Abstract
Objective
To assess the association of industry funding with the characteristics, outcome, and reported quality of randomized controlled trials (RCTs) of drug therapy for rheumatoid arthritis (RA).
Methods
MEDLINE and Cochrane Central Register of Controlled Trials databases were searched to identify original RA drug therapy RCTs published in 2002–3 & 2006–7. Two reviewers independently assessed each RCT for the funding source, characteristics, outcome [positive (statistically significant result favoring experimental drug for the primary outcome) or not positive], and reporting of methodological measures whose inadequate performance may bias treatment effect assessment. RCTs registered at ClinicalTrials.gov and completed in the study years were assessed for publication bias.
Results
103 eligible RCTs were identified with following funding sources: 58 (56.3%) industry; 19 (18.4%) non-profit; 6 (5.8%) mixed; and 20 (19.4%) unspecified. Industry funded RCTs had significantly more study centers and subjects; while non-profit funded RCTs had longer duration, and were more likely to study different treatment strategies. Outcome could be assessed for 86 (83.5%) RCTs. Funding source was not associated with higher likelihood of positive outcomes favoring the sponsored experimental drug [industry (75.5%), non-profit (68.8%), mixed (40%), and unspecified (81.2%); p = 0.37]. Industry funded RCTs had trend towards higher likelihood of non-publication (38.6% versus 16.7%, p = 0.093). Industry-funded RCTs reported more frequent performance of double-blinding, adequate participant flow description, and intention-to-treat analysis.
Conclusion
Industry funding was not associated with higher likelihood of positive outcomes of published drug therapy RCTs for RA, and reported better on some key RCT quality measures.
Keywords: Randomized Controlled trials, Industry Funding, Conflict of interest, Rheumatoid arthritis
A dramatic increase in pharmaceutical industry funding and support of biomedical research has occurred in the past few decades (1, 2). This has led to strong concerns regarding inappropriate influence of industry funding on the biomedical research (3). Preponderance of evidence shows that industry funded research is associated with increased likelihood of pro-industry results and conclusions (4–11).
Randomized controlled trials (RCTs) are considered the “gold standard” means to assess healthcare interventions. They are designed to eliminate bias by randomly distributing known and unknown confounding factors. RCTs need to be methodological sound to eliminate sources of bias that may appear at various stages. Bias make the results differ systematically from the truth through the combination of various study design, data analysis, and presentation factors (12). Substantial evidence shows that methodological quality of RCTs affects estimates of intervention efficacy (13–15). Limited data on the association of industry funding with RCT methodological quality shows conflicting results with some studies showing no difference (5, 16), while others showing either a trend towards higher quality (17, 18) or significantly higher quality compared to non-industry funded RCTs (8, 19–21).
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that chiefly manifests as inflammatory destructive arthritis, and affects 0.5–1% of the adults (22). Drug therapy options for RA treatment have remarkably improved over the past fifteen years.
In particular, the discovery and availability of biologics as therapeutic agents for RA treatment was facilitated by the funding of clinical trials from pharmaceutical companies. A study to assess secular changes in the methodological quality of published RCTs in rheumatology found no differences between industry and non-profit funded RCTs (23). However, this study included both drug and non-pharmaceutical therapy RCTs, and only 102 (42.5%) of the 240 study RCTs were for RA treatment. There are no data on the influence of industry funding on outcome of RA drug therapy RCTs. The objective of this study was to determine the association of industry funding with the characteristics, outcome and the reported methodological quality of drug therapy RCTs for RA.
METHODS
Study years
RA drug therapy RCTs published in the years 2002–2003 and 2006–2007 were studied. The study year’s selection was based upon the latest available Consolidated Standards of Reporting Trials (CONSORT) statement version (publication year 2001) at the time of data collection (24). The CONSORT statement, originally proposed in the year 1996, was developed to improve the quality of RCT reporting (25). The quality of RCTs in rheumatology before (1987–1988) and after (1997–1998) the original CONSORT statement has already been published (23). Hence, we choose an immediate (2002–2003) and a late [2006–2007, latest possible at the time of data collection in 2008] time periods after revised CONSORT statement publication.
Search strategy
Literature was searched using PubMed, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. Search terms used were “rheumatoid arthritis” and “arthritis, rheumatoid”. Limits used were “Clinical Trials” (in PubMed), “English” (in PubMed) and the years “2002–2003” and “2006–2007”. Retrieved reports were screened by review of the title and abstract. Full published report was retrieved when subject allocation method (random or non-random) remained unclear from the title and abstract. Full review was done for reports that randomly allocated patients to different intervention groups.
Inclusion and exclusion criteria
RCTs were selected if they met the following criteria: 1) original report of a single RCT; 2) pharmacological intervention; 3) parallel design; and 4) clinical primary end point(s). If multiple publications originated from a single RCT, the first published report with clinical primary end point(s) was selected. RCTs were excluded based on one or more of the following criteria: 1) phase 1 RCT; 2) reports of RCT’s open label extension phase; 3) non-English language; and 4) inclusion of non-RA subjects. We have referred to thus identified eligible studies as “published” RCTs, when needed, to distinguish them from “registered” RCTs (see below).
Data abstraction
A reference sheet that defined and listed criteria that would fulfill adequate description of each study variable, when applicable, was created. 15 RCTs (in sets of five each) published in non-study years were evaluated using the reference sheet to further clarify definitions of study variables. Two reviewers (NAK and JIL) independently assessed each eligible “published” RCT for outcomes of interest. Differences were resolved by consensus. All data were collected from the original RCT paper. An earlier publication (in a non-study year) was reviewed when the study authors specifically referred to that publication for the methodological details.
RCT characteristics
Data on the journal and year of publication, number of authors, total number of study subjects, duration of the study, number of study centers, and the number of study countries, study phase and the design of the study intervention arms were recorded. The study agent(s) were classified as experimental drug (ED), active comparator drug (ACD) or placebo. An RCT with ED that targeted a specific molecule in inflammatory pathway or specific receptor in circulation or on a particular cell surface was considered to be have used biological disease modifying anti-rheumatic agent (DMARD). RCTs with one or more of the following as ED were classified as having used traditional DMARD(s): methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, corticosteroids, azathioprine, cyclosporine, tacrolimus, or cyclophosphamide. The remaining RCTs were classified as using “other” drug as experimental intervention (such as non-steroidal anti-inflammatory drugs, bisphosphonates, antibiotics). Impact factor (IF) of the journal of study publication was obtained from 2007 Journal Citation Reports® Science Edition. RCTs were considered to be published in high or low impact journal if the publication journal had ≥ median or < median IF respectively.
Funding information
RCT funding source(s) were classified, based on the disclosure in the published manuscript, as one of the following: 1) industry (manufacturer of the ED); 2) nonprofit (such as National Institute of Health or Arthritis Foundation); 3) mixed (both industry and non-profit sources); or 4) unspecified (no funding source disclosure). We categorized RCTs with full or partial funding from industry as being “industry funded”, and those with non-profit or unspecified funding source as “non-profit funded” for presenting most of the analyses in this paper.
Outcome assessment
The RCT study intervention arms were classified as having ED, ACD(s) or placebo. The outcome assessment of the experimental intervention arm was based upon the designated primary outcome in the published RCT report. First reported outcome was used as the primary outcome if none was explicitly specified. Table 1 summarizes the criteria used for outcome designation for RCTs with different intervention arm structures. Broadly, an RCT with a statistically significant result for the primary outcome favoring the ED arm was classified as having a positive outcome. Outcome could not be assessed for RCTs with safety of an ED as the primary outcome or if no study intervention arm was declared as experimental a priori.
Table 1.
Outcome assessment criteria for the study RCTs
| Study intervention arms | Outcome classification
|
|
|---|---|---|
| Positive outcome | Non-positive outcome | |
| Experimental drug vs placebo or Experimental drug vs active comparator drug | Statistically significant result in favor of the experimental drug for the primary outcome | |
| Experimental drug vs active comparator drug vs placebo | Statistically significant result favoring experimental drug over placebo and no significant difference with the active comparator drug | Statistically significant result favoring active comparator drug over experimental drug |
| Experimental drug vs active comparator drug vs combination of experimental and active comparator drug | Any study arm with the experimental drug significantly better than the active comparator group | |
| Multiple doses of experimental drug vs placebo | At least one dose showed significant efficacy in primary outcome without increased adverse events | Significant safety concerns even if efficacy was found for primary outcome, and authors did not recommend use of the experimental drug |
Assessment of reported methodological quality of RCT
Individual quality measures that are important for RCT’s internal validity, and whose inadequate performance may bias treatment effect assessment were evaluated for adequate reporting (24, 26, 27). These include:
Randomization
It is a method for allocating study participants to different study intervention arms by chance alone. Randomization was considered adequate if an explicit appropriate description of the allocation method (such as random number table use, computer generated random sequence) was provided.
Allocation concealment
It is a method to prevent foreknowledge of treatment assignment from those who enroll RCT participants to shield them from being influenced by such knowledge. Adequate reporting of allocation concealment required explicit description of measures to conceal subject allocation to intervention groups from those responsible for assessing patients for the trial entry. Examples of such measures include central treatment arm allocation using telephone/automated assignment system, local pharmacy use, serially numbered sealed opaque envelopes, and number/coded bottles.
Blinding
An RCT was considered to have adequate reporting of “double-blinding” if specific measures (such as use of identical looking placebo) were described that would make the study subjects and health care-givers unaware of who received which study intervention. Reporting of outcome assessor blinding was noted for RCTs not conducted in a double-blind fashion.
Participant flow description
Description of flow of participants, either in the text or as a flow diagram, through each RCT stage was assessed for adequacy. Specifically, reporting of the number of participants who were randomized to each intervention arm, received the intended treatment, completed the study protocol, and analyzed for the primary outcome was noted.
Intention-to-treat (ITT) analysis
ITT analysis was deemed performed if all randomized patients were analyzed according to the original assigned intervention group. Exclusion of one or more of the following categories of study subjects from the RCT analyses including 1) those with no post-baseline assessment; 2) those found to be non-eligible for the RCT after randomization; and 3) those who never received any study intervention was not considered violation of the ITT principle (28, 29). ITT analysis is not applicable for the RCTs with safety of the ED as the primary end point.
Assessment of publication bias
Publication bias occurs when trials publish only positive results, partial results or with a primary outcome different than the original protocol (30, 31). Two authors (KDT and MS) performed publication bias assessment using following methods:
ClinicalTrials.gov (CTG) registry was searched for clinical trials (CTs) of RA. United States National Library of Medicine established CTG in the year 2000 as an Internet-based publicly accessible registry of CTs (32). Funding source information is mandatory for CTG registration. CTG search results were screened for CTs that were exclusively for RA, had drug therapy as intervention, specified “randomized” method for subject allocation, were described as phase 2 or more, and reported completion in the years 2002–2003 & 2006–2007. CTG defines a completed trial as a study that has concluded and participants are no longer being examined or treated. Since most “published” RCTs did not report their start and completion dates, we selected the completion years for “registered” RCTs identical to the publication years for “published” RCTs to closely approximate characteristics of “published” and “registered” RCTs. Lead study sponsors were classified as industry or non-profit (governmental organizations, universities etc). “Registered” RCTs were assessed for publication using a standardized search strategy. First, CTG record was searched for links to publications resulting from the registered RCT. PubMed, CENTRAL and Google Scholar databases were sequentially searched if CTG had no publication information. Publication of “registered” RCTs was confirmed by matching the study descriptions at CTG with that in the manuscript.
All “published” RCTs were assessed for CTG registration to study discordance in the published and the registered primary outcome. First, “published” RCT manuscripts were searched for CTG registration reporting. Second, PubMed listing of each “published” RCT was assessed for CTG registration information. Finally, we searched the CTG with terms “rheumatoid arthritis” and “name of the interventional drug(s).”
Statistical analysis
Categorical data were described as number and percentages. Since all the study continuous variables had non-normal distribution, they are described as median (inter quartile range, IQR). Association of RCT funding source with study outcome and quality parameters was assessed using Pearson’s Chi-square test. Fisher’s exact or likelihood ratio tests were used for contingency tables with 4 or > 4 cells respectively, when the expected cell count was < 5. Mann-Whitney U test was done to compare continuous data. Logistic regression was used to adjust for potential confounders when assessing the association between the funding source and RCT outcome. SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for data analysis.
RESULTS
Characteristics of the study RCTs
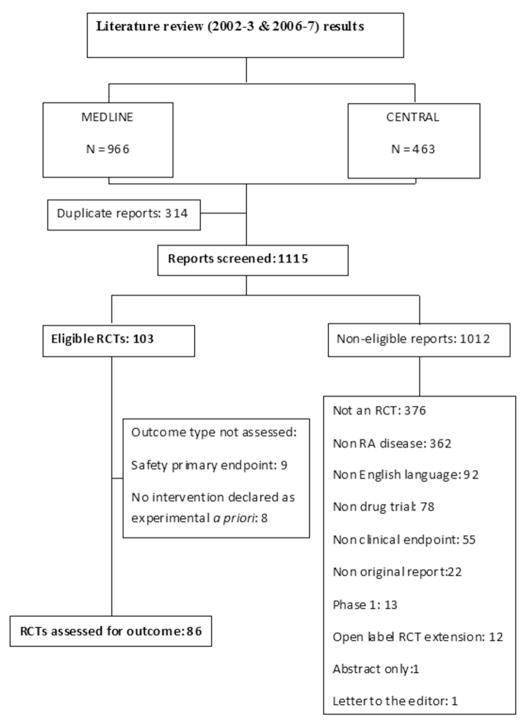
Figure 1 depicts the outcome of screening of the 1115 reports identified from the literature search. A total of 103 (9.2%) RCTs met the eligibility criteria. Most reports were excluded because of non-randomized study design or having non RA disease as the main focus. 58 (56.3%) RCTs reported funding by manufacturer of the ED; 19 (18.4%) by non-profit source(s); 6 (5.8%) had mixed funding sources while 20 (19.4%) RCTs had unspecified funding source. Hence, 64 (62.1%) RCTs had complete or partial industry funding.
Figure 1.
Flowchart depicting selection of eligible randomized control trials for the study.
No significant difference was found in the RCT funding source between the years 2002–3 and 2006–7. However, RCTs differed significantly on several characteristics according to the funding source (Table 2). Industry-funded RCTs had larger number of study subjects, and were more likely to be conducted in multiple centers and countries. Vast majority of the Phase 2 RCTs, and those with biological DMARD as ED were industry funded. Industry funding was also associated with publication in a higher IF journal. Non-industry funded RCTs were more likely to study traditional DMARD agents, and test different strategies using drug or combination of drugs for RA treatment. The study duration was significantly longer for the non-industry funded RCTs (Table 2). Even after exclusion of Phase 2 studies (which typically are of shorter duration), non-industry funding was associated with longer study duration [median (IQR) of 12 (5.6–21) months versus 6 (3.5–12) months, p = 0.046].
Table 2.
Characteristics of the study randomized controlled trials (RCTs).*
| Characteristics | All RCTs | Industry funded RCTs | Non-industry funded RCTs | P** |
|---|---|---|---|---|
| Year of publication | ||||
| 2002–2003 | 48 (46.6) | 28 (58.3) | 20 (41.7) | |
| 2006–2007 | 55 (53.4) | 36 (65.5) | 19 (34.5) | 0.46 |
| Number of study centers | ||||
| Single | 30 (29.1) | 9 (30) | 21 (70) | |
| Multiple | 73 (70.9) | 55 (75.3) | 18 (24.7) | <0.001 |
| Number of countries of study conduct | ||||
| Single | 66 (62.9) | 27 (40.9) | 39 (59.1) | |
| Multiple | 37 (37.1) | 37 (100) | 0 (0) | <0.001 |
| Number of study authors, median (IQR) | 9 (5–11) | 9 (7–11) | 6 (4–10) | 0.004 |
| Number of study patients, median (IQR) | 126 (53–393) | 295 (78–633) | 66 (45–151) | <0.001 |
| Duration of study in months, median (IQR) | 6 (3–12) | 5.6 (3–12) | 12 (4.7–18) | 0.012 |
| Type of study | ||||
| Phase 2 | 16 (15.5) | 14 (87.5) | 2 (12.5) | |
| Phase 3/unspecified | 87 (84.5) | 50 (57.5) | 37 (42.5) | 0.026 |
| Study agent | ||||
| Traditional DMARD(s) | 25 (24.3) | 5 (20) | 20 (80) | |
| Biologic DMARD(s) | 38 (36.9) | 36 (94.7) | 2 (5.3) | |
| Others | 40 (38.8) | 23 (57.5) | 17 (42.5) | <0.001 |
| Type of study intervention arms | ||||
| ED vs placebo | 34 (33) | 20 (58.8) | 14 (41.2) | |
| ED vs ACD | 19 (18.4) | 11 (57.9) | 8 (42.1) | |
| ED vs ACD vs placebo | 7 (6.8) | 6 (85.7) | 1 (14.3) | |
| ED vs ACD vs ED+ACD combination | 4 (3.9) | 4 (100) | 0 (0) | |
| Multiple doses of ED | 6 (5.8) | 4 (66.7) | 2 (33.3) | |
| Multiple doses of ED vs placebo | 19 (18.4) | 15 (78.9) | 4 (21.1) | |
| Different treatment strategies with drug(s) | 14 (13.6) | 4 (28.6) | 10 (71.4) | 0.02*** |
| Journal impact factor, median (IQR) | 4 (2.7–7.7) | 6.4 (3.1–7.7) | 2.9 (1.3–5.8) | <0.001 |
ED: Experiment drug(s); ACD: Active comparator drug(s)
Values represent n(%) unless specified otherwise.
p Values are for comparison between industry and non-industry funded RCTs using chi-square test for categorical variables and Mann-Whitney U test for continuous variables unless specified otherwise.
Maximum likelihood ratio
Funding source and study outcome
86 (83.5%) could be assessed for efficacy outcome. Nine RCTs had safety as the primary outcome while eight RCTs did not declare a specific intervention as experimental a priori, and hence could not be assessed for efficacy outcome. A large proportion of the RCTs, with the exception of mixed source funded RCTs, had positive outcome (Table 3). Industry funding of RCTs was not associated with higher likelihood of positive outcome [likelihood ratio = 3.28, df = 3, p Value = 0.35] favoring the ED. An association between industry funding and study outcome was not found when comparing RCTs with any industry funding (39/54, 72.2%) with those that had no declared industry funding (24/32, 75%) [χ2 = 0.08, df = 1, p = 0.77]; or when comparing only RCTs that were exclusively industry funded (37/49, 75.5%) with those exclusively non-profit source funded (11/16, 68.8%) [χ2 = 0.28, df = 1, p = 0.59]. No association between funding source and the study outcome was found after adjustment for the type of study drug used, number of study center, study phase, number of study subject or journal IF (data not shown).
Table 3.
Funding source and efficacy outcome of study randomized controlled trials (RCTs).*
| Funding source | RCTs with positive outcome | Total number of RCTs | % RCTs with positive outcome |
|---|---|---|---|
| Industry | 37 | 49 | 75.5 |
| Non-profit | 11 | 16 | 68.8 |
| Mixed | 2 | 5 | 40 |
| Unspecified | 13 | 16 | 81.2 |
86/103 (83.5%) RCTs could be assessed for efficacy outcome.
Funding source and reporting quality of RCTs
Several key methodological aspects of “published” RCTs were not adequately reported irrespective of the funding source (Table 4). This was particularly true for adequate reporting of random sequence generation and allocation concealment. Industry funded RCTs were significantly associated with more frequent reporting of several methodological quality measures such as double-blinding, adequate descriptions of participant flow during the study, and performance of ITT analysis (Table 4). Blinding of outcome assessor and RCT funding source showed no significant associations. Analyses using all 4 categories of funding (industry, non-profit, mixed, unspecified) or excluding RCTs with mixed funding or using RCTs with explicitly stated industry and non-profit funding source showed similar results for association of funding source with methodological quality of RCT reporting (data not shown). Adjustment for the individual quality measures did not change the results of association between funding source and the study outcome (data not shown).
Table 4.
Methodological quality measures of industry and non-industry funded randomized controlled trials (RCTs).
| Industry funded (N = 64), n (%) | Non-industry funded (N = 39), n (%) | P* | |
|---|---|---|---|
| Random sequence generation | 22 (34.4) | 14 (35.9) | 0.87 |
| Allocation concealment | 20 (31.2) | 11 (28.2) | 0.74 |
| Double-blinding | 55 (85.9) | 20 (51.3) | <0.001 |
| Blinding of outcome assessor** | 7/9 (77.8) | 8/19 (42.1) | 0.11 |
| Participant flow | 54 (84.4) | 26 (66.7) | 0.036 |
| Intention-to-treat analysis*** | 45/56 (80.4) | 21/38 (55.3) | 0.009 |
Chi-square or Fisher’s exact test
For RCTs that were not double-blind
Excluding RCTs with safety as primary outcome
Funding source and publication bias
62 RCTs registered at CTG (9 in the years 2002–2003 & 53 in the years 2006–2007) met the eligibility criteria. 44 (71%) had industry and 18 (29%) had non-profit source as the lead study sponsor. Industry sponsored RCTs showed a trend towards lower likelihood of publication than the non-profit sponsored RCTs [27 (61.4%) vs 15 (83.3%), (p = 0.093)]. Only 6 of the 103 (5.8%) “published” RCTs were registered at CTG. “Published” and “registered” primary outcomes were identical in 5 (83.3%) of these RCTs.
DISCUSSION
Our study found no association between the source of funding of “published” RCTs of drug therapy for RA and their outcome. A trend towards publication bias was found in the industry funded RCTs. Industry funded RCTs reported significantly better on the performance of certain methodological quality measures.
Our finding of industry as the funding source for majority of the published as well as registered RCTs is consistent with the trend for increased proportion of biomedical research to be industry funded (1, 2). The significant differences in RCT characteristics associated with funding source has important implications for the nature of RCTs conducted, and hence the evidence generated for clinical care of RA patients. While industry funded RCTs predominantly focused on assessment of efficacy and safety of newer therapeutic drugs, majority of non-profit funded RCTs evaluated established drugs and different strategies to use drugs for RA treatment. Evidently, industry funded RCTs had more financial resources as they were more likely to be multicenter, multinational and with higher subject enrollment. Despite the financial advantage, industry funded RCTs had shorter study duration than non-profit source funded RCTs. These differences clearly highlight the importance of both industry and non-profit sources for funding of RCTs to generate efficacy and safety evidence for newer as well as established drugs and strategies for their use in clinical care.
Though preponderance of data in medical literature shows that industry funding leads to higher chances of pro-industry results and conclusions (4–11), we did not find any association between the funding source and the study outcome of “published” RCTs of RA drug therapies. Adjustment for differences in RCT characteristics and reported methodological quality measures did not affect this finding. 1850 RCTs (with 80% power) would be needed to show a significant association between funding source and study outcome assuming relative frequency of explicitly stated industry and non-profit funding (about 3:1), and percentage with positive outcomes (75.5% and 68.8%) similar to our study. Hence, among “published” RA drug therapy RCTs relatively small difference exists in the study outcomes between those with industry and nonprofit funding source. One potential reason for the lack of association between funding source and study outcome could be publication bias. Indeed, we did find that industry funded “registered” RCTs at CTG had significant trend towards non-publication. Since these “registered” RCTs had investigator declared “completed” status, non-publication of their results suggests unfavorable outcome. We could not ascertain whether “published” RCTs more commonly presented outcomes that were favorable but different from the originally planned primary outcomes thus inflating the frequency of positive “published” RCTs as only few “published” RCTs had actually registered at CTG. Further studies are needed to address the extent and implications of publication bias in RA RCTs.
Nearly 75% of “published” RCTs had positive outcome. This could partly be from publication bias and partly from the difficulty in study outcome assignment due to the complex structure of study intervention arms. Majority of RCTs had > 2 intervention arms. The ED often showed positive results compared only to the placebo but not to the ACD, or only the combination of ED and ACD had positive results compared to ED or ACD alone. Most published RCT reports lacked clear description of the a priori intent of the RCT (superiority vs non-inferiority for different intervention arms). Thus in absence of such guidance, positive RCT outcome was assigned when any ED intervention arm (alone or in combination with ACD) showed a statistically significant result favoring the primary outcome. Finally, conducting RCTs with such high frequency of positive outcome raises ethical issues. An RCT should only be conducted if there is substantial uncertainty (equipoise) about the relative value of one treatment versus another (17). RCTs in which experimental intervention and control are thought to be non-equivalent based on the existing fund of knowledge may cause unnecessary harm to study subjects and waste precious resources.
A study of 240 RCTs of rheumatic diseases found no difference in any methodological quality measure between manufacturer-supported and non–manufacturer-supported RCTs (23). A more recent study of 64 systemic lupus erythematosus RCTs found a trend towards better study quality in pharmaceutical company-supported RCTs (18). However, our study found that industry funding was associated with better reporting of some key RCT methodological quality measures. This could be due to several potential reasons. First, availability of greater financial resources to industry funded RCT investigators may allow performance of more expensive measures such as double-blinding and more vigorous tracking and follow-up of study subjects. Second, non-profit funded RCTs studied strategies to use drug therapy for RA more often than industry funded RCTs [10 (25%) vs 4 (6%)], and double-blinding was considered impractical by the investigators for most such RCTs due to the complexity of study protocol requirements. Indeed, only one industry and one non-profit source funded strategy RCT was conducted in a double-blind fashion. Third, conceivably the mandates of the regulatory organizations such as Federal Drug Administration for methodologically rigorous RCTs to generate efficacy and safety data of a new drug may also account for better quality of the industry funded RCTs (33). Fourth, better reporting of methodological aspects in the “published” RCTs may also reflect attempts to dispel notions of bias that tend to be associated with industry funding. Finally, since we assessed the RCT methodological quality from the published manuscript, we cannot be certain whether our findings represent incomplete reporting or inadequate performance of these measures. However, measures such as ITT analysis can be performed without additional financial burden and can be ascertained from the published report itself. Yet, lower proportion of non-profit funded RCTs reported ITT analysis performance suggesting that funding source may be associated with real systematic differences in the RCT methodological quality measure performance.
The overall reporting of most RCT methodological quality measures, particularly for random sequence generation and allocation concealment, was suboptimal. Poor reporting/performance of RCT methodological quality measures have been reported across multiple specialties including rheumatology (19, 23, 34). Encouragingly, our study found improvement in several quality measures: randomization (35% vs 17.4%), allocation concealment (30.1% vs 19%), participant flow (77.7% vs 58.7%), and ITT analysis (64.1% vs 29.8%) when compared to 121 rheumatology RCTs published in the years 1997–1998 (23). However, only 38.8% were RA RCTs and non-drug therapy RCTs were included in the referenced study. Hence, the above comparison may not represent true secular changes in quality of reporting of RA RCTs.
The CONSORT statement was developed to promote standardized RCT reporting that would help readers assess its validity and interpret the results appropriately. It was originally proposed in 1996 with subsequent revisions in 2001 and 2010 (24, 25, 35). The current CONSORT statement has recommended list of 25 items and a flow diagram (36). CONSORT guidelines adoption by biomedical journals has been shown to improve reporting quality particularly of randomization and double-blinding (37–39). However, the improvements have been inconsistent with continued suboptimal reporting of measures like allocation concealment (37, 40, 41). Nonetheless, authors of RCT manuscript should be encouraged to strictly adhere to the CONSORT guidelines for improving RCT reporting quality.
Our study has some limitations. Nearly one-fifth of the “published” RCTs had no funding source disclosure. We considered these to be non-profit funding source for most analyses. Plausibly, some of these were industry funded. For sensitivity analysis, we reassessed our study results considering an extreme scenario of industry funding of all such RCTs. This did not alter our finding of lack of association of funding source with the RCT outcome. However, differences in the study quality measures were attenuated and remained significant only for ITT analysis performance in favor of industry funding. An improvement in funding source reporting is expected since this is mandatory for CTG registration, and the 2010 CONSORT statement has added an item explicitly for funding source reporting (32, 35).
We assessed the RCT methodological quality based upon its published report. Plausibly, authors may not have reported important quality measures despite their adequate performance causing underestimation of the study quality. In fact, discrepancy has been noted between methodological aspects of the “published” RCT reports and their study protocol or the RCT investigator’s report of the actual study methods (42–44). However, overwhelming majority of healthcare literature users rely upon RCT’s published report to assess its quality and validity, and do not have access to the study protocol or RCT investigators. Hence, inadequate RCT reporting hinders assessment of its quality and validity even though it may have been appropriately conducted.
We did not evaluate conclusion or recommendations offered by RCT’s authors in discussion section or abstract of the manuscript. Conclusions of RCTs with for-profit organization funding are more likely to recommend ED as the treatment of choice unrelated to the observed effect size (6). Finally, there is issue of the how best to assess quality of RCTs. Inadequate performance of the quality measures used in our study may bias estimates of treatment effect (13–15). However, the association with treatment effect size is not consistent across different specialties, varies for individual quality measures, and dependent upon whether the study outcome is subjective or not (45, 46). Moreover, different groups vary considerably in methods to assess RCT quality (47). Some groups assess quality measures individually, an approach recommended by the Cochrane Collaboration, while others use a composite quality scale (47).
In conclusion, industry funding of “published” RCTs of RA drug therapy was not associated with higher likelihood of positive outcomes favoring the sponsored ED. A trend towards higher non-publication rate of “registered” industry funded RCTs suggests that publication bias partially explains the observed lack of such association. Availability of adequate funds for RCT conduct from both industry and non-profit sources is essential to generate evidence for optimal advancement of RA treatment. Improvement in reporting of methodological quality measures is needed to enable better validity assessment of RCTs.
Acknowledgments
Grant or financial support: This study was supported, in part, by Award Number 1UL1RR029884 from the National Center For Research Resources.
Footnotes
None of the authors have any financial conflict of interest relevant to the manuscript.
References
- 1.Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Ann Pharmacother. 2004;38:579–85. doi: 10.1345/aph.1D267. [DOI] [PubMed] [Google Scholar]
- 2.Patsopoulos NA, Ioannidis JP, Analatos AA. Origin and funding of the most frequently cited papers in medicine: database analysis. BMJ. 2006;332:1061–4. doi: 10.1136/bmj.38768.420139.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontanarosa PB, Flanagin A, DeAngelis CD. Reporting conflicts of interest, financial aspects of research, and role of sponsors in funded studies. JAMA. 2005;294:110–1. doi: 10.1001/jama.294.1.110. [DOI] [PubMed] [Google Scholar]
- 4.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–65. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 5.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290:921–8. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170:477–80. [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, et al. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Control Clin Trials. 2004;25:598–612. doi: 10.1016/j.cct.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Chard JA, Tallon D, Dieppe PA. Epidemiology of research into interventions for the treatment of osteoarthritis of the knee joint. Ann Rheum Dis. 2000;59:414–8. doi: 10.1136/ard.59.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001;18:565–8. doi: 10.1093/fampra/18.6.565. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials. gov. Ann Intern Med. 2010;153:158–66. doi: 10.1059/0003-4819-153-3-201008030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalmers TC, Celano P, Sacks HS, Smith H., Jr Bias in treatment assignment in controlled clinical trials. N Engl J Med. 1983;309:1358–61. doi: 10.1056/NEJM198312013092204. [DOI] [PubMed] [Google Scholar]
- 14.Colditz GA, Miller JN, Mosteller F. How study design affects outcomes in comparisons of therapy. I: Medical Stat Med. 1989;8:441–54. doi: 10.1002/sim.4780080408. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 16.Kjaergard LL, Nikolova D, Gluud C. Randomized clinical trials in HEPATOLOGY: predictors of quality. Hepatology. 1999;30:1134–8. doi: 10.1002/hep.510300510. [DOI] [PubMed] [Google Scholar]
- 17.Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, et al. The uncertainty principle and industry-sponsored research. Lancet. 2000;356:635–8. doi: 10.1016/S0140-6736(00)02605-2. [DOI] [PubMed] [Google Scholar]
- 18.Yuen SY, Pope JE. Learning from past mistakes: assessing trial quality, power and eligibility in non-renal systemic lupus erythematosus randomized controlled trials. Rheumatology (Oxford) 2008;47:1367–72. doi: 10.1093/rheumatology/ken230. [DOI] [PubMed] [Google Scholar]
- 19.Montori VM, Wang YG, Alonso-Coello P, Bhagra S. Systematic evaluation of the quality of randomized controlled trials in diabetes. Diabetes Care. 2006;29:1833–8. doi: 10.2337/dc06-0077. [DOI] [PubMed] [Google Scholar]
- 20.Thomas O, Thabane L, Douketis J, Chu R, Westfall AO, Allison DB. Industry funding and the reporting quality of large long-term weight loss trials. Int J Obes (Lond) 2008;32:1531–6. doi: 10.1038/ijo.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rios LP, Odueyungbo A, Moitri MO, Rahman MO, Thabane L. Quality of reporting of randomized controlled trials in general endocrinology literature. J Clin Endocrinol Metab. 2008;93:3810–6. doi: 10.1210/jc.2008-0817. [DOI] [PubMed] [Google Scholar]
- 22.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 23.Hill CL, LaValley MP, Felson DT. Secular changes in the quality of published randomized clinical trials in rheumatology. Arthritis Rheum. 2002;46:779–84. doi: 10.1002/art.512. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 25.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3:i, iv, 1–98. [PubMed] [Google Scholar]
- 28.Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–4. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunz R, Guyatt G. Which patients to include in the analysis? Transfusion. 2006;46:881–4. doi: 10.1111/j.1537-2995.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet. 2005;365:1159–62. doi: 10.1016/S0140-6736(05)71879-1. [DOI] [PubMed] [Google Scholar]
- 31.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 32. [Accessed August 10, 2011];About the ClinicalTrials.gov Results Database. Available at: http://clinicaltrials.gov/ct2/info/results.
- 33. [Accessed August 19, Last accessed August 19, 2011];How drugs are developed and approved. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/default.htm.
- 34.Greenfield ML, Rosenberg AL, O’Reilly M, Shanks AM, Sliwinski MJ, Nauss MD. The quality of randomized controlled trials in major anesthesiology journals. Anesth Analg. 2005;100:1759–64. doi: 10.1213/01.ANE.0000150612.71007.A3. [DOI] [PubMed] [Google Scholar]
- 35.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed August 16, 2011];The CONSORT Statement. Available at: http://www.consort-statement.org/consort-statement/overview0/
- 37.Moher D, Jones A, Lepage L CONSORT Group (Consolitdated Standards for Reporting of Trials) Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285:1992–5. doi: 10.1001/jama.285.15.1992. [DOI] [PubMed] [Google Scholar]
- 38.Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185:263–7. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 39.Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ. 2010;340:c723. doi: 10.1136/bmj.c723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills EJ, Wu P, Gagnier J, Devereaux PJ. The quality of randomized trial reporting in leading medical journals since the revised CONSORT statement. Contemp Clin Trials. 2005;26:480–7. doi: 10.1016/j.cct.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ. 2005;330:1057–8. doi: 10.1136/bmj.38413.576713.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devereaux PJ, Choi PT, El-Dika S, Bhandari M, Montori VM, Schunemann HJ, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. J Clin Epidemiol. 2004;57:1232–6. doi: 10.1016/j.jclinepi.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Pildal J, Chan AW, Hrobjartsson A, Forfang E, Altman DG, Gotzsche PC. Comparison of descriptions of allocation concealment in trial protocols and the published reports: cohort study. BMJ. 2005;330:1049. doi: 10.1136/bmj.38414.422650.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill CL, LaValley MP, Felson DT. Discrepancy between published report and actual conduct of randomized clinical trials. J Clin Epidemiol. 2002;55:783–6. doi: 10.1016/s0895-4356(02)00440-7. [DOI] [PubMed] [Google Scholar]
- 45.Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA. 2002;287:2973–82. doi: 10.1001/jama.287.22.2973. [DOI] [PubMed] [Google Scholar]
- 47.Lundh A, Gotzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]