Abstract
Significance
Clinical healing by secondary intention frequently occurs in skin that is firmly anchored to underlying (human) connective tissue. Small animals (rodents) are extensively utilized to model human cutaneous wound healing, but they heal by wound contraction, a process that is limited in the human and confounds quantitative and qualitative evaluation of experimental wound repair.
Recent Advances
To alleviate wound contraction in loose-skinned species, practical solutions include choosing anatomical sites with firmly attached dermis and subcutis (e.g., rabbit ear) or performing mechanical fixation of the skin by using one of a number of devices or splints. In each case, the wound volume remains relatively constant, allowing the histomorphometric or biomolecular quantification of the cellular response under well-controlled, experimental conditions. In addition, the defined aperture of the splinted wound allows the placement of a variety of materials, including scaffolds, cells, and biologically active formulations into the wound site in an effort to potentiate the healing response and abrogate scarring. In contrast, production of larger experimental wounds or the deliberate distraction of wound margins can be used to model a hypertrophic response.
Critical Issues
Device design parameters should consider ease of application, durability, and lack of interference with the normal influx of local and circulating cells to the wound site.
Future Directions
Improved methods of securing flexible splints would provide a more efficient experimental platform. These devices could also incorporate optical or electronic sensors that report both the mechanical and physiological status of the healing.

Jeffrey M. Davidson, PhD
Scope
This review discusses the limitations of traditional excisional animal wound healing models and recent approaches that alleviate or exacerbate these drawbacks by utilizing endogenous and mechanical, device-based splinting or distraction.
Translational Relevance
Animal models are valuable to understanding basic mechanisms of wound healing under well-controlled, experimental conditions of biological replicates. Wounds that have well-defined, stable dimensions are optimal for determining biochemical, molecular, histologic, and histomorphometric aspects of repair. From such studies treatment regimes to potentiate repair can be validated as a precursor to human clinical trials.
Clinical Relevance
Wound contraction is one of the most significant limitations to using loose-skinned animals to model human wounds. These animals contain a panniculus carnosus, a thin sheet of striated muscle lying between the subcutaneous fat and dermal layer as well as a loosely organized subdermal fascial plane. Upon injury, this anatomy permits wound contraction that is often misrepresented as “wound closure” and therefore as “healing.” Contraction does not accurately reflect human wound healing by secondary intention, in which the wound margins remain separated and are not brought together by mechanical means (e.g., suture). Unrestrained contraction counteracts the accumulation of granulation tissue and the extent of epidermal migration and resurfacing. Because of the rapid contraction of unsplinted rodent wounds, data derived from their closure are more representative of healing by primary intention.
Practical Models
Rabbit ear “ulcers”
The Mustoe laboratory developed one of the earliest and most widely utilized models of the splinted wound, taking advantage of the anatomy of the rabbit ear.1 Both the inner and outer skin of this relatively large and easily accessible appendage are firmly attached to the underlying auricular cartilage, with no intervening muscle or adipose tissue. As a consequence, careful removal of the skin and underlying perichondrium results in a complete cutaneous defect with an avascular base (Fig. 1A). Granulation tissue fills the wound cavity to support subsequent epidermal resurfacing by migration and proliferation. This wound site, when covered with appropriate dressings, can be used as a test bed for a wide variety of wound healing agents. Further, to study ischemia on wound repair in the rabbit ear, the well-defined vascular supply of the pinna can be partially obliterated to create temporary ischemia that reverses over the course of ∼2 weeks by development of collateral circulation.1 The major limitations of the ear model are the process of healing over an avascular cartilage base and—with respect to materials testing—the moderate thickness of the skin as compared to the trunk.
Figure 1.
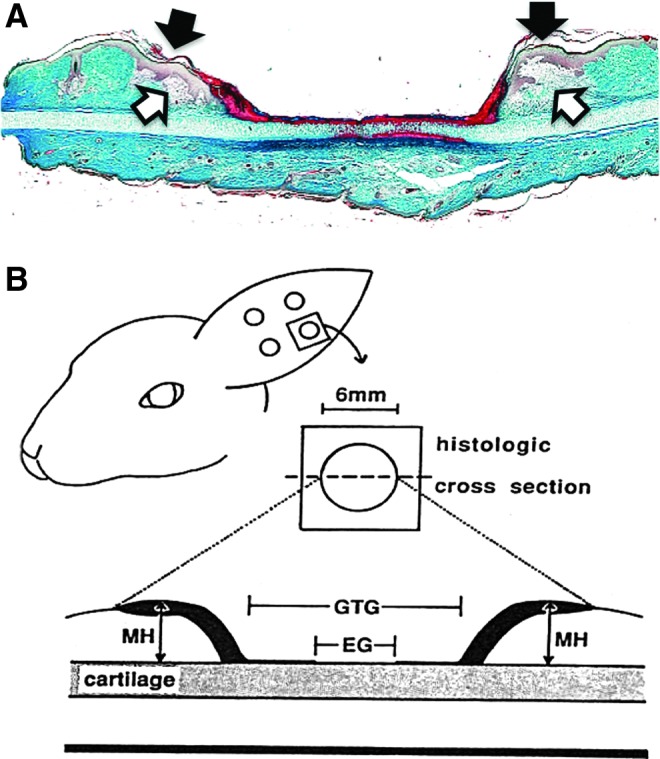
The rabbit ear model: a self-splinted wound. (A) Histological aspect of the ear wound 3 days after surgery (Masson's trichrome). Granulation tissue formation has initiated at the wound margins (open arrows), and there is a mild epidermal hyperplasia (solid arrows) that precedes migration over new granulation tissue. (B) Schematic representation of morphometric features that can be evaluated by a transverse section through the center of the excisional wound. EG, epithelial gap; GTG, granulation tissue gap; MH, maximum height. Reprinted from Ahn and Mustoe1 with permission. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The fixed dimensions of the rabbit ear model make quantitative morphometric analysis relatively straightforward (Fig. 1B), as long as the true diameter of the wound is visualized in tissue sections. Under standard conditions, there is a mild elevation of the resurfaced wound above the plane of the adjacent, normal skin, but this hypertrophy rapidly resolves, suggesting that the mechanical environment that is created by self-splinting does not produce a scar. In contrast, larger excisions, >7 mm in diameter, produce a hypertrophic response with significant thickening of the scar tissue for several weeks.2,3 This response may be due to the protracted time to reach complete reepithelization or the presence of a significant area of granulation tissue within an altered mechanical environment. This model has been used to investigate the anti-scarring activities of biologicals, drugs, and mechanical compression devices.3–6
Other naturally splinted wounds
While the rabbit ear model has numerous advantages, there are some limitations. The rabbit is not a useful genetic model, and ear skin is histologically simpler than trunk skin. As a result, a number of investigations have resulted in the development of two simple surgical models in the genetically tractable mouse that rely on the same principle of firmly anchored skin: cranial and tail excisions. These two approaches have been used to a limited extent in the mouse. The cranial model7 uses a (6 mm) biopsy punch to remove skin from the crown to the depth of the calvarial periosteum.8 Wounds in this area may also have the advantage of reduced self-grooming. The tail model9 involves the circumferential removal of a collar of both epidermis and dermis from a region between 2 and 3 cm from the base of the tail. In both of these cases as well as the rabbit ear model, the restoration of tissue integrity occurs in the absence of underlying soft connective tissue.
The presence of large deposits of subcutaneous fat also has a splinting effect in rats and mice. Indeed, excisional wounds in ob/ob and db/db mice actually increase in gape/diameter after surgery due to obesity10 and the extrusion of adipose tissue into the wound void. This phenomenon acts as a further retarding factor in the closure and resurfacing of the wound. The expansion of the wound can be limited by application of splinting devices, such as discussed in this review.
Mouse silicone rings
The silicone rubber “doughnut” is an innovative device that is now in wide use for reducing the contraction of rodent excisional wounds.11,12 The device is punched out of a 1 mm thick silicone rubber sheet, and an aperture matching the dimensions of the excisional wound is then cut in the middle of the circle to create a ring around the surgical site.13 The device is fixed in place on freshly shaved skin with or without depilation by a combination of adhesive and 6 or more sutures around the perimeter. One or more layers of dressing material may cover the attached device (Fig. 2). As a result, wound contraction is markedly reduced, and the wound cavity fills with granulation tissue and a migratory epidermis. Unless a durable adhesive is used, the anchoring sutures will become the main points of skin fixation, resulting in a somewhat stellate margin to the wound. Reduced contraction allows the investigator to produce reliable estimates of granulation tissue dimensions, cellularity, and epidermal migration.
Figure 2.
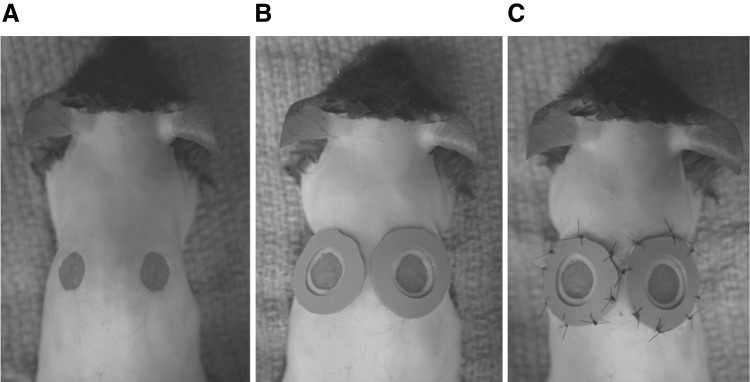
Mouse silicone splint model. The panels illustrate the preparation (A), application (B), and securing (C) of hand made silicone rings cut from a sheet of stock material. A covering film and a bandage or jacket are important further steps to keep the wound sites undisturbed. Reprinted from Galiano et al.13 with permission.
The main drawback of the silicone sutured ring, rarely discussed in publication, is the mutilation of the soft, thin material by self-grooming or interactions with cage mates. Using more protective dressings or a jacket with plastic wings that project caudally over the surgical site (Lomir Biomedical, Inc., Malone, NY) can ameliorate the former problem. However, because of the motion and activity of the animals, there can be a fairly high failure rate. In a recent report, silicone rings have been subcutaneously secured to reduce ring loss.14
Steel rings
A more durable approach to diminish excisional wound contraction is a metal frame.15 We have conducted a number of sponsored studies in the rat and mouse using a mass-produced, commercially available stainless steel lock washer to splint 6–10 mm full-thickness excisional wounds (Fig. 3A). The toothed, outer perimeter of the lock washer facilitates a secure suture placement and, unlike the silicone washer, animals have much more difficulty in dislodging metallic rings with toothed edges. No fabrication is necessary and sterilization is simple. Applying these rings takes less surgical skill than the silicone ring. As with the silicone rings, the healed wounds have a somewhat stellate outline derived from the interrupted sutures that secure the device (Fig. 3B). Additional sutures could be used at the expense of time in surgery. This device permits a more controlled examination of the wound healing response, with well-defined granulation tissue filling and epidermal resurfacing of the open wound.
Figure 3.
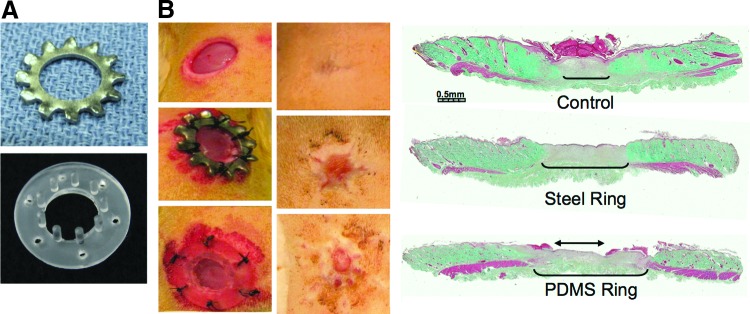
Comparison of metal and PDMS-based devices for wound splinting. (A) Devices. Examples of a commercial lock washer (top) and the molded PDMS device (bottom) with posts arranged around the inner diameter (10 mm) of the ring. The length and density of posts are typical for rat skin applications. (B) Ring application, 10 mm punch rat excisional wound. Top: surgery only control. Middle: steel ring. Bottom: PDMS device. Left: wound appearance, d0. Center: wound appearance, d14. Right: Histological aspect of d14 wounds, Masson's trichrome. The wound gap is marked by the margins of the (red) panniculus carnosus and the extent of new granulation tissue (brackets). Epithelization was completed in the blank and steel ring and slightly retarded by the larger gap (two-headed arrow) in the PDMS ring-splinted wounds. PDMS, polydimethylsiloxane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Polydimethylsiloxane devices
It is relatively easy to develop other types of wound restraints through microfabrication techniques that use molded polydimethylsiloxane (PDMS; Fig. 3A). Among the variations that have been tested in this laboratory, the most useful design is based on the silicone ring concept, with the addition of a set of perpendicular PDMS posts that project from the inner perimeter of the ring to the fascial plane at the base of the underlying wound.16 Since these are molded devices, there is no limitation to the actual geometry of the device or wound. Rectangular or other shaped cavities can easily be accommodated. During the course of development, we have optimized the depth, spacing, and thickness of the posts to avoid disturbance of subdermal tissues, to allow unrestrained ingression of granulation tissue/epidermis, and to provide adequate mechanical restraint of surrounding dermis to prevent contraction, creating a model of repair by secondary intention (Fig. 3B). The outer rim of the device has six holes for securing sutures to intact skin. The device dimensions can adapt to any defined wound area, and the architecture has been optimized specifically for application to rat and mouse excisional wounds.
The flange that circumscribes the wound cavity creates a controlled area that can be used to retain biomaterials or wound dressings in close contact with the underlying wound bed. It is also possible to retain the thin PDMS membrane that fills the aperture after molding as a window for observing the wound interior. However, the more typical approach is to occlude the open device with an adhesive, semipermeable membrane after application of an interventional treatment that will maintain a relatively high level of humidity over the wound site. If drier conditions are desirable, the membrane can be perforated. The flanged portion of the device can also be placed under the dermis with the perpendicular posts projecting upwards to the skin surface.
Splinting devices alter the mechanical environment of the wound by interrupting the tensile forces that are generated within and around the wound site.15,17 Indeed, there is reasonable evidence that various forms of restraint can reduce clinical scarring. Researchers at Stanford have shown that delayed application of a simple elastomeric polymer sheet can alter linear scar formation in the Duroc pig model by reducing mechanical forces of incisional wounds (stress shielding), and clinical testing for the device has been initiated.18 Use of the above described splint devices in rodent models may function similarly by offloading forces that lead to fibrosis and scar formation around excisional wounds that are exacerbated by injuries to the panniculus carnosus.
Distraction device
Unlike static splints, mechanical devices that actually distend the wound, and thus, exaggerate the typical forces at the wound margin can produce scar tissue in experimental models. For example, Aarabi et al.19 adapted the concepts of tissue expansion and distraction osteogenesis in a mouse model. A mechanical, screw-driven retractor with teeth projecting into the wound margin retracts the wound, by 2 mm, after 4 days of healing and subsequently by 4 mm on alternate days (Fig. 4). Under these conditions, these investigators reported the progressive development of a wound akin to a human hypertrophic scar. Interestingly, collagen production is apparently similar to normal wounds, and the data attribute the tissue response to a T-cell mediated process.
Figure 4.
Wound distraction. A screw-driven mechanical device can be used to elevate mechanical strain by progressive distraction of the wound margins. In the mouse, granulation tissue takes on a hypertrophic aspect. Reprinted from Aarabi et al.20 with permission.
Wound chambers
Enclosed chambers are useful for continuous observations for aspects of the healing process, such as angiogenesis and extravasation of blood-borne inflammatory cells into the wound site.20 In small rodents, these metal devices are typically implanted in the body wall by clamping a pair of metal rings together to enclose a skin fold in which one side of the fold includes a full-thickness excisional wound that has a transparent glass or plastic window. The rings both prevent wound contraction and epithelization to allow prolonged intravital microscopy of subcutaneous healing events21 or tumor vascularization.
Final Considerations
Healing by rapid wound contraction in small animals is not a fully informative preclinical model of human healing by secondary intention. Due to the very high lateral compliance provided by the panniculus carnosus and loose attachment of the dermis, forces generated within and around rodent excisional wounds rapidly bring the margins together; hence, epithelization occurs much more rapidly, even before the development of contractile myofibroblasts that are associated with scarring. Since most experimental wound models are developed with the intention of identifying genes, agents, or devices that benefit impaired wound healing, it is quite useful to examine wounds that maximize the demand for granulation tissue formation and epidermal resurfacing, rather than define healing as merely “closure” Splinting strategies have become widely accepted as more accurate preclinical models.
With regard to wound biomechanics, splinting devices interrupt the transmission of contractile forces at the interface between adjacent normal skin and newly formed granulation tissue.17 It is well-established from earlier splinting studies that the principal driving force for wound contraction is granulation tissue, with the rate dependent, in part, on the compliance of surrounding skin.22–24 Experimentally, when a splint is released after a point at which granulation tissue has had time to mature beyond the inflammatory phase, wounds will contract due to a higher tensional state within the granulation tissue. If the granulation tissue is released from the surrounding skin by lateral excision, the granulation tissue will contract inwards, while the surrounding skin will retract.15 In vitro, this rapid relaxation leads to a decrease in cell proliferation (BrdU incorporation) and an increase in apoptosis,15 much as has been observed in tethered vs. relaxed fibroblast populated collagen gels in vitro.25 As a result, splinting may create a higher and more persistent tensional state in these animal models.
Take-Home Messages.
Wound healing, “closure,” in loose-skinned small animal models occurs primarily through wound contraction.
Clinical healing in humans occurs predominantly through secondary intention as human skin is firmly anchored to underlying connective tissue.
Rodent wound contraction precludes controlled wound healing experiments.
Use of native or mechanical splinting alleviates contraction and simulates healing by secondary intention.
Splinted wounds produce a defined volume for presentation of therapeutics.
Wound distraction devices may be a useful model for hypertrophic scarring.
Apart from the contrasting biomechanical properties of rodent excisional wounds as compared to human wounds, the changing wound volume and area as a result of contraction frequently compromises accurate sampling and quantitative analysis of wound healing in animal models.26 Alternative models, such as subcutaneous wound chambers or porous implants do provide a fixed volume and prevent contraction. However, these devices exclude epithelial–mesenchymal interactions and markedly alter the mechanical environment. From an operational point of view, wound splinting devices for small animals should be easy to apply, tolerable to the subject, durable, inert, and compatible with histological and biochemical analysis.
Most splinting devices must be sutured or glued in place, and they are all susceptible to dislodging by animal activity. It would be useful to have a splinting design that could be rapidly and securely applied to the experimental animal. Given the increasing interest in the influence of the biomechanical environment on connective tissue repair, an adjustable splinting device, with the same design features mentioned above, would expand our experimental repertoire. In the future, a splint that could report—in real time—the chemical and mechanical wound environment, for example, pO2, blood flow, strain, lactate, pH, could become a valuable research tool.
Abbreviations and Acronyms
- EG
epithelial gap
- GTG
granulation tissue gap
- MH
maximum height
- PDMS
polydimethylsiloxane
Acknowledgments and Funding Sources
Development of the PDMS devices involved the efforts of Philip Samson, David Schaffer and John P. Wikswo from the Vanderbilt Institute for Integrative Biosystems Research and Education, and R. Michael Slowey from Vanderbilt University. Part of the work reported here was supported by NIH grants AR056138 (J.M.D., S.R.O.) and EB007611 (S.R.O.) and by the Department of Veterans Affairs (J.M.D.).
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of the article was expressly written by the authors listed. No ghostwriters were used to write this article.
About The Authors
Jeffrey M Davidson, PhD, is a Professor of Pathology, Microbiology, and Immunology at Vanderbilt University School of Medicine as well as a Senior Research Career Scientist in the Department of Veterans' Affairs. He is past president of the Wound Healing Society, president of the American Society for Matrix Biology, and the author of numerous publications. Fang Yu, MD, PhD, is a Staff Scientist at Vanderbilt University School of Medicine. She has co-authored several scientific articles. Susan R. Opalenik, PhD, is a Research Assistant Professor in the Department of Pathology, Microbiology, and Immunology at Vanderbilt University School of Medicine. She is the author or co-author of 21 publications.
References
- 1.Ahn ST. Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg. 1990;24:17. doi: 10.1097/00000637-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kloeters O. Tandara A. Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007;15(Suppl 1):S40. doi: 10.1111/j.1524-475X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 3.Saulis AS. Mogford JH. Mustoe TA. Effect of mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg. 2002;110:177. doi: 10.1097/00006534-200207000-00029. discussion 184. [DOI] [PubMed] [Google Scholar]
- 4.Brown RJ. Lee MJ. Sisco M. Kim JY. Roy N. Mustoe TA. High-dose ultraviolet light exposure reduces scar hypertrophy in a rabbit ear model. Plast Reconstr Surg. 2008;121:1165. doi: 10.1097/01.prs.0000302512.17904.2a. [DOI] [PubMed] [Google Scholar]
- 5.Jia S. Zhao Y. Mustoe TA. The effects of topically applied silicone gel and its silver derivative on the prevention of hypertrophic scarring in two rabbit ear-scarring models. J Plast Reconstr Aesthet Surg. 2011;64:e332. doi: 10.1016/j.bjps.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Ko JH. Kim PS. Zhao Y. Hong SJ. Mustoe TA. HMG-CoA reductase inhibitors (statins) reduce hypertrophic scar formation in a rabbit ear wounding model. Plast Reconstr Surg. 2012;129:252e. doi: 10.1097/PRS.0b013e31823aea10. [DOI] [PubMed] [Google Scholar]
- 7.Kim I. Mogford JE. Chao JD. Mustoe TA. Wound epithelialization deficits in the transforming growth factor-alpha knockout mouse. Wound Repair Regen. 2001;9:386. doi: 10.1046/j.1524-475x.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 8.Reid RR. Said HK. Mogford JE. Mustoe TA. The future of wound healing: pursuing surgical models in transgenic and knockout mice. J Am Coll Surg. 2004;199:578. doi: 10.1016/j.jamcollsurg.2004.05.262. [DOI] [PubMed] [Google Scholar]
- 9.Falanga V. Schrayer D. Cha J. Butmarc J. Carson P. Roberts AB. Kim SJ. Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen. 2004;12:320. doi: 10.1111/j.1067-1927.2004.012316.x. [DOI] [PubMed] [Google Scholar]
- 10.Buck DW., 2nd Jin da P. Geringer M. Hong SJ. Galiano RD. Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg. 2011;128:427e. doi: 10.1097/PRS.0b013e31822b7333. [DOI] [PubMed] [Google Scholar]
- 11.Reiss MJ. Han YP. Garcia E. Goldberg M. Yu H. Garner WL. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery. 2010;147:295. doi: 10.1016/j.surg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanik VD. Greives MR. Lerman OZ. Seiser N. Dec W. Chang CC. Warren SM. Levine JP. Saadeh PB. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 13.Galiano RD. Zhao LL. Clemmons DR. Roth SI. Lin X. Mustoe TA. Interaction between the insulin-like growth factor family and the integrin receptor family in tissue repair processes. Evidence in a rabbit ear dermal ulcer model. J Clin Invest. 1996;98:2462. doi: 10.1172/JCI119064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren R. Zhou B. Chen L. Silicone ring implantation in an excisional murine wound model. Wounds. 2012;24:36. [PubMed] [Google Scholar]
- 15.Carlson MA. Longaker MT. Thompson JS. Wound splinting regulates granulation tissue survival. J Surg Res. 2003;110:304. doi: 10.1016/s0022-4804(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 16.Yu F. Opalenik SR. Samson PC. Schaffer DK. Wikswo JP. Davidson JM. A novel PDMS device to prevent contraction in rodent excisional wounds. Wound Repair Regen. 2010;18:A43. [Google Scholar]
- 17.Gross J. Farinelli W. Sadow P. Anderson R. Bruns R. On the mechanism of skin wound “contraction”: a granulation tissue “knockout” with a normal phenotype. Proc Natl Acad Sci USA. 1995;92:5982. doi: 10.1073/pnas.92.13.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurtner GC. Dauskardt RH. Wong VW. Bhatt KA. Wu K. Vial IN. Padois K. Korman JM. Longaker MT. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 19.Aarabi S. Bhatt KA. Shi Y. Paterno J. Chang EI. Loh SA. Holmes JW. Longaker MT. Yee H. Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 20.Allison F., Jr. Smith MR. Wood WB., Jr. Studies on the pathogenesis of acute inflammation. I. The inflammatory reaction to thermal injury as observed in the rabbit ear chamber. J Exp Med. 1955;102:655. doi: 10.1084/jem.102.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorg H. Krueger C. Vollmar B. Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. J Anat. 2007;211:810. doi: 10.1111/j.1469-7580.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abercrombie M. James DW. Newcombe JF. Wound contraction in rabbit skin, studied by splinting the wound margins. J Anat. 1960;94:170. [PMC free article] [PubMed] [Google Scholar]
- 23.James DW. Newcombe JF. Granulation tissue resorption during free and limited contraction of skin wounds. J Anat. 1961;95:247. [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy DF. Cliff WJ. A systematic study of wound contraction in mammalian skin. Pathology. 1979;11:207. doi: 10.3109/00313027909061947. [DOI] [PubMed] [Google Scholar]
- 25.Grinnell F. Zhu M. Carlson MA. Abrams JM. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JM. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]