Abstract
Recent evidence suggests that GABAA receptor ligands may regulate ethanol intake via effects at both synaptic and extrasynaptic receptors. For example, the endogenous neurosteroid, allopregnanolone (ALLO) has a similar pharmacological profile as ethanol, and it alters ethanol intake in rodent models. Additionally, recent evidence suggests that δ-subunit containing extrasynaptic GABAA receptors may confer high sensitivity to both ethanol and neurosteroids. The purpose of the present study was to determine the effects of ganaxolone (GAN; an ALLO analogue) and gaboxadol (THIP; a GABAA receptor agonist with selectivity for the extrasynaptic δ-subunit) on ethanol intake, drinking patterns, and bout characteristics in operant and limited access self-administration procedures. In separate studies, the effects of GAN (0 – 10 mg/kg) and THIP (2 – 16 mg/kg) were tested in C57BL/6J male mice provided with two-hour access to a two-bottle choice of water or 10% ethanol or trained to respond for 30 minutes of access to 10% ethanol. GAN had no overall significant effect on operant ethanol self-administration, but tended to decrease the latency to consume the first bout. In the limited-access procedure, GAN dose-dependently decreased ethanol intake. THIP dose-dependently decreased ethanol intake in both paradigms, altering both the consummatory and appetitive processes of operant self-administration as well as shifting the drinking patterns in both procedures. These results add to literature suggesting time-dependent effects of neurosteroids to promote the onset, and to subsequently decrease, ethanol drinking behavior, and they support a role for extrasynaptic GABAA receptor activation in ethanol reinforcement.
1. Introduction
The γ-aminobutyric acidA (GABAA) receptor is one target of alcohol (ethanol) in the central nervous system (CNS; e.g., Kumar et al., 2009; Wallner et al., 2006), and many pharmacological agents that alter GABAA receptor function have been shown to alter ethanol intake in preclinical models (e.g., Chester and Cunningham, 2002; Finn et al., 2010). Additional evidence for a contribution of GABAA receptor signaling to ethanol drinking behaviors is provided by recent work utilizing site-specific knockdown of select GABAA receptor subunits. Knockdown of the α1 subunit in the ventral pallidum (Yang et al., 2011) and of the α2 subunit in the central nucleus of the amygdala (Liu et al., 2011) significantly decreased binge alcohol intake. Studies targeting extrasynaptic GABAA receptors found that knockdown of the α4 (Rewal et al., 2011, 2009) and δ (Nie et al., 2011) subunit in the nucleus accumbens shell significantly reduced moderate alcohol intake. Collectively, the available data suggest that GABAA receptors may regulate ethanol intake via effects at both synaptic and extrasynaptic receptors although the extent of the contribution of each class of receptors remains unclear.
Alcohol use disorders encompass a class of complex traits and behaviors including alcohol craving, escalation of alcohol use, tolerance, and development of physical dependence, making it difficult for animal models to capture the totality of this human disease. Therefore, it is important to test any potential therapeutic compound across a variety of procedural paradigms and animal models. Our recent work showed a shift in ethanol drinking patterns in mice across 24 hrs following administration of gaboxadol (THIP, a GABAA receptor agonist with selectivity for extrasynaptic receptors containing δ subunits; Chandra et al., 2006; Herd et al., 2009) and ganaxolone (GAN, a synthetic analogue of the neurosteroid allopregnanolone, ALLO; Carter et al., 1997); the drinking pattern changes following THIP and GAN were consistent with the involvement of synaptic and extrasynaptic GABAA receptors in the modulation of moderate ethanol intake (Ramaker et al., 2011). Thus, the purpose of the present study was to extend the examination of these two potent modulators of the GABAA receptor for their effects on limited access ethanol self-administration in two different paradigms that have been shown to produce physiologically relevant blood ethanol concentrations (BECs) in mice: operant self-administration and 2-bottle choice limited access drinking.
The neurosteroid ALLO is the most potent endogenous positive modulator of the GABAA receptor identified to date (e.g., Belelli and Lambert, 2005), and ALLO levels are increased in the periphery and brain following ethanol intake or administration (Barbaccia et al., 1999; Finn et al, 2004; Torres and Ortega 2004, 2003; VanDoren et al., 2000; but also see Holdstock et al., 2006; Porcu et al, 2010). ALLO has been shown to generalize to the discriminative stimulus effects of ethanol (Bowen et al., 1999; Grant et al., 1996), suggesting that the two compounds share similar subjective effects and that ALLO exhibits a similar behavioral, physiological, and pharmacological profile as ethanol. For instance, both compounds have anxiolytic, anticonvulsant, sedative-hypnotic, and muscle relaxant properties (Finn and Gee, 1994; Finn et al., 1997, Gasior et al., 1999). This relationship suggests that manipulation of neurosteroid levels may alter the reinforcing properties of ethanol, with subsequent effects on ethanol intake. Indeed, multiple rodent studies have shown that exogenous ALLO administration biphasically alters voluntary ethanol consumption (Finn et al., 2010; Ford et al, 2007a, 2005a; Janak et al., 1998; Janak and Gill, 2003; Sinnott et al., 2002). Similarly, administration of the 5α-reductase inhibitor finasteride, which decreases the metabolism of progesterone to ALLO, has been shown to decrease ethanol self-administration (Ford et al., 2008, 2005b). The inhibition of ethanol-induced increases in ALLO production by finasteride or adrenalectomy has also been shown to block some of the behavioral and physiological properties that normally follow ethanol administration (e.g., Khisti et al., 2003; Sanna et al., 2004; VanDoren et al., 2000), further indicating that the increase in ALLO levels following ethanol intake may impact sensitivity to some of ethanol’s reinforcing and behavioral properties.
One limitation to administering ALLO in an experimental procedure is its short half-life, which leads to a transient pharmacological effect (T ½ in plasma ~30 minutes in rodents and peak levels in brain within 10 min; maximum behavioral effect at 15 min post-injection is reduced to 50% effectiveness at 60–90 min post-injection; Mellon et al., 2008; Kokate et al., 1994). One way to circumvent this problem is by using GAN, a synthetic analogue with an added methyl group that extends the half-life to 3–4 fold that of ALLO, while retaining the primary neuropharmacological properties of this neurosteroid (Belelli and Herd, 2003; Carter et al., 1997). Though the research to date is limited, systemic GAN administration has been shown to alter ethanol consumption in two models of rodent self-administration. The first used rats in an operant self-administration paradigm (Besheer et al., 2010) and the second used mice in a 24-hour 2-bottle choice study (Ramaker et al., 2011). These studies support a similar biphasic effect on ethanol intake to that seen with ALLO, namely an enhancement of ethanol intake with low doses of the drug and suppression of ethanol intake with high doses of GAN. Therefore, the first goal of the present studies was to expand on these earlier findings by examining the effect of GAN on operant and limited access ethanol self-administration in mice.
The GABAA receptor is a pentaheteromeric chloride channel comprised of a combination of subunits consisting of α1–6, β1–3, γ1–3, δ, ε, θ, or Π. The specific combination of subunits that make up the channel alter the pharmacology, physiology, and subcellular localization of the receptors (e.g., Farrant and Nusser, 2005; Mody and Pearcy, 2004). For example, δ subunit-containing receptors are thought to occur exclusively outside of the synapse and to contribute to GABA-mediated tonic inhibition, in contrast to synaptic receptors which are responsible for phasic inhibition (Nusser et al., 1998). In vitro studies of the effects of ethanol on extrasynaptic GABAA receptors have produced inconsistent results. Some studies have shown a highly selective effect of ethanol at δ-subunit containing receptors, while other studies have been unable to replicate this effect (Borghese et al., 2006; Korpi et al., 2007; Olsen et al., 2007; Santhakumar et al., 2007). Recent in vivo work has shown that selectively modulating these extrasynaptic receptors either pharmacologically (Boyle et al., 1993, 1992; Moore et al., 2007; Ramaker et al., 2011), with viral knockdown (Nie et al., 2011; Rewal et al., 2011, 2009), or with subunit-specific knockout (Mihalek et al., 2001, 1999) can alter ethanol self-administration, indicating that these receptors may be important in mediating ethanol’s reinforcing effects.
Evidence shows that although ALLO can potentiate both tonic and phasic inhibition, extrasynaptic δ subunit-containing receptors may be especially sensitive to ALLO (Belelli and Lambert, 2005; Brown et al., 2002; Fodor et al., 2005). To better isolate the contribution of extrasynaptic GABAA receptors to ethanol’s reinforcing effects (which could eventually elucidate the contribution of each class of receptors to neurosteroid effects on ethanol intake), the second aim of this study was to utilize THIP, an agonist with selectivity for δ-subunit containing receptors, to examine the role of enhanced tonic inhibition on operant and limited access self-administration in mice.
In addition to examining effects on total ethanol intake, the present studies aimed to capture patterns of drinking that are not reflected by analysis of total consumption alone. In the current operant self-administration experiment, the use of a response requirement schedule (see Methods 2.5.1) enabled us to procedurally separate the appetitive and the consummatory phases of self-administration (e.g., Ford et al., 2007b; Samson et al., 1998). Further, analysis of bout and bin data in both procedures allowed for the examination of the shifts in the patterns of drinking and provided further insight into drug-induced effects on the microarchitecture of the drinking episodes. This may have important therapeutic relevance as human studies have shown that the pattern of drinking, for example, the size and frequency of drinking bouts, rather than overall intake, may be more informative when assessing problems associated with alcohol use and therapeutic efficacy of drugs (Anton et al., 2004; Boback et al., 2004; Gmel et al., 2001). Thus, the present studies add to the current literature by providing insight into the regulatory mechanisms in which GAN and THIP alter ethanol intake.
2. Materials and Methods
2.1. Animals
Fifty-four C57BL/6J (C57) male mice, approximately 8 weeks of age at the start of experiments, were used (Jackson Laboratory, Bar Harbor, ME). Twenty-four mice were single-housed in lickometer chambers (Exp 1; see below) and acclimated to a reverse 12-hour light: 12-hour dark cycle (lights off at 0600). Thirty mice were pair-housed in standard shoebox cages (Exp 2; see below) and acclimated to a 12-hour light: 12-hour dark cycle (lights on at 0600). All mice were provided ad libitum access to rodent chow and water (except where noted). Experiments were run Monday through Friday, and mice were weighed and handled daily on those days. All efforts were made to minimize animal suffering and to reduce the number of mice used. All procedures were approved by the local Institutional Animal Care and Use Committee and complied with NIH guidelines.
2.2. Drugs
Ethanol (200 proof; Pharmco Products, Brookfield, CT) and sucrose (Sigma-Aldrich Company, St. Louis, MO, USA) solutions were prepared by dilution in tap water. GAN was purchased from Dr. Robert Purdy (VA Research Foundation, San Diego, CA) and was solubilized in a solution of 3% (w/v) dimethyl sulfoxide (DMSO, Mallinckrodt Baker, Inc., Paris, KY) and 20% (w/v) 2-hydroxypropyl-β-cyclodextrin (β-cyclodextrin, Cargill Inc., Cedar Rapids, IA) in Millipore water. THIP (4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol hydrochloride) was purchased from Tocris Bioscience (Ellisville, MO) and dissolved in saline. Drugs were injected intraperitoneally (i.p) in a volume of 0.01 ml/g body weight.
2.3. Apparatus
Lickometer chambers were used as previously described by our lab (Ford et al., 2008, 2005a, 2005b; Ramaker et al., 2011). Briefly, the chamber was comprised of a 4-walled plexiglas insert that was positioned on an elevated wire grid floor inside a shoebox cage with bedding. The insert contained a hinged top and two small holes where the sippers of the drinking bottles protruded into the cage. The wire floor of the chamber and each sipper formed an open electrical circuit that was connected to a lickometer device (MED Associates Inc., St. Albans, VT). Each time the circuit was closed by animal contact, a lick was recorded for the respective sipper. Lickometers were interfaced to an IBM computer running MED-PC IV software (MED Associates Inc.) to record time-stamped licks for each individual animal and each fluid presented.
Operant self-administration sessions were carried out in eight sound and light-attenuating mouse chambers as described previously (Ford et al., 2007a, 2007b). Each chamber contained a stainless steel grid floor, house light, 2 retractable levers with a stimulus light above each one, and a retractable sipper apparatus. The retractable sipper consisted of a 10 ml graduated pipette with a double ball-bearing metal sipper tube. Each metal sipper was connected to a lickometer circuit, which interfaced to a computer operating with MED-PC software (Med-Associates Inc.).
2.4. Exp. 1: Two-hour Two-bottle Preference Procedure in Lickometer Chambers
2.4.1. Acclimation
Mice were acclimated to lickometer chambers for 2 weeks where they had ad libitum access to food and two 50 ml bottles of water. For the rest of the experiment, the 50 ml water bottles were removed and weighed 1.5 hours into the dark cycle, the respective injection was given, and 30 minutes later each mouse was given access to both a 10 ml bottle of a 10% (v/v) ethanol solution (10E) and a 10 ml bottle of tap water for the next 2 hours. The 10E bottles were counterbalanced between the left and right sides across lickometer chambers to minimize the influence of side preference; each individual animal had its ethanol bottle on the same side for the duration of the experiment to allow consistent drinking routines to become established. We have used this strategy consistently in our previous lickometers studies (Ford et al., 2008, 2005a, 2005b; Ramaker et al., 2011). At the conclusion of the 2 hours of access, fluid consumed was measured (to the nearest 0.05 ml), and the 10 ml bottles were replaced with 50 ml water bottles. To control for leakage, two control cages were set up with no animals present, and the amount lost from those bottles was averaged and subtracted from the amount consumed from each bottle. Mice received 2 hours of access to 10E or water for approximately 5 weeks, and were then divided into two groups, balanced for their ethanol intake and ethanol bottle side. Starting during the 8th week, group 1 (GAN group) received daily 20% β-cyclodextrin injections at 30 minutes prior to the session start, while group 2 (THIP group) received daily saline injections at 30 minutes prior to the session start. Daily 22-hour water intake was also measured throughout the experiment in order to assess any alterations in overall fluid balance following drug treatment.
2.4.2. Exp. 1a: GAN
Prior to drug treatment, mice (group 1; n = 12) received 14 days of i.p. 20% β-cyclodextrin injections in order to habituate them to injection and handling stress. Mice then received a weekly injection of GAN in 3% DMSO/20% β-cyclodextrin in the following order: 0 mg/kg, 5 mg/kg, 10 mg/kg, and 0 mg/kg. 20% β-cyclodextrin was given on all intervening days to maintain stable drinking routines under injection conditions. Baseline drinking values were calculated by averaging the two vehicle treatment (0 mg/kg, which contained 3% DMSO/20% β-cyclodextrin) days.
2.4.3. Exp. 1b: THIP
Following 14 days of saline injections, separate mice (group 2; n = 12) were given a weekly injection of THIP in the following order: 8 mg/kg, 4 mg/kg, 2 mg/kg, and 16 mg/kg. Saline was given on all intervening days. Baseline values (0 mg/kg) were calculated by averaging the 4 saline days that immediately preceded each THIP treatment day.
2.5. Exp. 2: Operant self-administration of ethanol or sucrose
2.5.1. Acquisition
Self-administration was assessed with the “sipper” procedure (Ford et al., 2007b; Samson et al., 1998). For 10 days mice were water restricted for 16 hours preceding the training sessions and were trained to respond for 10% sucrose (10S) on a Fixed Ratio (FR) 1 schedule during 60-minute sessions. A response on the “active” lever resulted in retraction of both levers, the house light turning off and the respective stimulus light turning on for 5 seconds, and the sipper extending into the chamber for 60 seconds. Responses on the “inactive” lever were recorded but had no scheduled consequence. The position of the active lever was counterbalanced between chambers. Sipper access was reduced from 60 to 30 seconds, and session length was decreased from 60 to 30 minutes over successive training sessions. After the initial 10 sessions, fluid restriction was lifted and the FR schedule was gradually increased across sessions until mice were consistently responding on FR-4. At this point, the animals were divided into two groups. An ethanol-reinforced group had 10% v/v ethanol (10E) added to the 10S solution and then sucrose was faded out in a step-wise manner to eventually yield a 10E solution (3 sessions each with 10S/10E, 5S/10E, and 2.5S/10E solutions). The sucrose-reinforced group had the 10S solution incrementally reduced to eventually yield a 2% sucrose solution (2S; 3 sessions each with 10S, 7.5S, 5S, 2.5S). Once the final concentration of each reinforcer solution was reached, the FR schedule was further raised in a step-wise fashion (3–4 sessions each at FR-4, FR-6, FR-8), and then a Response Requirement (RR) schedule was instituted, whereby all appetitive responding preceded a consummatory phase that involved 30 minutes of continuous fluid access. The RR schedule was also raised in a step-wise fashion (3–4 sessions each at RR-8, RR-10, RR-12, RR-14, RR-16). The final contingency was RR-16, where mice had up to 20 minutes to make 16 responses on the active lever, followed by 30 minutes of fluid access.
2.5.2. Exp. 2a: GAN
Mice were maintained on the RR-16 schedule for 3 weeks prior to any treatment to establish baseline drinking. During the third week, they received daily saline injections at 30 minutes prior to the start of the session to habituate them to handling. At this time, 9 mice in the 10E group and 14 mice in the 2S group consistently earned the reinforcer and licked over 100 times per session and were therefore included in the experiment. Mice who licked less than 100 licks on any baseline or pretreatment days (~ 0.25 g/kg) were excluded from statistical analysis as we estimated that this was the minimum an animal could drink to experience a pharmacological effect of the 10E (Ford et al., 2007a). Every 2–3 days, all mice received a systemic injection of GAN in 3% DMSO/20% β-cyclodextrin in the following order: 0 mg/kg, 5 mg/kg, 10 mg/kg, 0 mg/kg. 20% β-cyclodextrin was given on all intervening days. Baseline data was calculated by averaging the two vehicle treatment (0 mg/kg) days.
2.5.3. Exp. 2b: THIP
For 2 weeks following the conclusion of the GAN study, the same mice were weighed and run in a daily session without injections. After 2 weeks, daily saline injections with a 30 minute pretreatment time were resumed. In the week preceding THIP treatment and on all saline days, 12 mice in the 10E group and 17 mice in the 2S group consistently earned the reinforcer and licked over 100 times per session and were therefore used in the statistical analysis. One day each week, the saline injection was replaced with an injection of THIP in the following order: 2 mg/kg, 4 mg/kg, and 8 mg/kg. Baseline (0 mg/kg) data was calculated by averaging the 3 saline days that immediately preceded the THIP treatment days.
2.6. Blood Ethanol Concentrations
BECs were assessed at the end of the 2-hour lickometer experiment on a drug and vehicle day for each group (after 5 mg/kg GAN and the final vehicle treatment for group 1; after 4 mg/kg THIP and the final baseline treatment for group 2). A 20 μL sample was taken from the retro orbital sinus of each mouse. Blood samples were processed according to previously published methods (Finn et al., 2007). Briefly, each blood sample was added to 500 μL of a solution containing 4 mM n-propanol (internal standard) in deionized water, vortexed, and analyzed using head-space gas chromatography. Six pairs of external standards with known ethanol concentrations (from 0.5 to 3.0 mg/ml) were used in parallel to construct a standard curve from which unknown concentrations were interpolated. Blood samples were not taken in the operant mice as these cohorts were especially sensitive to any handling outside of the daily systemic injections.
2.7. Statistical Analysis
Ethanol intake (g/kg) was calculated based on the ml of 10E solution depleted and the pre-session body weight of each mouse. Patterns of 10E and water licks, as well as ethanol bout parameters were recorded using MED-PC IV software, and SoftCR version 4 (MED Associates, Inc.) was used to access the time-stamped data. A 10E bout was defined as a minimum of 20 licks with no more than a 60-s pause between successive licks (Ford et al., 2008, 2005a, 2005b; Ramaker et al., 2011). A similar strategy was used for the analysis of the 2S data. All statistical analyses were performed using SYSTAT 11. For intake and bout analysis, a repeated-measures ANOVA was used with dose as the factor. In the event of a significant main effect, pairwise differences against vehicle were determined by the Fisher’s Least Significance Difference multiple comparisons test. For bin analysis, a two-way repeated-measures ANOVA factored by bin and dose was conducted. For bin and bout analysis, data were excluded from statistical analysis for any animal whose licks/ml fell outside 2.5 standard deviations from the average across 5 pretreatment days. For all analyses, statistical significance was set at p ≤ 0.05. Graphs were made using GraphPad Prism 4 for Windows.
3. Results
3.1. Exp. 1a: Effect of GAN on limited access ethanol intake in lickometer chambers
3.1.1. Intake
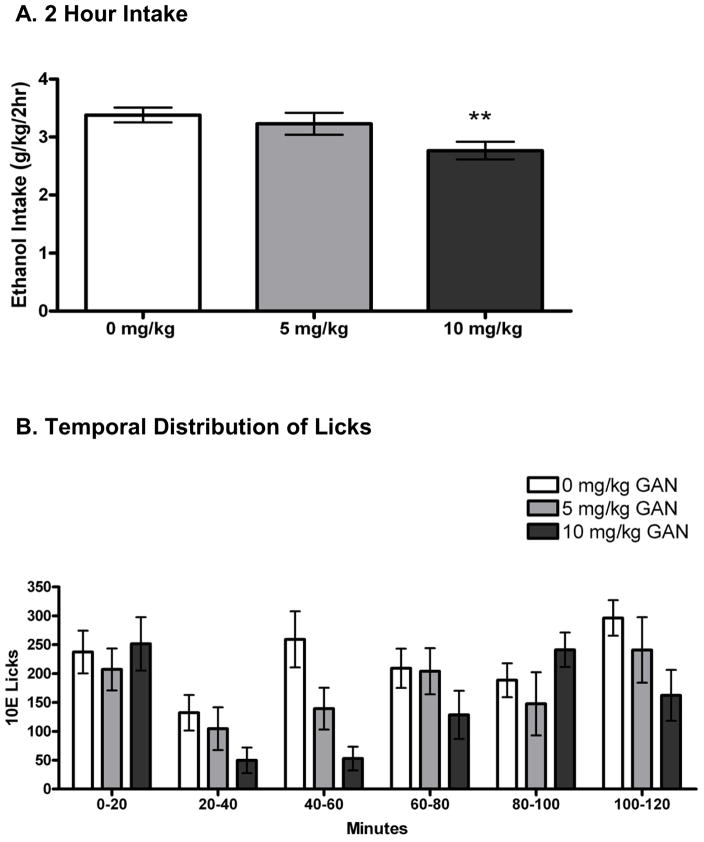
On the average of the 2 vehicle days, mice drank 3.39 ± 0.18 g/kg ethanol. Repeated-measures ANOVA revealed an effect of GAN treatment to significantly alter 10E intake versus vehicle [F(2,22) = 7.69, p < 0.01]; specifically, 10E intake was significantly decreased following 10 mg/kg GAN (p < 0.01; Fig. 1A). During the 2-hours of 2-bottle choice drinking, mice exhibited strong preference for the ethanol solution (>97% ethanol preference on all days). Two-hour water intake measurements were: 0.02 ± 0.01 ml (39 ± 13 licks) following 0 mg/kg GAN, 0.03 ± 0.01 ml (22 ± 7 licks) following 5 mg/kg GAN, and 0.02 ± 0.01 ml (32 ± 9 licks) following 10 mg/kg GAN. Because 2-hour water intake was negligible and below the level of reliable detection, statistical analyses were not performed on these data and they could not be used as a measurement of non-specific effects on fluid intake. There was a dose effect of GAN on 22-hour water intake [F(2,22) = 4.13, p < 0.05], with a slight increase following both doses (Table 1). BECs at the end of the 2 hr limited access session were 1.37 ± 0.17 mg/ml after DMSO vehicle and 1.22 ± 0.17 mg/ml after 5 mg/kg GAN. There was a significant positive correlation between BEC and g/kg consumed (r = 0.61, n = 24, p = 0.001; data not shown).
Figure 1.
Effect of ganaxolone (GAN) on 2 hour intake of 10% ethanol (10E), measured in A) g/kg and as B) number of licks per 20-minute bin. The 0 mg/kg treatment is the average of two vehicle injections. Values represent the mean ± SEM for 12 mice. **p < 0.01 versus within-subjects vehicle.
Table 1.
Effects of ganaxolone (GAN) on bout parameters for 2 hr limited access consumption of 10% ethanol (10E).
| DOSE GAN | 0 mg/kg | 5 mg/kg | 10 mg/kg |
|---|---|---|---|
| 2-hr 10E licks | 1322 ± 91 | 1043 ± 94** | 886 ± 93*** |
| 22-hr water intake (ml) | 3.25 ± 0.2 | 3.85 ± 0.2* | 3.58 ± 0.2+ |
| Latency to first lick (min) | 1.7 ± 0.7 | 4.7 ± 3.9 | 3.4 ± 2.3 |
| Latency to first bout (min) | 2.5 ± 1.1 | 4.7 ± 3.9 | 7.7 ± 4.4 |
| First bout size | 152 ± 23 | 133 ± 15 | 177 ± 20 |
| Ave bout size | 113 ± 6 | 116 ± 10 | 108 ± 17 |
| Bout frequency | 12.1 ± 1.1 | 9.6 ± 1.2 | 9.5 ± 1.7 |
| Ave lick rate (licks/min) | 366 ± 16 | 346 ± 21 | 324 ± 24 |
| Ave bout length (s) | 34.7 ± 4 | 34.0 ± 5 | 35.0 ± 8 |
| IBI (min) | 10.8 ± 1.0 | 14.8 ± 3.0 | 14.1 ± 1.9 |
Values represent the mean ± SEM for mice depicted in Fig. 1.
First and average bout size = licks/bout; bout frequency = bouts/session; IBI = interbout interval.
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001 versus within-subjects vehicle.
3.1.2 Bin analysis
Seven data points (out of 48) throughout the entirety of the experiment were removed from bin and bout analysis due to discordances in licks per ml correlation. For the remainder of the animals, licks and g/kg intake were highly correlated across all treatment days (Pearson’s r ranged from 0.86 to 0.95; p ≤ 0.001 for all days; data not shown). There was a dose-dependent effect of GAN on total 10E licks [F(2,16) = 12.17, p = 0.001; Table 1], consistent with the g/kg data. To better understand the pattern of ethanol intake, we examined lick patterns by sorting the data into 20-minute bins (Fig. 1B). There was a dose effect of GAN, consistent with the overall lick data, and no treatment by time interaction, suggesting that the GAN-induced decreases in 10E intake were consistent throughout the 2 hour time period (dose effect: [F(5,40) = 4.37; p < 0.01]). Based on our a priori hypothesis that GAN and ALLO would promote the onset of drinking, even when there was no change or a decrease in overall intake (Ford et al., 2005a; Ramaker et al., 2011, Sinnott et al, 2002), we examined the first 20 minutes of the session, split into 5-minute bins. The main effect of GAN dose did not reach statistical significance across the first 20-minute bin and there was no significant dose by bin interaction (data not shown), suggesting that GAN did not differentially alter intake across the first 20 minute bin.
3.1.3. Bout analysis
Analysis with ANOVA revealed a trend for GAN to decrease bout frequency [F(2,16) = 3.29, p =0.06], suggesting that the decrease in intake was primarily due to a 20% reduction in the number of bouts. There was no significant effect of GAN on latency to first bout, latency to first lick, first bout size, average bout size, average lick rate, average bout length, or average inter-bout interval (IBI; see Table 1).
3.2. Exp. 1b: Effect of THIP on limited access ethanol intake in lickometer chambers
3.2.1. Intake
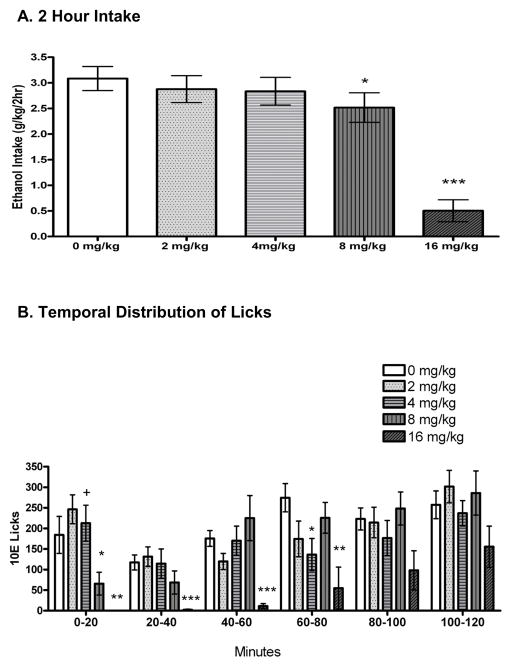
In the absence of THIP treatment (average of 4 baseline days), mice drank an average of 3.08 ± 0.21 g/kg over the 2-hour period. THIP dose dependently decreased 2-hour 10E intake [F(4,44) = 31.63, p < 0.001; Fig. 2A]. During the 2-hours of 2-bottle choice drinking, mice exhibited strong preference for the ethanol solution (>98% ethanol preference on all days). Water intake was: 0.013 ± 0.005 ml (65 ± 18 licks) following 0 mg/kg THIP, 0.001 ± 0.001 ml (36 ± 13 licks) following 2 mg/kg THIP, 0.004 ± 0.009 ml (62 ± 19 licks) following 4 mg/kg THIP, 0.008 ± 0.006 ml (106 ± 46 licks) following 8 mg/kg THIP, and 0.004 ± 0.004 ml (27 ± 14 licks) following 16 mg/kg THIP. As in the GAN study, water intake was below the level of reliable detection, and therefore not used in statistical analyses. There was a dose dependent increase in the 22-hour water intake following the conclusion of the 2-hour drinking session after THIP administration [F(4,44) = 2.53, p = 0.05; Table 2]. BECs upon the conclusion of a limited access session were 1.25 ± 0.21 mg/ml after one saline day and 0.98 ± 0.17 mg/ml after 4 mg/kg THIP. There was a significant positive correlation between BEC and g/kg consumed (r = 0.80, n = 24, p < 0.001; data not shown).
Figure 2.
Effect of gaboxadol (THIP) on 2 hour intake of 10% ethanol (10E), measured in A) g/kg and as B) number of licks per 20-minute bin. The 0 mg/kg treatment is the average of the four saline days that immediately preceded a drug day. Values represent the mean ± SEM for 12 mice. +p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001 versus within-subjects vehicle.
Table 2.
Effects of gaboxadol (THIP) on bout parameters for 2 hr limited access consumption of 10% ethanol (10E).
| DOSE THIP | 0 mg/kg | 2 mg/kg | 4 mg/kg | 8 mg/kg | 16 mg/kg |
|---|---|---|---|---|---|
| 2-hr 10E licks | 1246 ± 112 | 1072 ± 98 | 1047 ± 113 | 1120 ± 168 | 321 ± 123*** |
| 22-hr water intake (ml) | 3.32 ± 0.1 | 3.30 ± 0.2 | 3.10 ± 0.2 | 3.64 ± 0.1** | 3.68 ± 0.2* |
| Latency to first lick (min) | 3.0 ± 1.3 | 5.4 ± 3.0 | 0.43 ± 0.2+ | 4.2 ± 1.8 | 56.4 ± 8.5*** |
| Latency to first bout (min) | 4.9 ± 1.6 | 5.4 ± 3.0 | 5.3 ± 3.6 | 26.6 ± 7.5* | 80.1 ± 13.8*** |
| First bout size | 78 ± 12 | 79 ± 11 | 102 ± 32 | 71 ± 17 | 72 ± 29 |
| Ave bout size | 80 ± 5 | 83 ± 7 | 76 ± 5 | 81 ± 4 | 101 ± 31 |
| Bout frequency | 15.5 ± 1.0 | 13.0 ± 0.9* | 13.6 ± 1.1 | 13.4 ± 1.7 | 3.7 ± 1.4*** |
| Ave lick rate (licks/min) | 282 ± 21 | 327 ± 256 | 277 ± 26 | 282 ± 22 | 264 ± 44 |
| Ave bout length (s) | 45.1 ± 5.3 | 35.6 ± 6.6 | 40.1 ± 5.0 | 38.3 ± 4.9 | 54.3 ± 26 |
| IBI (min) | 7.5 ± 0.6 | 9.2 ± 0.8 * | 8.4 ± 0.7 | 7.5 ± 1.1 | 4.4 ± 1.0+ |
Values represent the mean ± SEM for mice depicted in Fig. 2.
First and average bout size = licks/bout; bout frequency = bouts/session; IBI = interbout interval. n = 12, but note that only 7 mice could be analyzed at 16 mg/kg THIP due to an absence of bouts for the other 5 mice.
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001 versus within-subjects vehicle.
3.2.2. Bin analysis
Four data points (out of 84) throughout the entirety of the experiment were removed from bin and bout analysis due to discordances in licks per ml data. For the remaining data points, there was a significant correlation between licks and g/kg on each baseline and drug day (Pearson r’s ranged from 0.76 to 0.95; p ≤ 0.01 for all days; data not shown). There was a dose-dependent effect of THIP on total 10E licks [F(4,36) = 10.29, p < 0.001; Table 2], consistent with the g/kg intake data. To better understand the pattern of ethanol intake, we examined lick patterns by 20-minute bins (Fig. 2B). Examining the data in 20-minute bins revealed a significant dose by bin interaction [F(20,180) = 1.65, p < 0.05]. Main effect analysis showed a significant effect of dose during bins 1 (minutes 0–20, p < 0.001), 2 (minutes 20–40, p < 0.001), 3 (minutes 40–60, p = 0.001), 4 (minutes 60–80, p = 0.05), and 6 (minutes 100–120, p = 0.005). Post hoc tests revealed that the 16 mg/kg THIP dose exerted a suppressive effect on 10E intake across bins 1–4. The 8 mg/kg THIP dose significantly decreased 10E intake during bin 1. There was a trend for an increase in licks during bin 1 with the 4 mg/kg THIP dose, and this dose significantly decreased licks during bin 4. Further analysis of the first 20 minutes examined by 5-minute intervals revealed a bin by dose interaction [F(12,108) = 2.19; p < 0.005]. Post hoc analysis revealed a dose effect during the first 5 minutes [F(4,36) = 6.91, p < 0.001] with 8 m/kg (p < 0.01) and 16 mg/kg (p < 0.001) significantly decreasing licks. There was also a dose effect during minutes 10–15 [F(4,36) = 3.28, p < 0.001] with a trend for a decrease in licks at 8 mg/kg (p = 0.10) and 16 mg/kg THIP (p = 0.08; data not shown).
3.2.3. Bout analysis
Following treatment with 16 mg/kg THIP, 5 mice did not drink a complete bout so their lick data was removed from all bout analysis (except bout frequency). THIP significantly increased latency to first 10E lick [F(4,20) = 34.38, p < 0.001] and latency to first 10E bout [F (4, 20) = 35.57, p < 0.001; Table 2]. The 16 mg/kg THIP dose increased latency to first lick and first bout and caused some noticeable sedative effects. Interestingly, 8 mg/kg THIP increased latency to first bout but had no effect on latency to first lick (Table 2), suggesting a lack of a confounding sedative effect. Further, 4 mg/kg THIP led to no change in latency to first bout, and a trend for a decrease in latency to first lick, again indicating a lack of a sedative effect at this dose. THIP significantly altered bout frequency [F (4, 36) = 17.50, p < 0.001] and IBI [F(4,20) = 5.92, p < 0.001]. There was no effect of THIP on average bout size, average bout length, lick rate, or first bout size.
3.3. Exp. 2a: Effect of GAN on operant self-administration of ethanol or sucrose
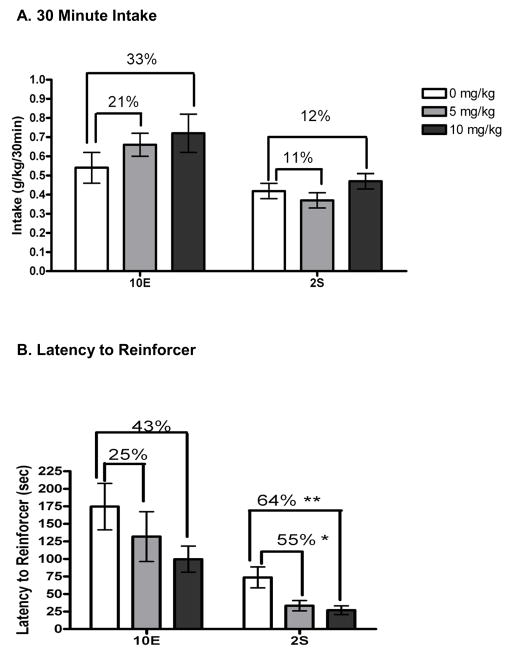
Pretreatment intakes were 0.70 ± 0.03 g/kg ethanol and 0.40 ± 0.03 g/kg sucrose for the 10E-and 2S-reinforced mice, respectively. Analyses of the 10E-reinforced mice indicated that GAN did not affect 10E intake to the level of significance, but caused a slight dose-dependent increase in intake (Fig. 3A); 5 mg/kg GAN increased 10E intake by 21% and 10 mg/kg GAN increased 10E intake by 33%. Latency to reinforcer (Fig. 3B) was decreased by 25% and 43% with 5 mg/kg and 10 mg/kg GAN, but this did not reach the level of significance. There was, however, a trend for GAN to decrease latency to first bout [F(2,16) = 2.65, p = 0.10; Table 3], consistent with a modest increase in the initiation of drinking. GAN also significantly reduced IBI [F(2,12) = 5.91, p < 0.05]. There was no effect of GAN on average bout size, first bout size, average lick rate, bout frequency, or average bout length.
Figure 3.
Effect of ganaxolone (GAN) on 30 minute operant self-administration of 10% ethanol (10E) or 2% sucrose (2S), measured in A) g/kg. B) The effect of GAN on latency to complete the RR-16 and acquire access to the reinforcer is shown in seconds. Values represent the mean ± SEM for 14 mice (10E) and 9 mice (2S). *p < 0.05, **p < 0.01 versus within-subjects vehicle.
Table 3.
Effects of ganaxolone (GAN) on bout parameters for 30-minute operant self-administration of 10% ethanol (10E) or 2% sucrose (2S).
| Reinforcer | 10E | 2S | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dose GAN | 0 mg/kg | 5 mg/kg | 10 mg/kg | 0 mg/kg | 5 mg/kg | 10 mg/kg |
| No. mice that earned reinforcer | 9 | 9 | 9 | 14 | 14 | 14 |
| Latency to first bout (s) | 191 ± 32 | 133 ± 35 | 100.8 ± 18 | 70.8 ± 16 | 38.4 ± 7+ | 25.8 ± 5** |
| Ave bout size | 62 ± 10 | 94 ± 20 | 76 ± 10 | 132 ± 20 | 100 ± 14 | 178 ± 38 |
| Bout frequency | 3.2 ± 0.6 | 2.9 ± 0.5 | 3.2 ± 0.5 | 5.8 ± 0.5 | 6.5 ± 0.5 | 4.9 ± 0.5+ |
| Ave lick rate (licks/min) | 222 ± 26 | 145 ± 54 | 98 ± 21 | 80 ± 16 | 101 ± 20 | 86 ± 26 |
| Ave bout length (min) | 0.83 ± 0.2 | 1.5± 0.5 | 1.5 ± 0.1 | 3.4 ± 0.5 | 2.84 ± 0.4 | 4.6 ± 0.7 |
| IBI (min) | 8.4 ± 1.4 | 3.0 ± 0.9* | 5.2 ± 1.0+ | 2.6 ± 0.3 | 3.0 ± 0.9 | 1.9 ± 0.2 |
| First bout size | 83 ± 14 | 120 ± 20 | 126 ± 16 | 307 ± 41 | 301 ± 59 | 497 ± 107* |
Values represent the mean ± SEM for mice depicted in Fig. 3.
First and average bout size = licks/bout; bout frequency = bouts/session; IBI = interbout interval.
p < 0.10,
p < 0.05,
p < 0.01 versus within-subjects vehicle.
Analyses of the 2S-reinforced mice indicated that GAN significantly altered 2S intake [F(2,26) = 4.83, p < 0.05]. However, no dose was significantly different than vehicle; 5 mg/kg GAN decreased 2S intake by 11%, whereas 10 mg/kg GAN increased 2S intake by 12% (Fig. 3A). There was a dose-dependent effect of GAN to decrease the latency to acquire the reinforcer [F(2,26) = 9.14, p = 0.001], which was decreased by 55% and 64% with 5 mg/kg and 10 mg/kg GAN, respectively (Fig. 3B). Similar to the effect on latency to acquire the reinforcer, GAN pretreatment decreased latency to the first bout [F(2,26) = 6.46, p < 0.01] and increased first bout size [F(2,26) = 4.604, p < 0.05], consistent with an enhancement in the initiation of drinking (see Table 3). There was also a significant effect of GAN on bout frequency [F(2,26) = 6.46, p < 0.01]. There was no effect of GAN on average bout size, lick rate, IBI, or average bout length.
3.4. Exp 2b: Effect of THIP on operant self-administration of ethanol or sucrose
Pretreatment intakes were 0.81 ± 0.08 g/kg ethanol and 0.41 ± 0.03 g/kg sucrose in the 10E- and 2S- reinforced mice, respectively. For both groups of mice, there was a dose-dependent effect of THIP on appetitive responding, which prevented some mice from attaining the reinforcer (see Table 4).
Table 4.
Effects of THIP on bout parameters for 30-minute operant self-administration of 10% ethanol (10E) or 2% sucrose (2S).
| Reinforcer | 10E | 2S | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Dose THIP | 0 mg/kg | 2 mg/kg | 4 mg/kg | 8 mg/kg | 0 mg/kg | 2 mg/kg | 4 mg/kg | 8 mg/kg |
| No. mice that earned reinforcer | 12 | 11 | 11 | 1 | 17 | 17 | 14 | 3 |
| Latency to first bout (s) | 234 ± | 286 ± 53 | 792 ± 126** | --- | 198 ± 54 | 157 ± 57 | 340 ± 78* | --- |
| Ave bout size | 67 ± 5 | 62 ± 6 | 53 ± 6 | --- | 126 ± 19 | 142 ± 30 | 70 ± 9* | --- |
| Bout frequency | 4.5 ± 0.5 | 4.6 ± 0.6 | 3.7 ± 0.5 | --- | 5.8 ± 0.5 | 5.5 ± 0.6 | 6.6 ± 0.5 | --- |
| Ave lick rate (licks/min) | 153 ± 30 | 103 ± 42 | 152 ± 37 | --- | 110 ± 18 | 106 ± 20 | 126 ± 23 | --- |
| Ave bout length (min) | 1.2 ± 0.2 | 1.5 ± 0.3 | 0.84 ± 0.2 | --- | 9.0 ± 5.9 | 4.1 ± 1.0 | 1.9 ± 0.3 | --- |
| IBI (min) | 4.8 ± 0.8 | 5.1 ± 0.8 | 7.7 ± 1.7 | --- | 3.4 ± 0.6 | 2.4 ± 0.4* | 3.4 ± 0.6 | --- |
| First bout size | 96 ± 10 | 92 ± 10 | 66 ± 12 | --- | 264 ± 33 | 266 ± 54 | 138 ± 33* | --- |
Values represent the mean ± SEM for mice depicted in Fig. 4.
First and average bout size = licks/bout; bout frequency = bouts/session; IBI = interbout interval.
p < 0.05,
p < 0.01,
p < 0.001 versus within-subjects vehicle.
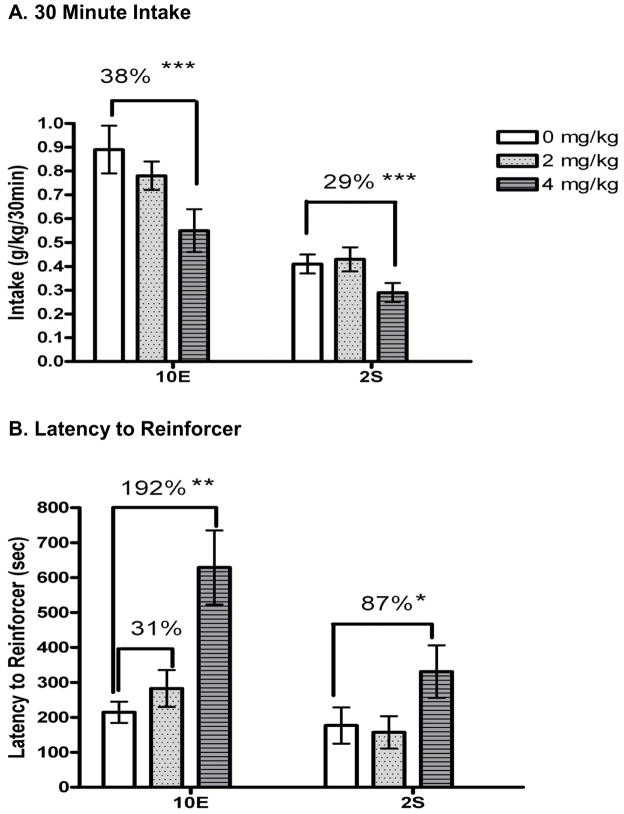
For the mice that earned the reinforcer, THIP also altered the consummatory phase of 10E self-administration; THIP dose dependently reduced g/kg 10E intake [F(2,18) = 16.49 p < 0.001; Fig. 4A]. The 12% decrease in intake following 2 mg/kg THIP was not significant, but 4 mg/kg THIP significantly decreased 10E intake by 38%. THIP also produced a dose dependent increase in the latency to acquire the 10E reinforcer [F(2,18) = 10.94, p = 0.001; Fig. 4B] and in latency to first bout [F(2,16) = 16.08, p < 0.001; Table 4]. The decrease in 10E intake could not be attributed to one specific parameter, but there was a trend for THIP to decrease first bout size [F(2,18) = 2.89; p = 0.08; Table 4]. There was no effect of THIP on average bout size, bout frequency, average lick rate, average bout length, or IBI.
Figure 4.
Effect of gaboxadol (THIP) on 30 minute operant self-administration of 10% ethanol (10E) or 2% sucrose (2S), measured in A) g/kg. B) The effect of THIP on latency to complete the RR-16 and acquire access to the reinforcer is shown in seconds. Values represent the mean ± SEM for 17 mice (10E) and 12 mice (2S) following vehicle (see Table 4 for n/group following each dose of THIP). *p < 0.05, **p < 0.01, ***p < 0.001 versus within-subjects vehicle.
Intake of 2S was also significantly altered by THIP [F(2,26) = 9.39, p = 0.001; Fig. 4A], with post-hoc tests revealing that 4 mg/kg THIP significantly decreased 2S intake by 29%. For the mice that earned the reinforcer, THIP significantly increased latency to acquire the reinforcer [F (2,26) = 6.44, p = 0.001; Fig. 4B] and latency to first bout [F (2,26) = 5.39, p < 0.01; Table 4]. The decrease in intake appeared to be due to a decrease in average bout size [F (2,26) = 7.02, p < 0.01]. THIP also exerted significant effects on IBI [F(2,26) = 7.02, p < 0.01], first bout size [F(2,26) = 6.39; p < 0.01], and bout frequency [F(2,26) = 4.10, p < 0.05]. There was no effect of THIP on average bout length or lick rate.
4. Discussion
The current study demonstrates that a neurosteroid analogue (GAN) and a GABAA receptor agonist with selectivity for extrasynaptic receptors (THIP) alter ethanol self-administration in mice, as measured with limited-access 2-bottle choice and operant self-administration paradigms. These studies expand on the existing literature by providing analysis of lick patterns and shifts in drinking patterns following administration of GAN and THIP, as well as providing insights into the appetitive and consummatory contributions of these drugs to ethanol self-administration.
Previous studies have shown a biphasic effect of ALLO on ethanol intake under limited access procedures; that is, low physiologically relevant doses of ALLO increased ethanol intake while supraphysiological doses of ALLO decreased ethanol intake (e.g., Finn et al., 2010; Ford et al., 2007a, 2005a, Janak et al., 1998). Besheer et al. (2010) reported a similar biphasic effect of GAN on operant self-administration in rats trained on a concurrent FR schedule. They found that 1 mg/kg and 3 mg/kg GAN produced a non-significant increase in ethanol intake (g/kg estimated by number of reinforcers) of 62% and 25%, respectively. Whereas the 10 mg/kg GAN dose produced a non-significant decrease in ethanol intake of 30% (Besheer et al., 2010), we found that 10 mg/kg GAN caused a significant 18% decrease in 2 hr ethanol intake in the present study. This pattern of decrease in ethanol consumption is consistent with data from a continuous access study in mice, where the 10 mg/kg GAN dose significantly decreased ethanol intake during the 2nd and 3rd hour of ethanol access (Ramaker, et al, 2011). Besheer and colleagues (2010) also found that 30 m/kg GAN significantly decreased ethanol intake by 87%, but that it also produced a motor-impairing effect. Thus, in order to prevent the motor impairment seen at high doses, the present studies used a more restricted dose-response range. Lick analysis at these doses revealed an effect of GAN throughout the entire 120 minute period, consistent with similar but extended actions as previously reported following ALLO administration (Ford et al., 2005a). A previous lickometer study using ALLO found that high doses of ALLO primarily decreased intake via reductions in bout frequency (Ford et al., 2005a). In the current study there was a trend for a reduction in bout frequency following GAN administration and a slight increase in time between bouts. These findings are consistent with our earlier work with ALLO and suggest that the significant decrease in 10E intake following GAN was primarily due to a 20% reduction in the number of bouts.
It should be noted that within the current 30 min operant self-administration sessions, ethanol intake was modestly increased by administration of 5 and 10 mg/kg doses of GAN (↑ 21% and 32%, respectively). This result is consistent with the significant increase in ethanol intake during the 1st hour of continuous ethanol access (Ramaker et al., 2011). Interestingly, previous studies have shown that ALLO promotes the initiation of drinking even at doses that produce no change or decreases in overall 2 hr ethanol intake (Ford et al., 2007a, 2005a; Ramaker et al., 2011). For example, our early work (Sinnott et al., 2002) found that ALLO injections in mice led to an increase in ethanol intake in the first hour of access and a decrease in the second hour. Likewise, our subsequent study that utilized lickometers (Ford et al., 2005a) revealed that ALLO exerted a biphasic and time-dependent effect on ethanol intake, with increases in ethanol intake primarily observed within the first 60 min of ethanol access (and in most cases, within the first 20 min of access). With this in mind, the slight increase in ethanol intake in the 30-minute operant session is therefore consistent with only capturing the initial increase in ethanol intake. In harmony with this idea was the current findings in the operant procedure that GAN trended to decrease latency to first bout, and appeared to modestly increase the appetitive drive to consume ethanol, as seen by a non-significant 25% and 43% decrease in time to acquire the reinforcer with 5 and 10 mg/kg GAN, respectively.
In addition to the different lengths of ethanol access in the current experiments, it is also possible that the different times of testing within the light/dark cycle contributed to the seemingly differing effects of GAN between the two current experimental paradigms. Analysis of the circadian rhythm of ALLO and other neurosteroids has shown that brain levels peak at the start of the dark cycle in rodents (Corpéchot et al., 1997). Because the limited-access experiment was conducted during the early phase of the circadian dark cycle (which should correspond to higher endogenous levels), it is possible that the addition of exogenous GAN produced the equivalent of supraphysiological levels of neurosteroid with a resultant decrease in 10E intake (Finn et al., 2010; Ford et al., 2005a). However, the operant experiment was run during the light cycle when endogenous ALLO levels should be at their lowest. Although speculative, the addition of exogenous GAN at this time point may have resulted in neurosteroid levels on the ascending portion of the biphasic dose and time response curve. Thus, it is possible that the divergent time of testing during the circadian cycle and interaction with fluctuations in endogenous neurosteroid levels, as well as the different length of experimental sessions, contributed to the dissimilar direction of GAN’s effect on 10E intake. Overall, the present results with GAN in two different procedures and lengths of ethanol access, in conjunction with existing information on the effects of GAN and ALLO on ethanol intake, provide strong support for time-dependent effects of neurosteroids to promote the onset, and to subsequently decrease, ethanol drinking behavior.
Research examining the role of extrasynaptic GABAA receptors in ethanol sensitivity and reinforcement has produced inconclusive results. Some in vitro studies suggest that these extrasynaptic receptors exhibit enhanced sensitivity to ethanol, while others have failed to show a direct action of physiologically-relevant levels of ethanol (Borghese et al., 2006; Korpi et al., 2007; Olsen et al., 2007; Santhakumar et al., 2007). The δ-subunit is thought to occur primarily outside of the synaptic space (Nusser et al., 1998), making it a potential target of pharmacological intervention to exclusively alter tonic inhibition. δ-subunit knockout mice show decreases in ethanol self-administration and preference, a decrease in ethanol’s anticonvulsant effect, and decreases in neurosteroid sensitivity (Mihalek et al., 2001,1999), indicating that the δ-subunit may be important for some of the reinforcing and behavioral properties of both ethanol and neurosteroids. Because ALLO and GAN can act at both synaptic and extrasynaptic GABAA receptors, experiments with THIP aimed to isolate the effects on ethanol intake that were specific to extrasynaptic GABAA receptors. If both THIP and GAN altered the microarchitecture of ethanol intake in a similar manner, this would provide some indirect evidence that neurosteroids were altering ethanol intake via an action at extrasynaptic GABAA receptors.
There have been contradictory results related to the effects of THIP on ethanol self-administration. Early work (Boyle et al., 1993, 1992) found that 16 mg/kg THIP increased the acquisition and intake of ethanol in male Long Evans rats. We recently found that 8 and 16 mg/kg THIP significantly decreased ethanol intake in C57BL/6J mice during the first 5 hrs of a continuous access procedure (Ramaker et al., 2011). A decrease in 1 hr binge ethanol intake following 8 and 16 mg/kg THIP also was reported in C57BL/6J mice (Moore et al., 2007). The current limited-access study showed no effect on 2 hr ethanol intake with 2 or 4 mg/kg THIP, and a decrease in intake with 8 and 16 m/kg THIP, while the current operant self-administration study revealed a significant decrease in ethanol intake following administration of 4 mg/kg THIP (and as mentioned previously, administration of 8 mg/kg resulted in only a small subset of mice gaining access to the sipper). The effect of the high THIP doses (e.g., 8 & 16 mg/kg) to suppress ethanol intake in various mouse models is therefore consistent across studies. Since the 4 mg/kg THIP dose only decreased operant ethanol self-administration, it is possible that the instrumental responding that was required to gain access to the ethanol solution enhanced the sensitivity to the behavioral effects of this dose of THIP. Certainly, the 38% decrease in ethanol self-administration corresponded to a 192% increase in the latency to acquire the reinforcer and a 238% increase in the latency to complete the first ethanol bout.
One potential explanation for this sensitivity shift between experiments is that local levels of GABA and extrasynaptic receptor density can alter the potency of THIP and neurosteroids (Houston et al., 2012). Thus, although speculative, it is possible that the different experimental parameters (i.e., instrumental versus free access) employ slightly divergent brain regions and neural networks and that local differences in GABA levels or receptor density may contribute to differences seen in sensitivity to drugs across paradigms. Another potential explanation for the variation between procedures in the effect of the 4 mg/kg THIP dose may be a shift in the dose-response curve due to differences in sensitivity to pharmacological manipulation across intake levels. Importantly, at the end of the 2 hr lickometer study, mice achieved physiologically relevant BECs of > 100 mg/dl that are associated with the criteria for binge drinking (i.e., > 80 mg/dl), whereas data from our laboratory suggest that BECs likely would be much lower following a 30 min operant self-administration session (e.g., average BECs of 30 or 37 mg/dl, range of BECs from 0 – 107 mg/dl; Ford et al., 2007a, 2007b). Further work is necessary to elucidate shifts in sensitivity between paradigms, but together, the current studies corroborate earlier findings of a suppressive effect of THIP on ethanol intake and support a role for the involvement of extrasynaptic GABAA receptors in ethanol reinforcement.
One of the advantages of the current experiments was the ability to examine bouts of consumption and individual lick patterns that have not been represented in previous paradigms that merely measure overall intake. Additionally, the RR schedule contingency provided insight into the appetitive and consummatory processes and their respective contributions to changes in ethanol intake. Specifically, in the limited access study, the latency to first bout was increased following 8 and 16 mg/kg THIP, indicating a decrease in the initiation of drinking following THIP treatment. Also, latency to acquire the reinforcer (and also latency to first bout) was increased by 4 mg/kg THIP in the operant study. Together these studies indicate that the decreases in intake following THIP in the current procedures may be partly explained by THIP’s effect to decrease the appetitive drive to consume ethanol, as well as to decrease the onset of ethanol consumption.
Potential complications that arise with the use of GABAA receptor-specific drugs involve sedative and non-specific motor effects that may confound interpretations of effects on ethanol drinking behavior. We deliberately limited our range of GAN doses to test, based on locomotor suppressive effects that were reported following 30 mg/kg GAN (Besheer et al., 2010). With regard to THIP, ataxia has been reported following 5 mg/kg THIP in CF1 mice (Madsen et al., 2011), while another study showed an effect of THIP on ataxia after 10 and 30 mg/kg, but not 5 mg/kg THIP (Herd et al., 2009). In the present studies, locomotor activity was not directly measured, and low baseline responding on the inactive lever (<1 per animal per session) precluded its use as a tool to assess non-specific motor effects, so we cannot exclude an effect of THIP-elicited motor impairment on the intake of ethanol in the operant paradigm. However, given that there was no effect on latency to first lick following 8 mg/kg THIP and a trend for a decrease in latency to first lick at 4 mg/kg THIP, it is unlikely that the decrease in intake in the lickometer experiment was confounded by locomotor suppression at either of these doses. On the other hand, administration of the 16 mg/kg THIP dose caused observable sedative effects that likely contributed to the decrease in ethanol intake.
Another consideration in the interpretation of the THIP data is whether the doses employed produced concentrations that would selectively activate extrasynaptic GABAA receptors. In the present study, injections of 16 mg/kg THIP caused a strong sedative effect and likely led to activation of both synaptic and extrasynaptic receptors, consistent with data showing that 15 mg/kg THIP caused ataxia, analgesia, and sedation in both wild-type and δ-subunit knockout mice (Chandra et al., 2006). On the other hand, injections of THIP doses of up to 10 mg/kg in rats produced peak CNS levels within minutes, and levels were in the low micromolar range by 30 minutes post-injection (Cremers and Ebert, 2007). Given that these low micromolar THIP concentrations (2–5 μM) act very selectively, if not exclusively, at δ-containing receptors (Stórustovu and Ebert, 2006), we are confident that the 2 – 8 mg/kg doses of THIP that were employed in the present study exerted selective effects at extrasynaptic GABAA receptors. Importantly, these studies extend existing information to indicate that doses of THIP that act preferentially at extrasynaptic GABAA receptors modulate ethanol intake in multiple paradigms, including a 24-hour access and limited-access 2-bottle choice procedure, a single bottle drinking in the dark procedure, and operant self-administration procedure (Moore et al., 2007; Ramaker et al., 2011).
One important consideration in studying drugs that affect ethanol intake is the generalizability to other calorie containing solutions or to sweetened solutions. In the present operant self-administration study, GAN appeared to enhance the appetitive phase of sucrose self-administration, based on the significant decrease in the latency to access the reinforcer. While sucrose intake was not systematically affected by GAN in the present study, a study in NSA mice found a biphasic effect of GAN on sucrose intake (↑ with a 4 mg/kg dose, no change with 8 mg/kg, and ↓ at a 16 mg/kg dose, Vanover et al., 2000). Thus, use of a larger range of doses in the present study may have revealed significant effects on sucrose intake. None the less, this is the first study directly comparing the effect of GAN on ethanol reinforcement to that of a non-drug natural reinforcer. Similarly, there is also conflicting evidence on the specificity of ALLO to alter intake of sucrose and saccharin, which may be influenced by species differences (rat versus mouse), procedural differences (operant versus 2-bottle choice), or availability of a concurrent reinforcer (Janak and Gill 2003; Sinnott et al., 2002). There is some evidence to suggest that local increases in GABA concentration and administration of GABAA receptor agonists stimulate feeding behavior, so the nonspecificity of drugs that potentiate the release of GABA could be due to stimulation of systems that trigger increases in caloric intake. Since sucrose has a caloric value similar to that of ethanol, potentiation of the GABAA receptor could be partially responsible for the non-specific effects on both ethanol and sucrose intake (Kelley et al., 2005; Stratford and Kelley, 1997). Similarly, the effect of THIP to decrease intake in the present study was not specific to ethanol reinforcement, as intake of sucrose and latency to acquire the sucrose reinforcer were altered by THIP in a comparable manner to that of ethanol. Consistent with this, Moore et al. (2007) also found that 8 and 16 mg/kg THIP decreased 1-hour water intake in mice. To our knowledge, this is the first study examining the generalizability of THIP to intake of a sweetened reinforcer. However, data following brain region-specific knockdown of extrasynaptic GABAA receptor subunits (i.e., α4 and δ) have illustrated that the subunit knockdown produced a selective decrease in ethanol intake that did not extend to sucrose intake in rats (Nie et al., 2011; Rewal et al., 2011, 2009). Clearly, further studies examining the specificity of the regulatory role of extrasynaptic GABAA receptors in ethanol versus general reinforcement are necessary.
4.1. Conclusions
The present results add to an abundance of data showing a relationship between neurosteroid levels, ethanol reinforcement and ethanol-induced behaviors. Further, these data corroborate a growing body of literature suggesting that extrasynaptic GABAA receptors may be an important target of ethanol and may regulate ethanol intake. Further investigation into the contribution of neurosteroids and extrasynaptic GABAA receptors to the reinforcing properties of ethanol are necessary. GAN is currently in phase II clinical trials for treatment of convulsions and post-traumatic stress disorder. Until recently, THIP was in phase III clinical trials for use as a treatment for insomnia, but it currently is in a phase II clinical trials as a combination therapy with escitalopram in major depressive disorder. Therefore, continued investigation of these (or similarly acting) drugs as possible therapeutic agents is valuable. A more complete profile of the manner in which GAN and THIP affect ethanol reinforcement and intake in various preclinical models could lead to novel therapeutic strategies for the treatment of alcohol use disorders.
Highlights.
THIP dose dependently decreased ethanol intake in two drinking paradigms in C57BL/6J male mice.
Ganaxolone shifted the pattern of drinking and time and dose-dependently altered ethanol intake.
GABAA receptors may regulate ethanol intake at both synaptic and extrasynaptic receptors.
Acknowledgments
We would like to thank Michelle Tanchuck and Chris Snelling for their technical assistance. Funding for these projects was provided by grants AA016981 and AA012439 and by grants and resources from the Department of Veteran Affairs. MJR was supported by an OHSU Scholarship, and MMF was supported by KO1 AA016849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O’Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bobak M, Room R, Pikhart H, Kubinova R, Malyutina S, Pajak A, Kurilovitch S, Topor R, Nikitin Y, Marmot M. Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. J Epidemiol Community Health. 2004;58:238–242. doi: 10.1136/jech.2003.011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Smith BR, Amit Z. Microstructural analysis of the effects of THIP, a GABAA agonist, on voluntary ethanol intake in laboratory rats. Pharmacol Biochem Behav. 1992;43:1121–1127. doi: 10.1016/0091-3057(92)90491-w. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Phamacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-Hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–80. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Cremers T, Ebert B. Plasma and CNS concentrations of gaboxadol in rats following subcutaneous administration. Eur J Pharmacol. 2007;562:47–52. doi: 10.1016/j.ejphar.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Lotrich F, Gallaher EJ. Genetic differences in behavioral sensitivity to a neuroactive steroid. J Pharmacol Exp Ther. 1997;280:820–828. [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–70. [PubMed] [Google Scholar]
- Fodor L, Biro T, Maksay G. Nanomolar allopregnanolone potentiates rat cerebellar GABAA receptors. Neurosci Lett. 2005;383:127–130. doi: 10.1016/j.neulet.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA. Inhibition of 5α-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007a;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007b;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005a;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005b;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gmel G, Klingemann S, Muller R, Brenner D. Revising the preventive paradox: the Swiss case. Addiction. 2001;96:273–284. doi: 10.1046/j.1360-0443.2001.96227311.x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for δ-GABAA receptors. Eur J Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, de Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Houston CM, McGee TP, MacKenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? J Neurosci. 2012;32:3887–3897. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O’Buckley T, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: Correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, Luddens H. Does ethanol act preferentially via selected brain GABAA receptor subtypes? The current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr, Aurelian L. Binge alcohol drinking is associated with GABAA α2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KK, Ebert B, Clausen RP, Krogsgaard-Larsen P, Schousboe A, White HS. Selective GABA transporter inhibitors tiagabine and EF1502 exhibit mechanistic differences in their ability to modulate the ataxia and anticonvulsant action of the extrasynaptic GABAA receptor agonist gaboxadol. J Pharmacol Exp Ther. 2011;338:214–219. doi: 10.1124/jpet.111.179671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–420. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABAA-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2 GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA. 2011;108:4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABAA receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35:1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. α4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Donahue R, Gill TM, Nie H, Ron D, Janak PH. α4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of alcohol. Addict Biol. 2011;17:309–321. doi: 10.1111/j.1369-1600.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stórustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Huber M, Wilent WB, Carter RB. Neuroactive steroids attenuate cocaine-induced sucrose intake in rats, but not cocaine-induced hyperactivity in mice. Psychopharmacology. 2000;149:269–276. doi: 10.1007/s002139900350. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABAA receptor subtypes. Pharmcol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AR, Liu J, Yi HS, Warnock KT, Wang M, June HL, Jr, Puche AC, Elnabawi A, Sieghart W, Aurelian L, June HL., Sr Binge drinking: in search of its molecular target via the GABAA receptor. Front Neurosci. 2011;108:4465–4470. doi: 10.3389/fnins.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]