Abstract
Atopic dermatitis (AD) is a chronic inflammatory disease characterized by the impairment of the skin-barrier function, increased oxidative cellular stress, and bacterial colonization. Hence, medical therapies of AD aim to control infection, reduce inflammation, and restore skin-barrier function by use of topical and systemic antibacterial drugs, topical corticosteroids, topical calcineurin inhibitors, and moisturizers. Textiles have the longest and most intense contact with the human skin, and functional textiles with intrinsic properties such as antioxidative capacity and antibacterial activity have been gaining in importance in medical applications. Specially designed textiles may support AD treatment and improve quality of life of AD. Here, we investigated the role of ZnO-functionalized textile fibers in the control of oxidative stress in AD in vitro and in vivo. In addition, the antibacterial effect and biocompatibility of the Zn textile was evaluated in vitro. We observed a rapid improvement of AD severity, pruritus, and subjective sleep quality when AD patients wore the ZnO textiles overnight on 3 consecutive days. This is possibly due to the high antioxidative capacity of the ZnO textile, as well as the allocation of strong antibacterial activity. Moreover, it was shown that the ZnO textiles possess very good biocompatibility and were well tolerated by AD patients.
Keywords: atopic dermatitis, antibacterial activity, biocompatibility, functionalized textiles, oxidative stress
Introduction
The skin is the external barrier of the human body. It is the most versatile human organ, plays a key role in protecting the body against environmental influences, and participates in the regulation of homeostasis and metabolic processes as well as immunological reactions. Exposure to ultraviolet light induces the generation of free radicals in the cells, like reactive oxygen species (ROS) and reactive nitrogen species (RNS). Increased oxidative stress has been documented in affected skin of individuals suffering from atopic dermatitis (AD)1–4 and seems to be involved in inflammation, skin-barrier impairment, and itch. Moreover, measurements of transepidermal water loss and corneometry have shown an inverse relation to AD severity.5 In addition, patients with AD display an augmented susceptibility to cutaneous bacterial, fungal, and viral infections.6,7 Microbial flora of atopic skin exhibits noticeable differences in contrast to normal skin, eg, more than 90% of patients with AD are colonized with Staphylococcus aureus, whereas it is found in less than 10% in healthy individuals.8 The carriage rate of AD patients for S. aureus was observed to be in inflammatory lesions and approximately 76% in uninvolved skin.9 Furthermore, the quantity of S. aureus could be related to the severity of AD.9,10 Hence, medical therapies of AD aim to control infection, reduce inflammation, and restore skin-barrier function by the use of topical and systemic antibacterial drugs,11 topical corticosteroids, topical calcineurin inhibitors, and moisturizers.12–14 Textiles have the longest contact with the human skin and have a major impact on its microenvironment. Hence, specially designed textiles may support medical treatment and improve quality of life of patients with AD.15
Here, we investigated the role of ZnO-functionalized textile fibers in the control of oxidative stress in AD in vitro and in vivo. In addition, the antibacterial effect and biocompatibility of the Zn textile was evaluated in vitro.
Material and methods
In vivo pilot trial
We investigated the in vivo effects of a special AD textile, Benevit Zink+ (Benevit Van Clewe, Dingden, Germany), which consists of 74% Lyocell fiber, 19% SmartCell sensitive fiber, and 7% spandex. In this uncontrolled pilot trial, adult patients with moderate to severe AD were enrolled after informed consent. Three days after individualized topical treatment with corticosteroids, calcineurin inhibitors, or coal tar, the ZnO textile was used for overnight underclothes with trousers and long-sleeve shirts (Figure 1) for three subsequent nights. AD diagnosis was performed according to the clinical criteria of Hanifin and Rajka.16 Severity was calculated by the three item severity score index before and after use of the Zn textile.17 The severity of pruritus was scored by the 5D itch scale.18 Subjective quality of sleep was assessed by a modified sleep-habits questionnaire.19
Figure 1.
Benevit Zink+ clothing contains 74% Lyocell fiber, 19% SmartCell sensitive fiber (comprised of ZnO), and 7% spandex.
Notes: Adult patients with moderate to severe atopic dermatitis were enrolled after informed consent. This ZnO textile was used for overnight underclothes in the form of trousers and long-sleeve shirts.
In vitro study
Measurement of antioxidative capacity
The antioxidative capacity (AOC) of the ZnO textile against ROS was assessed by an Abel antioxidant test kit with Pholasin for superoxide and other free radicals (Knight Scientific, Plymouth, UK) and scavenging of RNS was determined by Abel antioxidant test kits specific for peroxynitrite anion (Knight Scientific). The assays were performed as described earlier.20 In brief, to each ZnO-textile sample (0.25 cm2 and 0.5 cm2) the assay solutions were added. Subsequent to injecting the solution, generating free radicals, luminescence intensity was measured using the Novostar Galaxy plate reader (BMG Labtech, Ortenberg, Germany). A control without sample was included for each assay. Antioxidant activity impedes the development of the luminescence peak (peroxynitrite assay) and decreases its light intensity (peroxynitrite assay and superoxide assay). AOC of a sample was calculated as percentage reduction of peak luminescence, as given in equation (1). All experiments were carried out twice with a triplicate sample set. Results are expressed as means ± standard deviation. For statistical analysis, Student’s t-test was performed. Statistical significance was defined as P < 0.05. Statistically significant results are identified by asterisk symbols: ***P ≤ 0.001, **P ≤ 0.01, and *P ≤ 0.05.
| (1) |
Determination of antibacterial activity according to JIS L 1902
Testing for antibacterial activity was carried out in accordance with Japanese Industrial Standards L 1902: 2002, as reported previously.21Staphylococcus aureus (American Type Culture Collection 6538) and Klebsiella pneumoniae were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). For bacteria cultivation, special peptone and Lab-Lemco powder for fabrication of caso-bouillon and bacteriological agar were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Columbia agar plates with 5% sheep blood were acquired from BioMeriéux (Marcy l’Etoile, France) and 0.9% NaCl solution from Fresenius Kabi (Bad Homburg, Germany). Test microbes were cultivated in caso-bouillon for 24 hours at 37°C under aerobic conditions. For experiments, 400 mg samples of the ZnO textile were inoculated with 200 μL test microbe solution and incubated for 24 hours at 37°C under aerobic conditions. Polyester material (ITS Textilhandels, Rutzenham, Austria) was used as growth control. For determination of the germ number, the incubated samples were extracted in 0.9% NaCl solution supplemented with 0.2% Tween 20. Serial dilutions were plated onto Columbia agar plates and incubated for 24 hours at 37°C. Subsequently, colonies were counted, total colony-forming units (cfu) determined, and growth reduction calculated according to equation (2). A logarithmic microbial growth reduction of less than 0.5 represented no antibacterial activity. Values between 0.5 and 1 were rated as slight, values greater than 1 and less or equal to 3 as significant, and a log reduction greater than 3 as strong antibacterial activity.
| (2) |
Evaluation of biocompatibility according to DIN EN ISO 10993-5
For evaluation of biocompatibility, extracts of the ZnO textile were fabricated according to the standard used for evaluation of textile cytotoxicity (DIN EN ISO 10993-12). In brief, 1 g was incubated in 50 mL of Dulbecco’s modified Eagle’s medium (DMEM) in Erlenmeyer flasks (Greiner, Frickenhausen, Germany) at 37°C for 24 hours under shaking (ThermoBath, GFL, Großburgwedel, Germany). At all times, samples were handled under sterile conditions and extra care was taken for avoiding cross-contamination. Subsequently, extracts were centrifuged at 1000 rpm over gauze to dispose of insoluble material residues. The resulting filtrate was sterilized afterwards by passage through a 0.2 μm filter and classified as original extract (100%).
Human HaCaT keratinocytes were a kind gift from NE Fusenig of the German Cancer Research Center, Heidelberg, Germany. The cells are routinely cultured in DMEM supplemented with 1% antibiotic-antimycotic solution (10,000 U/mL penicillin, 10,000 μg/mL streptomycin, 25 μg/mL amphotericin; PromoCell, Heidelberg, Germany) and 10% fetal calf serum (PromoCell) for 7 days in 75 cm2 cell-culture flasks (Greiner) at 37°C in a humidified atmosphere containing 5% CO2. For biocompatibility experiments, cells were harvested by trypsin-ethylenediaminetetraacetic acid (PromoCell) treatment, seeded into 96-well plates (Greiner) at a density of 40,000 cells/cm2, and left for 48 hours. Then, culture medium was swapped for either fresh medium (negative control) or Zn-textile extracts in medium (original extract 100%, extraction ratio 1 g:50 mL) and serial dilutions of the original extract (75% at 0.75 g:50 mL, 50% at 0.5 g:50 mL, 25% at 0.25 g:50 mL, and 10% at 0.1 g:50 mL). As positive control for cytotoxicity, a Triton-X 100 (Merck, Darmstadt, Germany) was utilized. The HaCaT cells were afterwards incubated for 1, 24, and 48 hours followed by determination of cell viability and proliferation, which was performed on the basis of a luminometric adenosine triphosphate (ATP) assay (ATPLite M Assay; PerkinElmer, Waltham, MA, USA), as reported previously.22 Briefly, lysis solution was added to each well, followed by the substrate solution (luciferase/d-luciferin). After incubation, luminescence was determined using the Lumistar Galaxy microplate luminometer (BMG Labtech). Cellular ATP content was calculated on the basis of a standard curve. For illustration, the number of viable cells was expressed as a percentage of the control cells at the respective time point. Cytotoxic effects were analyzed by measurement of lactate dehydrogenase release using a cytotoxicity detection kit (Roche, Basel, Switzerland). The assay was run according to the manufacturer’s instructions. Optical density was determined at 490 nm with a reference measurement at 620 nm (Fluostar; BMG Labtech). Afterwards, cytotoxicity was evaluated by calculation of the percentage of the optical density of treated cells compared to control cells.
Results
In vivo results
The results of the in vivo pilot trial are summarized in Table 1. Twelve adult AD patients were included (five women, seven men) with a mean age of 60.6 ± 15.1 years (range 28–78 years). Before the use of the Zn textile, the average pruritus duration was 6–24 hours a day. Only four patients reported less than 6 hours of pruritus. After three nights with the Zn textile, ten patients reported less than 6 hours of pruritus. None of the patients suffered from pruritus all day long. Pruritus severity dropped from 3.5 ± 1.2 to 2.4 ± 1.1. Impairment by pruritus was scored 3.7 ± 0.7 and decreased to 2.1 ± 1.1.
Table 1.
Summary of the results of the in vivo pilot study
| Before trial | After trial | |
|---|---|---|
| Pruritus severity | 3.5 ± 1.2 | 2.4 ± 1.1 |
| Erythema | 2.2 ± 0.8 | 1.0 ± 0.8 |
| Edema and papules | 1.9 ± 0.8 | 0.8 ± 0.6 |
| Excoriations | 1.7 ± 1.1 | 0.6 ± 0.5 |
| Impairment by pruritus | 3.7 ± 0.7 | 2.1 ± 1.1 |
| Impairment of night sleep | 1.7 ± 1.4 | 1.4 ± 0.7 |
| Impairment at work | 1.4 ± 1.5 | 0.7 ± 1.2 |
| Impairment at leisure time | 1.4 ± 1.6 | 0.7 ± 1.2 |
| Impairment at housework | 1.4 ± 1.5 | 1.0 ± 1.3 |
The impairment of night sleep improved from 1.7 ± 1.4 to 1.4 ± 0.7. Impairment during professional activities improved from 1.4 ± 1.5 to 0.7 ± 1.2. Impairment of leisure time was reduced from 1.4 ± 1.6 to 0.7 ± 1.2. Housework impairment also decreased from 1.4 ± 1.5 to 1.0 ± 1.3.
Overall, sleep quality seemed to be better after use of the Zn textile. The number of patients suffering from wakefulness was reduced from five to two. Problems falling asleep were reported by six patients before use of the Zn textile and reduced to four patients after the use of the Zn textile overnight. Wake-up periods were noted by nine patients before and only six patients after the test period. Sleeping duration remained unchanged.
In addition, clinical cutaneous symptoms improved distinctly. Erythema decreased from 2.2 ± 0.8 to 1.0 ± 0.8, edema and papules decreased from 1.9 ± 0.8 to 0.8 ± 0.6, and excoriations reduced from 1.7 ± 1.1 to 0.6 ± 0.5 (Figures 2 and 3).
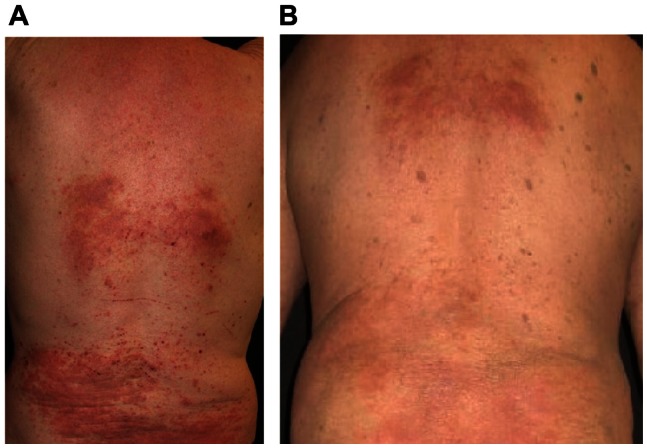
Figure 2.
Clinical improvement of nummular atopic dermatitis with adjuvant ZnO textile overnight (A) before and (B) after 4 days.
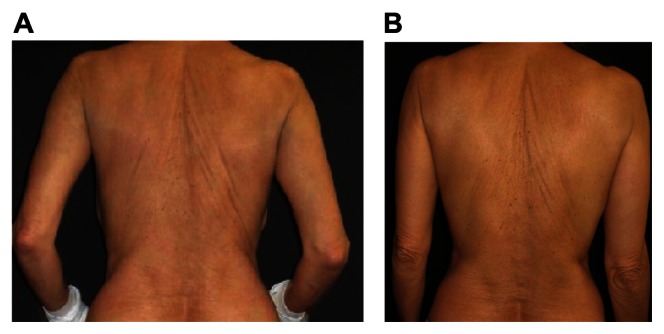
Figure 3.
Clinical improvement of disseminated atopic dermatitis with adjuvant ZnO textile overnight (A) before and (B) after 4 days.
In vitro effects
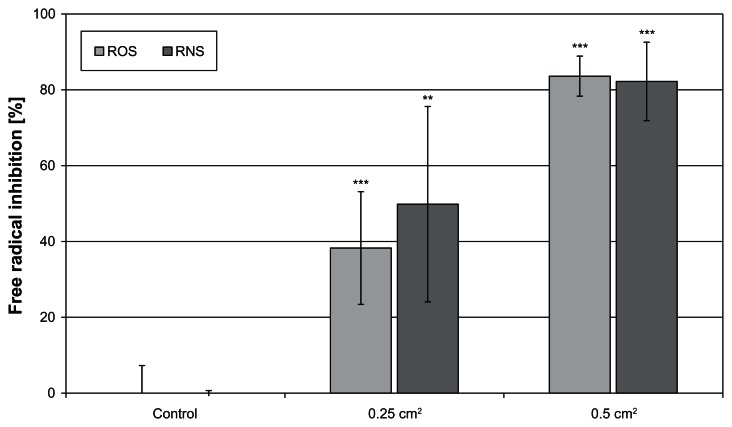
The Zn textile exhibited a significant antioxidant capacity against ROS as well as RNS in vitro (Figure 4). As illustrated, it exerted a dose-dependent effect. Moreover, it was shown that it is equally effective against ROS and RNS.
Figure 4.
A high antioxidative capacity was determined for the ZnO-textile.
Notes: It was equally effective in inhibiting reactive oxygen species (ROS) and reactive nitrogen species (RNS) formation in vitro. **P ≤ 0 0.01; ***P ≤ 0.001.
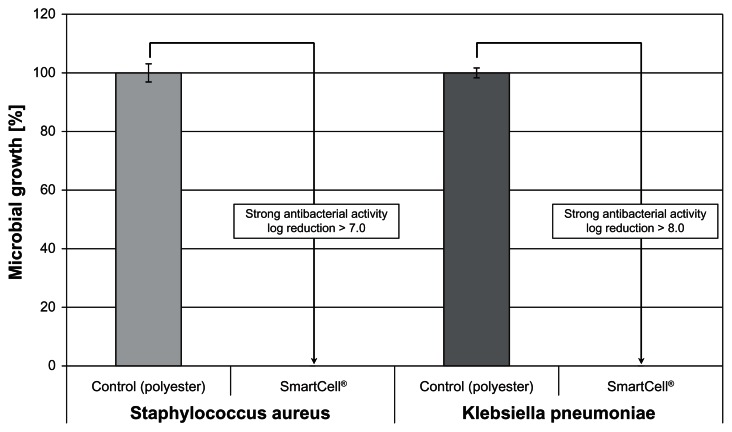
In addition, strong antibacterial activity against both Staphylococcus aureus and Klebsiella pneumoniae was observed, yielding log reductions > 3 (Figure 5). Bacterial growth was completely inhibited after a 24-hour incubation period.
Figure 5.
The ZnO textile possesses a strong antibacterial effect (log reduction > 3) against both Staphylococcus aureus and Klebsiella pneumoniae.
Note: Complete inhibition of microbial growth was achieved.
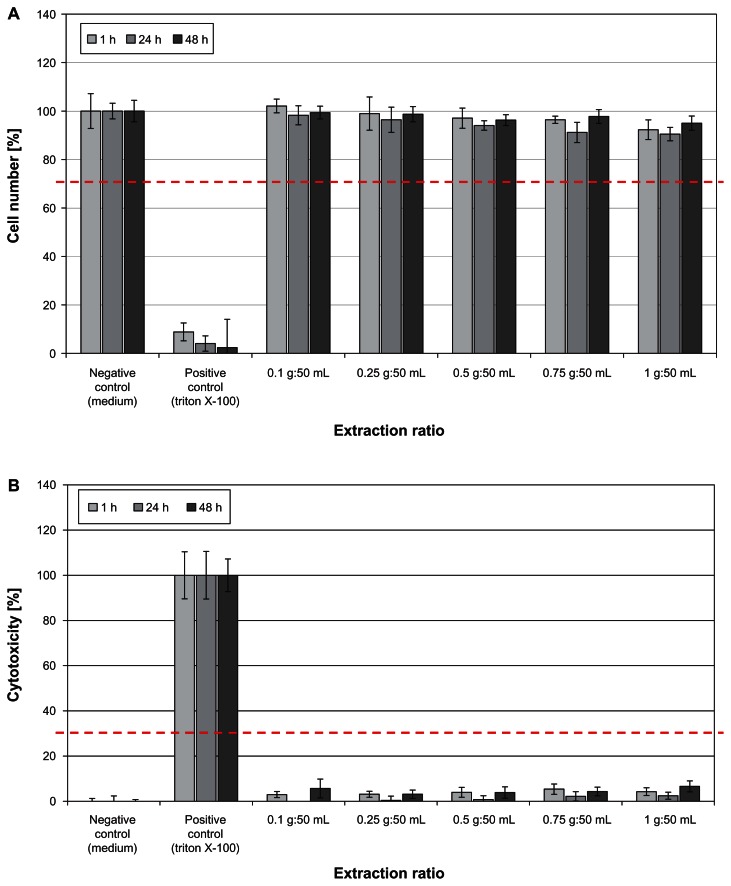
Figure 6 shows the results of the biocompatibility study performed according to DIN EN ISO 10993-5, used for evaluation of cytotoxic effects of textiles. Treatment of human HaCaT keratinocytes with Zn-textile extract had no negative influence on cell viability or cell proliferation (Figure 6A). In accordance, no cytotoxic potential, inducing cell death by necrosis, was observed (Figure 6B).
Figure 6.
(A) Determination of HaCaT keratinocyte cell viability and cell proliferation after 1, 24, and 48 hours of incubation with ZnO-textile extract. No negative effect on the cells was observed in vitro. At no time point or extraction ratio tested did cell numbers drop below the 70% threshold (red dotted line). (B) A significant release of lactate dehydrogenase was observed only for the positive control Triton X-100.
Notes: None of the ZnO textile-extraction ratios tested exhibited a cytotoxic potential. In accordance, cytotoxicity did not reach the threshold of 30% (red dotted line).
Discussion
AD is a chronic inflammatory disease characterized by the impairment of the skin-barrier function, increased oxidative cellular stress, and bacterial colonization. The development of new textiles with intrinsic properties such as antioxidative capacity and antibacterial activity presents a promising path in the treatment and maintenance of AD. Here, we observed a rapid improvement of AD severity, pruritus, and subjective sleep quality when AD patients wore the Benevit Zink+ clothing overnight. This textile comprises a specially coated ZnO fiber with high AOC against ROS and RNS, as well as strong antibacterial activity. In general, natural fibers like silk or cotton are preferred for individuals with sensitive skin or AD.23 These fibers can be functionalized by incorporation of silver or chitosan to reduce colonization of AD skin by staphylococci for improvement of disease severity.24–26 However, negative effects on cells of both silver ions21,27 and chitosan formulations22,28,29 have been observed in vitro. Moreover, the use of silver compounds has been associated with the risk of inducing bacterial adaptation.30–32 Hence, there is a demand for alternative effective compounds. ZnO may present an interesting option, as it provides sun protection33 and has been shown to improve hypertrophic scars.34 ZnO helps to restore the disturbed skin-barrier function in eczematous diseases35 and enhances wound healing.36 Moreover, ZnO is considered safe to use, since it does not penetrate the skin, even with disturbed barrier function.37,38 In accordance, high biocompatibility of the Benevit Zink+ was proven in this study in vitro. In addition, we were able to demonstrate that short time usage of this ZnO textile can contribute to improvement of night sleep, pruritus, and AD severity when used adjuvant to medical treatment. Hereby, an improvement of quality of life can be achieved for AD patients.
Acknowledgments
The authors would like to thank Dr Michael Zieger, Doreen Winter, Peggy Gasch, and Martina Grebner for excellent technical assistance.
Footnotes
Disclosure
The study was supported by an unrestricted grant of Benevit Van Clewe, Dingden, Germany. The authors report no other conflicts of interest in this work.
References
- 1.Kubo M, Kambayashi Y, Takemoto K, Okuda J, Muto M, Ogino K. Reactive nitrogen species formation in eosinophils and imbalance in nitric oxide metabolism are involved in atopic dermatitis-like skin lesions in NC/Nga mice. Free Radic Res. 2005;39(7):719–727. doi: 10.1080/10715760500139260. [DOI] [PubMed] [Google Scholar]
- 2.Korkina L, Pastore S. The role of redox regulation in the normal physiology and inflammatory diseases of skin. Front Biosci (Elite Ed) 2009;1:123–141. doi: 10.2741/E13. [DOI] [PubMed] [Google Scholar]
- 3.Chung J, Oh SY, Shin YK. Association of glutathione-S-transferase polymorphisms with atopic dermatitis risk in preschool age children. Clin Chem Lab Med. 2009;47(12):1475–1481. doi: 10.1515/CCLM.2009.336. [DOI] [PubMed] [Google Scholar]
- 4.Sapuntsova SG, Lebed’ko OA, Shchetkina MV, Fleyshman MY, Kozulin EA, Timoshin SS. Status of free-radical oxidation and proliferation processes in patients with atopic dermatitis and lichen planus. Bull Exp Biol Med. 2011;150(6):690–692. doi: 10.1007/s10517-011-1224-0. [DOI] [PubMed] [Google Scholar]
- 5.Addor FA, Takaoka R, Rivitti EA, Aoki V. Atopic dermatitis: correlation between non-damaged skin barrier function and disease activity. Int J Dermatol. 2012;51(6):672–676. doi: 10.1111/j.1365-4632.2011.05176.x. [DOI] [PubMed] [Google Scholar]
- 6.Melnik B. Disturbances of antimicrobial lipids in atopic dermatitis. J Dtsch Dermatol Ges. 2006;2(2):114–123. doi: 10.1111/j.1610-0387.2006.05902.x. German. [DOI] [PubMed] [Google Scholar]
- 7.Baker BS. The role of microorgamisms in atopic dermatitis. Clin Exp Dermatol. 2006;144(1):1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90(5):525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 9.Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113(6):780–782. [PubMed] [Google Scholar]
- 10.Roll A, Cozzio A, Fischer B, Schmid-Grendelmeier P. Microbial colonization and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004;4(5):373–378. doi: 10.1097/00130832-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Stinco G, Piccirillo F, Valent F. A randomized double-blind study to investigate the clinical efficacy of adding a non-migrating antimicrobial to a special silk fabric in the treatment of atopic dermatitis. Dermatol. 2008;217(3):191–195. doi: 10.1159/000141648. [DOI] [PubMed] [Google Scholar]
- 12.Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26(8):1045–1060. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 13.Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part II. J Eur Acad Dermatol Venereol. 2012;26(9):1176–1193. doi: 10.1111/j.1468-3083.2012.04636.x. [DOI] [PubMed] [Google Scholar]
- 14.Valdman-Grinshpoun Y, Ben-Amitai D, Zvulunov A. Barrier-restoring therapies in atopic dermatitis: current approaches and future perspectives. Dermatol Res Pract. 2012;2012:923134. doi: 10.1155/2012/923134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollina U, Abdel-Naser MB, Verma S. Skin physiology and textiles – consideration of basic interactions. Curr Probl Dermatol. 2006;33:1–16. doi: 10.1159/000093926. [DOI] [PubMed] [Google Scholar]
- 16.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. (Stockh) 1980;59(Suppl 92):44–47. [Google Scholar]
- 17.Wolkerstorfer A, de Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol (Stockh) 1999;79(5):356–359. doi: 10.1080/000155599750010256. [DOI] [PubMed] [Google Scholar]
- 18.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measurement of pruritus. Br J Dermatol. 2010;162(3):587–593. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unruh ML, Sanders MH, Redline S, et al. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52(2):305–313. doi: 10.1053/j.ajkd.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schönfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler UC. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005;26(33):6664–6673. doi: 10.1016/j.biomaterials.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Wiegand C, Heinze T, Hipler UC. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009;17(5):511–521. doi: 10.1111/j.1524-475X.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiegand C, Winter D, Hipler UC. Molecular-weight-dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Skin Pharmacol Physiol. 2010;23(3):164–170. doi: 10.1159/000276996. [DOI] [PubMed] [Google Scholar]
- 23.Ricci G, Neri I, Ricci L, Patrizi A. Silk fabrics in the management of atopic dermatitis. Skin Therapy Lett. 2012;17(3):5–7. [PubMed] [Google Scholar]
- 24.Fluhr JW, Breternitz M, Kowatzki D, et al. Silver-loaded seaweed-based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: safety assessment, mode of action and controlled, randomized single-blinded exploratory in vivo study. Exp Dermatol. 2010;19(8):e9–e15. doi: 10.1111/j.1600-0625.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 25.Park KY, Jang WS, Yang GW, et al. A pilot study of silver-loaded cellulose fabric with incorporated seaweed for the treatment of atopic dermatitis. Clin Exp Dermatol. 2012;37(5):512–515. doi: 10.1111/j.1365-2230.2011.04273.x. [DOI] [PubMed] [Google Scholar]
- 26.Tavaria FK, Soares JC, Reis IL, Paulo MH, Malcata FX, Pintado ME. Chitosan: antimicrobial action upon staphylococci after impregnation onto cotton fabric. J Appl Microbiol. 2012;112(5):1034–1041. doi: 10.1111/j.1365-2672.2012.05274.x. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo E, Bartolomé R, Barroso C, Moreno A, Domínguez C. Silver nitrate: antimicrobial activity related to cytotoxicity in cultured human fibroblasts. Skin Pharmacol Appl Skin Physiol. 1998;11(3):140–151. doi: 10.1159/000029820. [DOI] [PubMed] [Google Scholar]
- 28.Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials. 2001;22(3):261–268. doi: 10.1016/s0142-9612(00)00183-6. [DOI] [PubMed] [Google Scholar]
- 29.Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27(2–3):341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 31.Warriner R, Burrell R. Infection and the chronic wound: a focus on silver. Adv Skin Wound Care. 2005;18(Suppl 1):2–12. doi: 10.1097/00129334-200510001-00001. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand C, Abel M, Ruth P, Hipler UC. Analysis of the adaptation capacity of Staphylococcus aureus to commonly used antiseptics by microplate laser nephelometry. Skin Pharmacol Physiol. 2012;25(6):288–297. doi: 10.1159/000341222. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu I, MacFarlane D. Zinc oxide paste as sunscreen in the postoperative period. Dermatol Surg. 2012;38(6):965–966. doi: 10.1111/j.1524-4725.2012.02414.x. [DOI] [PubMed] [Google Scholar]
- 34.Aksoy B, Atakan N, Aksoy HM, et al. Effectiveness of topical zinc oxide application on hypertrophic scar development in rabbits. Burns. 2010;36(7):1027–1035. doi: 10.1016/j.burns.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Xhauflaire-Uhoda E, Henry F, Piérard-Franchimont C, Piérard GE. Electrometric assessment of the effect of a zinc oxide paste in diaper dermatitis. Int J Cosmet Sci. 2009;31(5):369–374. doi: 10.1111/j.1468-2494.2009.00505.x. [DOI] [PubMed] [Google Scholar]
- 36.Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15(1):2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 37.Zvyagin AV, Zhao X, Gierden A, Sanchez W, Ross JA, Roberts MS. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J Biomed Opt. 2008;13(6):064031. doi: 10.1117/1.3041492. [DOI] [PubMed] [Google Scholar]
- 38.Lin LL, Grice JE, Butler MK, et al. Time-correlated single photon counting for simultaneous monitoring of zinc oxide nanoparticles and NAD(P)H in intact and barrier-disrupted volunteer skin. Pharm Res. 2011;28(11):2920–2930. doi: 10.1007/s11095-011-0515-5. [DOI] [PubMed] [Google Scholar]