliferative and quiescent human VSMC. Eight miRNAs with fold change >1.3 were verified by qRT–PCR (Supplementary material online, Table S1).
2.3. Proliferation of VSMCs
Rat aortic VSMC were retro-transfected either with 30 nmol/L Pre-miR™ miRNA Precursor for miR-322 (Pre-322) or Pre-miR™ Negative control (Pre-Neg) (Life Technologies, Villebon sur Yvette, France) with Lipofectamine 2000 transfection reagent (Life Technologies) in 96-well plates according to the manufacturer's recommendations. Cells were cultured for 2 days in serum-free Dulbecco's Modified Eagle Medium. Then, 10% fetal bovine serum (FBS) or 0% FBS-medium was added for 24 h and Bromodeoxy-Uridine (BrdU) for the last 16 h. A colorimetric BrdU cell proliferation assay was performed, as recommended by the manufacturer (Roche Diagnostics, Meylan, France).
2.4. Migration
Confluent cell monolayer was wounded by scrapping with a 200 µL pipette tip and cell migration was stimulated with 10%-FBS medium, while proliferation was blocked by incubation with 40 µmol/L mitomycin C (Sigma-Aldrich). The distance of wound closure was photographed and measured over a 24 h period, using the Metamorph software (Roper Scientific, Evry, France).
2.5. Apoptosis
Apoptosis was determined using the NucView 488 Caspase-3 Assay kit for live cells (VWR International, Fontenay-sous-bois, France) according to the manufacturer's instructions.
2.6. RNA isolation
Total RNA including miRNAs from cultured VSMC was isolated with the mirVana miRNA isolation kit (Life technologies) and total RNA from rat carotid arteries using the RNeasy Mini kit from Qiagen (Courtaboeuf, France) according to the manufacturer's instructions.
2.7. RT–PCR
Total RNA reverse transcriptase-PCR analysis was performed using the Absolute QPCR SYBR green mix (ABgene, Courtaboeuf, France) on an MX3005P QPCR system (Stratagene, Agilent Technologies, Massy, France). The list of primers is included in Supplementary material online, Table S2. Transcript levels were normalized to the RPL32 mRNA. MicroRNA-specific RT–PCR was performed using specific Taqman miRNA assays (Life technologies) and normalized either to small RNA RNU6B (U6) probe for human samples or rat-specific U87. The relative transcript level between two samples was calculated using the 2−ΔΔCT method.
2.8. Protein preparation and western blot
Protein extracts were prepared using the Promokine Mammalian Whole Cell Extraction kit (PromoCell GMBH, Heidelberg, Germany) and phosphatase inhibitors (Sigma-Aldrich, Saint-Quentin Fallavier, France). The following antibodies were used: rabbit polyclonal antibody to GAPDH (ab9485, 1/2500, Abcam, Paris, France), anti-cyclin D1 (556470, 1/1000, BD Biosciences, Le Pont de Claix, France), and anti-stromal-interacting molecule 1 (STIM1) c-terminal (S6197,1/1000, Sigma-Aldrich); rabbit anti-calumenin (1/500) was a generous gift of Dr Kim Do.16 Densitometric analysis was performed with NIH Image/ImageJ, and the expression level of the various proteins was normalized to GAPDH.
2.9. Luciferase reporter constructs and miRNA target validation by luciferase assay
PsiCHECK-2 vector (Promega, Charbonnieres, France) containing both Firefly and Renilla luciferase genes was used to introduce 3′UTR sequence immediately downstream the stop codon of the Renilla luciferase gene. Various constructs of cyclin D1, calumenin, and STIM1 3′UTR (see oligonucleotides list in Supplementary material online, Table S3) surrounding the predicted miRNA binding sites were inserted. After 48 h of incubation, Firefly and Renilla luciferase activities were sequentially measured using the Dual-Glo Luciferase Assay system (Promega) as recommended.
2.10. Ca2+ transients measurements
Pre-322 or Pre-Neg (30 nmol/L final) was transfected in rat VSMC by Amaxa nucleofector using D-033 program. Three days post-transfection cells were loaded with 6 µmol/L FURA-2AM (Life Technologies) and store-operated Ca2+ entry (SOCE) was measured using the standard ‘Ca2+ off/Ca2+ on’ protocol.17
2.11. Generation of adenovirus vectors overexpressing miR-322
A miR-322 precursor DNA consisting of the mature miRNA with flanking sequences (400 bp upstream and 250 bp downstream miR-322) was PCR-amplified from rat genomic DNA. AdCMV-miR-322 efficiency was tested on VSMC and showed a strong overexpression (20–50-fold) of mature miR-322. An adenovirus control encoding beta-galactosidase under the cytomegalovirus (CMV) promoter (AdCMV-Bgal) was similarly generated.
2.12. In vivo experiments
Rat carotid artery injury, adenoviral infection, sacrifice, and tissue collection were performed as previously described.18 Adult male Wistar rats (Janvier, France) weighing 350–400 g were anaesthetized with sodium pentobarbital (50 mg/kg, one ip injection) and simultaneously received Meloxicam (1.5 mg/kg, one subcutaneous injection). Anaesthesia was monitored by periodic observation of respiration and pain response. The left external carotid artery from adult male Wistar rats was injured and infected using 1 × 1010 p.f.u. of AdCMV-miR-322 or AdCMV-Bgal diluted to a total volume of 100 μL in physiological serum. After surgery, the animals received furosemide (5 mg/kg, ip). All surgical procedures have been approved (Ministère de l'Agriculture, France, authorization for surgery C-75-665-R). Animals were sacrificed at 14 days (as described in rat VSMC culture paragraph) and carotid arteries were included in cryomatrix. Haematoxylin–eosin staining was performed on cross-sections.
2.13. MicroRNA in situ hybridization
Fluorescent in situ hybridizations of miR-322 were performed on 5 µm cryomatrix embedded arterial sections according to Exiqon's protocol and recommendations for miRcury LNA™ miRNA ISH (Exiqon, Vedbaek, Denmark) and Tyramide Signal Amplification (TSA)™ Plus Fluorescence system (Perkin-Elmer, Waltham, USA). MiR-322 (39520-15, Exiqon) 5′ and 3′-DIG-labelled LNA mercury probes were used at 100 nM. An anti-digoxigenin-POD antibody (Roche Diagnostics) was added at 1/400 for 1 h at room temperature. Signal was then amplified with a TSA plus Cy3 substrate (Perkin-Elmer).
2.14. Confocal microscopy
Immunohistochemistry was performed on methanol-fixed and 0.1% Triton-PBS-permeabilized sections according to a standard protocol. The following antibodies were used: anti-cyclin D1 (Ab7958 (Abcam), 1/50) and anti-STIM1 c-terminal (S6197, 1/1000, Sigma-Aldrich). Proteins were visualized using secondary antibodies conjugated to Alexa 594 (Life technologies). Sections were examined with a Leica TCS4D confocal scanning laser microscope using a Plan Apochromat 40× objective (NA 1.40, oil immersion). All settings were kept constant to allow comparison.
2.15. Statistical analysis
Data are expressed as means ± SEM. Experiments with two groups were analysed with the non-parametric Mann–Whitney test or two-sample t-test when indicated. Time course experiments were analysed with Kruskall–Wallis corrected by the Conover–Imam test. When P-values were below 0.05, differences were considered significant.
3. Results
3.1. Identification of miR-424/322 as a new miRNA modulated during VSMC proliferation
The quiescent-differentiated/proliferative-dedifferentiated status of primary human and rat VSMC was confirmed by RT–PCR using the differentiation markers genes calponin (CNN1), smooth muscle myosin heavy chain (MYH11), and smooth muscle alpha actin 2 (ACTA2). They were significantly down-regulated in proliferative cells (Supplementary material online, Figure S1).
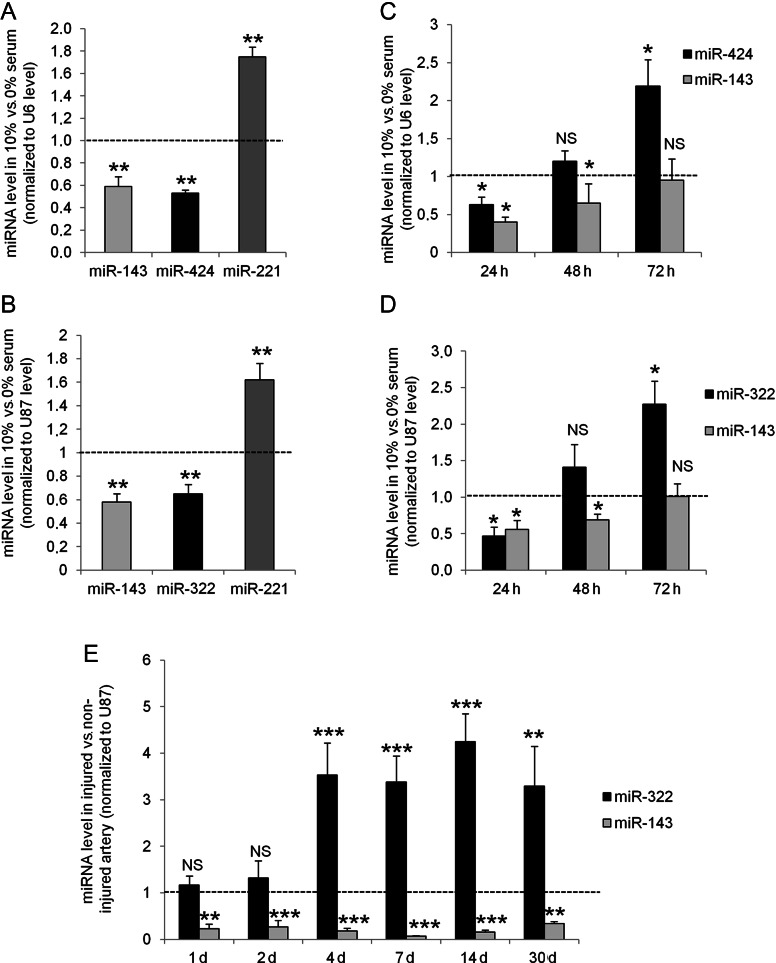
Using miRNAs microarray, we compared the expression of miRNAs in quiescent vs. 24 h serum-induced proliferative VSMC. A panel of miRNAs the expression of which was differentially expressed by at least 30% was identified. These differences were verified by qRT–PCR in human and rat VSMC (Supplementary material online, Table S1). Among them, modulations of the expression of miR-143 (×0.59) and miR-221 (×1.75) had already been described in vascular proliferative pathologies, which validated our experiments. Interestingly, miR-424 and miR-143 were lower in proliferative VSMC than in quiescent VSMC (×0.53) (Figure 1A). In rats, the ortholog of human miR-424, miR-322, which displays only one nucleotide difference A→G at the 3′ end of its sequence (Supplementary material online, Figure S1C), was similarly decreased 24 h after serum-induced proliferation (Figure 1B). We further checked the kinetic of expression of miR-424/322 in vitro after serum-induced proliferation. In human VSMC (Figure 1C) as well as in rat VSMC (Figure 1D), miR-424/322 expression was first decreased after 24 h, but then up-regulated at 72 h while miR-143 expression remained low throughout. In vivo miR-322 expression was also increased in rat carotid arteries after balloon injury (Figure 1E) after 4 days and stayed up-regulated for at least 30 days post-angioplasty. On the contrary, miR-143 expression was strongly down-regulated at the early-stage post-angioplasty and was maintained low up to 30 days. MiR-503, which is expressed in cluster with miR-424/322 was also up-regulated at Day 4 and Day 14 post-angioplasty, whereas miR-16, which share the same seed sequence, was unchanged (see Supplementary material online, Figure S2). In contrast to miR-424/322 expression, which was decreased after 24 h serum stimulation in vitro and only raised after 4 days post-angioplasty in vivo, cyclin D1 mRNA was already up-regulated 24 h after proliferation stimulation or angioplasty (see Supplementary material online, Figure S3). Despite miR-322 up-regulation later on, cyclin D1 mRNA stayed increased up to 30 days post-angioplasty. We hypothesized that the increase in miR-424/322 could represent a new mechanism to limit proliferation, although not sufficient to completely block vascular proliferation.
Figure 1.
miR-424/322 is a new miRNA modulated during VSMC proliferation. miR-143, miR-221, and miR-424 quantification in proliferative human VSMC (n = 5) (A) or rat VSMC (n = 5) (B) compared with quiescent VSMC (**P < 0.01). Time course of miR-143 and miR-424 expression in cultured human VSMC (n = 3) (C) and rat VSMC (n = 4) (D) upon serum induction compared with quiescent VSMC. Cells were serum starved for 48 h then proliferation was induced by serum for 24–72 h (*P < 0.05; **P < 0.01). (E) Time course of miR-143 and miR-322 expression after balloon injury of the rat left carotid artery compared with the level in the right non-injured carotid artery. Rats were sacrificed at 1, 2, 4, 7, 14, and 30 days post-angioplasty (n = 6 in each group except for 30 days group, n = 4) (**P < 0.01; ***P < 0.0001).
3.2. miR-424/322 inhibits proliferation and migration, has no effect on apoptosis and induces redifferentiation of VSMC
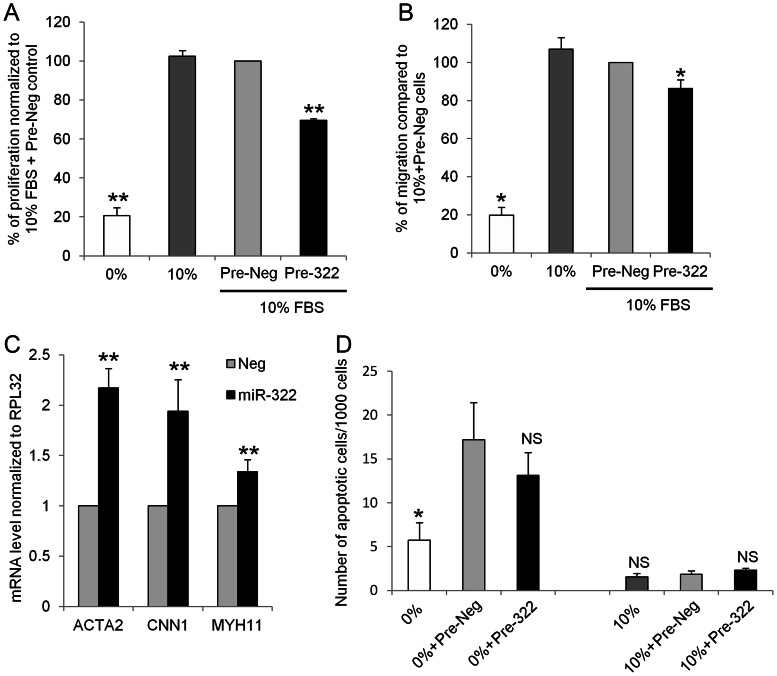
To test the effect of miR-322 overexpression on VSMC proliferation, rat aortic VSMC were transfected with Pre-322 or a negative control Pre-miR (Pre-Neg). Cells were serum starved for 2 days and then proliferation was stimulated by serum. In cells overexpressing miR-322, proliferation was reduced by 30% when compared with Pre-Neg-transfected cells (Figure 2A). On the contrary, using anti-miR molecules (100 nM, Life technologies), we observed a 60% decrease in miR-322 expression compared with cells transfected with anti-Neg (negative control) 72 h post-transfection (Supplementary material online, Figure S4A). This decrease was associated with a significant (+15%) increase in proliferation (Supplementary material online, Figure S4B). Similarly, migration was significantly decreased when miR-322 was overexpressed, as determined by a wound assay on confluent cells (Figure 2B). Furthermore, miR-322 overexpression led to a significant increase in the expression of differentiation markers such as ACTA2, CNN1, and MYH11 (Figure 2C). Apoptosis was not affected by miR-322 overexpression (Figure 2D and Supplementary material online, Figure S5).
Figure 2.
miR-322 inhibits VSMC proliferation (A) and migration (B) and promotes VSMC differentiation (C) without affecting apoptosis (D). (A) Measurement of BrDU incorporation in rat VSMC transfected with Pre-322 or Pre-Neg (control). Results are expressed as a percentage of Pre-Neg + 10% FBS (n = 5; **P < 0.01). (B) VSMC were transfected or not with Pre-Neg or Pre-322 for 72 h and migration was stimulated by 10% serum for 24 h while proliferation was blocked with 40 µmol/L mitomycin (C). Wound scrap were performed and migration measured after 24 h using the Metamorph software (n = 4; *P < 0.05). (C) mRNA levels of differentiation markers ACTA2, CNN1, and MYH11 in rat VSMC after 48 h of Pre-322 or Pre-Neg overexpression (n = 5; **P < 0.01). (D) VSMC transfected with Pre-Neg or Pre-322 for 72 h and cultured in 0 or 10% serum for 24 h were incubated 30 min with NucView 488 caspase-3 substrate. Each experiment was performed in triplicate. Apoptotic nuclei, which showed a strong green fluorescence, and total cells were counted on similar areas for each condition. The number of apoptotic cells for 1000 cells are presented (n = 3; *, P < 0.05).
3.3. miR-424/322 inhibits STIM1, calumenin, and cyclin D1 gene expression
Potential miR-424/322 targets were identified using the algorithms Targetscan (http://www.targetscan.org), MicroCosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/), and PicTar (http://pictar.mdc-berlin.de/). Among miR-424/322 targets, were found pro-proliferative cyclin D1 (CCND1 gene) and Ca2+-regulating proteins calumenin (calu), known to interact with and inhibit SERCA2a activity16 and stromal interaction protein 1 (STIM1) known to inhibit VSMC proliferation19–21(Supplementary material online, Figure S5). Cyclin D1 has previously been described as a miR-424 direct target in other tissues.22,23
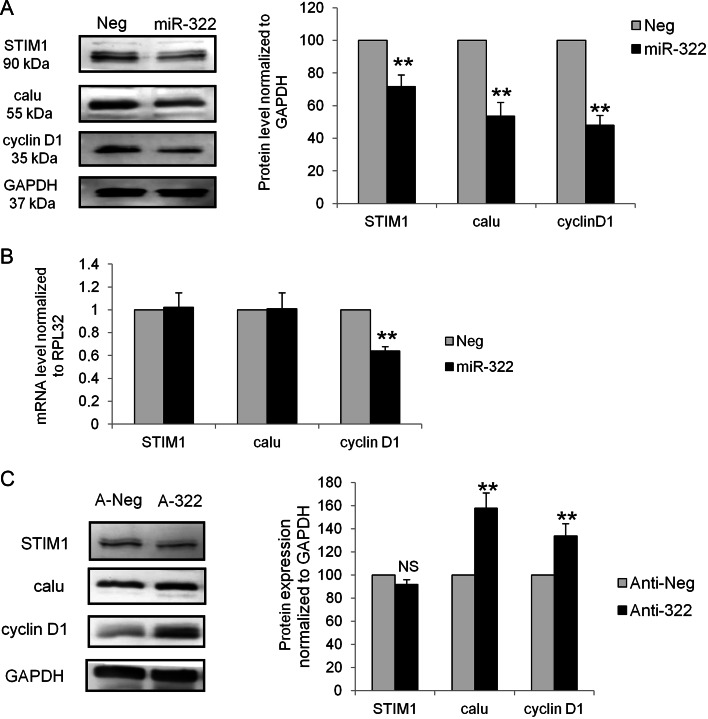
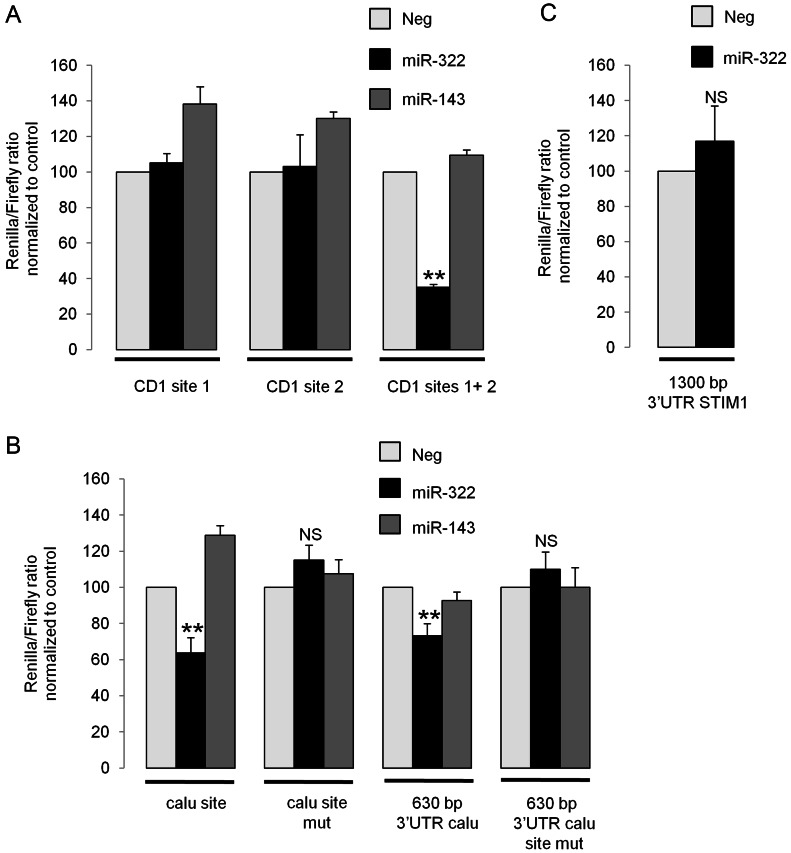
Overexpressing miR-322 in rat VSMC led to a decrease in STIM1, calumenin and cyclin D1 protein levels after 72 h (Figure 3A), but only the cyclin D1 mRNA level was decreased (Figure 3B). Alternatively, decreasing miR-322 resulted in an overexpression of calumenin and cyclin D1 protein expression but no change in the STIM1 protein level (Figure 3C). A slower rate of synthesis of STIM1 expression, which is a membrane protein, may explain this result. We then performed luciferase assays by adding various 3′UTR constructs to luciferase gene to determine whether cyclin D1, calumenin, and STIM1 mRNA were directly targeted by miR-322. Two close and highly conserved miR-424/322 recognition target sites (see Supplementary material online, Figure S6A) are present in the cyclin D1 3′UTR. Fragments including either site 1 or site 2 or both sites have been inserted in a luciferase reporter vector downstream the Renilla luciferase. Interestingly, an overexpression of miR-322 was unable to decrease Renilla luciferase activity when either site was present alone but a strong inhibitory effect (−65%) was observed when both sites were present in the 3′UTR (Figure 4A). Moreover, this inhibitory effect was specific for miR-322, since miR-143 failed to affect the luciferase activity.
Figure 3.
miR-424/322 predicted targets cyclin D1, calumenin and STIM1 are regulated by miR-322. (A) Rat VSMC were transfected with the negative control Pre-Neg (Neg) or with Pre-322 (miR-322) for 72 h. Representative western blots showing the expression of STIM1, calumenin (calu), cyclin D1, and GAPDH used as loading control. Quantification of the western blot: the level of each protein was normalized to GAPDH and presented as a percentage of the value observed in cells transfected with Pre-Neg (n = 5; **P < 0.01). (B) mRNA levels, relative to RPL32 mRNA, expressed as the percentage of the value observed in cells transfected with Pre-Neg (n = 5; **, P < 0.01). (C) Rat VSMC were transfected with anti-Neg (negative control, A-Neg) or anti-322 (anti-miR-322, A-322). Representative western blots showing expression of STIM1, calu, cyclin D1, and GAPDH used as a loading control. Quantification of the western blot: the level of each protein was normalized to GAPDH and presented as a percentage of the value observed in anti-Neg-transfected cells (n = 5; **P < 0.01).
Figure 4.
miR-424/322 directly targets cyclin D1 and calumenin 3′UTR but not STIM1 3′UTR. Luciferase assays were performed 48 h after co-transfection of psiCheck2 vector encoding Renilla luciferase with various 3′UTR constructs and Pre-322 or Pre-Neg. Renilla luciferase activity was quantified and normalized to Firefly luciferase activity. The Renilla/Firefly ratio is expressed as a percentage of the value observed in cells transfected with Pre-Neg. (A) Cyclin D1 3′UTR constructs corresponding to miR-322-binding site 1 (CD1 site 1), site 2 (CD1 site 2), or including both sites were tested (CD1 sites 1+2) (n = 5; **P < 0.01). (B) Calumenin 3′UTR region surrounding miR-322-binding site (calu site) or a large 630 bp region of calumenin 3′UTR including miR-322-binding site (630 bp 3′UTR calu) were tested as well as the same constructs with mutations in the sequence complementary to miR-322 seed sequence (site mut) (n = 5; **P < 0.01). (C) A construct with 1300 bp of STIM1 3′UTR including miR-322 binding site was tested (n = 5).
The region surrounding the miR-424/322 recognition target site (1138–1144) in the calumenin 3′UTR and a long 630 bp-fragment of this 3′UTR were also tested for direct binding, since secondary structures could occur in the 3′UTR that either prevent or facilitate accessibility of the miRNA on its target sequence. With both constructs, an overexpression of miR-322 but not of miR-143 significantly decreased luciferase activity (Figure 4B). Moreover, constructs with mutation of miR-322 seed sequence-binding site (TGCTGCT→TCGACGA, see Supplementary material online, Table S3) abolished miR-322 inhibitory effect on luciferase activity, thus, indicating that calumenin is a direct target of miR-322. Similar experiments were performed with a vector containing 1300 bp of the STIM1 3′UTR that includes miR-424/322 recognition target site (226–232). However, no inhibitory effect on luciferase activity was observed with this reporter construct when miR-322 was overexpressed (Figure 4C), indicating that STIM1 protein inhibition by miR-322 was not due to a direct interaction between STIM1 mRNA and miR-322.
3.4. miR-424/322 inhibits SOCE
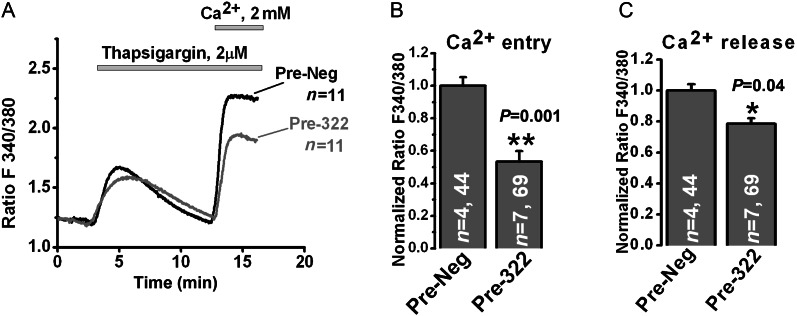
As miR-322 inhibited the expression of Ca2+ regulating proteins calumenin and STIM1, Ca2+ measurements were performed in rat VSMC to determine whether Ca2+ release from the endoplasmic reticulum induced by passive store depletion using the SERCA pump inhibitor thapsigargin or the subsequent activation of SOCE upon store depletion could be affected by miR-322 overexpression. Using a standard Ca2+ off/Ca2+ on the protocol,17 we showed that miR-322 overexpression in rat VSMC inhibited significantly SOCE upon Ca2+ restoration to the extracellular medium (∼40% inhibition) when compared with Pre-Neg-transfected cells, (Figure 5A and B), consistent with the decrease in the STIM1 protein level which is caused by miR-322 overexpression. In addition, we also observed a modest decrease in Ca2+ released by thapsigargin (Figure 5C).
Figure 5.
miR-424/322 inhibits thapsigargin-induced SOCE in VSMC. Pre-Neg or Pre-322 were transfected in rat VSMC by Amaxa nucleofector using D-033 program. Three days post-transfection, cells were loaded with 6 µM FURA-2AM and SOCE was measured using standard ‘Ca2+ off/Ca2+ on’ protocol. (A) Representative traces from Pre-Neg and Pre-miR-322 transfected cells; n = number of cells imaged. (B) Bar graphs depicting thapsigargin-mediated SOCE data obtained from two separate transfections. n = number of coverslips and total number of cells imaged per condition (**P < 0.01). (C) Bar graphs depicting thapsigargin-mediated Ca2+ release obtained from two separate transfections. n = number of coverslips and total number of cells imaged per condition (*P < 0.05).
3.5. miR-424/322 overexpression limits restenosis after balloon angioplasty of the rat carotid artery
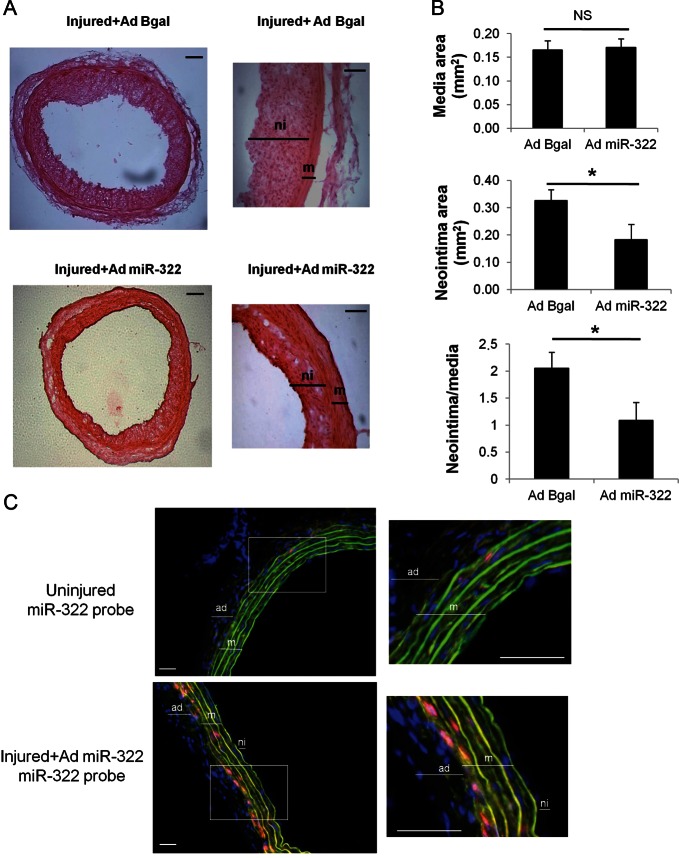
In light with our in vitro results that pointed towards an anti-proliferative role of miR-424/322, and our in vivo results which showed delayed miR-322 increase after balloon angioplasty (Figure 1E), we hypothesized that an early and potent increase in miR-322 in vessels could prevent VSMC proliferation and neointima formation after balloon angioplasty. We produced an adenovirus with a CMV promoter that unabled a strong overexpression of miR-322 in VSMC. Our in vitro results using this AdCMV-miR-322 on VSMC (MOI of 200) showed a 30-fold overexpression 3 days after infection, which correlated with a decrease by 30% of cyclin D1 mRNA level (Supplementary material online, Figure S7A). Moreover, miR-322 overexpression was stable as we observed the same level of up-regulation after 6 days (× 34±1.8, n = 2). The virus expressed a sequence specific to miR-322 which did not encode miR-503. Immediately after angioplasty of the left carotid artery, rats were infected either with AdCMV-miR-322 or with a control adenovirus encoding the beta-galactosidase gene (AdCMV-Bgal). The Ad-CMV virus allows a rapid (from Days 2 or 3) and strong (>30×) overexpression of the transgene (Lompré et al., under revision). Fourteen days after angioplasty and infection, carotids were removed. Infection of left carotids by AdCMV-miR-322 was verified by PCR (data not shown). MiR-322 expression in the injured carotids was still two times higher in AdCMV-miR-322 animals than in AdCMV-Bgal animals (Supplementary material online, Figure S7B). Interestingly, there was a smaller neointima (sometimes barely visible) in AdCMV-miR-322 infected carotids compared with AdCMV-Bgal infected ones (Figure 6A). Morphometric analysis showed no difference in the media size between AdCMV-miR-322 and AdCMV-Bgal infected carotids, but the size of the neointima and the neointima/media ratio were significantly reduced in AdCMV-miR-322 animals (Figure 6B). Therefore, miR-322 overexpression immediately after balloon injury was able to limit VSMC proliferation in vivo. Furthermore, in agreement with an inhibitory effect on proliferation, cyclin D1 was almost absent from AdCMV-miR-322 infected carotids compared with AdCMV-Bgal infected ones (Supplementary material online, Figure S8A). STIM1 was found expressed in the media and the neointima with as a stronger pattern in the neointima as previously reported.19 However, we could not detect any difference in the level of STIM1 in AdCMV-BGal and AdCMV-miR-322 infected carotids at the cellular level possibly due to the limitation of the technique. Nevertheless, as the neointima was thinner in AdCMV-miR-322 infected carotids, the global STIM1 expression was reduced (Supplementary material online, Figure S8A).
Figure 6.
miR-424/322 overexpression limits neointima formation after angioplasty of the rat carotid artery. Angioplasty followed by infection with AdCMV-BGal or AdCMV-miR-322 was performed on rat left carotid arteries. Rats were sacrificed 14 days after injury. (A) Representative haematoxylin–eosin staining (left images, scale bar, 100 µm; right images, scale bar, 50 µm) and (B) morphometric analysis of rat carotid cross-sections. Graphs represent mean ± SEM of media area, intima area, and intima/media areas ratio (n = 5 for Ad CMV-Bgal, n = 6 for AdCMV-miR-322 (*P < 0.05). (C) In situ hybridization of miR-322 in right uninjured carotid and in left carotid 14 days post-injury and infection with AdmiR-322. miR-322 was detected using 40 nM 5′–3′DIG-labelled Probe hybridized at 47°C and TSA plus Cy3 substrate. Representative images showing miR-322 (red), green autofluorescence of the elastic lamina (green), and cell nuclei (DAPI, blue). Media (m), neointima (ni), and adventitia (ad) are indicated (scale bars: 50 µM).
Finally, miR-322 gene transfer was assessed by in situ hybridization, which showed a stronger expression of miR-322 in the left injured carotid of AdCMV-miR-322 animals compared with the uninjured right carotid (Figure 6C). As expected, miR-322 was found expressed in the media, in particular, in the internal layers indicating that miR-322 was present at basal state and overexpressed in VSMC, whereas U6, a small nucleolar RNA was found more ubiquitously expressed (Supplementary material online, Figure S8B).
4. Discussion
Recent studies have highlighted the role of miRNAs in vascular tissues and how deregulation of their expression can contribute to proliferative diseases such as atherosclerosis and restenosis.3–5 In this study, we have demonstrated that miR-424/322 is a novel regulator of VSMC proliferation, which acts by modulating Ca2+ regulating proteins such as calumenin and STIM1 and by targeting cyclin D1. First, we identified miR-424/322 as a miRNA induced during VSMC proliferation in vitro and in vivo. We showed that miR-424/322 stays up in the carotid artery for at least 30 days after the injury and is highly expressed when the remodelling of the tissue occurs. In addition, we demonstrated that increasing the level of miR-424/322 prevents VSMC proliferation in vitro and injury-induced remodelling in vivo. This mechanism of action is different from what was previously shown for deregulated vascular miRNAs. MiR-145,24 miR-133,9 and miR-19510 are highly expressed in quiescent VSMC and down-regulated when cells proliferate. Overexpressing these miRNAs inhibits proliferation indicating that the down-regulation was a pathological response to proliferation. On the contrary, previous miRNAs up-regulated in the balloon-injured carotid artery model of restenosis such as miR-21,11 miR-221/222,12 and miR-146a13 were thought to participate to neointimal hyperplasia as neointima formation was prevented by inhibiting these up-regulations using antagomiRs. In this study, we show that another miRNA, miR424/322, is up-regulated in proliferative VSMC and after vascular injury, but interestingly, it has an anti-proliferative and anti-dedifferentiation effect. Thus, miR-424/322 up-regulation could be considered as an adaptive response to counteract proliferation.
In agreement with our results showing that miR-322 is induced during proliferation, miR-322 expression was also increased in another vascular proliferative disease, pulmonary hypertension: its expression was induced in the rat by hypoxia or monocrotalin treatment. However, in these experiments, the increased miR-424/322 expression was transient: it was up-regulated after 7 days but returned to the basal level after 21 days.25
The mechanism leading to the induction of miR-424 during VSMC proliferation is not known. The mir-424/322 promoter was first studied by Rosa et al.,26 which focused on a haematopoietic transcription factor and identified a PU.1 binding site (at position −1681/−1671) responsible, at least in part, for miR-424/322 induction during monocyte/macrophage differentiation. Ghosh et al.27 also concluded that PU.1 was responsible for miR-424 induction by hypoxia in endothelial (HUVEC) cells. However, using a computational analysis approach, Schmeier et al.28 identified other transcription factors implicated in miR-424 regulation in monocytes/macrophages; in particular, they showed that in addition to PU.1, ELK1, USF2, CEBPB, and HOXA4 were likely to be responsible for regulating the expression of miR-424.
In VSMC PU.1 and HOXA4 transcription factors seem not expressed but ELK1, USF2, and CEBPB are present and could be implicated in miR-424/322 regulation upon proliferation. We analysed miR-424/322 human and rat promoters using MatInspector (www.genomatix.de). Various predicted binding sites were also found for CREB, p53, and NFAT on the region covering 1.6 kb upstream of a pre-miR-424 sequence. In particular, the predicted binding sites for p53 (−1326/−1303) and for NFAT (−1444/−1425) are well conserved between humans and rats and could affect miR-424/322 levels, as their expression and activity are modulated during VSMC proliferation. Kim et al.29 demonstrate that, in pulmonary endothelial cells, apelin regulates the expression of miR-424/503. However, the link between apelin signalling and miR-424/503 was not clarified and this effect seemed not similar in VSMC (from our experiments, data not shown). More experiments are needed to decipher which transcription factors are responsible for miR-424/322 regulation during VSMC proliferation.
Among the multiple predicted targets of miR-424/322 were cell- cycle regulator cyclin D1 and calcium-handling proteins such as calumenin and STIM1. STIM1 is now well established as a Ca2+ sensor in the endoplasmic reticulum, which upon Ca2+ depletion activates SOCE, an event of major importance in VSMC proliferation. We and others have previously shown that inhibiting STIM1 using shRNA prevented VSMC proliferation and in vivo vascular remodelling induced in the rat by balloon injury of the carotid artery.19–21 Our data indicate that miR-424/322 effectively decreases STIM1 protein expression but that miR-424/322 does not bind to the 3′UTR of STIM1. This indirect regulation may explain the absence of STIM1 protein increase when miR-322 was decreased in our experiments, as the dynamic of regulation should take more time than miR-322 direct targets. It remains unclear whether this indirect effect is a specific effect of miR-424/322 on STIM1 expression or the consequence of a global redifferentiation of VSMC as miR-322 also induces the expression of VSMC differentiation markers. It could also be due to an increased degradation of the protein by the proteasome. Indeed, a study by Zhang et al.30 has shown similarly that STIM1 protein, but not mRNAs was decreased by retinoic acids in rat mesangial cell. An inhibitor of the 26S proteasome abolished the retinoic acids effects. Whether a similar increase in the proteasome activity is induced by miR-424/322 remains to be determined. Regardless, the effect on STIM1expression is in agreement with the reduction in SOCE observed in miR-424/322-overexpressing cells.
Calumenin is a Ca2+-binding protein localized in the endoplasmic reticulum as well as the Golgi complex and can be secreted. Recent publications have shown that calumenin is a regulator of SERCA2a, able to inhibit its activity in cardiac myocytes.16 It is also involved in pathological activities such as malignant cell transformation.31,32 Here, we showed that calumenin is a real target of miR-424/322, which is able to bind to calumenin 3′UTR and inhibit calumenin protein expression. This decrease could improve SERCA2a activity and allow a better Ca2+ cycling in VSMC. However, in our experiments, the SR Ca2+ content (released by thapsigargin) is only marginally affected by mir-424/322 overexpression. This is in agreement with a previous study showing that silencing calumenin does not alter the caffeine-evoked Ca2+ transients in HL-1 cells.16
Cyclin D1 is also a very important downstream target of miR-424/322: as it controls the cell-cycle entry, its level has to be finely regulated. Cyclin D1 was previously shown as a direct target of miR-424 in HepG2 cells and myoblasts.22,23 We showed that the presence of the two 3′UTR-binding sites of cyclin D1 mRNA is essential to obtain the inhibition of protein expression by miR-424/322, whereas in a previous study the authors concluded that only site 2 was necessary for the inhibitory effect on cyclin D1.23 The discrepancy may reside in the difference in the cell type used and/or the fact that site 2 alone was not tested in this previous set of experiments. It is also possible that a secondary structure of cyclin D1 mRNA is necessary to allow a good fixation of miR-424/322 to its binding sites.
Although we focused our attention on cyclin D1, we cannot exclude that some of the effects of miR-322 overexpression on VSMC proliferation could also be due to other targets. Indeed, miR-424/322 is a member of miR-15/107 family (miR-15a, 15b, 16, 103, 107, 195, 424, 497, 503)33 also known as miR-16 family,23 which share similar seed sequences and common targets implicated in cell-cycle regulation. These miRNAs induce G1 cell-cycle arrest by regulating multiple downstream effectors of cell-cycle including cyclin D1, cyclin E1, cyclin-dependent kinase 6, and cell division cycle homologue 25a (Cdc25a). MiR-195 has just been shown to reduce VSMC proliferation by targeting Cdc42, cyclin D1, and fibroblast growth factor 1 and to limit neointimal formation when overexpressed using an adenovirus in balloon-injured carotid arteries.10 Interestingly, miR-503, which is the only miRNA of this family also found in a cluster with miR-424/322, was also up-regulated during restenosis although its expression was much lower (mean Ct of miR-322 = 26, mean Ct of miR-503 = 31), whereas miR-16 expression was unchanged. The transcription(s) factor(s) implicated remained to be identified.
Other studies have emphasized a similar negative feedback loop between miRNAs and a cell-cycle regulator: this is the case for miR-146a and Kruppel-like factor 4 during VSMC proliferation13 and for cyclin D1/miR-17/20 in breast cancer cell proliferation.34 The negative effect of miR-424/322 on cyclin D1 could allow fine control of cell-cycle under physiological conditions but may be overwhelmed in pathological situations such as restenosis. In addition to its role in proliferation, miR-322 also leads to the re-expression of VSMC differentiation markers. A similar role was also demonstrated in other tissues: miR-424 was up-regulated and necessary for monocyte/macrophage differentiation26 and miR-424/322 and miR-503 were induced during skeletal muscle differentiation (myogenesis) to promote cell-cycle quiescence and differentiation by down-regulating Cdc25a.35 Interestingly, miR-424 was also induced in endothelial cells by vascular endothelial growth factor (VEGF)/basic fibroblast growth factor, and inhibited the downstream components of pro-angiogenic pathways involving VEGF receptor 2 and FGF receptor 1. Overexpression of miR-424 in endothelial cells reduced proliferation, migration, and cord formation.36 Furthermore, the recent study by Kim et al.29 is also in favour of an anti-proliferative effect of miR-424 in pulmonary endothelial cells, mediated by a regulation of its targets FGF2 and FGFR1. Altogether, these results suggest that miR-424 is an important regulator of both VSMC and endothelial cells functions, which is up-regulated under proliferation/stress signals to prevent/limit vascular dysfunction.
In summary, we identified an anti-proliferative and pro-differentiation role for miR-424/322 in the vasculature. MiR-424/322 is likely induced as a signal to counteract proliferation but the level of induction is clearly not sufficient to completely block cell-cycle progression in a pathological situation such as injury-induced restenosis. Nevertheless, early and ample introduction of miR-424/322 in vivo in balloon-injured vessels greatly reduces neointima formation in a rat model of restenosis. Thus, miR-424/322 could prove to be a useful therapeutic tool to inhibit VSMC dedifferentiation during vascular occlusive disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Acknowledgements
We thank the Service de Chirurgie Cardiothoracique, Pôle de cardiologie, Hôpital Pitié-Salpêtrière, 47–83, boulevard de l'Hôpital, 75013, Paris, France, for providing human artery mammary samples and A. Dauphin from Plate-forme d'Imagerie Cellulaire Pitié—Salpêtrière for confocal microscopy experiments. We are grateful to H. Kim Do, Department of Life Science and Systems Biology Research Center, Gwangju Institute of Science and Technology (GIST), Gwangju- China for providing the calumenin antibody.
Conflict of interest: none declared.
Funding
This work was supported by Association Française contre les Myopathies (AFM), and by the Leducq Foundation (Caerus network) and in part by grant HL097111 from the National Institutes of Health and a visiting professorship from ‘la mairie de Paris’ to M.T.; E.M. was supported by the French Ministry of Research and Education.
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. doi:10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. doi:10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. doi:10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z, Li G. Role of specific microRNAs in regulation of vascular smooth muscle cell differentiation and the response to injury. J Cardiovasc Transl Res. 2011;3:246–250. doi: 10.1007/s12265-010-9163-0. doi:10.1007/s12265-010-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zernecke A. MicroRNAs in the regulation of immune cell functions – implications for atherosclerotic vascular disease. Thromb Haemost. 2012;107:626–633. doi: 10.1160/TH11-08-0603. doi:10.1160/TH11-08-0603. [DOI] [PubMed] [Google Scholar]
- 6.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. doi:10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. doi:10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. doi:10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. doi:10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 10.Wang YS, Wang HY, Liao YC, Tsai PC, Chen KC, Cheng HY, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotypes and prevents neointimal formation. Cardiovasc Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 11.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. doi:10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. doi:10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. doi:10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchand A, Abi-Gerges A, Saliba Y, Merlet E, Lompre AM. Calcium signaling in vascular smooth muscle cells: from physiology to pathology. Adv Exp Med Biol. 2012;740:795–810. doi: 10.1007/978-94-007-2888-2_35. doi:10.1007/978-94-007-2888-2_35. [DOI] [PubMed] [Google Scholar]
- 15.Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, et al. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. doi:10.1161/01.ATV.20.5.1225. [DOI] [PubMed] [Google Scholar]
- 16.Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim do H. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem. 2009;284:31109–31121. doi: 10.1074/jbc.M109.031989. doi:10.1074/jbc.M109.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, et al. Evidence for STIM1–and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. doi:10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. doi:10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 19.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2009;17:455–462. doi: 10.1038/mt.2008.291. doi:10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, et al. An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc Res. 2009;81:660–668. doi: 10.1093/cvr/cvn338. doi:10.1093/cvr/cvn338. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, et al. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. doi:10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest AR, Kanamori-Katayama M, Tomaru Y, Lassmann T, Ninomiya N, Takahashi Y, et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia. 2010;24:460–466. doi: 10.1038/leu.2009.246. doi:10.1038/leu.2009.246. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. doi:10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. doi:10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. doi:10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 26.Rosa A, Ballarino M, Sorrentino A, Sthandier O, De Angelis FG, Marchioni M, et al. The interplay between the master transcription factor PU.1 and miR-424 regulates human monocyte/macrophage differentiation. Proc Natl Acad Sci USA; 2007. pp. 19849–19854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. doi:10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmeier S, MacPherson CR, Essack M, Kaur M, Schaefer U, Suzuki H, et al. Deciphering the transcriptional circuitry of microRNA genes expressed during human monocytic differentiation. BMC Genomics. 2009;10:595. doi: 10.1186/1471-2164-10-595. doi:10.1186/1471-2164-10-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. doi:10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Meng H, Li ZH, Shu Z, Ma X, Zhang BX. Regulation of STIM1, store-operated Ca2+ influx, and nitric oxide generation by retinoic acid in rat mesangial cells. Am J Physiol Renal Physiol. 2007;292:F1054–F1064. doi: 10.1152/ajprenal.00286.2006. doi:10.1152/ajprenal.00286.2006. [DOI] [PubMed] [Google Scholar]
- 31.Honore B. The rapidly expanding CREC protein family: members, localization, function, and role in disease. Bioessays. 2009;31:262–277. doi: 10.1002/bies.200800186. doi:10.1002/bies.200800186. [DOI] [PubMed] [Google Scholar]
- 32.Yabe D, Nakamura T, Kanazawa N, Tashiro K, Honjo T. Calumenin, a Ca2+-binding protein retained in the endoplasmic reticulum with a novel carboxyl-terminal sequence, HDEF. J Biol Chem. 1997;272:18232–18239. doi: 10.1074/jbc.272.29.18232. doi:10.1074/jbc.272.29.18232. [DOI] [PubMed] [Google Scholar]
- 33.Finnerty JR, Wang WX, Hebert SS, Wilfred BR, Mao G, Nelson PT. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010;402:491–509. doi: 10.1016/j.jmb.2010.07.051. doi:10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. doi:10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkar S, Dey BK, Dutta A. MiR-322/424 and -503 are induced during muscle differentiation and promote cell cycle quiescence and differentiation by down-regulation of Cdc25A. Mol Biol Cell. 2010;21:2138–2149. doi: 10.1091/mbc.E10-01-0062. doi:10.1091/mbc.E10-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. doi:10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.