Abstract
To better understand the role of transcription factor NF-E2-related factor (NRF) 2 in the human and its contribution to cancer chemoprevention, we have knocked down its negative regulators, Kelch-like ECH-associated protein 1 (KEAP1) and broad-complex, tramtrack and bric à brac and cap'n'collar homology 1 (BACH1), in HaCaT keratinocytes. Whole-genome microarray revealed that knockdown of KEAP1 resulted in 23 messenger RNAs (mRNAs) being up-regulated ≥2.0-fold. mRNA for aldo-keto reductase (AKR) 1B10, AKR1C1, AKR1C2 and AKR1C3 were induced to the greatest extent, showing increases of between 12- and 16-fold, whereas mRNA for glutamate-cysteine ligase catalytic and modifier subunits, NAD(P)H:quinone oxidoreductase-1 and haem oxygenase-1 (HMOX1) were induced between 2.0- and 4.8-fold. Knockdown of BACH1 increased HMOX1 135-fold but induced the other genes examined to a maximum of only 2.7-fold. Activation of NRF2, by KEAP1 knockdown, caused a 75% increase in the amount of glutathione in HaCaT cells and a 1.4- to 1.6-fold increase in their resistance to the electrophiles acrolein, chlorambucil and cumene hydroperoxide (CuOOH), as well as the redox-cycling agent menadione. Inhibition of glutathione synthesis during KEAP1 knockdown, by treatment with buthionine sulfoximine, abrogated resistance to acrolein, chlorambucil and CuOOH, but not to menadione. In contrast, knockdown of BACH1 did not increase glutathione levels or resistance to xenobiotics. Knockdown of NRF2 in HaCaT cells decreased glutathione to ∼80% of normal homeostatic levels and similarly reduced their tolerance of electrophiles. Thus, the KEAP1–NRF2 pathway determines resistance to electrophiles and redox-cycling compounds in human keratinocytes through glutathione-dependent and glutathione-independent mechanisms. This study also shows that AKR1B10, AKR1C1 and AKR1C2 proteins have potential utility as biomarkers for NRF2 activation in the human.
Introduction
The ability to adapt to environmental stress and perturbations in normal metabolic processes is a fundamentally important characteristic of all living cells. In mammals, many antioxidant proteins, such as the glutamate-cysteine ligase catalytic (GCLC) and glutamate-cysteine ligase modifier (GCLM) subunits, thioredoxin reductase (TXNRD1), sulfiredoxin (SRXN1), the ferritin heavy (FTH1) and ferritin light (FTL) chains and haem oxygenase-1 (HMOX1), along with drug-metabolizing enzymes such as aldo-keto reductase (AKR), NAD(P)H:quinone oxidoreductase-1 (NQO1) and glutathione S-transferase (GST), are co-ordinately induced in response to redox and electrophile stressors through the presence of antioxidant response elements (AREs, 5′-A/GTGAC/GNNNGCA/G-3′) in their gene promoters (1–5). A principal regulator of ARE-driven gene expression is NF-E2-related factor (NRF) 2, a cap'n'collar (CNC) basic-region leucine zipper (bZIP) transcription factor. Under normal homeostatic conditions, NRF2 is a short-lived protein that is readily ubiquitylated by CUL3-RBX1 and degraded by the 26S proteasome (6–9). The ubiquitylation of NRF2 depends on Kelch-like ECH-associated protein 1 (KEAP1) that functions as a substrate adaptor for CUL3-RBX1 (8,9). KEAP1 is a dimeric protein, comprising a broad complex, tramtrack and bric à brac (BTB) domain, an intervening region (or linker region) and a Kelch-repeat domain (7,8,10). It is considered to be the major negative regulator of NRF2 under non-stressful conditions (6). Upon redox and electrophile stress, KEAP1 is inactivated, thus allowing NRF2 to evade ubiquitylation by CUL3-RBX1 and accumulate in the nucleus, where it is recruited to gene promoters as a heterodimer with small musculoaponeurotic fibrosarcoma (MAF) proteins and transactivates ARE-driven genes (3,10).
Cancer chemopreventive agents inhibit carcinogenesis because they up-regulate the ARE gene battery (1–4). At least nine different classes of chemical have been reported to induce ARE-driven gene expression (11). They all share the property of either being thiol reactive themselves or they generate such metabolites following biotransformation within the cell. It is thought that these agents activate NRF2 because they modify Cys residues in KEAP1, thereby preventing it from serving as an ubiquitin ligase substrate adaptor (12–14). While it is clear that NRF2 can be activated, and cytoprotection conferred, by inhibition of KEAP1 (15,16), the contribution of other mechanisms to the up-regulation of the ARE gene battery and chemoprevention is less certain.
Multiple copies of ARE-like sequences are present in the gene promoters of mouse and human HMOX1, where they have been variously called haem response elements or stress response elements or MAF recognition elements (17,18). Compelling data have been presented that mouse Hmox1 is repressed by broad-complex, tramtrack and bric à brac and cap'n'collar homology 1 (Bach1) protein, a DNA-binding protein that lacks a transactivation domain, presumably because it prevents NRF2 from binding to the multiple ARE-like sequences in its upstream regulatory region (19,20). Similarly, the human HMOX1 gene is repressed by BACH1 (21). Under normal physiological circumstances, inhibition of Hmox1 expression by Bach1 is overcome by haem and involves loss of DNA binding by the BTB-CNC transcriptional repressor and its export from the nucleus (19,22). As HMOX1 is a member of the ARE gene battery, these observations raise the question of whether other ARE-driven genes are repressed by BACH1. For example, it has been proposed that the expression of FTH1, FTL, NQO1 and TXNRD1 might also be negatively regulated by BACH1 (23,24). If true, inactivation of the BTB-CNC repressor protein may represent another route by which the ARE gene battery could be up-regulated and a chemopreventive response elicited. At present it is, however, unclear whether BACH1 negatively regulates all ARE-driven genes or only certain members of the battery.
To date, the battery of genes regulated by NRF2 in the human has not been well characterized. Such information is urgently required to help design clinical chemopreventive intervention trials that incorporate biomarkers for NRF2 activation. Using a candidate approach, luciferase reporter assays have revealed that the gene promoters of human AKR1C2, GCLC, GCLM and NQO1 contain functional AREs (25–28). Essentially, all attempts to identify human ARE-driven genes have employed inducing agents such as sulforaphane (SFN), tert-butyl hydroquinone or β-naphthoflavone, and these have indicated that certain AKR isoenzymes are particularly inducible (29–34). In man, this family comprises the enzymes AKR1A1, AKR1B1, AKR1B10, AKR1C1, AKR1C2, AKR1C3, AKR1C4, AKR1D1, AKR7A2 and AKR7A3, as well as the potassium voltage-gated β-subunits AKR6A3, AKR6A5 and AKR6A9 that seem to lack obvious catalytic activity (35). It is not clear, however, how many human AKR genes are regulated by NRF2.
The functional consequences of activation of human NRF2 need to be documented more precisely than has hitherto been achieved using inducing agents because these xenobiotics are unlikely to be specific for the CNC-bZIP factor alone. To help define the ARE gene battery in man, we have knocked down KEAP1 and performed a whole-genome microarray analysis to identify NRF2 target genes. Furthermore, to test the hypothesis that BACH1 also regulates ARE-driven genes, we have also knocked down this BTB-CNC transcriptional repressor protein and measured its effect on expression of 15 of the detoxication and antioxidant genes that were substantially induced by KEAP1 knockdown. As a model system, we have employed the spontaneously immortalized human HaCaT keratinocytes because they are non-tumorigenic, have a stable chromosome content but possess TP53 harbouring a C→T mutation in codon 179 and a CC→TT mutation in codons 281 and 282 (36,37). Using HaCaT cells, we have examined the contribution made by NRF2 to drug resistance and the involvement of glutathione in this process. Our data reveal that activation of NRF2 by antagonism of KEAP1, rather than by antagonism of BACH1, provides protection against the electrophilic compounds acrolein, chlorambucil and cumene hydroperoxide (CuOOH) as well as the redox-cycling chemical menadione. We have also shown that resistance against the electrophiles is dependent on glutathione, whereas resistance against the redox-cycling agent is not dependent on the antioxidant tri-peptide.
Materials and methods
Chemicals
These were of the highest quality that was readily commercially available. The short inhibitory RNA (siRNA) employed against KEAP1 was Target#1 described by Devling et al. (33). The siRNA against NRF2 was purchased from QIAGEN (product no. 5100657944; Crawley, UK) and that against BACH1 was obtained from Ambion, Austin, TX (AM16810).
Cell culture
The human keratinocyte HaCaT cell line was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mM L-glutamine (Invitrogen, Paisley, UK). Transfection of HaCaT cells with siRNA was carried out when they were 30–40% confluent using Lipofectamine 2000 (Invitrogen). To induce ARE-driven gene expression, the keratinocytes were treated with 5 μM SFN (in 0.1% dimethyl sulfoxide) for 24 h prior to analysis. To inhibit glutathione synthesis, HaCaT cells were treated with 50 μM buthionine sulfoximine (BSO) for 4 h before they were analysed.
Microarray analysis
The microarray experiments involved two colour hybridizations of biological replicates of labelled RNA from HaCaT cells transfected with either KEAP1 siRNA or scrambled (Scrm) siRNA against RNA isolated from HaCaT cells transfected with lipofectamine alone (i.e. control): to this end, the fibroblasts were transfected with 100 nmol/l of Target#1 siRNA against KEAP1 or were transfected with 100 nmol/l of a Scrm siRNA or were mock transfected as described previously (33). Forty-eight hours after transfection, total RNA was isolated from the HaCaT cells and labelled with either cyanine 3 (Cy3)-cytidine triphosphate (CTP) or cyanine 5 (Cy5)-CTP fluorescent dyes. Labelled RNA was hybridized to separate arrays in competition with RNA from mock-transfected cells. The hybridization reactions were performed in duplicate, with additional dye-swap replicates, giving a total of four arrays per treatment. The analyses were performed using the Whole Human Genome Array from Agilent Technologies, which comprised a set of 44 000 60mer oligonucleotide probes. Genes that were significantly (P < 0.01) altered in their expression were selected by Agilent feature extraction (v7.1) software using an Agilent error model (Agilent Feature Extraction User Manual G2566-90012). Rosetta Luminator software (Rosetta Biosoftware, Kirkland, WA) was used to generate signature lists of significantly (P < 0.01) regulated genes derived from replicate microarray hybridizations by calculating an error weighted mean of the data from the four replicate arrays (38).
Measurement of mRNA levels
HaCaT cells were transfected with siRNA at a final concentration of either 100 nmol/l (in the microarray experiments) or 50 nmol/l (in all other instances). Total cellular RNA was extracted using the RNeasy Mini Kit (QIAGEN), with on-column DNase treatment. The relative levels of messenger RNA (mRNA) species were determined by TaqMan® reverse transcription–polymerase chain reaction (RT–PCR) using 18S RNA as an internal standard. The primer and probe sets used to measure human KEAP1, GCLM and NQO1 have been described elsewhere (33). The primer and probe sets for AKR1C1 and AKR1C2 are shown in supplementary Table 1 (available at Carcinogenesis Online). All TaqMan assays were performed in triplicate on three separate occasions. Data were normalized and then combined and mean values ± SEM are presented.
Biochemical assays
Protein concentration was measured using the Coomassie dye-binding method. Total glutathione was measured by the method of Tietze (39).
Western blotting
Equal portions (typically 10 μg protein) of 10 000g supernatants prepared from whole-cell lysates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis using 10% polyacrylamide resolving gels. These were immunoblotted onto Immobilon-P membranes (40). When available, a recombinant protein was run in lane 1 as a positive control. Equal sample loading was confirmed by probing for GAPDH or actin. Polyclonal rabbit antisera raised against human AKR1A1, AKR1B1, AKR1C1 and AKR1C4 have been reported previously (41). Antiserum against human AKR1B10 was raised during the present study in New Zealand White female rabbits against a purified full-length N-terminally His-tagged recombinant reductase that had been expressed from a pET-15b (Clontech, Mountain View, CA) plasmid in Escherichia coli BL21 (DE) pLysS cells (Novagen, Merck, Darmstadt, Germany). A mouse monoclonal antibody against human AKR1C3 was also employed that has been characterized previously (42). The antisera used against rat NQO1, human GCLC and human GCLM have been reported earlier (33,40). Antiserum against SRXN1 was kindly provided by Dr Lesley I.McLellan (University of Dundee). Antibodies against HMOX1 were from BioVision Research Products (Mountain View, CA). Each western blot is representative of at least three independent experiments. The levels of HMOX1 protein were quantified by chemiluminescence on a LAS-3000 mini Imager (Fujifilm), and the data were analysed using AIDA software (Raytest).
Determination of cell viability
Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (43). Briefly, 96-well plates were seeded with 1 × 104 HaCaT cells per well and they were allowed to adhere for at least 24 h before transfection with siRNA for 48 h or pre-treatment (‘priming’) for 24 h with SFN or pre-treatment for 4 h with the glutamate-cysteine ligase inhibitor BSO. Immediately thereafter, the keratinocytes were placed in media with reduced serum (0.1%) and exposed to a range of concentrations of acrolein, chlorambucil, CuOOH or menadione for 24 h. Following challenge with these xenobiotics, 25 μl of MTT (5 mg/ml in phosphate-buffered saline) was added to each well of the microtitre plate and the HaCaT cells were incubated at 37°C for 90 min. Media was removed by inversion of the plate and was replaced with 200 μl of MTT lysis buffer [20% (wt/vol) sodium dodecyl sulphate dissolved at 37°C in aqueous 50% N,N-dimethylformamide, pH 4.7]. The plates were further incubated for 2–3 h at 37°C prior to measuring OD570 using a Benchmark microplate reader (Bio-Rad, Hemel Hempstead, UK). The OD570 value was used as a measure of viability, and the concentration of xenobiotic that reduced the OD570 to 50% of that observed from cells treated with vehicle (i.e. dimethyl sulfoxide alone) is referred to as the lethal dose 50 (LD50) value (i.e. a dose of xenobiotic that kills 50% of cells) (43). Reactions were performed in triplicate on three separate occasions. Data were normalized and then combined and mean values ± SEM are presented.
Data analysis
This was performed using the Prism 4 software package (Graphpad Software, La Jolla, CA). Statistical significance of the fold-induction values for TaqMan® RT–PCR, and of differences in intracellular glutathione levels, were calculated using the unpaired t-test. The MTT survival data were analysed using non-linear regression to determine mean LD50 values and 95% confidence intervals, and the statistical significance of these values were tested using two-way analysis of variance. The statistical evaluation of data is indicated as follows: P > 0.05, not significant (ns); P = 0.05–0.01, * or §; P = 0.01–0.001, ** or §§; P < 0.001, *** or §§§ (with an asterisk indicating a significant increase and the symbol § indicating a significant decrease).
Results
Identification of genes in HaCaT cells that are negatively regulated by KEAP1
To identify NRF2 target genes in HaCaT cells, we first knocked down its negative regulator, KEAP1. Previously, we reported that transfection of the keratinocytes with 100 nmol/l of Target#1 siRNA reduced the level of mRNA for KEAP1 to ∼30% of its level in mock-transfected cells (33). Using the Agilent Whole Human Genome Array, we found that knockdown of KEAP1 with 100 nmol/l Target#1 siRNA for 48 h increased the expression of 671 mRNA species >1.3-fold in HaCaT cells and that during the microarray experiment, KEAP1 mRNA was reduced to 40% of the level in mock-transfected cells. The gene signature list has been deposited with the National Center for Biotechnology Information (accession number GSE17011). Among the induced mRNAs, 95 were up-regulated >1.5-fold, 43 were increased >1.7-fold, and 23 were up-regulated ≥2.0-fold. The most inducible transcripts included those for enzymes involved in the oxidation and reduction of xenobiotics, the synthesis of glutathione (GSH), the generation of NADPH, the reduction of thiols, the binding of iron and the catabolism of haem.
Of the 23 mRNAs that were identified by Agilent Whole Human Genome Array to be up-regulated ≥2.0-fold following KEAP1 knockdown, 12 are listed in Table I. The Agilent software calculated the increases in these transcripts, in decreasing order, as follows: AKR1C3 (NM_003739), 7.27-fold; AKR1B10 (NM_020299), 7.27-fold; AKR1C1 (NM_001353), 6.48-fold; HMOX1 (NM_002133), 3.34-fold; SRXN1 (NM_080725), 2.91-fold; FTL (NM_000146), 2.48-fold; GCLC (NM_001498), 2.28-fold; AKR1B1 (NM_001628), 2.27-fold; NQO1 (NM_000903), 2.23-fold; GCLM (NM_002061), 2.09-fold; phosphogluconate dehydrogenase (NM_002631), 2.05-fold and TXNRD1 (NM_003330), 2.0-fold. It should be noted that two of these mRNAs appeared twice in the gene signature list, AKR1C1 appeared as NM_001353 and BC040210, and GCLC appeared as NM_001498 and M90656; neither of these were included twice in our final count of inducible genes. In addition, increases in the following eight transcripts were observed upon KEAP1 knockdown: complementary DNA DKFZp686B14224 (BX640843), 2.73-fold; IMAGE clone 5756011 (BC035691), 2.44-fold; solute carrier family 7 member 11 (BC041925), 2.4-fold; complementary DNA DKFZp761K058 (AL833940), 2.24-fold; zinc finger, CysCysHisCys domain containing 2 (NM_017742), 2.16-fold; uridine diphosphate-glucose dehydrogenase (NM_003359), 2.11-fold; desmuslin, transcript variant A (NM_145728), 2.11-fold; cofilin 2, transcript variant 1 (NM_021914), 2.03-fold. Lastly, the following three unknown transcripts were also elevated by KEAP1 knockdown: A_24_P152845, 7-31-fold; A_24_P451992, 2.08-fold and ENST00000313481, 2.01-fold.
Table I.
KEAP1 knockdown up-regulates mRNA for cytoprotective proteins
| Gene function | Gene name | Accession number | Fold change |
||
| Microarray |
RT–PCR |
||||
| KEAP1 siRNA | KEAP1 siRNA | SFN | |||
| Xenobiotic metabolism | AKR1A1 | NM_006066 | — | 1.0 ± 0.1ns | 0.8 ± 0.1ns |
| AKR1B1 | NM_001628 | 2.3 | 1.5 ± 0.2* | 1.4 ± 0.2* | |
| AKR1B10 | NM_020299 | 7.3 | 13.2 ± 1.7*** | 1.7 ± 0.1*** | |
| AKR1C1 | NM_001353 | 6.5 | 12.1 ± 1.4*** | 4.8 ± 0.5*** | |
| AKR1C2 | NM_001354 | — | + | + | |
| AKR1C3 | NM_003739 | 7.3 | 12.3 ± 1.1*** | 3.1 ± 0.2*** | |
| AKR1C4 | NM_001818 | — | — | — | |
| NQO1 | NM_000903 | 2.2 | 3.9 ± 0.1*** | 3.0 ± 0.3** | |
| PTGR1 | NM_012212 | 1.8 | 1.6 ± 0.3ns | 1.5 ± 0.1ns | |
| GSH biosynthesis | GCLC | NM_001498 | 3.1 | 4.2 ± 0.6*** | 5.2 ± 1.2** |
| GCLM | NM_002061 | 2.1 | 2.0 ± 0.1** | 1.6 ± 0.2* | |
| GSR | NM_000637 | 2.2 | 1.7 ± 0.1*** | 1.3 ± 0.1** | |
| NADPH generation | G6PD | NM_000402 | 1.9 | 2.9 ± 0.2*** | 2.6 ± 0.4*** |
| ME1 | NM_002395 | 1.8 | 1.8 ± 0.1*** | 2.3 ± 0.1*** | |
| PGD | NM_002631 | 2.1 | 2.5 ± 0.2*** | 2.0 ± 0.3*** | |
| Antioxidant | HMOX1 | NM_002133 | 3.3 | 2.8 ± 0.4*** | 1.5 ± 0.1*** |
| SRXN1 | NM_080725 | 2.9 | 2.4 ± 0.1** | 1.0 ± 0.1ns | |
| TXNRD1 | NM_003330 | 2.0 | 2.6 ± 0.3*** | 2.2 ± 0.2*** | |
| Iron metabolism | FECH | NM_000140 | 2.0 | 2.0 ± 0.4* | 1.8 ± 0.2* |
| FTL | NM_000146 | 2.5 | 2.7 ± 0.1*** | 3.2 ± 0.5** | |
| FTH1 | NM_002032 | — | 1.3 ± 0.2ns | 1.1 ± 0.2ns | |
| Gene transcription | MAFG | NM_002359 | 1.6 | 2.0 ± 0.1** | 1.1 ± 0.1ns |
The HaCaT cells were transfected with 100 nM KEAP1 siRNA and the fold change in levels of transcripts, relative to mock-transfected control cells, which were arbitrarily ascribed a value of 1.0 (data not shown), were determined by Agilent microarray and by TaqMan RT–PCR. Transfection with 100 nM Scrm siRNA was included as an additional control (data not shown). For the RT–PCR analysis, SFN pre-treatment was included as a positive control. Statistical significance for KEAP1 knockdown and SFN pre-treatment were calculated relative to mock transfection and dimethyl sulfoxide pre-treatment (data not shown), respectively, and is indicated by single, double and triple asterisk symbols as *, P = 0.05–0.01; **, P = 0.01–0.001; ***, P <0.001. In the case of the AKR1C2 transcript, this was detected in cells transfected with KEAP1 siRNA or pre-treated with SFN, but not in control cells; as the fold-induction could not be calculated, the presence of mRNA is simply indicated by the symbol ‘+’. ns, Not significant.
The authenticity of microarray data for the most inducible detoxication and antioxidant genes on the signature list was checked independently using TaqMan® RT–PCR. We measured the mRNA levels of all members of the human AKR1 family because it was considered possible that the high degree of sequence identity between individual species might have lead to cross-hybridization of one mRNA with more than one oligonucleotide probe or vice versa. As a positive control, we treated HaCaT cells with 5 μM SFN for 24 h. Analysis by TaqMan® RT–PCR revealed that among the genes selected for further examination, AKR1B10, AKR1C1, AKR1C2 and AKR1C3 were up-regulated to the greatest extent following KEAP1 knockdown (Table I). The AKR mRNA species were also induced by SFN. As anticipated, RT–PCR confirmed that NQO1 was over-expressed upon KEAP1 knockdown but not to the same extent as the AKR isoenzymes. Also, RT–PCR confirmed that mRNA for the GCLC and GCLM subunits were substantially up-regulated, as was that for the FTL subunit, but to a more modest degree than was observed for the AKRs. TaqMan® analyses supported the microarray data which indicated that mRNA for enzymes involved in NADPH generation and antioxidant stress proteins, including G6PDH, 6-phosphogluconate dehydrogenase, HMOX1, SRXN1 and TXNDR1, were also up-regulated following KEAP1 knockdown.
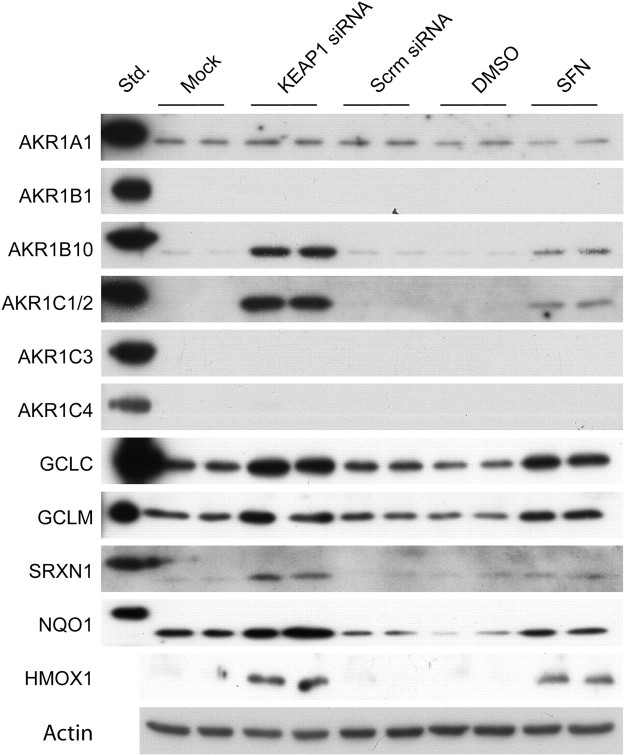
To assess whether the increase in mRNAs following knockdown of KEAP1 translated into an increase in protein, a series of western blotting experiments were performed. Using antibodies specific for individual AKR subfamilies, immunoblotting showed that AKR1B10, AKR1C1 and/or AKR1C2, but not AKR1C3, were increased by transfection of Target#1 into HaCaT cells (Figure 1). Western blotting also revealed that knockdown of KEAP1 increased the levels of GCLC, GCLM, SRXN1, NQO1 and HMOX1 protein.
Fig. 1.
Detoxification and antioxidant proteins are increased in keratinocytes following KEAP1 knockdown. HaCaT cells were transfected with siRNA or treated with SFN, as described in the text. Protein samples were prepared from whole-cell lysates for western blotting and probed with polyclonal antisera as indicated.
Besides the genes listed in Table I, microarray also revealed a number of inducible mRNA species whose murine orthologues are subjected to NRF2-mediated induction by xenobiotics (1–3) or are up-regulated in livers of hepatocyte-specific Keap1-null mice (15,16). These transcripts included the following: transketolase (NM_001064), increased 1.95-fold; chemokine (C-C motif) ligand 26 (NM_006072), increased 1.8-fold; ATP-binding cassette, subfamily C, member 3 (ABCC3, or CFTR/MRP) (NM_003786), increased 1.74-fold and solute carrier family 26, member 11 (NM_173626), increased 1.67-fold. Other substantially induced genes included: D4, zinc and double plant homeo domain fingers family 2 (NM_006268), increased 1.99-fold; Lin11, Isl-1 and Mec-3 domain kinase 1, variant 1 (NM_002314), increased 1.92-fold; poly(A)-binding protein, cytoplasmic 4 (NM_003819), increased 1.9-fold; H-rev107-like protein 5 (BC034222), increased 1.82-fold; aspartate β-hydroxylase (NM_032466), increased 1.77-fold; peptidylprolyl isomerase F (cyclophilin F) (NM_005729), increased 1.73-fold; apoptosis-inducing factor-homologous mitochondrion-associated inducer of death (NM_032797), increased 1.72-fold.
KEAP1 knockdown decreased the expression of keratin and other genes in HaCaT cells
In the oesophagus of Keap1−/− mice, the expression of keratin 6 is greatly increased in an NRF2-dependent manner (44). Surprisingly, examination of the microarray gene signature list indicated that knockdown of KEAP1 produced decreases of between 15 and 40% in mRNA for keratins 1, 4, 5, 6A, 6E, 13, 14, 16, 17 and 19, as well as HA3A and K6HF (data not shown). None of the keratins were induced by KEAP1 knockdown. Interestingly, a number of other transcripts were down-regulated following knockdown of KEAP1, suggesting that NRF2 may control certain repressor activities. The mRNAs whose expression decreased most included the following: a disintegrin and metalloproteinase domain 12 (metric alpha), transcript variant 2 (NM_021641) which reduced to 4.9%; complementary DNA FLJ37003 fis clone BRACE2008500, highly similar to creatine kinase, ubiquitous mitochondrial precursor (EC 2.7.3.2) (AK094322) which reduced to 6.6%; C-terminal PSD95, DlgA and zo-1 domain ligand of neuronal nitric oxide synthase (NM_014697) which reduced to 12%; matrix metalloproteinase 13 (collagenase 3) (NM_002427) which reduced to 38% and serine (or cysteine) proteinase inhibitor, clade B (ovalbumin), member 2 (BC012609) which reduced to 39%.
NRF2 contributes to the basal and inducible expression of ARE-driven genes in HaCaT cells
Following our demonstration that knockdown of KEAP1 in HaCaT cells resulted in the up-regulation of members of the ARE gene battery, we wished to determine whether NRF2 controlled both the basal and inducible expression of these genes. As these experiments required the simultaneous knockdown of two target genes, we decided to reduce the amount of siRNA for transfection from 100 nmol/l, used to define the gene signature list, to a total of 50 nmol/l, with the amount of each siRNA species being set at 25 nmol/l; in the case of single-knockdown experiments, 25 nmol/l Scrm siRNA was also added in order to maintain the final siRNA concentration of 50 nmol/l. Transfection of HaCaT cells with 25 nmol/l KEAP1 siRNA gave similar increases in mRNAs as were observed previously using 100 nmol/l KEAP1 siRNA in the microarray experiment (Table II).
Table II.
NRF2 regulates basal and inducible expression of multiple transcripts, whereas BACH1 specifically inhibits expression of HMOX1
| Gene function | Gene name | Accession Number | siRNA Species |
|||||
| KEAP1 |
BACH1 |
NRF2 |
KEAP1/BACH1 |
BACH/NRF2 |
KEAP1/NRF2 |
|||
| Transcription Fold Change | ||||||||
| Xenobiotic metabolism | AKR1A1 | NM_006066 | 1.1 ± 0.1ns | 0.9 ± 0.1ns | 0.7 ± 0.1§§§ | 1.2 ± 0.1ns(ns) | 0.9 ± 0.1ns | 1.2 ± 0.1ns |
| AKR1B1 | NM_001628 | 1.6 ± 0.1*** | 1.1 ± 0.1ns | 0.8 ± 0.1§§ | 1.8 ± 0.2***(ns) | 0.7 ± 0.1§§§ | 0.9 ± 0.1ns | |
| AKR1B10 | NM_020299 | 16.2 ± 2.1*** | 1.1 ± 0.1ns | 0.6 ± 0.1§§§ | 25.8 ± 1.8***(ns) | 0.5 ± 0.1§§§ | 0.9 ± 0.1ns | |
| AKR1C1 | NM_001353 | 14.4 ± 3.0*** | 3.2 ± 1.4ns | 0.2 ± 0.1§§§ | 18.2 ± 2.5***(ns) | 0.2 ± 0.1§§§ | 0.4 ± 0.1§ | |
| AKR1C2 | NM_001354 | + | — | — | + | — | — | |
| AKR1C3 | NM_003739 | 15.4 ± 2.7*** | 1.8 ± 0.1*** | 0.2 ± 0.1§§§ | 23.8 ± 3.6***(ns) | 0.3 ± 0.1§§§ | 0.4 ± 0.1§§§ | |
| AKR1C4 | NM_001818 | — | — | — | — | — | — | |
| NQO1 | NM_000903 | 2.4 ± 0.4** | 0.9 ± 0.1ns | 0.3 ± 0.1§§§ | 2.6 ± 0.5**(ns) | 0.3 ± 0.1§§§ | 0.5 ± 0.1§ | |
| PTGR1 | NM_012212 | 1.5 ± 0.1** | 0.9 ± 0.1ns | 0.8 ± 0.1ns | 2.5 ± 0.3***(*) | 0.8 ± 0.1ns | 1.4 ± 0.2§ | |
| GSH biosynthesis | GCLC | NM_001498 | 4.8 ± 0.9*** | 1.5 ± 0.1*** | 0.5 ± 0.1§§§ | 5.8 ± 0.6***(ns) | 0.6 ± 0.1§§§ | 0.9 ± 0.1ns |
| GCLM | NM_002061 | 2.4 ± 0.6* | 1.8 ± 0.5ns | 0.6 ± 0.2ns | 4.3 ± 0.8**(ns) | 0.9 ± 0.2ns | 0.6 ± 0.1§§§ | |
| GSR | NM_000637 | 1.7 ± 0.2** | 1.4 ± 0.2ns | 1.1 ± 0.2ns | 2.7 ± 0.1***(***) | 1.2 ± 0.1ns | 1.2 ± 0.1ns | |
| NADPH generation | G6PD | NM_000402 | 2.2 ± 0.1*** | 1.2 ± 0.1ns | 0.6 ± 0.1§§§ | 3.7 ± 0.4***(**) | 0.7 ± 0.1§§§ | 1.1 ± 0.1ns |
| ME1 | NM_002395 | 1.5 ± 0.2* | 1.3 ± 0.1ns | 0.8 ± 0.2ns | 2.1 ± 0.2***(*) | 0.8 ± 0.1§§§ | 0.8 ± 0.1§§ | |
| PGD | NM_002631 | 2.1 ± 0.2*** | 1.1 ± 0.1ns | 0.7 ± 0.1§§ | 4.2 ± 1.1*(ns) | 0.8 ± 0.1§§ | 1.4 ± 0.1§§§ | |
| Antioxidant | HMOX1 | NM_002133 | 2.3 ± 0.3*** | 136.4 ± 8.5*** | 0.4 ± 0.1§§§ | 388.0 ± 51.3***(***) | 11.8 ± 0.6*** | 0.3 ± 0.1§§§ |
| SRXN1 | NM_080725 | 1.8 ± 0.1*** | 0.9 ± 0.1ns | 0.9 ± 0.1ns | 3.3 ± 0.3***(***) | 0.9 ± 0.1ns | 1.2 ± 0.1ns | |
| TXNRD1 | NM_003330 | 2.2 ± 0.2*** | 2.7 ± 0.6* | 0.5 ± 0.1§§§ | 4.7 ± 1.0**(*) | 0.6 ± 0.1§§§ | 0.9 ± 0.1ns | |
| Iron metabolism | FECH | NM_000140 | 1.9 ± 0.1*** | 0.9 ± 0.1ns | 0.8 ± 0.1§ | 2.4 ± 0.6*(ns) | 0.6 ± 0.1§§§ | 1.1 ± 0.1ns |
| FTL | NM_000146 | 2.4 ± 0.1*** | 1.5 ± 0.2** | 0.5 ± 0.1§§§ | 4.7 ± 0.5***(***) | 0.5 ± 0.1§§§ | 1.0 ± 0.4ns | |
| FTH1 | NM_002032 | 1.3 ± 0.1** | 2.0 ± 0.2*** | 0.9 ± 0.1ns | 2.3 ± 0.1***(***) | 1.7 ± 0.1*** | 1.4 ± 0.2ns | |
| Gene transcription | MAFG | NM_002359 | 1.7 ± 0.4* | 1.3 ± 0.1ns | 0.9 ± 0.2ns | 2.3 ± 0.3**(ns) | 1.4 ± 0.3ns | 1.3 ± 0.2ns |
| CRABPII | NM_001878 | 0.8 ± 0.1ns | 0.8 ± 0.1ns | 0.6 ± 0.1§ | 0.9 ± 0.1ns(ns) | 0.8 ± 0.1ns | 0.9 ± 0.1ns | |
TaqMan RT–PCR was performed on a selection of NRF2-dependent genes, following transfection with combinations of siRNA (to give a final concentration of 50 nmol/l). Statistical significance was calculated relative to mock-transfected cells with the single, double and triple * or § symbols indicating different significance levels of increases or decreases in data as described in the Materials and methods section. Statistical significance of the difference between KEAP1/BACH1 and KEAP1 fold-change values was also calculated, with the results depicted in parenthesis. Where a fold-induction value could not be derived, an increase in transcript is indicated by ‘+’. ns, Not significant.
Transfection of HaCaT cells with 25 nmol/l NRF2 siRNA reduced the mRNA for the CNC-bZIP protein to 15% of the level observed following mock transfection. Further TaqMan® experiments showed that knockdown of NRF2 was accompanied by substantial decreases in the expression of AKR1C1, AKR1C2, AKR1C3, NQO1 and HMOX1 that were up-regulated by KEAP1 knockdown (Table II). It was also noted that among the known ARE gene battery members, the expression of some was more modestly reduced by NRF2 knockdown, whereas others, such as PTGR1, GCLM, GSR, ME1, SRXN1, FTH1 and MAFG, showed no significant change in expression upon NRF2 knockdown. Thus, NRF2 exerts a variable level of control on the basal transcription rates of different members of the ARE gene battery.
To explore whether NRF2 is necessary for the up-regulation of all the genes in the signature list, we performed KEAP1/NRF2 double-knockdown experiments. Transfection of HaCaT cells with siRNAs against KEAP1 and NRF2 resulted in their mRNAs being reduced to 60 and 35%, respectively. As shown in Table II, knockdown of NRF2 abolished up-regulation of members of the ARE gene battery upon the simultaneous knockdown of KEAP1.
BACH1 negatively regulates HMOX1, but not all ARE-driven genes
It is unclear how many ARE-driven genes are negatively regulated by BACH1. To address this question, we knocked down BACH1. mRNA for BACH1 was readily detected by TaqMan® chemistry in HaCaT cells, and 48 h after transfection with 25 nmol/l of a pre-designed siRNA, the level of BACH1 mRNA was reduced to ∼30% of the level observed following mock transfection. Western blotting also showed that BACH1 protein was reduced to a similar extent by the siRNA (data not shown).
The effect that knockdown of BACH1 had on ARE-driven gene expression was first examined by studying HMOX1 because BACH1 is known to repress this oxygenase gene (20,21). TaqMan® RT–PCR and western blotting revealed that HMOX1 was up-regulated 136-fold following BACH1 knockdown and is therefore negatively controlled by the BTB-CNC protein to a much greater extent than had been observed for any of the genes by KEAP1. We also examined whether BACH1 contributed to the negative regulation of FTH1, FTL, NQO1 and TXNRD1 as the BTB-CNC protein has been implicated in their repression (23,24). Table II shows that relative to HMOX1, only very modest increases of 1.5- to 2.7-fold in the mRNA were observed for AKR1C3, GCLC, TXNRD1, FTL and FTH1 upon BACH1 knockdown, and no increase was observed in NQO1 mRNA (Table II).
KEAP1 and BACH1 repress ARE-driven genes by separate mechanisms, but NRF2 is required for induction of HMOX1
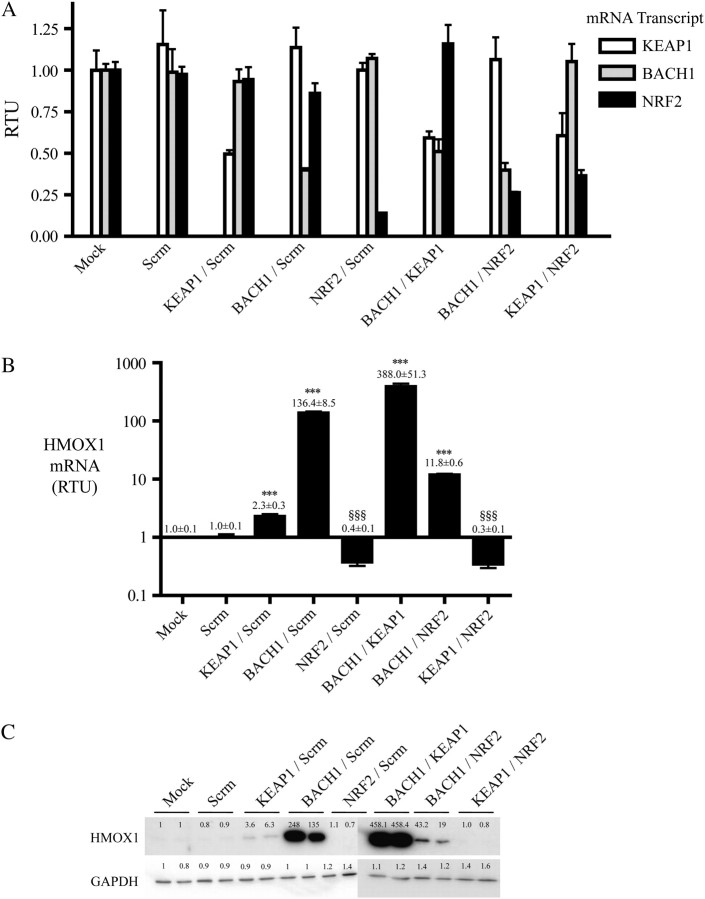
In order to examine whether KEAP1 and BACH1 might repress members of the ARE gene battery synergistically, and to determine the contribution made by NRF2 to the activation of these genes following de-repression, we performed double-knockdown experiments. Figure 2A shows that combinations of two siRNA species against KEAP1, BACH1 or NRF2 (each at 25 nmol/l) reduced the levels of their target mRNAs in HaCaT cells to between 15 and 60% of the levels observed following mock transfection. A combined knockdown of KEAP1 with BACH1 resulted in a substantially greater induction of HMOX1 mRNA than was obtained by transfection with KEAP1 siRNA or BACH1 siRNA alone. This increase in induction of HMOX1 following double knockdown was also apparent at the protein level. Augmented induction upon knockdown of both BACH1 and KEAP1 was also observed for GSR, G6PD, SRXN1, FTL and FTH1, but the synergistic effects were not so pronounced as was observed for HMOX1.
Fig. 2.
Knockdown of KEAP1, NRF2 and BACH1 in keratinocytes reveals a hierarchy in the regulation of HMOX1 expression. (A) TaqMan RT–PCR analyses of KEAP1, BACH1 and NRF2 mRNA levels following transfection of HaCaT cells with combinations of siRNAs. The final siRNA concentration in each transfection was adjusted to give a total of 50 nmol/l; the Scrm control transfection experiment contained 50 nM Scrm siRNA, whereas all other transfections contained two siRNA species, each at 25 nmol/l. (B) TaqMan RT–PCR of HMOX1 mRNA following transfection of HaCaT cells with combinations of siRNA. Statistical significance was calculated relative to mock-transfected cells, with the exception of double KEAP1/BACH1 and KEAP1/NRF2 siRNA transfections, where significance was calculated relative to KEAP1 siRNA-transfected cells. (C) Western blotting for HMOX1 protein following transfection of HaCaT cells with combinations of siRNA species, with GAPDH being employed as a loading control. The fold increases were calculated relative to the mock-transfected sample (lane 1).
Double knockdown of BACH1 and NRF2 substantially diminished the induction of HMOX1, when compared with its up-regulation following knockdown of BACH1 alone, indicating that NRF2 mediates the majority of HMOX1 induction under such circumstances. It is possible that NRF2 is solely responsible for induction of HMOX1 upon BACH1 knockdown and that 100% efficient knockdown of the CNC-bZIP factor might completely ablate HMOX1 induction. The double knockdown of BACH1 and NRF2 also abolished the modest induction of AKR1C3, GCLC, FLT and TXNRD1, but not of FTH1. These data indicate that BACH1 is the principal negative controller of HMOX1, but it might also modestly repress AKR1C3, GCLC, FLT, FTH1 and TXNRD1 because significant increases in expression of these genes were observed upon knockdown of BACH1 alone. In all cases, NRF2 was found to be the major positive regulator of genes that are repressed by BACH1.
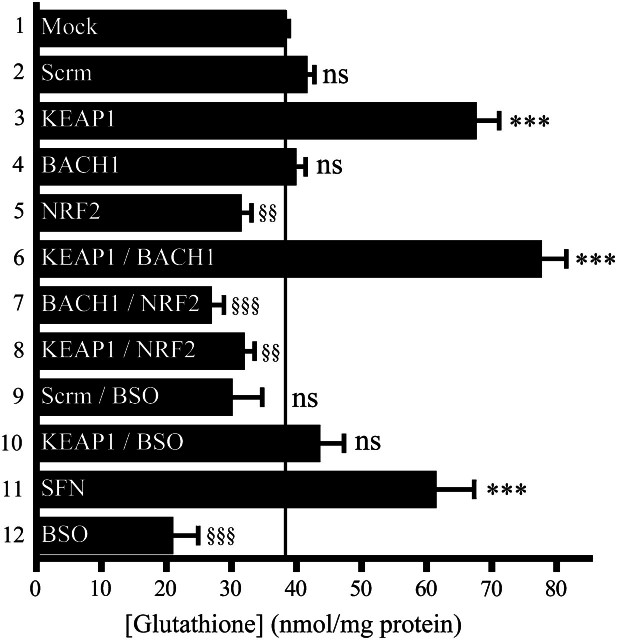
The KEAP1–NRF2 pathway controls intracellular glutathione
As NRF2 regulates GCLC and GCLM, we tested whether knockdown of KEAP1, NRF2 and BACH1 influenced glutathione levels in HaCaT cells. Figure 3 shows that KEAP1 knockdown stimulated a 1.75-fold increase in the amount of glutathione, whereas NRF2 knockdown decreased the antioxidant to ∼80% of its normal level, and BACH1 knockdown had no effect. Double knockdown of KEAP1 and NRF2 also decreased glutathione levels. In contrast, treatment of HaCaT cells with 5 μM SFN for 24 h, which was used as a positive control, increased glutathione 1.6-fold, whereas treatment of HaCaT cells with 50 μM BSO for 4 h, which was used as a negative control, decreased the antioxidant to 50% of normal levels.
Fig. 3.
NRF2 regulates intracellular glutathione levels. The HaCaT cells were mock transfected (lane 1) or transfected with various siRNA species at a total concentration of 50 nmol/l (lanes 2–10), treated for 24 h with SFN (lane 11) and/or treated for 4 h with BSO (lanes 9, 10 and 12) as described in Materials and methods. Data from repeat experiments were combined and are presented as nanomole glutathione/milligram of protein. Statistical significance for lanes 2–12 was calculated relative to lane 1.
The KEAP1–NRF2 pathway controls resistance to electrophiles and redox-cycling agents
The biological consequences of up-regulating ARE-driven genes in human cells are poorly understood. To test the hypothesis that this gene battery determines the ability of keratinocytes to withstand electrophiles, we knocked down KEAP1 and/or NRF2 in HaCaT cells before challenging them for 24 h with xenobiotics and measuring their survival. Given the marked up-regulation of AKR1C1 following KEAP1 knockdown, we were particularly interested in examining whether keratinocytes that over-expressed AKR1C1 were resistant to α,β-unsaturated aldehydes because this reductase exhibits activity towards such compounds (45).
Firstly, we genetically activated NRF2 by KEAP1 knockdown to determine the contribution that the CNC-bZIP factor could make to inducible drug resistance in HaCaT cells. Table III, panel B shows that constitutively activated NRF2 afforded ∼1.6-fold resistance against the α,β-unsaturated aldehyde acrolein and ∼1.5-fold resistance against the nitrogen mustard chlorambucil and the organic hydroperoxide CuOOH. Activation of NRF2 conferred ∼1.4-fold resistance against the redox-cycling agent menadione. In contrast, knockdown of NRF2 made HaCaT cells 65–78% less tolerant of acrolein, chlorambucil and CuOOH but only modestly more sensitive to menadione (Table III, panel G).
Table III.
KEAP1 and NRF2 determine cellular susceptibility to chemical toxicity, primarily through modulation of glutathione
| Chemical | LD50 |
|||
| Mean | (95% CI) | FC | P | |
| A—Scrm siRNA | ||||
| Acrolein | 194.2 | (186.6–202.0) | 1.00 | ns |
| Chlorambucil | 697.4 | (658.0–739.2) | 1.00 | ns |
| CuOOH | 685.8 | (644.2–730.1) | 1.00 | ns |
| Menadione | 28.34 | (26.84–29.91) | 1.00 | ns |
| B—KEAP1 siRNA | ||||
| Acrolein | 316.1 | (281.2–355.3) | 1.63 | *** |
| Chlorambucil | 1009 | (902.9–1128) | 1.45 | *** |
| CuOOH | 1026 | (821.9–1282) | 1.50 | *** |
| Menadione | 38.59 | (36.32–40.11) | 1.36 | *** |
| C—SFN | ||||
| Acrolein | 318.8 | (266.0–382.2) | 1.88 | *** |
| Chlorambucil | 998.6 | (914.3–1196) | 1.25 | *** |
| CuOOH | 829.1 | (790.8–869.3) | 1.31 | *** |
| Menadione | 40.1 | (38.50–41.75) | 1.40 | *** |
| D—Scrm siRNA/BSO | ||||
| Acrolein | 52.75 | (23.3–71.9) | 0.27 | §§§ |
| Chlorambucil | 388.2 | (337.4–446.7) | 0.56 | §§§ |
| CuOOH | 421.5 | (395.6–449.0) | 0.61 | §§§ |
| Menadione | 30.39 | (28.88–31.99) | 1.05 | ns |
| E—KEAP1 siRNA/BSO | ||||
| Acrolein | 119.8 | (106.5–134.9) | 0.62 | §§§ |
| Chlorambucil | 646.1 | (613.9–679.9) | 0.93 | ns |
| CuOOH | 641.6 | (611.5–673.1) | 0.94 | ns |
| Menadione | 41.83 | (38.96–44.91) | 1.48 | *** |
| F—SFN/BSO | ||||
| Acrolein | 101.7 | (79.2–156.4) | 0.52 | §§§ |
| Chlorambucil | 566.8 | (531.3–604.6) | 0.81 | §§§ |
| CuOOH | 462.2 | (439.2–486.3) | 0.67 | §§§ |
| Menadione | 47.39 | (43.86–51.21) | 1.67 | *** |
| G—NRF2 siRNA | ||||
| Acrolein | 132.9 | (122.0–144.7) | 0.68 | §§§ |
| Chlorambucil | 541.2 | (494.2–592.6) | 0.78 | §§§ |
| CuOOH | 443.5 | (429.6–458.0) | 0.65 | §§§ |
| Menadione | 26.8 | (25.65–28.00) | 0.92 | ns |
| H—KEAP1 siRNA/BACH1 siRNA | ||||
| Acrolein | 297.4 | (265.6–332.9) | 1.53 | *** |
| Chlorambucil | 1097 | (923.1–1201) | 1.57 | *** |
| CuOOH | 901.4 | (857.0–948.2) | 1.31 | *** |
| Menadione | 37.81 | (34.89–39.62) | 1.33 | *** |
| I—BACH1 siRNA | ||||
| Acrolein | 204.2 | (186.9–223.1) | 1.05 | ns |
| Chlorambucil | 626.2 | (583.3–672.3) | 0.90 | ns |
| CuOOH | 605.1 | (574.7–637.1) | 0.88 | ns |
| Menadione | 30.31 | (28.49–32.24) | 1.10 | ns |
The HaCaT cells were transfected/pre-treated as follows: (A) Scrm siRNA, (B) KEAP1 siRNA, (C) SFN, (D) Scrm siRNA with BSO, (E) KEAP1 siRNA with BSO, (F) SFN with BSO, (G) NRF2 siRNA, (H) KEAP1 and BACH1 siRNA, (I) BACH1 siRNA. These cells were then exposed to a range of concentrations of acrolein, chlorambucil, CuOOH or menadione. The mean LD50 of chemical compounds, with 95% confidence intervals is presented. The fold change in LD50 and significance of data are calculated relative to those transfected with Scrm siRNA (A), as described in Materials and methods. ns, Not significant; FC, Fold change.
To test the possibility that cytoprotection conferred by genetic activation of NRF2 is due to over-production of glutathione, we treated HaCaT cells with 50 μM BSO for 4 h immediately before challenge with xenobiotics to reduce the increase in glutathione biosynthesis that occurred upon KEAP1 knockdown. Table III, panels D and E show that treatment of HaCaT cells with BSO made them between 1.5- and 3-fold more sensitive to acrolein, chlorambucil and CuOOH but not to menadione, when compared with Table III, panels A and B, respectively. However, BSO-treated HaCaT cells in which NRF2 was activated by KEAP1 knockdown were not as sensitive to any of the four test chemicals as were BSO-treated HaCaT cells that had been transfected with Scrm siRNA (Table III, panels B and E). These results suggest that the cytoprotective effects resulting from genetic activation of NRF2 might not be solely attributed to over-production of glutathione.
Besides carrying out KEAP1 knockdown as a means of activating NRF2, we also ‘primed’ HaCaT cells for 24 h with 5 μM SFN immediately before challenge for 24 h with various toxicants (Table III, panel C). This type of pre-conditioning conferred on the keratinocytes between 1.25- and 1.9-fold resistance to the four test xenobiotics. However, if the cells were co-treated with 50 μM BSO during the last 4 h of priming with SFN, resistance to acrolein, chlorambucil and CuOOH was abolished, but not resistance to menadione (Table III, panels C and F). It is also interesting to note that HaCaT cells treated with both BSO and SFN were less sensitive to acrolein, chlorambucil and CuOOH than were the fibroblasts treated with BSO alone (Table III, panels C and D), a finding which again suggests that factors other than glutathione contribute to drug resistance.
Knockdown of BACH1 does not confer protection against electrophiles and redox-cycling agents
As BACH1 knockdown greatly increased the expression of HMOX1, we wished to determine if this was sufficient to afford protection against xenobiotics. Table III, panel I shows that knockdown of BACH1 alone in HaCaT cells did not influence their sensitivity to acrolein, chlorambucil, CuOOH or menadione. Furthermore, knockdown of both BACH1 and KEAP1 did not increase the level of drug resistance observed beyond that observed following knockdown of KEAP1 alone (Table III, panels B and H).
Discussion
To date, the human ARE gene battery has been poorly characterized. Through studying genes whose orthologues are inducible in rodents or genes known to be over-expressed in drug-resistant cell lines, it has been shown previously that AKR1C1, AKR1C2, NQO1, GCLC and GCLM are induced by SFN, tert-butyl hydroquinone or β-naphthoflavone in a wide range of human cell lines including retinal pigment epithelial ARPE-19, colon CaCo-2, HT29 and LS-174, keratinocyte HaCaT, liver HepG2, mammary MCF7, neuroblastoma IMR-32 and prostate LNCaP cells (25–34,46,47). Although gene microarray analyses have been carried out using RNA from CaCo-2 cells treated with SFN (48), and on gastric mucosal RNA samples taken from human volunteers 6 h after they had consumed broccoli soup (49), a clear picture has not emerged about the targets of NRF2 in man.
In order to avoid non-specific effects associated with chemical inducers, we have utilized a genetic approach to identify members of the human ARE gene battery. Our microarray experiments revealed 23 genes from the whole genome that were up-regulated ≥2-fold upon knockdown of KEAP1 in HaCaT cells. These included AKR1C1, AKR1C2, AKR1C3, NQO1, GCLC, GCLM and HMOX1 that were previously thought by many researchers to be regulated by NRF2. However, it also included AKR1B10, G6PD, ME1, PGD and SRXN1 that are not widely recognized to be members of the human ARE gene battery. A surprising feature of our results is that all the proteins involved in xenobiotic metabolism that were up-regulated in HaCaT cells catalyse drug oxidation or reduction reactions; we found no evidence for the up-regulation of drug-metabolizing enzymes that catalyse conjugation reactions. In particular, while Alpha-class GST subunits are inducible in mouse skin (50), we found no evidence that this transferase class is inducible in HaCaT cells. The human keratinocyte cell line expresses significant amounts of class Pi GST, but its level was not obviously increased by SFN or knockdown of KEAP1 (data not shown). Our microarray data indicated GST Mu-class 3 was induced 1.24-fold in HaCaT cells, but this was not evaluated further. Other researchers have found that GCLC, GCLM, HMOX1 and NQO1 can be induced in human keratinocytes and human melanocytes by treatment with SFN or tert-butyl hydroquinone (51–53), but there are no reports of cytosolic GST isoenzymes being induced in these cells.
We have shown previously that knockdown of KEAP1 in HaCaT cells results in an increase in NRF2 protein and ARE-driven luciferase reporter activity (33). These data suggested that induction of gene expression in HaCaT cells upon KEAP1 knockdown is mediated by NRF2. Our finding that double knockdown of NRF2 and KEAP1 prevented up-regulation of genes on the signature list supports the hypothesis that NRF2 mediates gene induction. It is also in keeping with the notion that KEAP1 does not repress NRF1 (54). In the present study, single knockdown of NRF2 revealed that in addition to mediating inducible gene expression, the CNC-bZIP factor also regulates the normal basal expression of a substantial number of genes. The most obvious of these were AKR1C1, AKR1C2, AKR1C3 and NQO1. Presumably the basal expression of other members of the ARE gene battery is controlled in part by additional transcription factors such as NRF1, c-Jun or nuclear factor-kappaB (55).
We found that knockdown of KEAP1 produced a gradation in the induction of AKR1C1, GCLC, GCLM, HMOX1 and NQO1. The reason why NRF2 transactivates certain members of the ARE gene battery to a greater extent than others is not well understood. A probable explanation is that NRF2 engages in interactions with various co-activators and co-repressors, in order to recruit polymerase II to the promoters of its target genes, and that some of these protein–protein interactions may be gene specific. The Neh5 domain of NRF2 is capable of interacting with Brahma-related gene 1, a protein involved in chromatin remodelling (56). In the case of HMOX1, its promoter contains 30 TG repeats, situated approximately −260 nucleotides from the transcriptional start site. In the presence of chromatin remodelling complexes, this TG repeat sequence possesses the ability to form a Z-DNA structure that can stimulate transcription. Evidence from knockdown experiments has been presented that Brahma-related gene 1 increases NRF2-mediated transcription of HMOX1, but not the transcription of GCLC, GCLM and NQO1, suggesting that it may contribute to the variation in induction of ARE-driven genes upon activation of NRF2 (56). Conversely, knockdown of Brahma-related gene 1 resulted in an increased induction of AKR1C1 (56). Thus, chromatin remodelling may both augment and repress NRF2-mediated induction of ARE-driven genes.
Knockdown of BACH1 stimulated a substantially greater induction of HMOX1 in HaCaT cells than was observed for other ARE-driven genes. The HMOX1 gene promoter contains numerous ARE-like sequences that are largely clustered at −4.0 and −9.0 kb from the transcriptional start site called enhancer 1 and enhancer 2, respectively (18). Studies in the mouse have shown that repression of Hmox1 by Bach1 through it binding to enhancer 2 can only occur in a chromatin environment (57). The enhancer 2 contains three ARE-like motifs, and repression of Hmox1 by Bach1 was found to be inefficient when two of these cis-elements were mutated (57). This suggests that multiple ARE-like sequences, each of which serves as a Bach1-binding site, are required for correct repression of Hmox1. Our results suggest that among the human ARE gene battery, only HMOX1 contains the necessary multiple cis-elements that allow it to be repressed efficiently by BACH1. In HaCaT cells, we observed modest increases in expression of AKR1C3, GCLC, FTL, FTH1 and TXNRD1 upon BACH1 knockdown. This may reflect the presence of multiple AREs in their gene promoters, but further work is required to clarify this point.
Our experiments have provided convincing evidence that in human keratinocytes NRF2 mediates both intrinsic and inducible resistance against electrophiles. Thus, knockdown of KEAP1 in HaCaT cells increased their tolerance of acrolein, chlorambucil and CuOOH, whereas knockdown of NRF2 increased their sensitivity to these xenobiotics. The protection provided by NRF2 against electrophiles could be attributed in part to its ability to up-regulate glutathione because we found BSO treatment overcame resistance to these compounds. We have also provided evidence that NRF2 mediates resistance against the redox-cycling agent menadione, but in this case, resistance was independent of glutathione and can probably be attributed to induction of NQO1 (43). In contrast, we found that knockdown of BACH1 made no impact on the sensitivity of HaCaT cells to acrolein, chlorambucil, CuOOH or menadione. This finding is consistent with our observation that while BACH1 is the principal negative regulator of HMOX1 it does not repress other ARE-driven genes to anything like the same extent.
We interpret the finding that NRF2 mediates protection of the non-transformed HaCaT keratinocytes against the cytotoxic effects of electrophiles and redox-cycling compounds as evidence for its involvement in cancer chemoprevention. However, the discovery that ∼20% of patients with lung cancer possess tumour-specific constitutive activation of NRF2 as a consequence of somatic mutations in either KEAP1 or NRF2 (58–60) suggests that the CNC-bZIP factor may contribute to the promotion of carcinogenesis under certain circumstances (reviewed in ref. 61). The finding that AKR1B10, AKR1C1, AKR1C2 and AKR1C3 are strongly induced upon KEAP1 knockdown is noteworthy because these enzymes exhibit catalytic properties that are relevant to carcinogenesis. AKR1B10 is a highly efficient retinal reductase converting retinal to retinol, thereby preventing the formation of retinoic acid (62). It is therefore plausible that in carcinomas and dysplastic cells, the increased expression of AKR1B10, upon activation of NRF2, could promote cell proliferation. Indeed, it has been reported that knockdown of AKR1B10 in colorectal cells made them more susceptible to cell death by acrolein and crotonaldehyde (63). Members of the AKR1C family are involved in the metabolic activation of polycyclic aromatic hydrocarbons, which are tobacco carcinogens that arise by the conversion of polycyclic aromatic hydrocarbon-trans-dihydrodiols to electrophilic and redox-active polycyclic aromatic hydrocarbon-ortho-quinones (64). In human lung adenocarcinoma A549 cells, this pathway leads to the production of reactive oxygen species and the formation of the highly mutagenic lesion 8-oxo-deoxyguanosine (65). These findings suggest that up-regulation of AKR1B10 and AKR1C isoenzymes in smokers susceptible to lung cancer would be deleterious since this may lead to an increased activation of tobacco carcinogens.
The finding that many AKR isoenzymes are up-regulated upon KEAP1 knockdown suggests that they could represent biomarkers in two different patho-physiological settings. Firstly, in non-transformed cells, high levels of AKR1B10, AKR1C1 or AKR1C2 could reflect cellular adaptation to redox stress, possibly due to inflammation or a variety of metabolic disturbances. Secondly, in transformed cells, high levels of these AKRs may result from NRF2 being dys-regulated within the lesion. Although it has only recently been recognized that KEAP1 and NRF2 are subject to somatic mutations, the older literature contains reports of AKR up-regulation in neoplastic lesions. For example, proteomic and microarray analyses have shown that AKR1B10 and AKR1C family members are over-expressed in non-small-cell carcinoma and squamous cell carcinoma of the lung, in oral dysplastic cells treated with cigarette smoke condensate and in bronchial epithelial cells of smokers (66–68). Besides their up-regulation in lung tissue, AKR1B10, AKR1C1 and AKR1C2 have also been reported to be over-expressed in hepatocellular carcinoma, inflammatory breast cancer, intestinal metaplasia and Barrett's mucosa (69–71). Given the range of conditions associated with over-expression of AKR1B10, AKR1C1 and AKR1C2, it will be important to establish whether their over-expression can serve as biomarkers for circumstances in which oxidative stress arises, such as uncoupling of mitochondrial oxidative phosphorylation, inflammatory disease and tumour promotion. In addition, further work is required to establish to what extent AKR1B10, AKR1C1 and AKR1C2 are up-regulated in tumours with somatic mutations in KEAP1 or NRF2. The use of AKR proteins as biomarkers would be of considerable practical value because activation of NRF2 stimulates GSH over-production, which confers protection against cytotoxic agents and increases cell proliferation. Thus, tumours with constitutively active NRF2 probably ought not to be treated with chemotherapeutic agents that are either inactivated or eliminated in a GSH-dependent fashion (for a review of such drugs, see ref. 72).
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
Cancer Research-UK (C4909/A5942); Association for International Cancer Research (05-154).
Acknowledgments
We thank Dr Albena T.Dinkova-Kostova, Dr Graham Betton and Professor Masayuki Yamamoto for their helpful advice and Dr Lesley I.McLellan for the gift of antibodies. A.K.M. was supported by a Biotechnology and Biological Sciences Research Council (UK) Collaborative Awards in Science and Engineering PhD studentship with AstraZeneca.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AKR
aldo-keto reductase
- ARE
antioxidant response element
- BACH1
broad-complex, tramtrack and bric à brac and cap'n'collar homology 1
- BSO
buthionine sulfoximine
- BTB
broad-complex, tramtrack and bric à brac
- bZIP
basic-region leucine zipper
- CNC
cap'n'collar
- CuOOH
cumene hydroperoxide
- FTH1
ferritin heavy
- FTL
ferritin light
- GCLC
glutamate-cysteine ligase catalytic
- GCLM
glutamate-cysteine ligase modifier
- GSH
glutathione
- GST
glutathione S-transferase
- HMOX1
haem oxygenase-1
- KEAP1
Kelch-like ECH-associated protein 1
- LD50
lethal dose 50
- mRNA
messenger RNA
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NQO1
NAD(P)H:quinone oxidoreductase-1
- NRF
NF-E2-related factor
- RT–PCR
reverse transcription–polymerase chain reaction
- Scrm
scrambled
- SFN
sulforaphane
- siRNA
short inhibitory RNA
- SRXN1
sulfiredoxin
- TXNRD1
thioredoxin reductase
References
- 1.Thimmulappa RK, et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 2.Kwak MK, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 3.Nioi P, et al. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem. J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu R, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6JNrf2 (-/-) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Kensler TW, et al. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 6.McMahon M, et al. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang DD, et al. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang DD, et al. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon M, et al. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 11.Dinkova-Kostova AT, et al. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi N, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggler AL, et al. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl Acad. Sci. USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, et al. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okawa H, et al. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 16.Osburn WO, et al. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inamdar NM, et al. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors. Biochem. Biophys. Res. Commun. 1996;221:570–576. doi: 10.1006/bbrc.1996.0637. [DOI] [PubMed] [Google Scholar]
- 18.Reichard JF, et al. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa K, et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichard JF, et al. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J. Biol. Chem. 2008;283:22363–22370. doi: 10.1074/jbc.M801784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, et al. Heme regulates gene expression by triggering Crm1-dependent nuclear export of Bach1. EMBO J. 2004;23:2544–2553. doi: 10.1038/sj.emboj.7600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhakshinamoorthy S, et al. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J. Biol. Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 24.Hintze KJ, et al. Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro and coordinates expression with heme oxygenase1, β-globin, and NADP(H) quinone (oxido) reductase1. J. Biol. Chem. 2007;282:34365–34371. doi: 10.1074/jbc.M700254200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, et al. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J. Biol. Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 26.Mulcahy RT, et al. Constitutive and β-naphthoflavone-induced expression of the human γ-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J. Biol. Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 27.Moinova HR, et al. An electrophile responsive element (EpRE) regulates β-naphthoflavone induction of the human γ-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J. Biol. Chem. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 28.Lou H, et al. Induction of AKR1C2 by phase II inducers: identification of a distal consensus antioxidant response element regulated by NRF2. Mol. Pharmacol. 2006;69:1662–1672. doi: 10.1124/mol.105.019794. [DOI] [PubMed] [Google Scholar]
- 29.Ciaccio PJ, et al. Regulation of human dihydrodiol dehydrogenase by Michael acceptor xenobiotics. J. Biol. Chem. 1994;269:15558–15562. [PubMed] [Google Scholar]
- 30.Burczynski ME, et al. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res. 1999;59:607–614. [PubMed] [Google Scholar]
- 31.Bonnesen C, et al. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 32.Li J, et al. Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J. Biol. Chem. 2002;277:388–394. doi: 10.1074/jbc.M109380200. [DOI] [PubMed] [Google Scholar]
- 33.Devling TW, et al. Utility of siRNA against Keap1 as a strategy to stimulate a cancer chemopreventive phenotype. Proc. Natl Acad. Sci. USA. 2005;102:7280A–7285A. doi: 10.1073/pnas.0501475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XJ, et al. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006;66:10983–10994. doi: 10.1158/0008-5472.CAN-06-2298. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, et al. Aldo-keto reductases and bioactivation/detoxication. Annu. Rev. Pharmacol. Toxicol. 2007;47:263–292. doi: 10.1146/annurev.pharmtox.47.120505.105337. [DOI] [PubMed] [Google Scholar]
- 36.Lehman TA, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- 37.Boukamp P, et al. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer. 1997;19:201–214. doi: 10.1002/(sici)1098-2264(199708)19:4<201::aid-gcc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Weng L, et al. Rosetta error model for gene expression analysis. Bioinformatics. 2006;22:1111–1121. doi: 10.1093/bioinformatics/btl045. [DOI] [PubMed] [Google Scholar]
- 39.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 40.Kelly VP, et al. Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H:quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- 41.O'Connor T, et al. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999;343:487–504. [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HK, et al. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Higgins LG, et al. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi N, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 45.Burczynski ME, et al. The reactive oxygen species and Michael acceptor-inducible human aldo-keto reductase AKR1C1 reduces the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. J. Biol. Chem. 2001;276:2890–2897. doi: 10.1074/jbc.M006655200. [DOI] [PubMed] [Google Scholar]
- 46.Gao X, et al. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukaemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane. Proc. Natl Acad. Sci. USA. 2001;98:15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks JD, et al. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol. Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 48.Traka M, et al. Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J. Nutr. 2005;135:1865–1872. doi: 10.1093/jn/135.8.1865. [DOI] [PubMed] [Google Scholar]
- 49.Gasper AV, et al. Consuming broccoli does not induce genes associated with xenobiotic metabolism and cell cycle control in human gastric mucosa. J. Nutr. 2007;137:1718–1724. doi: 10.1093/jn/137.7.1718. [DOI] [PubMed] [Google Scholar]
- 50.Dinkova-Kostova AT, et al. Induction of the Phase 2 response in mouse and human skin by sulforaphane-containing Broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 51.Durchdewald M, et al. Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo. J. Invest. Dermatol. 2007;127:646–653. doi: 10.1038/sj.jid.5700585. [DOI] [PubMed] [Google Scholar]
- 52.Marrot L, et al. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 53.Wagner AE, et al. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp. Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00928.x. DOI: 10.1111/j.1600-0625.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, et al. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem. J. 2006;399:373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wild AC, et al. Regulation of γ-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, et al. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol. Cell. Biol. 2006;26:7942–7952. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dohi Y, et al. Heme oxygenase-1 gene enhancer manifests silencing activity in a chromatin environment prior to oxidative stress. Antioxid. Redox Signal. 2006;8:60–67. doi: 10.1089/ars.2006.8.60. [DOI] [PubMed] [Google Scholar]
- 58.Singh A, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta T, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 60.Shibata T, et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl Acad. Sci. USA. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes JD, et al. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Gallego O, et al. Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem. J. 2006;399:101–109. doi: 10.1042/BJ20051988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan R, et al. Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: implication for cancer intervention. Int. J. Cancer. 2007;121:2301–2306. doi: 10.1002/ijc.22933. [DOI] [PubMed] [Google Scholar]
- 64.Palackal NT, et al. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J. Biol. Chem. 2002;277:24799–24808. doi: 10.1074/jbc.M112424200. [DOI] [PubMed] [Google Scholar]
- 65.Park JH, et al. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc. Natl Acad. Sci. USA. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukumoto S, et al. Overexpression of the aldo-keto reductase family protein AKR1B10 is highly correlated with smokers’ non-small cell lung carcinomas. Clin. Cancer Res. 2005;11:1776–1785. doi: 10.1158/1078-0432.CCR-04-1238. [DOI] [PubMed] [Google Scholar]
- 67.Woenckhaus M, et al. Smoking and cancer-related gene expression in bronchial epithelium and non-small-cell lung cancers. J. Pathol. 2006;210:192–204. doi: 10.1002/path.2039. [DOI] [PubMed] [Google Scholar]
- 68.Penning TM, et al. Genomics of smoking exposure and cessation: lessons for cancer prevention. Cancer Prev. Res. 2008;1:80–83. doi: 10.1158/1940-6207.CAPR-08-0047. [DOI] [PubMed] [Google Scholar]
- 69.Zeindl-Eberhart E, et al. Detection and identification of tumor-associated protein variants in human hepatocellular carcinomas. Hepatology. 2004;39:540–549. doi: 10.1002/hep.20060. [DOI] [PubMed] [Google Scholar]
- 70.Gomes LI, et al. Expression profile of malignant and nonmalignant lesions of esophagus and stomach: differential activity of functional modules related to inflammation and lipid metabolism. Cancer Res. 2005;65:7127–7136. doi: 10.1158/0008-5472.CAN-05-1035. [DOI] [PubMed] [Google Scholar]
- 71.Dressman HK, et al. Gene expression profiles of multiple breast cancer phenotypes and response to neoadjuvant chemotherapy. Clin. Cancer Res. 2006;12:819–826. doi: 10.1158/1078-0432.CCR-05-1447. [DOI] [PubMed] [Google Scholar]
- 72.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]