Abstract
Objective
To mechanically control the wound environment and prevent cutaneous scar formation.
Approach
We subjected various material substrates to biomechanical testing to investigate their ability to modulate skin behavior. Combinations of elastomeric materials, adhesives, and strain applicators were evaluated to develop topical stress-shielding devices. Noninvasive imaging modalities were utilized to characterize anatomic site-specific differences in skin biomechanical properties in humans. The devices were tested in a validated large animal model of hypertrophic scarring. Phase I within-patient controlled clinical trials were conducted to confirm their safety and efficacy in scar reduction in patients undergoing abdominoplasty surgery.
Results
Among the tested materials and device applicators, a polymer device was developed that effectively off-loaded high tension wounds and blocked pro-fibrotic pathways and excess scar formation in red Duroc swine. In humans, different anatomic sites exhibit unique biomechanical properties that may correlate with the propensity to form scars. In the clinical trial, utilization of this device significantly reduced incisional scar formation and improved scar appearance for up to 12 months compared with control incisions that underwent routine postoperative care.
Innovation
This is the first device that is able to precisely control the mechanical environment of incisional wounds and has been demonstrated in multiple clinical trials to significantly reduce scar formation after surgery.
Conclusion
Mechanomodulatory strategies to control the incisional wound environment can significantly reduce pathologic scarring and fibrosis after surgery.

Geoffrey C. Gurtner, MD, FACS
Introduction
Wound healing proceeds through overlapping stages of inflammation, proliferation, and remodeling.1 All wounds heal with some degree of scar formation, but the mechanisms that govern whether the result will be a fine thin scar, a prominent hypertrophic scar, or a tumor-like keloid remain unclear.2,3 It is estimated that more than 230 million major surgical procedures are performed around the world each year, all of which result in cutaneous wounds which heal with scars.4 In addition, fibrotic complications after injury can cause considerable dysfunction and disfigurement, costing more than $4 billion yearly in healthcare costs in the United States alone.5 Modern multimodality regimens have produced inconsistent outcomes, and recombinant cytokine-based strategies have failed to prevent scar formation in phase III clinical trials (www.renovo.com).6 The current challenge for researchers and health care providers is to develop highly effective approaches that combat this significant biomedical problem.
The role of mechanical force in wound healing was observed by anatomists and surgeons more than a century ago.7 Subsequent clinical observations and reports have substantiated the importance of tension in scar formation after injury. For example, sternotomy wounds develop greater fibrosis in the lower half of the incision that is subjected to higher tensional forces.8 In breast surgery, the amount of tension required to close an incision directly correlates with the amount of scar widening at 1 year.9 The development of keloids has also been linked to high tension regions in the body.10,11 Based on these concepts, reconstructive surgeons align incisions along topographical maps to minimize mechanical forces and to reduce subsequent scar formation.12 Taken together, these studies and observations suggest that strategies to control the mechanical environment have the potential to block wound fibroproliferation.
Clinical Problem Addressed
Inflammatory pathways have long been the focus of attack for anti-scarring therapy, but strategies that block inflammation alone have proved suboptimal with significant side effects. Recent studies have demonstrated that wound repair is also regulated by multiple noninflammatory mediators, including, but not limited to, mechanical force, extracellular matrix (ECM) dynamics, and oxygen tension.13,14 Our group and others have shown that mechanical tension plays a critical role in pathologic scar formation on a cellular, tissue, and organ level.15–19 Complex networks in mechanotransduction (i.e., the conversion of mechanical stimuli into biologic responses) have been linked to biologic pathways that drive fibrosis, including inflammation, cell survival, and matrix production.20–25
Using a mouse model of hypertrophic scar formation, we demonstrated that mechanical forces can activate intracellular pro-survival pathways during repair.15 We have also shown that mechanical forces can maintain a “chronic” inflammatory state which is modulated by T-lymphocyte signaling and systemic recruitment of pro-fibrotic cell populations.26 Microarray analysis of scar samples obtained from this model revealed a major role for inflammatory signaling and matrix deposition regulated by focal adhesion kinase (FAK), a nonreceptor tyrosine kinase mechanosensor.25 The activation of fibrotic mechanotransduction pathways was critically dependent on fibroblast-specific FAK, a target we effectively blocked via both transgenic and small molecule approaches. These small animal studies provided a mechanistic framework for understanding how biophysical signaling pathways activate scar formation in vivo.
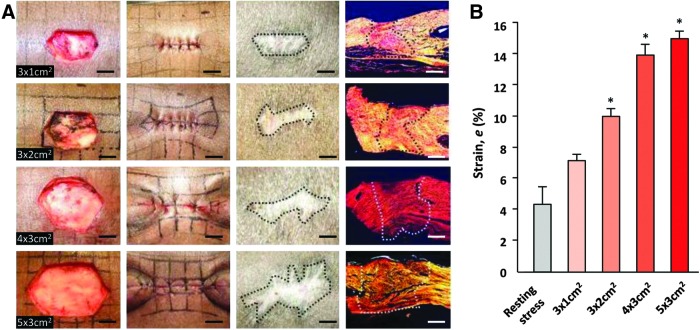
However, significant differences exist between mouse and human skin, including thickness, biomechanics, histological architecture, and mechanisms of repair.27 Pigs have been described as an ideal large animal model for studying human-like wound healing. In particular, the red Duroc pig has been proposed as a robust model for studying hypertrophic scarring.28,29 Our group developed a hypertrophic-like scar model in red Duroc pigs by creating full-thickness excisional wounds that were sutured closed under high tension.30 The degree of scar formation directly correlated with the amount of tension required to close the wound, strongly suggesting that mechanical forces have a direct effect on pathologic fibrosis (Fig. 1). Resultant scars were elevated, hypervascular, and exhibited increased cellularity and expression of alpha-smooth muscle actin, all features of human hypertrophic scarring.3 These findings validated the role of mechanical force in scar formation in a large animal known to form human-like scars.
Figure 1.
Development of a hypertrophic-like scar model in red Duroc pigs. (A) Wounds of increasing dimensions were created and sutured closed to generate increasing levels of tension. Sutures were removed at postsurgery day 4, and wounds were followed for up to 8 weeks, resulting in varying degrees of gross and histological scar formation. (B) Calculated strains were significantly elevated with larger wound dimensions. *p<0.05. Figure reprinted with permission from Gurtner et al.30 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Based on anecdotal evidence and our previous studies, we hypothesized that dynamic off-loading of mechanical forces within the incision would effectively reduce scar formation. After injury, the main load-bearing components of skin (i.e., dermal collagen fibers) should be rebuilt in the setting of active physiologic stresses. If the immature structures cannot offset the physiologic stresses, the scar will either dehisce or spread over time (referred to as “creep” in materials science). In injured human skin, stress results in scar hypertrophy, an active fibroproliferative process that is directly induced by mechanical force.31 Thus, we sought to develop a novel device that is capable of stress-shielding the wound, promoting skin repair, and blocking wound fibrosis.
Materials and Methods
Combinations of polymer backing materials and pressure-sensitive adhesives were constructed and subjected to biomechanical testing. Variable amounts of deformation (prestrain) were applied, and constructs were photographed over time to detect material relaxation and skin irritation. Deformation was detected by displacement of inked grids stamped on the skin surface. Biomechanical properties of fabricated polymers were compared with commercially available dressing products: EpiDerm (Biodermis, Henderson, NV), Epi-Tape (Biodermis), Neosporin ScarSolution (Johnson & Johnson, New Brunswick, NJ), Curad Scar Therapy (Medline Industries, Inc., Mundelein, IL), and NuSil silicone sheets (NuSil Technology LLC, Carpinteria, CA).
The initial applicator was designed using two binder clips coupled to the ends of a flexible polymer material of set dimensions. A spring-loaded applicator was fabricated using 301 stainless steel that incorporated a latch mechanism. The spring force stretched the dressing to generate targeted levels of prestrain. A manual cam-driven applicator was designed for the use of longer dressings via a flexible interface that improved conformance to nonplanar body geometry. Memory foam stamper applicators employed a three-link armature and locking mechanism to prestrain the polymer dressings. Various design elements were integrated to construct a self-straining stainless steel spring handle equipped with a memory foam applicator. A hinged, self-release applicator and dressing was also developed to facilitate dressing separation from the applicator. Timed rollers with hook and loop fastening technology enabled facile engagement/disengagement of the dressing. The book-style applicator was constructed as a scalable, multi-hinged device that generated consistent strains when opened and maintained these forces throughout activation of its pressure sensitive adhesive.
We utilized computational finite element analysis (FEA; ADINA software v8.3; ADINA R&D, Inc., Watertown, MA) based on nonlinear three-dimensional analysis to model the different stress states applied by dressings to various wound configurations. Digital image speckle correlation (DISC; 3D DISC system Q-400; Dantec Dynamics, Skovlunde, Denmark) was used to investigate region-specific differences in human skin biomechanics, as previously described.32 Briefly, DISC is a noncontact technique that is used to optically determine strain levels based on displacement of random dot patterns on a material surface after loading forces are applied. This noninvasive technique is highly valuable to examine material behaviors on dynamic surfaces such as the human body.
Results
Initially, we developed a silicone sheet-based polymer dressing with a Teflon applicator (Dupont, Hayward, CA) to apply ∼45% prestrain to the dressing (Fig. 2A). After device deployment on skin, ∼20% compressive stress-shielding was applied at the dressing center. To test the polymer device in a preclinical setting, we utilized the previously described scar model in red Duroc pigs.30 Full-thickness excisional wounds on the dorsa of Red Duroc swine were sutured closed and stress-shielded for up to 8 weeks. The application of the polymer device to the wound surface results in controlled compressive forces based on the material properties of the polymer itself and the degree of prestrained applied. This provides off-loading forces to counteract distractive forces caused by the configuration and natural recoil of the wound itself. Compared with unshielded incisions that healed under high tension, stress-shielded incisions demonstrated significantly reduced scar hypertrophy, matrix deposition, and epithelial thickening (Fig. 3). Further, stress-shielded incisions demonstrated features that recapitulated the histological architecture of unwounded skin, including restoration of epithelial rete pegs and return of skin adnexae (Fig. 3). These preclinical studies indicate that device stress-shielding of incisions is a safe and effective approach that is used for reducing wound fibrosis and for promoting regenerative repair pathways.
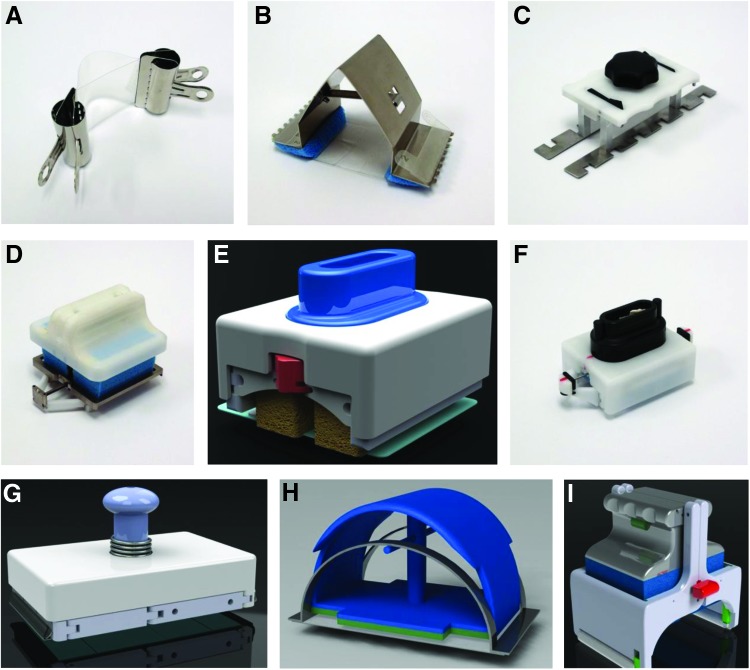
Figure 2.
Early-generation polymer device. Photographs of various applicators constructed during the development of the stress-shielding device. (A) A binder clip-based applicator. (B) A metal spring-loaded applicator. (C) A cam-driven applicator. (D–G) Various integrated foam stamper applicators. (H) A self-straining spring handle applicator. (I) A hinged self-release applicator. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.
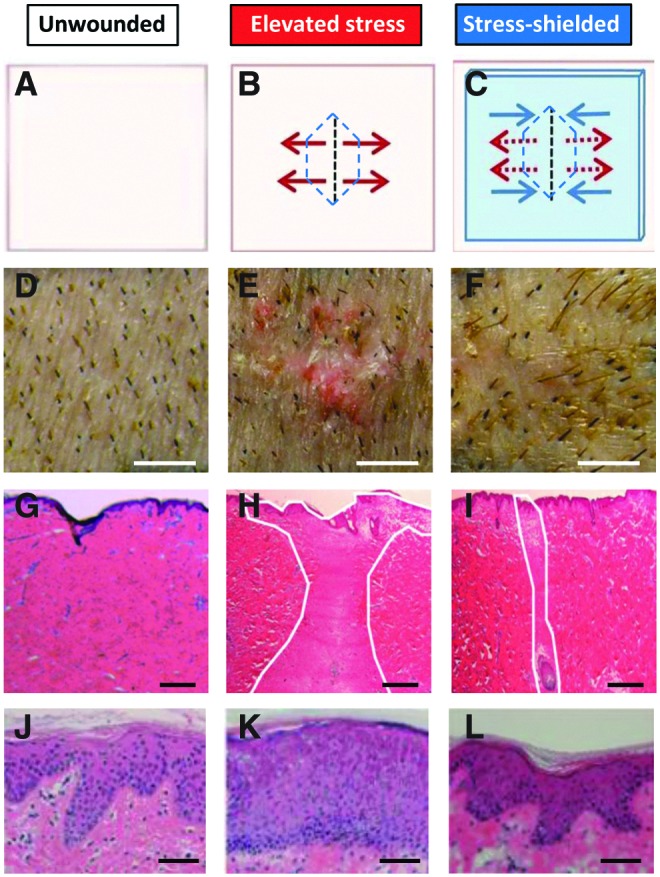
Device validation in a large animal model. Schematic of wound model (A–C), photographs (D–F), scar histology (G–I), and epithelial histology (J–L) of pig skin under different mechanical tension conditions. (A, D, G, J) Unwounded skin subjected to physiologic stresses. (B, E, H, K) Elevated stress incisions after closure of large excisional wounds. (C, F, I, L) The same elevated stress incision, but off-loaded with the stress-shielding device during repair. Note the recapitulation of unwounded epithelial architecture in stress-shielded wounds (J–L), suggesting that regenerative wound healing pathways may be activated. Figure reprinted with permission from Gurtner et al.30 Scale bars: (D–F) 5 mm; (G–I) 500 μm; (J–L) 50 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
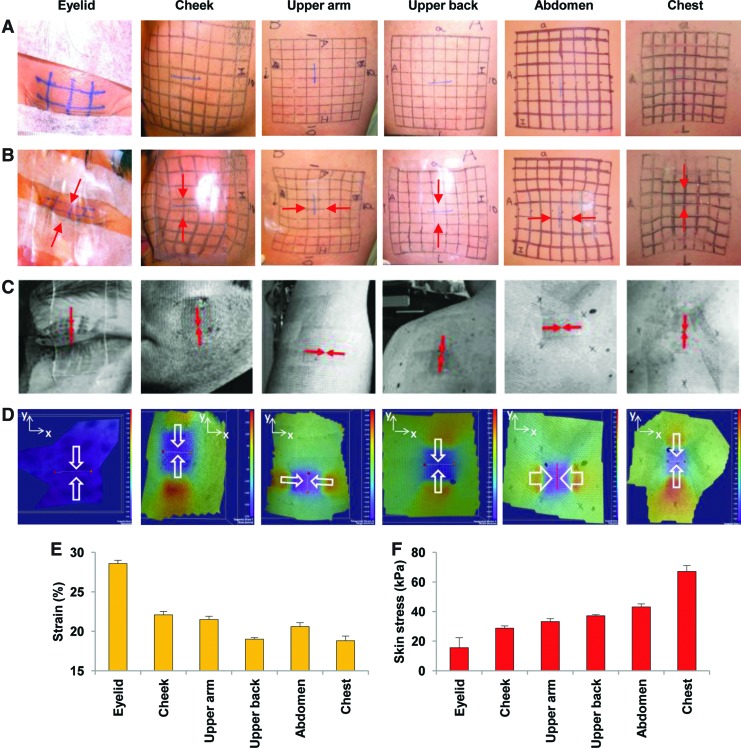
In continued studies of the in vivo biomechanical behavior of human skin, we developed a second-generation applicator device based on a metal spring-loaded applicator to ensure more reliable prestrain (Fig. 2B). We tested this spring-loaded polymer device on human volunteers and studied its material properties using noninvasive technologies. After device application, compressive strains were measured using FEA and DISC analysis based on line deformation of inked grids drawn on various anatomic sites: eyelid, cheek, upper arm, upper back, abdomen, and chest. Color heatmaps were generated to visualize tensile forces applied to skin beneath and adjacent to the polymer devices. Both FEA and DISC analyses demonstrated that deformational strains were highest in the eyelid and cheek, and lowest in the torso region (Fig. 4). These findings are consistent with described anatomic site differences in human skin stiffness, strongly suggesting that biomechanical properties may dictate the propensity to form pathologic scars in humans.33 Further, these data suggest that region- and wound-specific off-loading devices may be essential for optimizing scar appearance.
Figure 4.
Biomechanical analyses of human skin behavior in vivo. (A, B) Strain analyses were conducted based on grid line displacement before (A) and after (B) device application. Red arrows indicate axis of device compression. (C) Digital image speckle correlation analysis was used to measure skin strains non-invasively. Red/white arrows indicate axis of device compression. (D) Tensile (red/orange color) and compressive (blue/purple color) skin strains were mapped after device application. (E) Measured strains and (F) calculated stresses on different anatomic sites. Data are means±standard deviations from three healthy adult men. Figure reprinted with permission from Wong et al.32 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
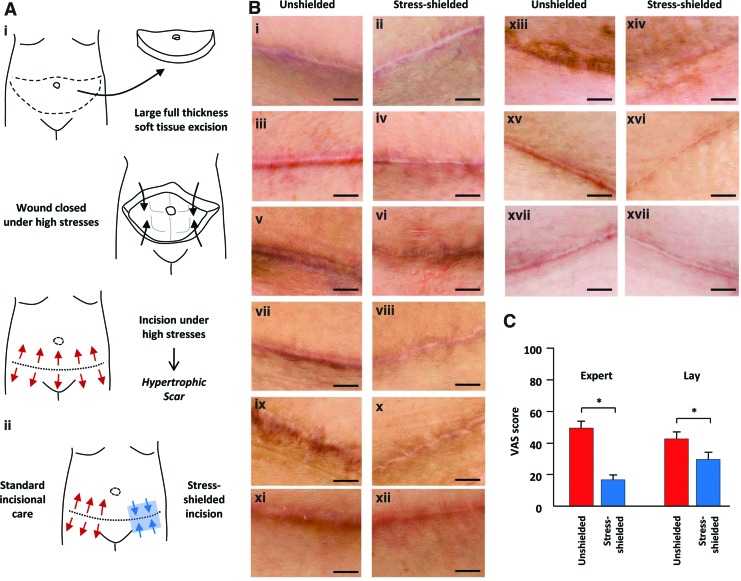
In the phase I clinical trial, nine female patients who underwent abdominoplasty surgery (which involves surgical closure of large soft tissue excisions) were followed for up to 12 months, as previously published (Fig. 5).30 One side of the incision was treated in standard fashion and served as the within-patient control. A portion of the contralateral side was treated with the stress-shielding polymer device starting at approximately 1 week postsurgery and was replaced weekly for at least 7 weeks. Professional photographs were taken of the treatment and control incisions over time. After 6–12 months, lay and expert panels blindly rated the scar appearance of paired incisions using a validated visual analog scale. Device off-loading of incisions significantly improved scar appearance by up to 63% compared with within-patient controls (14;p=0.004). The device was well tolerated and was not associated with any major side effects such as dehiscence or delayed healing. Importantly, this study demonstrated the safety of the polymer device for postsurgical wound healing, suggesting that the mechanical modulation of wounds has tremendous potential to reduce scar formation.
Figure 5.
Phase I clinical trial in abdominal surgery patients. (A) Schematic of abdominoplasty surgery (i) involving the excision of skin and subcutaneous fat and surgical closure under high mechanical forces that predispose wounds to robust scar formation. Clinical study schematic (ii) demonstrates application of the stress-shielding polymer to one side of the incision, whereas the within-patient control side is left unshielded. (B) Photographs of paired abdominal incisions at 6–12 months postsurgery (paired rows). Note the significant scar elevation, widening, discoloration, and irregularity in unshielded control incisions (i, iii, v, vii, ix, xi, xiii, xv, xvii) compared with device stress-shielded treatment incisions (ii, iv, vi, viii, x, xii, xiv, xvi, xviii). (C) Quantification of expert and lay panel analyses using a visual analog scale (VAS), with lower scores indicating improved scar appearance. Data are means±standard errors of the mean. Scale bar is 1 cm. *p<0.01. Figure reprinted with permission from Gurtner et al.30 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Based on the impressive early results with the device, we implemented clinically informed device improvements toward the consistent delivery of predetermined strains. A reusable spring-type applicator was developed but proved difficult to manipulate and required precision mounting of the polymer onto the metal spring device. We prototyped other reusable applicator models to enhance the consistency and ease of prestrain application and to improve the commercial viability of the product. However, prototypes based on a cam-driven applicator were too rigid for nonplanar surfaces and difficult to apply a consistent strain (Fig. 2C). Various foam stamper applicators were also hard to use on nonplanar surfaces and had high part counts that were not amenable for efficient large-scale production (Fig. 2D–G). A spring handle applicator was found to be cost effective and imparted consistent strains, but was limited by its abrupt polymer release mechanism (Fig. 2H). A hinged self-release applicator applied even pressures to nonplanar surfaces but had a high part count and was too complex to use (Fig. 2I).
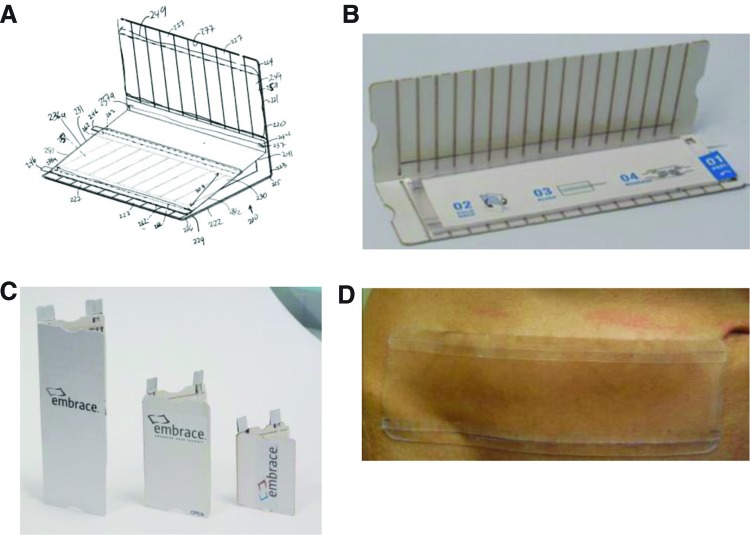
After extensive testing and device modification, we developed a highly novel book-type applicator with the polymer sheet incorporated into the device (Embrace™ Advanced Scar Therapy, Neodyne Biosciences, Inc., Menlo Park, CA; Fig. 6). The disposable, single-use applicator applies a consistent strain on the polymer when opened. The flexibility and transparency of the applicator allows for easy deployment of the polymer device onto nonplanar surfaces. Disengagement of the polymer device from the applicator is highly intuitive and readily achieved by the action of two pull strings. Feedback from patients and healthcare providers has confirmed a high level of satisfaction with device comfort and ease of use.
Figure 6.
Development of current stress-shielding polymer device. (A) Blueprint schematic and (B) photograph of the polymer device loaded into the book-style strain applicator. (C) The polymer device has been developed in several sizes to accommodate various size wounds in different body regions. (D) Photograph of the polymer device after application to the lower abdomen. Images courtesy of Neodyne Biosciences, Inc. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Discussion
The biomedical market for postsurgical wound management devices is immense and includes various types of topical dressings, adhesives, and tapes.34 However, these products have been designed to “manage wounds” and not to prevent scarring. The aggregate body of scientific literature and clinical experience strongly supports a primary role for mechanical forces in fibrogenesis and cutaneous scar formation. Thus, we hypothesized that an active, stress-shielding device based on elastomeric polymer technology would be able to prevent excess scar formation by controlling wound mechanical forces.
Based on our testing results, we developed a polymer device that precisely offloaded pro-fibrotic tensile forces throughout the remodeling phase of repair, resulting in scars that are markedly less hypertrophic and wide. The preclinical and early clinical results led us to propose a conceptual model for scar formation based on mechanical homeostasis. We proposed that wound fibrosis is a direct biological response for restoring mechanical equilibrium across the wound after injury. Elevated levels of distractive forces are counteracted by the deposition of stiffer and stronger scar matrix to maintain skin integrity and re-establish mechanical homeostasis.
It is possible that by altering the mechanical environment throughout wound remodeling (i.e., offloading the dermal matrix and minimizing collagen deposition), resultant wounds may be weaker and prone to failure. However, in our extensive preclinical and clinical studies, we did not observe any instances of wound dehiscence or delayed healing for more than a year. This strongly indicates that the off-loading forces applied by the polymer device do not have negative clinical consequences on wound healing and dermal strength. In addition, as the wound matures, its intrinsic mechanical properties also change, suggesting that the stress-shielding requirements may likewise change. Our data suggest that the off-loading demands of a wound only decrease over time and that any additional compressive forces applied by the polymer do not adversely impact healing. However, we are currently investigating an expanded range of products for the unique biomechanical demands among different anatomic regions, patient characteristics, wound dimensions, and repair stages.
In addition to physically blocking mechanical forces with a device, recent studies suggest that inhibiting mechanotransduction pathways via biochemical means may also prove effective. These nondevice strategies would be highly relevant in massively injured (e.g., burn wound) or irregularly contoured wounds (e.g., hand or face) that are not amenable to a topical device. For example, small interfering RNA, transgenic techniques, and small-molecule inhibitors of the cell-matrix mechanosensor FAK have been successfully used to reduce skin, pulmonary, and cardiac fibrosis in mouse models.35,36 These studies demonstrate proof of principle that targeted blockade of scar mechanotransduction pathways with molecular pharmaceuticals may prove effective clinically.
Key Findings.
Mechanical forces are a major determinant of pathologic scar formation after surgery.
Different regions of the body exhibit unique biomaterial properties that may predispose wounds to form exuberant scars.
A topical stress-shielding polymer can safely control the biophysical wound environment and block pro-fibrotic pathways.
Another promising approach to control scar formation is the use of scaffolds. The ECM transmits mechanical forces to fibroblasts (the end effector of matrix production) and other wound cells, and is known to regulate cell function based on its physical properties and specific matrix ligands.37–39 Novel wound scaffolds that can regulate the biomechanical and architectural environment during repair have considerable potential to reduce scarring, whether used alone or in conjunction with delivery of molecular anti-fibrotics. As the complex pathways linking mechanotransduction, inflammation, and fibrosis are further elucidated, researchers will be able to develop targeted therapies to modulate specific aspects of the wound repair process.
Innovation
Current strategies to reduce fibroproliferative wound healing remain suboptimal. The novel stress-shielding technology used in these studies is safe, well tolerated, and highly effective in improving incisional scar appearance after surgery. To our knowledge, this is the first demonstration that physically off-loading wounds with a topical device can dramatically reduce fibroproliferative scarring. More broadly, these findings substantiate the importance of mechanical forces in cutaneous disease and highlight the therapeutic potential of mechanomodulatory approaches to control wound healing.
Abbreviations and Acronyms
- DISC
digital image speckle correlation
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FEA
finite element analysis
- VAS
visual analog scale
Acknowledgments and Funding Sources
These studies were supported by a U.S. Armed Forces Institute of Regenerative Medicine grant (DOD #W81XWA0-08-2-0032) and a Wallace H. Coulter Translational Partners Grant.
Author Disclosure and Ghostwriting
All authors have an equity position in Neodyne Biosciences, Inc. R.H.D., P.G.Y., M.T.L., and G.C.G. are founders of Neodyne Biosciences, Inc. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About The Authors
Victor W. Wong, MD, is a postdoctoral research fellow in surgery at Stanford University under the mentorship of Drs. Geoffrey Gurtner and Michael Longaker. He completed his undergraduate work with Honors at University of California-Berkeley and received his medical degree from Boston University. His research interests include scar mechanotransduction, skin biomaterials, and bioreactor-based organ engineering. He has received prestigious research awards from the American College of Surgeons, the Wound Healing Society, and the Plastic Surgery Research Council. In addition, Dr. Wong has published more than 40 peer-reviewed articles (Nature Medicine, Annals of Surgery, Biomaterials, Tissue Engineering) and several book chapters, and has been an invited speaker at international conferences. Dr. Wong is completing his general surgery residency at Oregon Health & Science University and will be starting a plastic and reconstructive surgery fellowship at Johns Hopkins this summer. Bill Beasley has served as President and Chief Operating Officer of Neodyne Biosciences since June 2008. Mr. Beasley holds a B.S. in Pre-Professional Studies from the University of Notre Dame. John Zepeda is Director of Engineering for Neodyne Biosciences and has been with the company since July 2008. Mr. Zepeda holds a B.S. in Mechanical Engineering from Santa Clara University. Reinhold H. Dauskardt, PhD, is Professor and Associate Chair of Materials Science and Engineering at Stanford University. His laboratory focuses on nanomaterials design for thin-film structures, high-performance laminates, and biomaterials for regeneration and wound healing. Paul G. Yock, MD, is the Weiland Professor of Bioengineering and Medicine at Stanford University. He has invented a number of technologies that are in widespread clinical use, including the Rapid Exchange angioplasty and stenting system, which is the primary approach used worldwide. Dr. Yock founded and directs the Stanford Program in Biodesign. Michael T. Longaker, MD, MBA, is the Deane P. and Louise Mitchell Professor and Vice Chair of Surgery, Co-Director of the Institute for Stem Cell Biology and Regenerative Medicine, and Professor of Bioengineering and Materials Science and Engineering at Stanford University. His research experience includes the cellular and molecular biology of extracellular matrix, the biology of keloids and hypertrophic scars, and the cellular and molecular events that surround distraction osteogenesis with regard to craniofacial development. Recently, his focus has been on multipotent mesenchymal cells derived from adipose tissue and their applications for tissue repair, replacement, and regeneration. Geoffrey C. Gurtner, MD, is Professor of Surgery and Materials Science Engineering at Stanford University. He also serves as the Associate Chairman for Research in the Department of Surgery. Dr. Gurtner is a magna cum laude graduate of Dartmouth College and an AOA graduate of the University of California–San Francisco School of Medicine. He completed a general surgery residency at the Massachusetts General Hospital/Harvard Medical School program, a plastic surgery residency at the New York University School of Medicine, and received advanced training in microsurgery at the University of Texas-M.D. Anderson Cancer Center. He is the author of more than 120 peer-reviewed publications in both scientific and surgical literature. He is an editor for two major text books in the field: Grabb & Smith's Plastic Surgery and Plastic Surgery (Third Edition). Dr. Gurtner runs a National Institutes of Health– and Department of Defense–funded laboratory that seeks to understand the importance of physical stimuli (mechanical and chemical) in the human response to injury. This has led to the development of novel technologies in a wide variety of clinical areas.
References
- 1.Gurtner GC. Werner S. Barrandon Y. Longaker MT. Wound repair and regeneration. Nature. 2008;453:314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Gauglitz GG. Korting HC. Pavicic T. Ruzicka T. Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kose O. Waseem A. Keloids and hypertrophic scars: are they two different sides of the same coin? Derm Surg. 2008;34:336. doi: 10.1111/j.1524-4725.2007.34067.x. [DOI] [PubMed] [Google Scholar]
- 4.Weiser TG. Regenbogen SE. Thompson KD. Haynes AB. Lipsitz SR. Berry WR. Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 5.Aarabi S. Longaker MT. Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 2007;4:e234. doi: 10.1371/journal.pmed.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustoe TA. Cooter RD. Gold MH. Hobbs FD. Ramelet AA. Shakespeare PG. Stella M. Téot L. Wood FM. Ziegler UE. International Advisory Panel on Scar Management: International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Langer K. On the anatomy and physiology of the skin. I. The cleavability of the cutis. [Translated from Langer K: Zur Anatomie und Physiologie der Haut. I. Uber die Spaltbarkeit der Cutis. Sitzungsbericht der Mathematisch-naturwissenschaftlichen Classe der Kaiserlichen Academie der Wissenschaften. Br J Plast Surg. 1861;1978;4431:19. 3. [PubMed] [Google Scholar]
- 8.Durkaya S. Kaptanoglu M. Nadir A. Yilmaz S. Cinar Z. Dogan K. Do absorbable sutures exacerbate presternal scarring? Tex Heart Inst J. 2005;32:544. [PMC free article] [PubMed] [Google Scholar]
- 9.Wray RC. Force required for wound closure and scar appearance. Plast Reconstr Surg. 1983;72:380. doi: 10.1097/00006534-198309000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa R. Okai K. Tokumura F. Mori K. Ohmori Y. Huang C. Hyakusoku H. Akaishi S. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- 11.Akaishi S. Akimoto M. Ogawa R. Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445. doi: 10.1097/SAP.0b013e3181238dd7. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelmi BJ. Blackwell SJ. Phillips LG. Langer's lines: to use or not to use. Plast Reconstr Surg. 1999;104:208. [PubMed] [Google Scholar]
- 13.Wong VW. Akaishi S. Longaker MT. Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol. 2011;131:2186. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- 14.Schultz GS. Davidson JM. Kirsner RS. Bornstein P. Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarabi S. Bhatt KA. Shi Y. Paterno J. Chang EI. Loh SA. Holmes JW. Longaker MT. Yee H. Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 16.Abrams JM. Carlson MA. Zhu M. Grinnell F. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 17.Cerda E. Mechanics of scars. J Biomech. 2005;38:1598. doi: 10.1016/j.jbiomech.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Costa AM. Peyrol S. Porto LC. Comparin JP. Foyatier JL. Desmouliere A. Mechanical forces induce scar remodeling. Study in non-pressure-treated versus pressure-treated hypertrophic scars. Am J Pathol. 1999;155:1671. doi: 10.1016/S0002-9440(10)65482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmouliere A. Chaponnier C. Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Rep Regen. 2005;13:7. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 20.Alenghat FJ. Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- 21.Gieni RS. Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 22.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 23.Jaalouk DE. Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichelt J. Mechanotransduction of keratinocytes in culture and in the epidermis. Eur J Cell Biol. 2007;86:807. doi: 10.1016/j.ejcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Wong VW. Rustad KC. Akaishi S. Sorkin M. Glotzbach JP. Januszyk M. Nelson ER. Levi K. Paterno J. Vial IN. Kuang AA. Longaker MT. Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW. Rustad KC. Glotzbach JP. Sorkin M. Inayathullah M. Major MR. Longaker MT. Rajadas J. Gurtner GC. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol Biosci. 2011;11:1458. doi: 10.1002/mabi.201100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong VW. Sorkin M. Glotzbach JP. Longaker MT. Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harunari N. Zhu KQ. Armendariz RT. Deubner H. Muangman P. Carrougher GJ. Isik FF. Gibran NS. Engrav LH. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos MLC. Gragnani A. Ferreira LM. Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res. 2008;29:363. doi: 10.1097/BCR.0b013e3181667557. [DOI] [PubMed] [Google Scholar]
- 30.Gurtner GC. Dauskardt RH. Wong VW. Bhatt KA. Wu K. Vial IN. Padois K. Korman JM. Longaker MT. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelmi BJ. Blackwell SJ. Mancoll JS. Phillips LG. Creep vs. stretch: a review of the viscoelastic properties of skin. Ann Plast Surg. 1998;41:215. doi: 10.1097/00000637-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Wong VW. Levi K. Akaishi S. Schultz G. Dauskardt RH. Scar zones: region-specific differences in skin tension may determine incisional scar formation. Plast Reconstr Surg. 2012;129:1272. doi: 10.1097/PRS.0b013e31824eca79. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 34.Lionelli GT. Lawrence WT. Wound dressings. Surg Clin North Am. 2003;83:617. doi: 10.1016/S0039-6109(02)00192-5. [DOI] [PubMed] [Google Scholar]
- 35.Lagares D. Busnadiego O. Garcia-Fernandez RA. Kapoor M. Liu S. Carter DE. Abraham D. Shi-Wen X. Carreira P. Fontaine BA. Shea BS. Tager AM. Leask A. Lamas S. Rodríguez-Pascual F. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemente CF. Tornatore TF. Theizen TH. Deckmann AC. Pereira TC. Lopes-Cendes I. Souza JR. Franchini KG. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res. 2007;101:1339. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- 37.Bornstein P. Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 38.Daley WP. Peters SB. Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 39.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]