Abstract
The process of angiogenesis requires endothelial cells (ECs) to undergo profound changes in shape and polarity. Although this must involve remodelling of the EC cytoskeleton, little is known about this process or the proteins that control it. We used a co-culture assay of angiogenesis to examine the cytoskeleton of ECs actively undergoing angiogenic morphogenesis. We found that elongation of ECs during angiogenesis is accompanied by stabilisation of microtubules and their alignment into parallel arrays directed at the growing tip. In other systems, similar microtubule alignments are mediated by the formin family of cytoskeletal regulators. We screened a library of human formins and indentified formin-like 3 (FMNL3; also known as FRL2) as a crucial regulator of EC elongation during angiogenesis. We showed that activated FMNL3 triggers microtubule alignment and that FMNL3 is required for this alignment during angiogenic morphogenesis. FMNL3 was highly expressed in the ECs of zebrafish during development and embryos that were depleted for FMNL3 showed profound defects in developmental angiogenesis that were rescued by expression of the human gene. We conclude that FMNL3 is a new regulator of endothelial microtubules during angiogenesis and is required for the conversion of quiescent ECs into their elongated angiogenic forms.
Key words: Angiogenesis, Endothelial cell, Formin, Cytoskeleton
Introduction
Endothelial cells (ECs) in quiescent vasculature form a tightly connected sheet of extremely flattened cells. They are unusual in exhibiting two concurrent polarities: apical-basolateral polarity and planar cell polarity. The apical surface of the EC is always oriented towards the lumen of the vessel, whereas the axis of planar cell polarity is aligned with the direction of blood flow (Rogers et al., 1986). During angiogenesis, ECs undergo extreme changes in morphology and polarity. Cells convert to an invasive form and burrow through the basement membrane surrounding the vessel to form an angiogenic sprout. As the new vessel forms, ECs migrate outwards as a collective chord of highly elongated cells (Carmeliet, 2000). Although much is known about the control of shape and polarity in ECs in stable vessels, little is known about the mechanisms controlling the morphological changes that underpin the angiogenic process.
In other biological systems that involve cell elongation, the cytoskeleton plays the major part in controlling morphogenesis and polarity. Cell elongation in neurons during axonal outgrowth is accompanied by reorganisation of the microtubule cytoskeleton to form parallel tracks that align with the long axis of the axon shaft (Dent and Gertler, 2003). Similar reorganisations of microtubules accompany cell elongation in diverse biological systems, from the elongation of myotubes during skeletal muscle formation (Gundersen et al., 1989; Bugnard et al., 2005) through to the extension of the hyphal tip in fungi (Steinberg et al., 2001). In these situations, realignment of the microtubules defines the polarity of the elongating cell and directs the transport of material to the growing tip.
Recent studies have defined roles for members of the formin family in the regulation of cell elongation (Bartolini and Gundersen, 2010). Formins are regulators of both the actin and microtubule cytoskeletons. The family is defined by a conserved formin homology 2 (FH2) domain, which catalyses the nucleation of actin filaments (Pruyne et al., 2002; Sagot et al., 2002). The FH2 domain also binds to the growing (‘barbed’) end of the actin filament, preventing it from being capped and so promoting filament elongation (Zigmond et al., 2003; Harris et al., 2004). The actin filaments formed by formins are characteristically long and unbranched, and form the structural building blocks for actin stress fibres (Young et al., 2008). The actin filaments made by formins are also used to construct filopodia, which are sensory projections that enable migrating cells to sense their external environment (Mellor, 2010). In addition to regulating the actin cytoskeleton, certain formins have been shown to control microtubule alignment and stability. In migrating fibroblasts, a subset of microtubules becomes selectively stabilised and aligned towards the protrusive leading edge (Gundersen and Bulinski, 1988). This realignment of the microtubules is required for polarised migration. Initial studies showed that the formin mDia2 is localised to stable microtubules in these cells and that microtubule stabilisation is induced by its activation (Palazzo et al., 2001). Subsequent studies showed that the formins mDia1, mDia3 and inverted formin 1 (INF1) are also able to interact with microtubules and promote stabilisation (Bartolini and Gundersen, 2010). In addition to promoting microtubule stabilisation, some formins mediate the alignment of microtubules into parallel arrays. In budding yeast, the formins bud neck involved 1 (Bni1) and Bni1 related (Bnr1) produce actin cables that orientate microtubules towards the bud for polarised growth (Pruyne et al., 2004). In migrating fibroblasts, activation of mDia2 promotes the formation of a parallel array of stable microtubules that are aligned towards the protrusive front of the cell (Palazzo et al., 2001). mDia1 can also trigger the formation of parallel arrays of microtubules, and mutations in mDia1 that block this alignment of microtubules also block cell elongation, suggesting that the two processes are inherently linked (Ishizaki et al., 2001). In keeping with this, mDia1 is required for axonal elongation (Arakawa et al., 2003). Similar effects are seen in HeLa cells expressing the formins INF1 (Young et al., 2008) or formin homology 2 domain containing 1 (FHOD1) (Gasteier et al., 2005). In each case, the formin can trigger the formation of parallel arrays of microtubules in a subset of cell types and this is associated with cell elongation. The mechanisms involved in microtubule alignment by formins are still unclear. In several cases, microtubules are aligned along the actin filaments that are produced by the formins, although it is not clear whether direct interactions occur between the microtubules and actin (Bartolini and Gundersen, 2010). Some formins can interact with the tips of elongating microtubules, suggesting that selective capture at the plasma membrane also contributes to microtubule realignment (Wen et al., 2004).
The role of the microtubule cytoskeleton in mediating cell elongation in diverse systems prompted us to investigate microtubule function in angiogenesis. Here, we show that EC elongation during angiogenesis is accompanied by profound changes in the microtubule cytoskeleton. Microtubules become stabilised and aligned into a parallel cytoskeletal array directed at the growing tip. Through screening the human formin family, we show that the formin-like 3 (FMNL3; also known as FRL2) is required for microtubule reorganisation during angiogenesis and for efficient EC elongation. In keeping with this, depletion of FMNL3 in zebrafish embryos leads to profound defects in developmental angiogenesis.
Results
EC microtubules undergo realignment and stabilisation during angiogenesis
ECs participating in angiogenesis must undergo profound shape changes, converting from the highly flattened morphology of ECs in quiescent vasculature to an elongated, invasive form. Currently, little is known about how this morphological conversion is achieved. To investigate this, we used a well-established three-dimensional (3D) co-culture assay of angiogenesis to examine the architecture of the microtubule cytoskeleton as ECs underwent the morphological conversions of angiogenesis. In this assay, ECs are co-cultured with primary dermal fibroblasts. The fibroblasts secrete a complex 3D extracellular matrix (ECM), rich in collagen I, through which the ECs grow. The mature culture is three to five cells deep, with the ECs embedded in the ECM (Sorrell et al., 2007; supplementary material Fig. S1). Importantly, ECs in this assay recapitulate the key morphological changes of angiogenesis, with the ECs migrating collectively to form a branching network of chords that undergo anastomosis. As the vessels mature, they form a patent lumen and secrete a basement lamina, rich in laminin and collagen IV (Bishop et al., 1999; Donovan et al., 2001; Sorrell et al., 2007).
We compared the microtubule cytoskeleton of ECs undergoing angiogenic morphogenesis in the co-culture with that of ECs grown in monoculture. ECs grown as a quiescent monolayer displayed a radial array of microtubules focussed on a microtubule-organising centre (MTOC) located next to the nucleus (Fig. 1A). A similar distribution of microtubules has been reported in ECs in stable vasculature in vivo (McCue et al., 2006). Detyrosinated tubulin accumulates in microtubules over time and so acts as a marker of microtubule stability (Schulze et al., 1987). The microtubules in quiescent ECs were highly dynamic, as judged by the very low levels of staining for detyrosinated tubulin (glu-tubulin; Fig. 1A). In marked contrast, ECs undergoing angiogenesis stained intensely for stable microtubules (Fig. 1B). High-resolution imaging of the stable microtubules showed that they were aligned in parallel along the long axis of the elongating EC (Fig. 1C). In agreement with their heavy staining for glu-tubulin, these microtubules did not label for end-binding 1 (EB1), a protein that associates with the tips of actively growing microtubules (Vaughan, 2005) (Fig. 1C). Similar parallel alignments of stable microtubules are found in elongating neurons (Witte et al., 2008). Intriguingly, similar to neurons, the stable microtubules in angiogenic ECs terminated before the growing tip, and often looped backwards in this region (Fig. 1C). The upregulation of stable microtubules was not simply a general feature of cells in 3D culture, as the surrounding fibroblasts had only a few stable microtubules, which were restricted to the primary cilia (Fig. 1C).
Fig. 1.
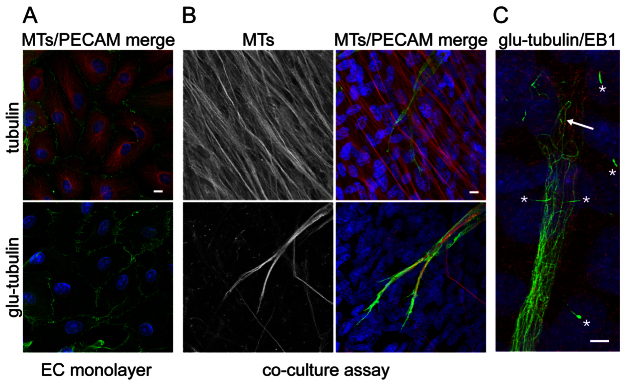
The microtubule cytoskeleton is reorganised and stabilised during angiogenesis. (A) ECs were grown in monoculture and stained for PECAM-1 (green) and either total microtubules (top panel; red) or for detyrosinated, stable microtubules (bottom panel; glu-tubulin, red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). ECs in monoculture displayed a radial array of microtubules with little or no stable microtubule staining. (B) ECs were grown in an organotypic co-culture to stimulate angiogenic morphogenesis and stained identically and in parallel to the cells in (A). The cells were imaged in parallel and under the same settings. ECs undergoing angiogenic morphogenesis showed strong staining for stable microtubules. The surrounding fibroblasts contained dense microtubules (top panels) but few stable microtubules. (C) High-magnification image of the tip of an EC tube in the organotypic assay. Stable microtubules (green) were aligned along the long axis of the body of the tube. At the growing tip, they looped backwards (arrow). Cells were also stained for EB1 (red), which marks the tips of dynamic microtubules. EB1 staining was not detected at the tips of the stable microtubules, but was apparent at the leading edge of the EC tube. The cilia of the surrounding fibroblasts can be seen clearly (asterisks). Scale bars: 10 μm. MT, microtubule.
Screening of the formin family identifies FMNL3 as a regulator of angiogenic morphogenesis
Stabilisation and alignment of microtubules is important for the polarisation of migrating cells (Gundersen and Bulinski, 1988) and also for the elongation of neurons and myotubules (Gundersen et al., 1989; Witte et al., 2008). In these cell types, the realignment of the microtubule cytoskeleton is coordinated by formins (Bartolini and Gundersen, 2010). The stabilisation and alignment of microtubules that we observed in ECs undergoing angiogenic morphogenesis suggested that formins have a role in angiogenesis. To examine this, we used the co-culture assay to screen a library of small interfering RNA (siRNA) targeting the human formins. We used a modification of the assay described by Mavria and colleagues in which ECs are transfected with siRNA before being added to the assay, which ensures that gene silencing is restricted to the ECs (Mavria et al., 2006). Representative images for each formin screened are shown in (Fig. 2). We quantified vessel formation in terms of the total length of vessels per unit area (Fig. 3A). In neurons (Arakawa et al., 2003) and migrating fibroblasts (Palazzo et al., 2001; Wen et al., 2004; Bartolini et al., 2008), the alignment and stabilisation of microtubules is regulated by two closely related formins; mDia1 and mDia2. Surprisingly, neither mDia1 nor mDia2 were required for the formation of vessels in the assay (Fig. 3A). We confirmed the efficiency of silencing of these two formins, and of the related mDia3 protein, by western blotting (supplementary material Fig. S2). Although most other formins also showed no significant effect, silencing of FMNL3 and dishevelled associated activator of morphogenesis 2 (DAAM2) severely inhibited vessel formation (Fig. 3A). We also observed a smaller but statistically significant effect of silencing FHOD1 (P<0.05) (Fig. 3A). FMNL3 and DAAM2 are uncharacterised members of the formin family and little is known of their biological function. We focussed on FMNL3 (also known as FRL2) (Harris et al., 2004) because it gave the strongest phenotype. FMNL3 was easily detected in ECs and we were able to silence expression by >90% with each of two independent siRNAs (Fig. 3B). Depletion of FMNL3 had no effect on the growth, viability or morphology of ECs grown in monoculture (data not shown); however, depletion of FMNL3 with either siRNA led to strong inhibition of vessel formation in the co-culture assay (Fig. 3E). Quantification of the assay also revealed a significant reduction in branch formation with depletion of FMNL3; however, this effect was smaller than the overall effect on vessel length (Fig. 3E). Interestingly, examination of the co-cultures showed that ECs were able to proliferate in the assay, but were unable to undergo conversion to an angiogenic morphology and grew instead mainly as islands of cells (Fig. 3C). Higher resolution confocal imaging showed some stunted EC tubes growing from the islands of ECs (Fig. 3C).
Fig. 2.

Screening for formin function in angiogenesis. ECs were treated with siRNA SmartPools for the human formins and then transferred to the co-culture angiogenesis assay. Control cells were treated with siRNA to a control gene encoding lamin. ECs were fixed and stained for PECAM-1 after 6 days. Representative images for each formin are shown. Scale bar: 500 μm.
Fig. 3.
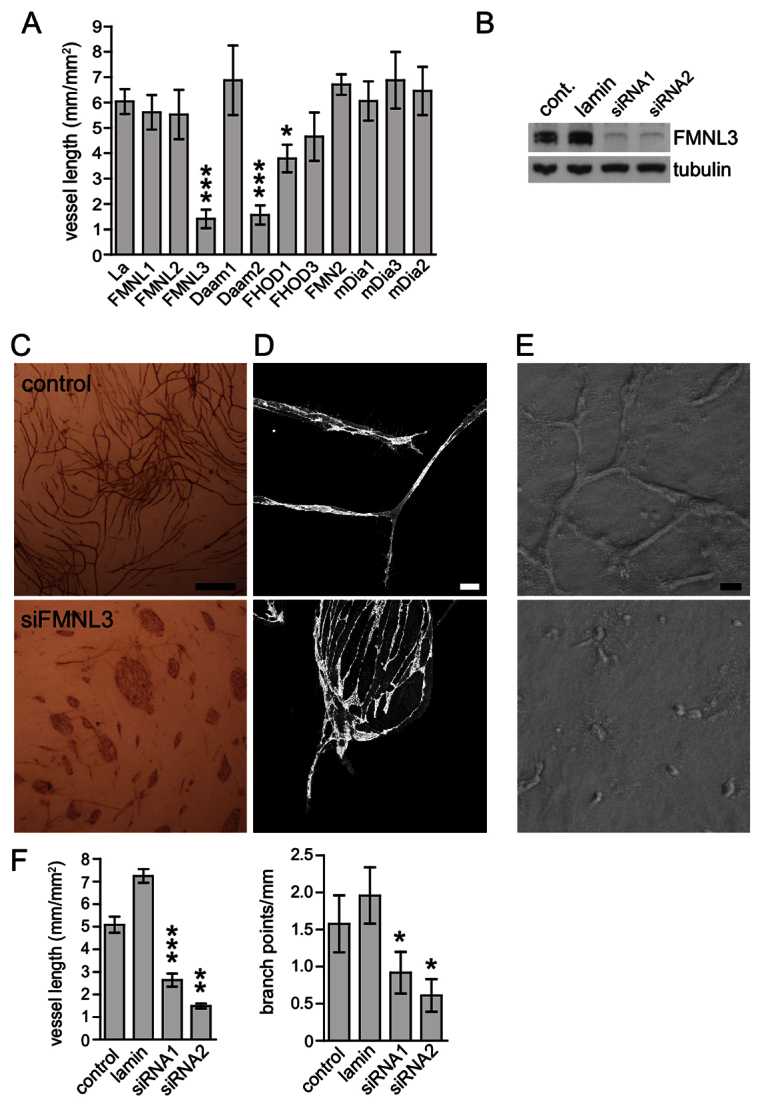
FMNL3 is required for angiogenic morphogenesis. (A) ECs were treated with siRNA SmartPools for the human formins and then transferred to the co-culture angiogenesis assay. Control cells were treated with siRNA to a control gene encoding lamin. ECs were fixed and stained after 6 days and the density of endothelial tubes was measured (i.e. total length of tubes per unit area). Data show the mean ± s.e.m. of three independent experiments, except for lamin, where n=6. The effects of formin silencing were analysed using the Student's t-test (unpaired, two-tailed). Silencing of FMNL3 and DAAM2 both led to significant inhibition of vessel formation (P<0.001). Silencing of FHOD1 gave a smaller but still significant inhibition (P<0.05). (B) Silencing of FMNL3 in ECs using two independent single siRNAs. Each siRNA gave >90% silencing of FMNL3 expression by western blotting, compared with untransfected controls, or ECs transfected with a siRNA to a control gene (encoding lamin). The requirement of FMNL3 for angiogenic morphogenesis was confirmed by using these two independent FMNL3 siRNAs. (C) Representative images of the co-cultures stained for PECAM-1. Silencing of FMNL3 inhibited the formation of EC tubes and led the cells to grow as islands. Scale bar: 1 mm. (D) Confocal imaging of the co-culture assay stained for PECAM-1 at higher magnification. ECs treated with FMNL3 siRNA produced only short tubes that emerged from islands of cells with strong cell–cell junctions. Scale bar: 10 μm. (E) Phase contrast images of in vitro angiogenesis assays in 3D collagen gels. At 48 h, the control ECs had formed a network of tubes, whereas ECs treated with FMNL3 siRNA had failed to extend. (F) Quantification of the co-culture assays with FMNL3 siRNA treatment. ECs were treated without siRNA (control), with lamin siRNA or with the two separate FMNL3 siRNAs. Graphs show the mean ± s.e.m. of three independent experiments. Data were analysed using the Student's t-test (paired, two-tailed). Treatment with either FMNL3 siRNA led to strong inhibition of vessel formation. FMNL3 silencing also led to an inhibition of vessel branching. ***P<0.001; **P<0.01; *P<0.05. Cont., control; La, lamin.
One possible explanation for the defect in EC elongation in the co-culture assay was that depletion of FMNL3 disrupted some physical interaction between the ECs and fibroblasts. To examine this, we performed a second in vitro angiogenesis assay in which ECs are cultured between two layers of collagen I gel in the presence of vascular endothelial growth factor (VEGF). In this assay, the ECs form a network of vessels with a rudimentary lumen over a period of 24–48 hours (Matsumoto et al., 2002; Bohman et al., 2005). As with the co-culture assay, silencing of FMNL3 strongly inhibited vessel formation. ECs depleted of FMNL3 showed a general lack of extension and were rounded in morphology (Fig. 3D). We conclude that the requirement for FMNL3 for EC elongation in angiogenesis does not depend on the presence of fibroblasts.
FMNL3 is highly expressed in zebrafish vasculature and is required for efficient developmental angiogenesis
To examine the role of FMNL3 in angiogenesis in a physiological context, we used the well-established zebrafish model of vascular development (Ellertsdottir et al., 2010). The first vessels to appear in the zebrafish embryo are the dorsal aorta and the posterior cardinal vein, which form by vasculogenesis. This process is morphologically distinct from angiogenesis, and involves angioblasts migrating to the midline and then coalescing. As in other vertebrates, this primitive vasculature is elaborated on by sprouting angiogenesis. At ~24 hours post-fertilisation (24 hpf), the intersomitic vessels (ISV) begin to sprout from the dorsal artery, followed closely by sprouting from the posterior cardinal vein. The ISV elongate outwards to the dorsal side of the embryo, where they establish connections with neighbouring ISV to form the dorsal longitudinal anastomosing vessel (DLAV), which closes the circulatory loop (Ellertsdottir et al., 2010). The rapid development of the vasculature in zebrafish and the ease of imaging the whole animal have made it a valuable system for studying both vasculogenesis and angiogenesis.
Database analysis revealed that zebrafish have a single orthologue of FMNL3, which shows a high degree of conservation to the human protein (72% identity). Nothing is known of the tissue distribution of FMNL3 in adult zebrafish or its expression pattern during development. We examined FMNL3 expression in zebrafish embryos by in situ hybridisation at 24 hpf, when the major vessels are present and the ISV are actively forming. Interestingly, expression of FMNL3 mRNA was almost entirely restricted to the ECs of the developing vasculature in these embryos. High expression was seen in the major head and trunk vessels, and also in the ISV (Fig. 4). We conclude that FMNL3 is an endothelial formin.
Fig. 4.
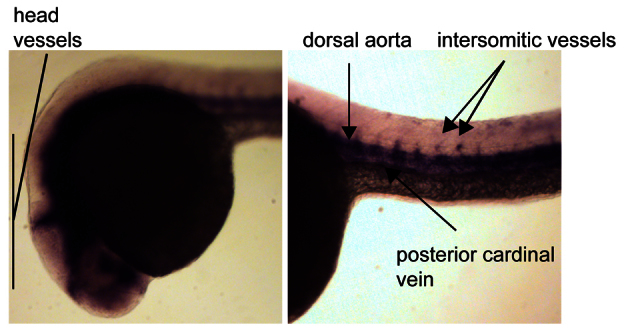
FMNL3 is highly expressed in the developing zebrafish vasculature. Zebrafish embryos were fixed at 24 hpf and FMNL3 expression was localised by in situ hybridisation with an FMNL3 mRNA probe. FMNL3 expression was generally restricted to the vasculature. No staining was seen with the corresponding negative (sense) probe (data not shown).
To examine the requirement for FMNL3 during developmental angiogenesis, we used the fli:gfp zebrafish line, which expresses GFP in ECs under the Fli promoter, enabling visualisation of the vasculature through development (Lawson and Weinstein, 2002). We silenced FMNL3 expression by injection of a specific morpholino oligonucleotide and visualised the developing vasculature by fluorescence imaging of the whole embryo. The control morphant embryos showed normal vascular development, whereas FMNL3 morphant embryos showed no obvious defect in the formation of the major trunk vessels; however, depletion of FMNL3 led to a profound defect in angiogenesis in >80% of these embryos (Fig. 5A,B,C). Although sprouting of the ISV occurred, the ECs failed to extend fully across the intersomic region and there was no apparent formation of the DLAV (Fig. 5A,B). No other obvious defect was observed in the development of the embryos. To test the specificity of the FMNL3 morpholino oligonucleotide, we attempted a rescue by co-injection into the embryos of the human FMNL3 mRNA. Despite the species distance, human FMNL3 was able to mediate a significant rescue of the developmental angiogenesis defect (Fig. 5A,D). We conclude that FMNL3 is an EC-localised formin that is required for developmental angiogenesis.
Fig. 5.
FMNL3 is required for developmental angiogenesis. (A) fli:GFP zebrafish embryos were injected with FMNL3 morpholino oligos (MO) to silence expression. Control embryos were injected with a control MO. The formation of ISV (arrows) by angiogenesis was assessed at 30 h. Panels show inverted grey-scale images of the GFP fluorescence. Silencing of FMNL3 strongly inhibited ISV formation, resulting in short and disorganised sprouts. The DLAV (arrowhead) did not form. Co-injection of human FMNL3 mRNA with the zebrafish FMNL3 MO rescued the phenotype (bottom panel). (B) Magnified section of the embryo trunk. (C,D) Quantification of the effects of FMNL3 depletion on formation of the ISV. In each case, ISV formation was scored in three independent experiments. A minimum of 30 embryos were scored for each condition in each experiment. Data are means ± s.e.m. Statistical significance was analysed using Student's t-test (unpaired, two-tailed). (C) Silencing of FMNL3 led to a defect in ISV formation in >80% of embryos (P<0.01). (D) Co-injection of human (h) FMNL3 mRNA with the zebrafish FMNL3 MO led to significant rescue of ISV formation (P<0.01). Cont., control.
FMNL3 is required for microtubule alignment during angiogenesis
The role of formins in microtubule alignment and stabilisation in other systems prompted us to examine the role of FMNL3 in microtubule reorganisation during angiogenesis. We first examined whether overexpression of FMNL3 in ECs grown in monoculture would be sufficient to trigger microtubule reorganisation and cell elongation. FMNL3 showed a plasma membrane localisation in ECs, with an unusual concentration specifically at the adhesive edge of the cell (Fig. 6A,B). The related formin FMNL1 has been shown to have an N-terminal myristoylation site that targets it to the plasma membrane (Han et al., 2009). Examination of the FMNL3 sequence revealed that FMNL3 also has a consensus site for N-terminal myristoylation, providing a potential explanation for its membrane localisation. In keeping with this, mutation of the myristoylation site led to loss of plasma membrane association (data not shown). Overexpression of full-length FMNL3 had no apparent effect on the radial distribution of microtubules in ECs grown as monolayers (Fig. 6A); neither did it affect the low level of stable microtubule staining, which was concentrated almost entirely in the centrosomes (Fig. 6B). Overexpression of full-length FMNL3 did have a profound effect on the actin cytoskeleton. ECs expressing exogenous FMNL3 lost their actin stress fibres and the cell body was filled with a fine skein of actin filaments (Fig. 6C). Similar rearrangements of the actin cytoskeleton are seen in cells expressing mDia1 (Watanabe et al., 1999). Unlike the actin filaments produced by mDia1 (Ishizaki et al., 2001) or FHOD1 (Gasteier et al., 2005), there was no apparent alignment of microtubules with the actin filaments produced by FMNL3 (data not shown).
Fig. 6.

Full-length FMNL3 regulates the EC actin cytoskeleton. (A,B) ECs were grown in monolayers on fibronectin-coated coverslips and transfected with GFP-tagged FMNL3 (green). Cell nuclei were stained with DAPI (blue). (A) Staining for total tubulin (red) showed that the total microtubule population was unaffected by FMNL3 overexpression. FMNL3 was localised to the plasma membrane and concentrated to a rim around the periphery of the cells. (B) FMNL3 overexpression did not induce stable microtubule formation, as shown by staining for glu-tubulin (red). (C) Overexpression of FMNL3 caused a profound reorganisation of the actin cytoskeleton, as shown by staining with fluorescent phalloidin (red). Actin stress fibres were lost and replaced with a fine skein of F-actin filaments. Scale bars: 10 μm.
Formins are regulated by an autoinhibitory interaction between the N terminus and the C terminus (Goode and Eck, 2007). Previous studies of the role of mDia1 in microtubule stabilisation and alignment used a constitutively active truncation mutant comprising the central FH1 and FH2 domains of mDia1. This active mutant triggered both microtubule stabilisation and alignment in cells (Ishizaki et al., 2001). We made the equivalent mutant of FMNL3 and expressed it in ECs in monoculture. Expression of activated FMNL3 had no effect on microtubule stabilisation (data not shown); however, it triggered the reorganisation of the radial EC microtubules into parallel arrays. Interestingly, the tips of these aligned microtubules contacted patches of bright FMNL3 staining at the plasma membrane (Fig. 7A). Some formins are able to bind to the tip of elongating microtubules, suggesting that selective capture at the plasma membrane contributes to microtubule alignment (Wen et al., 2004). We used total internal reflection (TIRF) microscopy of GFP–FMNL3 and mCherry–tubulin to examine the spatial relationships of full-length FMNL3 and microtubules in live ECs. Imaging by TIRF showed the plasma membrane localisation of FMNL3 clearly and revealed the presence of discrete patches of concentrated FMNL3 at the adhesive rim of the cell (Fig. 7). These patches of plasma membrane FMNL3 were frequently contacted by microtubules tips (Fig. 7; supplementary material Movie S1).
Fig. 7.

Activated FMNL3 triggers microtubule alignment. (A) ECs were transfected with an activated FMNL3 mutant comprising the FH2 and FH3 domains (green). Expression of activated FMNL3 (ΔFMNL3) led to reorganisation of the radial array of microtubules (red) into a parallel array. The tips of these microtubules contacted bright patches of FMNL3 staining at the plasma membrane. The right-hand panel shows a close-up of the area highlighted in the middle panel. Scale bar: 10 μm. (B) ECs were co-transfected with GFP–FMNL3 (full-length) and mCherry–tubulin. Cells were imaged using live cell TIRF to determine the spatial relationship between FMNL3 and microtubules. The panel shows a frame from a movie (supplementary material Movie S1). FMNL3 was tightly localised to the adhesive edge of the cell, where it was present in patches of focussed staining. Elongating microtubules frequently contacted these bright, plasma membrane patches of FMNL3 (asterisks).
As expression of activated FMNL3 led to the reorganisation of microtubules in quiescent ECs, we were interested to see whether the loss of FMNL3 had any effect on the microtubule cytoskeleton in ECs undergoing angiogenesis. To examine this, we used the co-culture assay to study the few, short EC tubes that formed when cells were treated with FMNL3 siRNA. In keeping with the effects of activated FMNL3, depletion of FMNL3 had a profound and significant effect on the ability of ECs to reorganise the microtubules into parallel arrays (Fig. 8). The EC tubes that did occur contained sparse, disorganised microtubules that frequently formed knots or tangles in the body of the vessel (Fig. 8). Similar microtubule tangles have been reported in HeLa cells expressing defective mutants of mDia1 (Bartolini et al., 2008). We conclude that FMNL3 is required for the realignment of the microtubule cytoskeleton during angiogenesis.
Fig. 8.
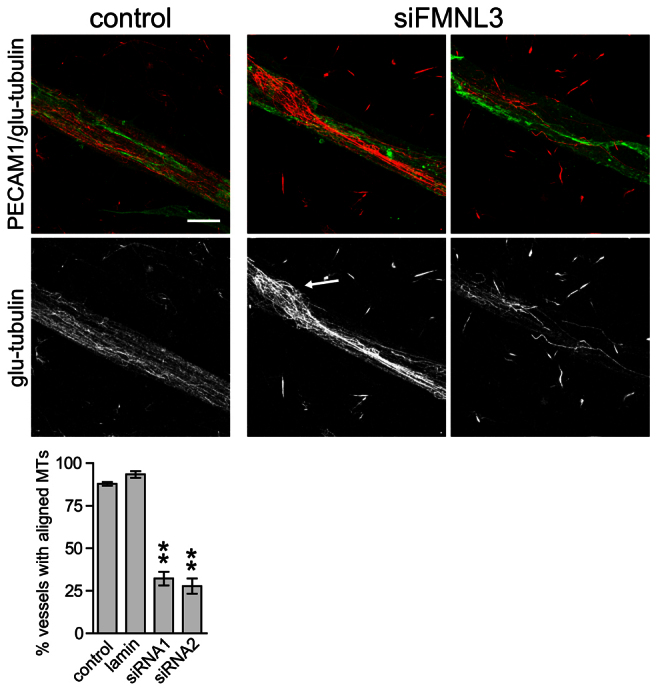
FMNL3 is required for microtubule realignment during angiogenic morphogenesis. ECs were treated with or without FMNL3 siRNA and then transferred to the co-culture angiogenesis assay. Cells were stained for PECAM-1 (green) and glu-tubulin (red). ECs in co-culture contained stable microtubules aligned along the length of the tubule (left panels). In the few tubules that formed with FMNL3-depleted ECs, the microtubules still stained for stable microtubule markers; however, alignment was lost. The stable microtubules that were present were sparse, disorganised and often seen in knotted structures (arrow). Scale bar: 10 μm. The percentage of vessels containing aligned stable microtubules was quantified from three independent experiments and the data analysed using Student's t-test (unpaired, two-tailed). Depletion of FMNL3 using either siRNA led to a significant loss of microtubule alignment (P<0.01).
Discussion
In this study, we showed that ECs undergoing angiogenic morphogenesis reorganise their microtubule cytoskeleton to form a parallel array of stable microtubules aligned with the long axis of the elongating cells. This situation is reminiscent of axonal outgrowth in neurons, where stable microtubules are aligned towards the growing tip of the cell (Conde and Caceres, 2009). Many parallels have been drawn between angiogenesis and neurogenesis (Zacchigna et al., 2008). In both cases, cells must convert to a highly elongated morphology. In neurons, microtubule stabilisation precedes initial extension of the axon (Witte et al., 2008), suggesting that early events in elongation require the microtubule cytoskeleton. Microtubules are also required for the subsequent elongation of the axon shaft and for its morphology (Conde and Caceres, 2009). The growing tip of the neuron is a flattened region called the growth cone, which acts to sense directional cues and to guide the direction and rate of extension (Dent and Gertler, 2003). Whereas the axon shaft contains a parallel array of stable microtubules, the growth cone contains highly dynamic microtubules that are required for the guidance of the growth cone towards chemotactic signals. Stable microtubules from the axon terminate just within this region and often display a bent or looped-back appearance (Conde and Caceres, 2009). Intriguingly, the tips of elongating ECs showed the same pattern: stable microtubules terminated before the tip and showed looped-back structures indistinguishable from those of active growth cones (Fig. 1C). These data suggest that the mechanisms of cell elongation in angiogenesis and axonogenesis share a similar cytoskeletal basis.
In elongating neurons, the stable microtubule array acts as a train track to direct protein trafficking to the growing tip of the cell. This is crucial for the establishment and maintenance of cell polarity (Tahirovic and Bradke, 2009). In addition, modifications of stable microtubules, including detyrosination, allow for selective recruitment of the microtubule-based motor protein kinesin-1 (Larcher et al., 1996; Liao and Gundersen, 1998). In neurons, this recruitment directs kinesin-1 to the stable axonal microtubules (Hammond et al., 2009). In turn, kinesin-1 binds to signalling proteins, including the C-Jun-amino-terminal kinase-interacting protein 1 (JIP-1) signalling scaffold, and directs them to the axon (Dajas-Bailador et al., 2008). Thus, the stable microtubule array allows for selective trafficking of cargo towards the tip of the elongating axon. At the head of the angiogenic sprout is the tip cell, a specialised EC that detects the gradient of growth factor and signals to the stalk cells behind it (Phng and Gerhardt, 2009). We know that VEGF receptor 2 is concentrated at the front edge of the tip cell during angiogenesis in vivo and that this polarised distribution of the receptor is required for effective gradient sensing (Gerhardt et al., 2003). Recent studies have shown that VEGF receptor 3 is localised in the same fashion (Nilsson et al., 2010). It is tempting to speculate that realignment of the microtubule cytoskeleton functions to allow delivery of these receptors to the leading edge of the tip cell through mechanisms of polarised traffic.
The formin family of proteins has widespread roles in the alignment and stabilisation of microtubules in elongating cells (Bartolini and Gundersen, 2010). In neurons, this function is dependent on mDia1, which is also required for axonal elongation (Arakawa et al., 2003). Here, we find no requirement for mDia1, 2 or 3 for the elongation of ECs during angiogenesis, but instead find that the uncharacterised formin FMNL3 is crucial for this process. Depletion of FMNL3 leads to a loss of microtubule alignment in ECs undergoing angiogenesis and overexpression of a constitutively active mutant of FMNL3 triggers microtubule alignment in quiescent ECs. Interestingly, although full-length FMNL3 triggers rearrangements of the actin cytoskeleton in quiescent ECs, it does not alter microtubule alignment. This suggests that upstream signals are required for this function. Similar to many other formins, FMNL3 contains a conserved binding site for Rho GTPases, which can mediate the activation and localisation of formins in cells (Baarlink et al., 2010). It seems probable that Rho GTPases have an important role in controlling FMNL3 activity during angiogenesis and it will be important to uncover the angiogenic signalling pathways controlling FMNL3 function in this process. It will also be important to pursue the roles of DAAM2 and FHOD1, two formins that are additional hits in our screen of angiogenic morphogenesis. One possibility is that these formins control the stabilisation of EC microtubules, a function that is independent of FMNL3.
FMNL3 is dispensable for vasculogenesis during zebrafish development, but is required for angiogenesis. This is consistent with a role for FMNL3 in EC elongation, as the processes of vasculogenesis are morphologically distinct to those of angiogenesis and do not require the same polarised elongations (Carmeliet, 2000). Interestingly, although FMNL3 is highly conserved between humans and fish (72% identity), it is absent from Drosophila, which do not carry out angiogenesis and have a single, distantly related FMNL-like protein (Liu et al., 2010). Ciona, which sit at the bottom of the chordate clade, also lack the gene encoding FMNL3 (data not shown). It would seem that the duplication of the ancestral gene encoding FMNL that gave rise to FMNL3 occurred in the same bracket of evolutionary time as the development of angiogenesis. In zebrafish, FMNL3 expression is almost entirely restricted to the endothelium during development, and embryos depleted of FMNL3 show no obvious secondary defects. We conclude that FMNL3 is a specialised formin with a role that is focussed on angiogenesis.
Materials and Methods
Materials
Monoclonal mouse anti-α-tubulin (clone B-5-1-2) was from Sigma and rabbit polyclonal anti-glu-tubulin was from Chemicon. Monoclonal mouse (clone 9G11) and polyclonal sheep antibodies to platelet EC adhesion molecule 1 (PECAM-1) were from R&D Systems. Monoclonal mouse anti-myc-epitope (9E10) was from Santa Cruz Biotechnology and rabbit polyclonal antibodies to collagen I and to mDia1 were from Abcam. Rabbit polyclonal antibodies to mDia2 and mDia3 were from Protein Tech Group and Bethyl Laboratories, respectively. Rabbit anti-FMNL3 was a gift from John Copeland. Mouse monoclonal anti-EB1 was from BD Transduction Laboratories. Alexa Fluor-conjugated fluorescent secondary antibodies were purchased from Invitrogen, as was Alexa-Fluor-594–phalloidin. Horseradish-peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch.
Plasmids
Human full-length FMNL3 was cloned into the expression vector pEGFP–N3 (Clontech) to make a C-terminal fusion with GFP. The activated FMNL3 mutant comprised the FH1 and FH2 domains (amino acids 484–964) and was cloned into the same vector. The mCherry–tubulin expression vector was a generous gift from Roger Tsien (Shaner et al., 2004).
Cell culture
Human umbilical vein ECs (HUVEC) were collected from umbilical cords as described previously (Van Hinsbergh and Draijer, 1996). ECs were maintained in Dulbecco's modified Eagle's medium/Ham's F12 Nutrient mixture (DMEM/F12) containing 2% heat-inactivated fetal calf serum (Sigma), 1 μg/ml hydrocortisone, 5ng/ml epidermal growth factor (R&D Systems), 10 ng/ml recombinant basic fibroblast growth factor (R&D Systems), 20 μg/ml heparin sulphate (Sigma), 250 ng/ml insulin, 100 U/ml penicillin and 100 μg/ml streptomycin. Normal human dermal fibroblasts (NHDF, PromoCell) were maintained in DMEM with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 292 μg/ml L-glutamine.
siRNA oligonucleotides and cell transfection
A SmartPool siRNA oligonucleotide library for the human formin family was designed and synthesised by Dharmacon (Thermo Fisher Scientific). Additional siRNAs were synthesised by Eurofins. Sequences of all oligonucleotides used are detailed in supplementary material Table S1. HUVEC were transfected with siRNA oligonucleotides and/or expression vectors using GeneFECTOR (VennNova), according to the manufacturer's instructions.
In vitro angiogenesis assays
A modified version of the co-culture assay of Bishop et al. (Bishop et al., 1999; Mavria et al., 2006) was used to study angiogenesis in vitro. NHDF were seeded onto glass coverslips at 3×104 cells/ml in endothelial growth media (EGM, Lonza) and grown until confluent over 5 days. On day 4, ECs were seeded at 4×104 cells/ml in fibronectin-coated six-well plates and incubated overnight. On day 5, ECs were transfected with siRNA, as described above. After incubation for 3 hours, ECs were harvested and seeded onto the confluent NHDF at 3×104 cells/ml in EGM. Medium was refreshed every 2 days. For quantification of vessel formation, cells were fixed on day 11 in 70% ethanol at −20°C for 30 minutes. Cells were treated with 0.3% hydrogen peroxide in methanol for 15 minutes to remove endogenous alkaline phosphatase activity. ECs were labelled with mouse anti-PECAM-1 antibody in 1% BSA and incubated for 1 hour at 37°C. The labelled endothelial tubes were stained using an alkaline phosphatase-conjugated secondary antibody and BCIP/NBT substrate (Sigma). In addition to this co-culture angiogenesis assay, some experiments used an in vitro assay of angiogenesis in a collagen gel, as described by Bohman et al. (Bohman et al., 2005). Briefly, serum-starved ECs were seeded at 8×105 cells/ml onto a 0.23% (w/v) collagen I gel (PureCol™, Inamed Biomaterials, The Netherlands). After 2 hours, the cells were overlaid with a second layer of collagen and then the collagen gel was overlaid with complete growth medium and supplemented with 50 ng/ml VEGF. The 3D culture was maintained for up to 48 hours before being fixed in paraformaldehyde and then processed for immunofluorescence microscopy. For siRNA experiments, ECs were transfected with the relevant siRNA 24 hours before plating on collagen.
Immunofluorescence microscopy
Cells were prepared for confocal immunofluorescence microscopy by fixation in paraformaldehyde, except for imaging of microtubules, where the cells were fixed in methanol. Confocal microscopy was performed using a Leica AOBS SP2 confocal laser-scanning microscope with an attached Leica DMIRE2 inverted microscope. Confocal sections were taken across the z-plane and processed to form a 2D projection representing the full depth of the cell culture.
TIRF microscopy
ECs were co-transfected with GFP-tagged FMNL3 and mCherry-tagged tubulin. Live cell imaging of the relationship between FMNL3 localisation and microtubules was performed using a Leica AM TIRF MC system set at a 70-nm penetration depth with a 100× NA 1.47 lens. Images were acquired at two frames per second using a Hamamatsu C9100 EM-CCD camera. Image stacks were converted into movies using ImageJ (National Institutes of Health, Bethesda).
Zebrafish angiogenesis
Zebrafish FMNL3 cDNA was amplified from embryo cDNA using PCR. Digoxigenin-labelled probes for in situ hybridisation were generated by in vitro transcription using an RNA labelling kit (Roche). Antisense probe was generated by linearising the plasmid with BamH1 and transcribing with T7. In situ hybridisation was carried out following previously described methods (Thisse and Thisse, 2008). To silence FMNL3 expression, a specific morpholino antisense oligonucleotide was synthesised by Gene Tools LLC, USA. A standard negative morpholino control was used for comparison. The sequence of each oligo is given in supplementary material Table S1. Morpholino antisense oligonucleotides were microinjected into single-cell zebrafish embryos. For rescue experiments, human FMNL3 mRNA was synthesised by linearising a hFMNL3/pCS2+ plasmid and transcribing with SP6 polymerase using the mMessage mMachine Kit (Ambion). This human FMNL3 mRNA was co-injected with the morpholino oligonucleotides, where indicated.
Footnotes
Funding
This work was supported by a British Heart Foundation project grant to H.M. and by a Cancer Research UK programme grant to R.B.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.091066/-/DC1
References
- Arakawa Y., Bito H., Furuyashiki T., Tsuji T., Takemoto-Kimura S., Kimura K., Nozaki K., Hashimoto N., Narumiya S. (2003). Control of axon elongation via an SDF–1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 161, 381-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarlink C., Brandt D., Grosse R. (2010). SnapShot: Formins. Cell 142, 172-172 e1 [DOI] [PubMed] [Google Scholar]
- Bartolini F., Gundersen G. G. (2010). Formins and microtubules. Biochim. Biophys. Acta 1803, 164-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F., Moseley J. B., Schmoranzer J., Cassimeris L., Goode B. L., Gundersen G. G. (2008). The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 181, 523-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop E. T., Bell G. T., Bloor S., Broom I. J., Hendry N. F., Wheatley D. N. (1999). An in vitro model of angiogenesis: basic features. Angiogenesis 3, 335-344 [DOI] [PubMed] [Google Scholar]
- Bohman S., Matsumoto T., Suh K., Dimberg A., Jakobsson L., Yuspa S., Claesson-Welsh L. (2005). Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J. Biol. Chem. 280, 42397-42404 [DOI] [PubMed] [Google Scholar]
- Bugnard E., Zaal K. J., Ralston E. (2005). Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskeleton 60, 1-13 [DOI] [PubMed] [Google Scholar]
- Carmeliet P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389-395 [DOI] [PubMed] [Google Scholar]
- Conde C., Caceres A. (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319-332 [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F., Jones E. V., Whitmarsh A. J. (2008). The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr. Biol. 18, 221-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent E. W., Gertler F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209-227 [DOI] [PubMed] [Google Scholar]
- Donovan D., Brown N. J., Bishop E. T., Lewis C. E. (2001). Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis 4, 113-121 [DOI] [PubMed] [Google Scholar]
- Ellertsdottir E., Lenard A., Blum Y., Krudewig A., Herwig L., Affolter M., Belting H. G. (2010). Vascular morphogenesis in the zebrafish embryo. Dev. Biol. 341, 56-65 [DOI] [PubMed] [Google Scholar]
- Gasteier J. E., Schroeder S., Muranyi W., Madrid R., Benichou S., Fackler O. T. (2005). FHOD1 coordinates actin filament and microtubule alignment to mediate cell elongation. Exp. Cell Res. 306, 192-202 [DOI] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode B. L., Eck M. J. (2007). Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 76, 593-627 [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. (1988). Selective stabilization of microtubules oriented toward the direction of cell migration. Proc. Natl. Acad. Sci. USA 85, 5946-5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. (1989). Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 109, 2275-2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. W., Huang C. F., Kaech S., Jacobson C., Banker G., Verhey K. J. (2009). Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Eppinger E., Schuster I. G., Weigand L. U., Liang X., Kremmer E., Peschel C., Krackhardt A. M. (2009). Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J. Biol. Chem. 284, 33409-33417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. S., Li F., Higgs H. N. (2004). The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 279, 20076-20087 [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Morishima Y., Okamoto M., Furuyashiki T., Kato T., Narumiya S. (2001). Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3, 8-14 [DOI] [PubMed] [Google Scholar]
- Larcher J. C., Boucher D., Lazereg S., Gros F., Denoulet P. (1996). Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site. Regulation by polyglutamylation. J. Biol. Chem. 271, 22117-22124 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307-318 [DOI] [PubMed] [Google Scholar]
- Liao G., Gundersen G. G. (1998). Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 273, 9797-9803 [DOI] [PubMed] [Google Scholar]
- Liu R., Linardopoulou E. V., Osborn G. E., Parkhurst S. M. (2010). Formins in development: orchestrating body plan origami. Biochim. Biophys. Acta 1803, 207-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Turesson I., Book M., Gerwins P., Claesson-Welsh L. (2002). p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J. Cell Biol. 156, 149-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavria G., Vercoulen Y., Yeo M., Paterson H., Karasarides M., Marais R., Bird D., Marshall C. J. (2006). ERK–MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 9, 33-44 [DOI] [PubMed] [Google Scholar]
- McCue S., Dajnowiec D., Xu F., Zhang M., Jackson M. R., Langille B. L. (2006). Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ. Res. 98, 939-946 [DOI] [PubMed] [Google Scholar]
- Mellor H. (2010). The role of formins in filopodia formation. Biochim. Biophys. Acta 1803, 191-200 [DOI] [PubMed] [Google Scholar]
- Nilsson I., Bahram F., Li X., Gualandi L., Koch S., Jarvius M., Soderberg O., Anisimov A., Kholova I., Pytowski B., et al. (2010). VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 29, 1377-1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A. F., Cook T. A., Alberts A. S., Gundersen G. G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723-729 [DOI] [PubMed] [Google Scholar]
- Phng L. K., Gerhardt H. (2009). Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196-208 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. (2002). Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612-615 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Legesse-Miller A., Gao L., Dong Y., Bretscher A. (2004). Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 20, 559-591 [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Boden P., Kalnins V. I., Gotlieb A. I. (1986). The distribution of centrosomes in endothelial cells of non-wounded and wounded aortic organ cultures. Cell Tissue Res. 243, 223-227 [DOI] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D. (2002). Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42-50 [DOI] [PubMed] [Google Scholar]
- Schulze E., Asai D. J., Bulinski J. C., Kirschner M. (1987). Posttranslational modification and microtubule stability. J. Cell Biol. 105, 2167-2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567-1572 [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Baber M. A., Caplan A. I. (2007). A self-assembled fibroblast–endothelial cell co-culture system that supports in vitro vasculogenesis by both human umbilical vein endothelial cells and human dermal microvascular endothelial cells. Cells Tissues Organs 186, 157-168 [DOI] [PubMed] [Google Scholar]
- Steinberg G., Wedlich-Soldner R., Brill M., Schulz I. (2001). Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J. Cell Sci. 114, 609-622 [DOI] [PubMed] [Google Scholar]
- Tahirovic S., Bradke F. (2009). Neuronal polarity. Cold Spring Harb. Perspect. Biol. 1, a001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Thisse B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59-69 [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W. M., Draijer R. (1996). Culture and characterization of human endothelial cells. In Epithelial Cell Culture: A Practical Approach (ed. Shaw A. J.). Oxford: Oxford University Press; [Google Scholar]
- Vaughan K. T. (2005). TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J. Cell Biol. 171, 197-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Kato T., Fujita A., Ishizaki T., Narumiya S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136-143 [DOI] [PubMed] [Google Scholar]
- Wen Y., Eng C. H., Schmoranzer J., Cabrera-Poch N., Morris E. J., Chen M., Wallar B. J., Alberts A. S., Gundersen G. G. (2004). EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820-830 [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. (2008). Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. G., Thurston S. F., Copeland S., Smallwood C., Copeland J. W. (2008). INF1 is a novel microtubule-associated formin. Mol. Biol. Cell 19, 5168-5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S., Ruiz de Almodovar C., Carmeliet P. (2008). Similarities between angiogenesis and neural development: what small animal models can tell us. Curr. Top. Dev. Biol. 80, 1-55 [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., Pring M. (2003). Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13, 1820-1823 [DOI] [PubMed] [Google Scholar]



