Abstract
Background
Biomarkers for predicting cardiovascular events in community-based populations have not consistently added information to standard risk factors. A limitation of many previously studied biomarkers is their lack of cardiovascular specificity.
Methods and Results
To determine the prognostic value of 3 novel biomarkers induced by cardiovascular stress, we measured soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I in 3,428 participants (mean age 59, 53% women) in the Framingham Heart Study. We performed multivariable-adjusted proportional hazards models to assess the individual and combined ability of the biomarkers to predict adverse outcomes. We also constructed a “multimarker” score composed of the 3 biomarkers, in addition to B-type natriuretic peptide and high-sensitivity C-reactive protein. During a mean follow-up of 11.3 years, there were 488 deaths, 336 major cardiovascular events, 162 heart failure events, and 142 coronary events. In multivariable-adjusted models, the 3 new biomarkers were associated with each endpoint (p<0.001) except for coronary events. Individuals with multimarker scores in the highest quartile had a 3-fold risk of death (adjusted hazard ratio, 3.2, 95% CI, 2.2–4.7; p<0.001), 6-fold risk of heart failure (6.2, 95% CI, 2.6–14.8; p<0.001), and 2-fold risk of cardiovascular events (1.9, 95% CI, 1.3–2.7; p=0.001). Addition of the multimarker score to clinical variables led to significant increases in the c-statistic (p=0.007 or lower) and net reclassification improvement (p=0.001 or lower).
Conclusions
Multiple biomarkers of cardiovascular stress are detectable in ambulatory individuals, and add prognostic value to standard risk factors for predicting death, overall cardiovascular events, and heart failure.
Keywords: biomarkers, risk assessment, risk prediction
Background
The prediction of cardiovascular events in low to intermediate risk individuals is an important challenge. Such individuals are unlikely to be targeted for preventive therapies, but as a group, they account for the majority of cardiovascular events in the population.1 Although the use of circulating biomarkers to aid risk prediction is attractive, prior studies have not consistently demonstrated value of biomarkers beyond standard risk factors in low to intermediate risk, individuals in the community.2–5 Indeed, the U.S. Preventive Services Task Force recently concluded that cardiovascular biomarkers provide limited clinical utility.6 This conclusion highlights the need to identify better biomarkers in the community-based setting.
A limitation of many previously studied biomarkers is their lack of cardiovascular specificity. For instance, high-sensitivity C-reactive protein (hsCRP), the most widely-studied biomarker in general populations, is secreted by the liver and may reflect inflammation from a variety of causes. In recent years, several newer biomarkers have emerged, including soluble ST2 (sST2), growth differentiation factor-15 (GDF-15), and the high-sensitivity troponins. Each is expressed or released by cardiovascular tissue in response to mechanical or pathological stress.7–12 Studies have highlighted the prognostic utility of these biomarkers in individuals with acute coronary syndromes and heart failure.13–22 Recent data suggest that these biomarkers could be prognostically informative in ambulatory individuals as well.23–26 However, sST2 has not been studied in a community-based cohort, the biomarkers have not been examined in combination, and there is limited information about their association with specific outcomes such as heart failure.
We therefore examined the individual and collective utility of sST2, GDF-15, and high-sensitivity troponin I for predicting cardiovascular outcomes in the community. We postulated that a panel of these cardiac-derived biomarkers would be capable of identifying individuals in the pre-clinical setting with an elevated risk of future cardiovascular disease, and add to existing risk prediction algorithms.
Methods
Study Sample
In 1971, 5,124 individuals were enrolled into the prospective cohort called the Framingham Offspring Study.27 The sixth examination, which occurred between 1995 and 1998, was used for the present analysis. Of the 3,532 attendees, we excluded those with serum creatinine > 2.0 mg/dl (n=21), or missing biomarker or follow-up data (n=83). After these exclusions, 3,428 individuals (97% of attendees) remained eligible for the present investigation. The protocol was approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
Participants underwent a standardized evaluation that included a medical history and physician-administered physical examination. Diabetes mellitus was defined by a fasting glucose ≥126 mg/dl or the use of insulin or other hypoglycemic medication.12 Participants were considered current cigarette smokers if they reported having smoked cigarettes regularly during the year preceding the Heart Study examination.
Biomarker Measurements
Blood biomarkers were measured in all participants using morning samples collected after an overnight fast. Participants were supine for approximately five to ten minutes prior to phlebotomy. Blood samples were immediately centrifuged, and plasma and serum were stored at −70°C. The samples did not undergo any freeze-thaw cycles prior to the performance of the assays below.
The concentration of sST2 was determined using a high-sensitivity, second-generation enzyme-linked immunosorbent assay with a detection limit of 2 ng/mL (Presage®ST2, Critical Diagnostics).28 sST2 values above 35 ng/mL have been linked to adverse outcomes in the setting of overt heart failure.18,29 Quantification of hsTnI was performed with an ultra-sensitive immunoassay for cardiac troponin I that utilizes a novel, single-molecule counting technology (Erenna hsTnI, Singulex).30 The limit of detection is 0.2 pg/mL, with an assay range of 0.5 to 70 pg/mL. The 99th percentile value for this assay has not been well-established, but in small studies of normal subjects ranges from approximately 7 pg/mL to 10 pg/mL.30–32 hsCRP and BNP were measured as previously described.2
GDF-15 levels were measured with a pre-commercial, automated electrochemiluminescent immunoassay on a Cobas e 411 analyzer (Roche Diagnostics). The assay has a limit of detection below 10 ng/L, a linear measuring range up to 20,000 ng/L, and an inter-assay imprecision of 2.3% and 1.8% at GDF-15 concentrations of 1,100 ng/L and 17,200 ng/L, respectively (Roche Diagnostics, data on file). GDF-15 values obtained with the electrochemiluminescent assay correlate closely with the values measured with our previously described immunoradiometric assay33 (r = 0.980, slope 1.049, intercept −136 ng/L, n = 45 samples with GDF-15 concentrations ranging from 567 to 13,334 ng/L). Using the immunoradiometric assay, 1200 ng/L was previously proposed as the upper limit of the reference interval in apparently healthy elderly individuals.33
Outcomes
During follow up, all suspected cardiovascular events were reviewed by a committee of three experienced investigators, using hospital records, physician office notes, and pathology reports. The definition of a major cardiovascular event in Framingham has been detailed previously,2 and comprises recognized myocardial infarction, coronary insufficiency (prolonged angina with documented ECG changes), coronary heart disease death, heart failure, and stroke. Major coronary events were defined as recognized myocardial infarction, coronary insufficiency, and coronary heart disease death. As in prior studies,2,34 we classified events that were based on history only (e.g. symptoms of intermittent claudication or transient ischemic attack, or typical chest pain without ECG evidence of ischemia or injury) as “non-major” and did not include them in the primary endpoint or multivariable regression models.
Statistical Analyses
Before inferential analyses, biomarker values were natural log transformed due to highly skewed distributions. We estimated partial correlations among biomarkers accounting for sex and age. We examined the association between the biomarkers and the risk of all-cause mortality, heart failure, first major cardiovascular events, and first major coronary events using multivariable proportional hazards (Cox) models.35 Log-transformed biomarker distributions were standardized to mean 0 and standard deviation [SD] 1, to facilitate comparison of effect sizes between biomarkers. The proportionality assumption was verified by testing the interaction of the biomarkers with follow-up time. Participants with prior heart failure, major cardiovascular events, or coronary heart disease, respectively, were excluded from analyses of those endpoints.
We first assessed the biomarkers individually in models containing the following standard cardiovascular risk factors: age, sex, systolic blood pressure, use of anti-hypertensive therapy, total cholesterol, HDL cholesterol, regular cigarette smoking, body mass index, and presence of diabetes. For analyses of incident heart failure, in accordance with prior studies, we also included electrocardiographic left ventricular hypertrophy, prevalent atrial fibrillation, prevalent major cardiovascular disease, and presence of a murmur (grade 3 out of 6 or greater systolic murmur, or grade 1 out of 4 or greater diastolic).36 Covariates for analyses of all-cause death were the same as for heart failure, except heart murmur. Sex-pooled analyses were performed after confirming that multiplicative interaction terms for biomarker and sex were statistically non-significant for all endpoints. We then performed analyses incorporating all biomarkers together, and added hsCRP and BNP, given their presence in prior “multimarker” panels for predicting cardiovascular risk.2 The identification of the most strongly associated biomarkers for each endpoint was confirmed using a backwards elimination model, with the clinical covariates forced into the model and using a retention p-value of 0.05. Though the urinary albumin excretion ratio (UACR, in mg/g) also predicts heart failure in Framingham,37 urine was not available on the full sample and the association was non-significant after inclusion of the newer biomarkers. Thus, we restricted the analyses to plasma markers.
The joint predictive utility of the five biomarkers (sST2, GDF-15, hsTnI, hsCRP, BNP) was evaluated by constructing a “multimarker” risk score. This score was defined as:
with β1, β2, and β3 denoting proportional-hazards regression coefficients for biomarkers A, B, and C, respectively, from a multivariable model for the outcome of interest. Participants were categorized according to sex-specific quartiles of this multimarker score.
We compared clinical and multimarker score models with the “best-fit” clinical models, based on models containing the conventional risk factors applied to the current study sample. We assessed performance using current methods.38,39 First, we evaluated model discrimination by calculating c-statistics for models including “base” clinical predictors listed with and without biomarkers.40 We then calculated the integrated discrimination improvement (IDI), a measure of a model’s ability to improve average sensitivity without reducing average specificity.41 Last, we evaluated the ability of biomarkers to reclassify risk, by examining the proportion of individuals reclassified correctly using the biomarkers using the net reclassification improvement (NRI) metric.41 We estimated both the “category-free” NRI and conventional NRI.42 The category-free NRI is useful for endpoints such as death and heart failure for which established risk categories do not exist. It was calculated using 0%, 1%, and 2%, as thresholds for minimum change in predicted risk required to indicate a change in classification. The corresponding indices are denoted as NRI(>0) (the statistic suggested by Pencina and colleagues42), NRI(>0.01), and NRI(>0.02). For the conventional NRI, we defined low, intermediate, and high risk as 10-year predicted risks of 0% to <10%, 10% to <20%, and ≥20% for first major cardiovascular events, coronary events, and death.43 For heart failure, we used categories that have been used previously in this cohort: 0% to <3%, 3% to <8%, and ≥8%.37 We also calculated the conventional NRI restricted to individuals in the intermediate-risk group, which has been referred to as the “clinical NRI.”44
In additional analyses, we assessed whether the association of biomarkers with outcomes differed by sex (using multiplicative interaction terms), and repeated the Cox proportional hazards models adjusting for prior non-major cardiovascular events or restricted to individuals without prior major or non-major cardiovascular events. We repeated the analyses excluding all individuals with diabetes. Further, we re-analyzed death and heart failure including a time-dependent covariate for interim myocardial infarction or heart failure (in death analyses). All analyses were performed using SAS software, version 9.1.3 (SAS Institute, Inc., Cary, NC). A two-sided p-value < 0.05 was considered statistically significant.
Results
Characteristics of the study sample are shown in Table 1. The mean age of the study sample was 59 years, and 53% of participants were women. Circulating sST2, GDF-15, and hsTnI concentrations were detectable in 100%, 100%, and 81% of participants, respectively. Characteristics of the sample, by biomarker quartiles, are detailed in Supplemental Table 1.
Table 1.
Baseline characteristics.
| Characteristic | Men (n=1,608) | Women (n=1,820) |
|---|---|---|
| Age, years | 59 ± 10 | 59 ± 10 |
| Body-mass index, kg/m2 | 28.5 ± 4.4 | 27.4 ± 5.7 |
| Systolic blood pressure, mm Hg | 130 ± 17 | 127 ± 20 |
| Use of anti-hypertensive therapy, % | 31 | 25 |
| Diabetes, % | 14 | 10 |
| Cigarette smoking, % | 15 | 16 |
| Total cholesterol, mg/dL | 199 ± 41 | 212 ± 39 |
| HDL cholesterol, mg/dL | 44 ± 12 | 58 ± 16 |
| Prevalent major CVD*, % | 8 | 3 |
| ECG LVH, % | 1 | 0.2 |
| Significant murmur, % | 3 | 2 |
| Biomarkers (median, IQR) | ||
| Soluble ST2, ng/mL | 23.6 (9.9) | 18.8 (7.9) |
| Growth differentiation factor-15, ng/L | 1066 (594) | 1022 (492) |
| High-sensitivity troponin I, pg/mL | 1.63 (1.59) | 1.15 (1.12) |
| B-type natriuretic peptide, pg/mL | 6.6 (12.7) | 10.0 (16.2) |
| High-sensitivity C-reactive protein, mg/L | 1.81 (2.91) | 2.38 (4.75) |
HDL: high density lipoprotein; CVD: cardiovascular disease; ECG: electrocardiography; LVH: left ventricular hypertrophy. For continuous variables, values are mean ± SD or medians (interquartile range).
Participants with prevalent CVD were included only in analyses of death and heart failure.
Age- and sex-adjusted correlations between sST2 and GDF-15 (r = 0.22, p<0.001), GDF-15 and hsTnI (r = 0.13, p<0.001), and sST2 and hsTnI (r = 0.09, p <0.001) were low in magnitude. Similarly, correlations of BNP and hsCRP with the three biomarkers were modest (all r < 0.4).
Prediction of death and cardiovascular events with new biomarkers
During a mean follow up of 11.3 years, 488 (14%) individuals died, 162 (5%) experienced a first heart failure event, 336 (10%) experienced a first major cardiovascular event, and 142 (5%) had a first major coronary event. Associations of sST2, GDF-15, and hsTnI with death, heart failure, major cardiovascular events, and coronary events are shown in Table 2 (for continuous values of each biomarker) and Supplemental Table 2 (for quartile results). In models adjusting for age, sex, body mass index, and conventional cardiovascular risk factors, the biomarkers were strongly associated with death, heart failure, and major cardiovascular events (p<0.001 for each biomarker-endpoint combination), but not with coronary heart disease events.
Table 2.
Association of new cardiac biomarkers with death and cardiovascular events.
| Death | Heart Failure | Major cardiovascular events | Coronary heart disease events | |||||
|---|---|---|---|---|---|---|---|---|
| Events/No. at risk* | 474/3358 | 152/3316 | 334/3228 | 173/3265 | ||||
| Multivariable- adjusted HR, per SD | p | Multivariable- adjusted HR, per SD | p | Multivariable- adjusted HR, per SD | p | Multivariable- adjusted HR, per SD | p | |
| Individual biomarkers | ||||||||
| sST2 | 1.32 (1.20–1.46) | <0.001 | 1.45 (1.23–1.70) | <0.001 | 1.23 (1.10–1.39) | <0.001 | 1.08 (0.92–1.27) | 0.37 |
| GDF-15 | 1.66 (1.51–1.81) | <0.001 | 1.52 (1.29–1.78) | <0.001 | 1.26 (1.12–1.41) | <0.001 | 1.10 (0.93–1.30) | 0.27 |
| hsTnI | 1.16 (1.07–1.26) | <0.001 | 1.28 (1.14–1.45) | <0.001 | 1.18 (1.07–1.29) | <0.001 | 1.02 (0.87–1.18) | 0.84 |
| Biomarker combination | 463/3252 | 149/3212 | 324/3124 | 169/3162 | ||||
| sST2 | 1.12 (1.02–1.24) | 0.02 | 1.29 (1.08–1.53) | 0.005 | 1.15 (1.02–1.30) | 0.03 | 1.03 (0.87–1.22) | 0.76 |
| GDF-15 | 1.52 (1.37–1.67) | <0.001 | 1.23 (1.03–1.47) | 0.03 | 1.13 (1.00–1.28) | 0.046 | 1.06 (0.88–1.26) | 0.55 |
| hsTnI | 1.06 (0.97–1.16) | 0.19 | 1.20 (1.04–1.38) | 0.01 | 1.13 (1.02–1.24) | 0.01 | 0.99 (0.85–1.16) | 0.93 |
| BNP | 1.13 (1.02–1.24) | 0.02 | 1.29 (1.10–1.52) | 0.002 | 1.15 (1.03–1.28) | 0.02 | 1.07 (0.91–1.26) | 0.42 |
| hsCRP | 1.18 (1.07–1.30) | 0.001 | 1.17 (0.97–1.40) | 0.11 | 1.11 (0.98–1.26) | 0.10 | 1.21 (1.02–1.44) | 0.03 |
Values are hazards ratios (HR), with 95% confidence interval, from models adjusting for age, sex, body mass index, systolic blood pressure, hypertension therapy, diabetes, cigarette smoking, total cholesterol, HDL cholesterol, atrial fibrillation (heart failure and death analyses only), major cardiovascular disease (heart failure and death analyses), ECG left ventricular hypertrophy (heart failure and death analyses), and heart murmur (heart failure analysis only). Results in top panel are from separate models for each biomarker. Results in bottom panel are from a single model adjusted for all biomarkers together. Biomarkers are log transformed; for hsTnI and BNP, values below the detection limit were assigned the detection threshold.
Because individual biomarker data was missing for some individuals (in all cases, no more than 3% of the total sample), the number of events is shown for the model with the fewest missing values. Numbers of individuals at risk differ from the overall sample size due to individuals with missing covariates for the multivariable models. SD: standard deviation. The SDs for each biomarker, in the sample for the death analyses, are as follows: log sST2, 0.36; log GDF-15, 0.41; log hsTnI, 0.75; log BNP, 0.91; log hsCRP, 1.16.
Table 2 also contains results from models incorporating all three novel biomarkers in addition to BNP and hsCRP. In models for death, heart failure, and major cardiovascular events, 4 of the 5 biomarkers remained significant: death (sST2, GDF-15, BNP, hsCRP), heart failure (sST2, GDF-15, hsTnI, BNP), and first major cardiovascular events (sST2, GDF-15, hsTnI, BNP). These findings were confirmed using backwards elimination models for each endpoint. For death, the magnitude of association with GDF-15 was particularly high (multivariable-adjusted hazards ratio per SD increment, 1.52, p<0.001) in comparison with the other biomarkers. For heart failure, hazards ratios for sST2, GDF-15, and hsTnI were comparable to those seen with BNP (hazards ratios per SD between 1.20 and 1.29).
Multimarker score
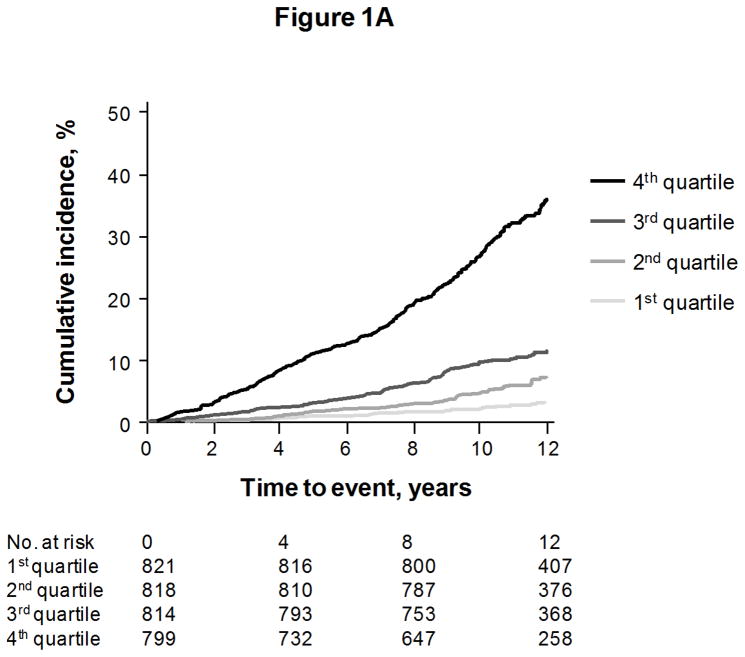
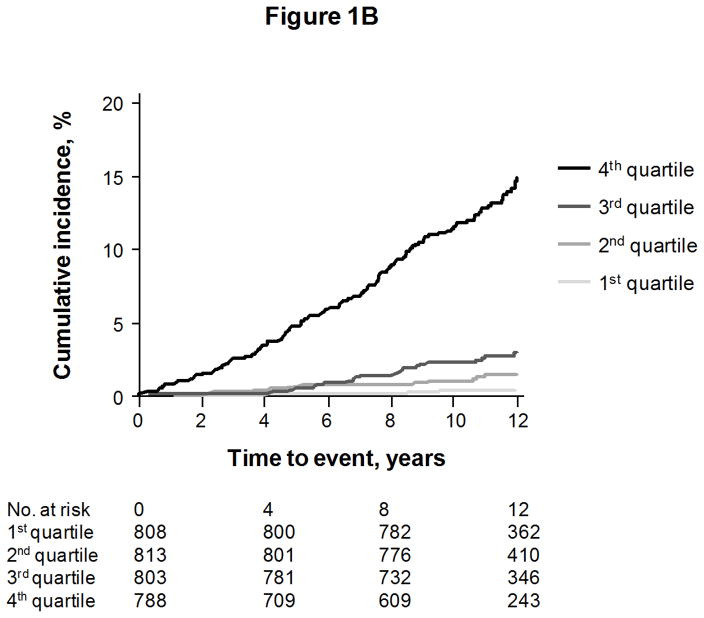
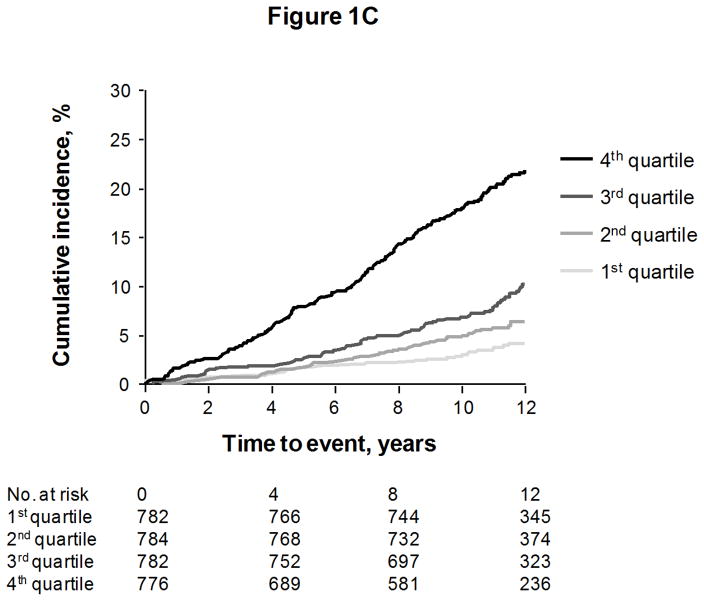
We constructed a “multimarker score” with sST2, GDF-15, hsTnI, BNP, and hsCRP, for each endpoint except coronary heart disease events (for which the biomarkers were not significant predictors). The cumulative risks of events according to quartiles of the multimarker score are shown in Figures 1A to 1C, for death, heart failure, and major cardiovascular events, respectively.
Figure 1.
Cumulative incidence of death (Figure 1A), heart failure (Figure 1B), and first major cardiovascular events (Figure 1C), according to quartile of a multimarker score consisting of sST2, GDF-15, hsTnI, BNP, and hsCRP. Curves for heart failure and major cardiovascular events are adjusted for the competing risk of death.
The incremental predictive values of the score on top of clinical risk factors are shown in Table 3. Individuals with multimarker scores in the highest quartile had an approximately 3-fold risk of death, 6-fold risk of heart failure, and 2-fold risk of major cardiovascular events, compared with individuals in the lowest quartile. Results were unchanged when restricting models to individuals without diabetes or electrocardiographic left ventricular hypertrophy (Supplemental Table 3).
Table 3.
Multimarker score and prediction of future events.
| Death | Heart failure | Major cardiovascular events | |
|---|---|---|---|
| Score, per 1-unit increment | 1.80 (1.64–1.98) | 1.83 (1.55–2.16) | 1.43 (1.28–1.61) |
| p-value | <0.001 | <0.001 | <0.001 |
| By quartile of score | |||
| 1st quartile | Referent | Referent | Referent |
| 2nd quartile | 1.26 (0.82–1.92) | 1.52 (0.57–4.06) | 1.01 (0.67–1.53) |
| 3rd quartile | 1.55 (1.04–2.30) | 2.19 (0.88–5.42) | 1.10 (0.74–1.63) |
| 4th quartile | 3.20 (2.18–4.70) | 6.25 (2.63–14.82) | 1.87 (1.28–2.73) |
| P for trend | <0.001 | <0.001 | <0.001 |
| c-statistics | |||
| Best-fit clinical model | 0.787 | 0.846 | 0.779 |
| Best-fit clinical model + multimarker score | 0.810 | 0.870 | 0.790 |
| p-value | <0.001 | 0.002 | 0.007 |
| IDI | 0.05 (0.04–0.07) | 0.04 (0.02–0.06) | 0.02 (0.01–0.03) |
| p-value | <0.001 | <0.001 | <0.001 |
| NRI(>0)*, vs. best-fit clinical model | 0.42 (0.31–0.54) | 0.39 (0.21–0.57) | 0.21 (0.08–0.34) |
| p-value | <0.001 | <0.001 | 0.001 |
Values for continuous score and quartile are hazards ratios, with 95% CI, from multivariable models adjusting for age, sex, body mass index, systolic blood pressure, hypertension therapy, diabetes, cigarette smoking, total cholesterol, HDL cholesterol, atrial fibrillation (heart failure and death analyses only), major cardiovascular disease (heart failure and death analyses), ECG left ventricular hypertrophy (heart failure and death analyses), and heart murmur (heart failure analysis only). SD: standard deviation; IDI: integrated discrimination improvement; NRI: net reclassification improvement; CVD: cardiovascular disease.
NRI(>0) denotes category-free NRI, using a threshold of 0% for the minimum change in predicted risk necessary to change classification. Values for NRI(>0.01) and NRI(>0.02) correspond to category-free NRI with thresholds of 1% and 2%, and are shown in the text.
The addition of the multimarker score led to an improvement in discrimination for all 3 endpoints, as evidenced by significant increases in the c-statistic (p=0.007 to p<0.001) and IDI (all p<0.001). The multimarker score was also superior to a model containing clinical risk factors, hsCRP, and BNP (higher c-statistics for death, p<0.001; heart failure, p<0.001; and major cardiovascular events, p=0.017). The multimarker score led to significant improvements in classification accuracy for all endpoints, when compared with the best-fit covariate models for these endpoints. “Category-free” NRI values were 0.42 (p<0.001) for death, 0.39 (p<0.001) for heart failure, and 0.21 (p=0.001) for major cardiovascular events. The NRI remained significant when different minimum thresholds for change in predicted risk were used. For death, NRI(>0.01) was 0.29, and NRI(>0.02) was 0.24 (both p<0.001). For heart failure, NRI(>0.01) was 0.34, and NRI(>0.02) was 0.26 (both p<0.001). For major cardiovascular events, NRI(>0.01) was 0.15 (p=0.006) and NRI(>0.02) was 0.16 (p=0.001).
The conventional NRI with categories was also significant for the addition of the multimarker score to “best-fit” clinical models for heart failure (0.13, p<0.001) and death (0.06, p=0.02). The NRI with categories was not significant for major cardiovascular events (p=0.16). Reclassification tables for all 3 endpoints are shown in Supplemental Tables 4 through 6. For heart failure, 524 (16%) of individuals were reclassified. Reclassification was driven largely by people with events. Among individuals in this group, 13% were correctly up-classified, versus only 4% who were incorrectly down-classified. Notably, among 22 “low-risk” individuals who developed heart failure, 7 (36%) were correctly up-classified by the multimarker score. The proportion of individuals reclassified for death was similar (16%). The score correctly up-classified 21% (18 of 87) of low-risk individuals who died.
NRI results in intermediate-risk individuals (“clinical NRI”) were significant for all 3 endpoints: heart failure (0.42, p<0.001), death (0.28, p<0.001), and major cardiovascular events (0.22, p=0.002). The proportion of intermediate-risk individuals reclassified ranged from 37% to 47% for the 3 endpoints (Supplemental Tables 4 through 6). Correct reclassification was evenly split among those with and without events. For heart failure, 23% of those with events were correctly up-classified, versus only 3% incorrectly down-classified. Among those without heart failure events, 34% were correctly down-classified, versus 11% incorrectly up-classified. For individuals who died, 27% were correctly up-classified and 20% incorrectly down-classified. Among those who did not die, 34% were correctly down-classified and 13% incorrectly up-classified. For overall cardiovascular events, 22% of those with events were correctly up-classified, versus 11% incorrectly down-classified. Among those without events, 24% were correctly down-classified, and 13% were incorrectly up-classified.
Secondary analyses
Because hazard ratios across quartiles of the multimarker score for death appeared comparable to those for GDF-15 alone (Supplemental Table 2), we performed additional analyses to assess whether the full multimarker panel was superior to GDF-15 alone for predicting death. The multimarker model provided a better fit compared with GDF-15 alone, as evidenced by a significant improvement in the log-likelihood ratio test statistic (p<0.001). On the other hand, the IDI was of borderline significance (p=0.04), as was the category-free NRI (0.10, p=0.09) for death. The c-statistic was not significantly higher with the full multimarker score compared with GDF-15 alone.
In other secondary analyses, there was no significant interaction between sex and the multimarker score in predictive models, or with sex and any of the individual biomarkers. Additional adjustment for interim myocardial infarction or heart failure (in the death analyses) or interim myocardial infarction alone (in the heart failure analyses) did not alter the results. Similarly, because the primary analyses for death and heart failure included some individuals with prior cardiovascular events (with this status entered as a covariate), we repeated the analyses after excluding all such individuals. As shown in Supplemental Table 7, results were materially unchanged, with nearly identical hazard ratios. The improvement in discrimination remained significant when restricted to individuals with no prior cardiovascular disease (death, c-statistic 0.80 vs. 0.76 for models with and without biomarkers, respectively, p=0.001; heart failure, c-statistic 0.85 vs. 0.83, p=0.005).
Discussion
Concentrations of multiple biomarkers of cardiovascular stress are detectable in individuals in the general population, and provide prognostic information above and beyond traditional cardiovascular risk factors. The present investigation was enabled by the recent availability of 3 newer-generation assays: “high-sensitivity” assays for sST2 and hsTnI capable of detecting extremely low concentrations of the biomarkers, and a novel, automated electrochemiluminesence assay for GDF-15. From a pathophysiological perspective, our findings support the concept that cardiovascular dysfunction or injury can exist for many years before the onset of overt disease in ambulatory individuals. Indeed, the upper quartiles of sST2 and GDF-15 in our sample overlapped substantially with ranges observed in the setting of overt heart failure.16,18 Lastly, our data indicate that sST2, GDF-15, and hsTnI predict risk on top of established biomarkers such as hsCRP in the general population.
sST2 is an emerging biomarker that has been shown to predict adverse outcomes and death in individuals with established heart failure.17–19,45–47 The present study is the first to examine the prognostic value of sST2 measurements in the general population, showing that higher levels of circulating sST2 (comparable to those found in hospitalized patients18,29) can be detected in apparently healthy individuals and precede adverse outcomes. Circulating sST2 is a sensitive marker of cardiac stress, as suggested by experimental studies showing marked upregulation of myocardial ST2 gene expression induced by myocyte stretch in a manner reminiscent of BNP.8 Although other conditions, such as severe pulmonary disease or sepsis, have been associated with elevated sST2, such diagnoses are rare in an ambulatory cohort such as Framingham.
A member of the interleukin (IL)-1 receptor family, ST2 exists in both membrane-bound and soluble forms. The functional ligand of sST2 is IL-33, a cardiac fibroblast protein produced by myocyte stretch with known anti-hypertrophic and anti-fibrotic actions. It has been speculated that sST2 functions as a soluble ‘decoy’ receptor, preventing binding of IL-33 to a membrane-bound receptor version of ST2. In in vivo studies, infusion of large amounts of sST2 results in adverse cardiac remodeling, heart failure, and premature death.48 In clinical studies of heart failure, elevated values of sST2 have been associated with greater decompensation, abnormalities in systolic and diastolic function, and poorer long-term outcomes.18,47
We found that concentrations of GDF-15 were strongly associated with the risk of death and heart failure. GDF-15 is a distant member of the transforming growth factor-βcytokine superfamily. While GDF-15 is weakly expressed in most tissues under physiological conditions, its expression may significantly increase in response to cardiovascular inflammation and tissue injury.9,11,49,50 Ischemia, mechanical stretch, neurohormones, and pro-inflammatory cytokines stimulate the expression of GDF-15 in cardiac myocytes.49,51 Increased cardiac expression of GDF-15 has been observed in murine models of myocardial infarction, pressure overload, and heart failure.49,50,52 While the myocardium produces GDF-15, other cardiovascular cell types, including endothelial cells,53 vascular smooth muscle cells,54 and adipocytes55 have been shown to produce the biomarker under stressful conditions. GDF-15 has also been detected in atherosclerotic plaque macrophages.9,11 Its prominent anti-apoptotic, anti-hypertrophic, and anti-inflammatory actions in cardiovascular disease models suggest that GDF-15 may play a counter-regulatory role in the context of cardiovascular injury.49,50,52
In patients with acute coronary syndrome or chronic heart failure, GDF-15 concentrations are markedly elevated and correlate with both disease severity and mortality risk.14,16 Emerging data suggest that GDF-15 concentrations may also be prognostic in unselected populations. In a nested case-control sample from the Women’s Health Study, concentrations of GDF-15 detected with an early assay were associated with the risk of future cardiovascular events.56 In elderly, higher-risk individuals, GDF-15 has been related to subclinical cardiovascular disease57 and all-cause mortality.26 The present study reports the first experience with an automated assay for GDF-15, and extends the results of prior studies by focusing on a larger cohort of predominantly middle-aged individuals, with prospectively adjudicated cardiovascular events (including heart failure) and concurrent measurement of other biomarkers such as BNP and hsTnI.
We also investigated a novel, “ultra-sensitive” troponin I assay that detects troponin concentrations up to an order of magnitude lower than those detected by other “highly-sensitive” assays.58 The cardiac troponins are structural proteins involved in contraction and relaxation of the cardiomyocyte. Troponin assays are widely used for the detection of acute myocardial infarction, but measurement of troponins may also play a role in screening and diagnosis of cardiovascular dysfunction in a broader range of individuals. For instance, in the context of established heart failure, troponins are frequently elevated, almost always in the absence of overt myocardial infarction, and troponin elevation in this setting is a strong predictor of prognosis.20,21,59,60
The advent of “highly-sensitive” troponin assays has made investigation of this biomarker possible in ambulatory cohorts. Recent studies have reported the presence of low levels of troponin T in anywhere from 25% to 67% of ambulatory, older individuals, in whom levels are associated with all-cause and cardiovascular mortality.23–25 The current study extends these observations with the use of a “single-molecule” assay that detects circulating troponin I in more than 80% of ambulatory individuals with a generally lower risk than in other population studies. Although occult coronary artery disease could explain some of the troponin detected by highly sensitive assays in asymptomatic individuals,23 it is unlikely to account fully for our findings. Circulating troponins could reflect proteolysis and turnover of myocardial contractile proteins, which may be accelerated in the setting of myocardial stretch, oxidative stress, or neurohormonal activation.60
In contrast to their strong associations with heart failure and death, none of the 3 biomarkers were significantly associated with coronary heart disease events (myocardial infarction or unstable angina), after adjustment for conventional risk factors. This finding supports the hypothesis that the predictive value of the biomarkers arises more from their link with myocardial stress than with vascular stress or inflammation.
Additionally, sST2, GDF-15, and hsTnI each retained their association with heart failure or death when combined in risk models. Thus, although the predictive value of the biomarkers may stem from their correlation with cardiovascular stress, each appears to capture a distinct aspect of pathophysiology. This premise is further supported by their relatively low correlation, indicating that they provide non-overlapping information.61 Uncorrelated biomarkers are particularly attractive candidates for combining into “multimarker” panels.62 Several studies have examined the performance of multimarker panels in primary prevention cohorts, including a previous report from Framingham.2,3,5 These studies, which largely emphasized biomarkers of inflammation, hemostasis, or oxidative stress, found little improvement in discrimination or reclassification metrics when biomarkers were added to conventional risk algorithms. In contrast, we found that the 3 novel biomarkers, which increase in the context of cardiovascular dysfunction or injury, improved discrimination for the major endpoints studied. The increased cardiovascular specificity could account for the apparent superiority of the current multimarker panel over others that have been studied in this cohort.2
Increases in the c-statistic, while moderate, were statistically significant, and comparable to or greater than that observed in prior studies in middle-aged, primary prevention cohorts.3,5 This improvement was also accompanied by significant improvements in risk classification, which were particularly robust for heart failure and death. Approximately one-third of intermediate-risk individuals were correctly reclassified, with about half due to an increase in risk category. Correct up-classification may be of particular interest given its potential to alter treatment. Because the biomarkers reflect cardiovascular dysfunction or injury, elevated concentrations could motivate pharmacologic interventions to forestall or prevent the onset of cardiovascular disease. In the case of individuals at risk for heart failure, such interventions might include drugs with anti-remodeling effects on the myocardium (as recommended in clinical practice guidelines for at-risk patients).63 Given the value of the markers presently studied, this hypothesis may warrant testing in future clinical studies.
In conclusion, concentrations of sST2, GDF-15, and hsTnI predict the future risk of death, heart failure, and overall cardiovascular events, even in the context of robust clinical risk models. Addition of these biomarkers improves discrimination and leads to potentially relevant changes in risk classification. Our findings highlight the distinct prognostic value of newer biomarkers of underlying cardiovascular stress and injury in apparently healthy individuals.
Supplementary Material
Clinical perspective.
Biomarkers for predicting cardiovascular events in community-based populations have not consistently added information to standard risk factors. A limitation of many previously studied biomarkers is their lack of cardiovascular specificity. We examined 3 novel biomarkers induced by cardiovascular stress (soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I) in 3,428 participants in the Framingham Heart Study followed for a mean of 11.3 years. After adjustment for traditional risk factors, the 3 new biomarkers were associated with each endpoint (p<0.001) except for coronary events. Individuals with multimarker scores in the highest quartile had a 3-fold risk of death (p<0.001), 6-fold risk of heart failure (p<0.001), and 2-fold risk of cardiovascular events (p=0.001). Addition of the multimarker score to clinical variables led to significant increases in the c-statistic (p=0.007 or lower) and net reclassification improvement (p=0.001 or lower). Our findings support the concept that cardiovascular dysfunction or injury can exist for many years before the onset of overt disease, and highlight the distinct prognostic value of biomarkers of cardiovascular stress in apparently healthy individuals.
Acknowledgments
Funding Sources: The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195). This manuscript has been reviewed by the Framingham Heart Study for scientific content and consistency of data interpretation with previous Framingham Heart Study publications. Additional support was provided by NIH R01-HL-08675. Dr. Wollert was supported by the German Ministry of Education and Research (BMBF, BioChancePlus). Dr. Cheng is supported by a grant from the Ellison Foundation. Assays for sST2 were provided by Critical Diagnostics. Assays for hsTnI were provided by Singulex, Inc. GDF-15 assays were provided by Roche Diagnostics, Inc. These companies did not have access to study data and had no input into the data analyses, interpretation, or preparation of the manuscript for submission. Dr. Wang had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest Disclosures: Dr. Wang has received research or assay support from Diasorin, Brahms, LabCorp, and Siemens Diagnostics, and honoraria from Roche, Diasorin, and Quest Diagnostics. Drs. Wang, Larson, and Vasan are named as co-inventors on patent applications relating to the use of metabolomic or neurohormonal biomarkers in risk prediction. Dr. Januzzi has received research grant funding from Roche Diagnostics, Siemens Diagnostics, and Critical Diagnostics. Drs. Wollert and Kempf are named as co-inventors on a patent for the use of GDF-15 for cardiovascular applications, and have a contract with Roche Diagnostics for the development of a GDF-15 assay. Dr. Wollert has received research grant funding from Roche Diagnostics.
References
- 1.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 3.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Munzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The monica, risk, genetics, archiving, and monograph (morgam) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 5.Kim HC, Greenland P, Rossouw JE, Manson JE, Cochrane BB, Lasser NL, Limacher MC, Lloyd-Jones DM, Margolis KL, Robinson JG. Multimarker prediction of coronary heart disease risk: The women’s health initiative. J Am Coll Cardiol. 2010;55:2080–2091. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Using nontraditional risk factors in coronary heart disease risk assessment: U S. Preventive services task force recommendation statement. Ann Intern Med. 2009;151:474–482. doi: 10.7326/0003-4819-151-7-200910060-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Ford L. Release of cardiac troponin in acute coronary syndromes: Ischemia or necrosis? Clin Chim Acta. 1999;284:161–174. doi: 10.1016/s0009-8981(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of st2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (gdf-15/mic-1) in oxldl-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 10.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. Il-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jager SC, Bermudez B, Bot I, Koenen RR, Bot M, Kavelaars A, de Waard V, Heijnen CJ, Muriana FJ, Weber C, van Berkel TJ, Kuiper J, Lee SJ, Abia R, Biessen EA. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating ccr2-mediated macrophage chemotaxis. J Exp Med. 2011;208:217–225. doi: 10.1084/jem.20100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY, Ramji DP. Il-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–1229. doi: 10.4049/jimmunol.1000520. [DOI] [PubMed] [Google Scholar]
- 13.Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L. Growth-differentiation factor-15 improves risk stratification in st-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–2865. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 14.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth-differentiation factor-15 in patients with non-st-segment elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 15.Kempf T, Sinning JM, Quint A, Bickel C, Sinning C, Wild PS, Schnabel R, Lubos E, Rupprecht HJ, Munzel T, Drexler H, Blankenberg S, Wollert KC. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: Results from the atherogene study. Circ Cardiovasc Genet. 2009;2:286–292. doi: 10.1161/CIRCGENETICS.108.824870. [DOI] [PubMed] [Google Scholar]
- 16.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the valsartan heart failure trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 17.Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, Maisel AS, Fitzgerald RL. Serial sampling of st2 predicts 90-day mortality following destabilized heart failure. J Card Fail. 2008;14:732–738. doi: 10.1016/j.cardfail.2008.06.415. [DOI] [PubMed] [Google Scholar]
- 18.Rehman SU, Mueller T, Januzzi JL., Jr Characteristics of the novel interleukin family biomarker st2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT. Complementary roles for biomarkers of biomechanical strain st2 and n-terminal prohormone b-type natriuretic peptide in patients with st-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN. Prognostic value of very low plasma concentrations of troponin t in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 21.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 22.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High-sensitivity st2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The rancho bernardo study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: The framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 28.Dieplinger B, Januzzi JL, Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble st2 in human plasma--the presage st2 assay. Clin Chim Acta. 2009;409:33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High-sensitivity st2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2012;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, Goix P. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007;53:1990–1995. doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Fukushima N, Puskas R, Todd J, Goix P. Development and preliminary clinical validation of a high sensitivity assay for cardiac troponin using a capillary flow (single molecule) fluorescence detector. Clin Chem. 2006;52:2157–2159. doi: 10.1373/clinchem.2006.073163. [DOI] [PubMed] [Google Scholar]
- 32.Apple FS, Simpson PA, Murakami MM. Defining the serum 99th percentile in a normal reference population measured by a high-sensitivity cardiac troponin i assay. Clin Biochem. 2010;43:1034–1036. doi: 10.1016/j.clinbiochem.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 35.Cox DR. Regression models and life tables. J R Stat Soc [B] 1972;34 (Series B):187–220. [Google Scholar]
- 36.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PWF, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 37.Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, Vasan RS. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–1706. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand S-LT, O’Donnell CJ, Smith SC, Jr, Wilson PWF Diseases obotAHAEPoSA, Factors ER the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the american heart association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 accf/aha guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 40.Pencina MJ, D’Agostino RB. Overall c as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 41.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 42.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 44.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: The reynolds risk score for men. Circulation. 2008;118:2243–2251. 2244. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member st2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble st2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 47.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O’Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd-Jones DM, Wu AH. Measurement of the interleukin family member st2 in patients with acute dyspnea: Results from the pride (pro-brain natriuretic peptide investigation of dyspnea in the emergency department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through st2 signaling. Circ Heart Fail. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 49.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 50.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. Gdf-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 51.Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, Frey N. Gene expression pattern in biomechanically stretched cardiomyocytes: Evidence for a stretch-specific gene program. Hypertension. 2008;51:309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. Gdf15/mic-1 functions as a protective and antihypertrophic factor released from the myocardium in association with smad protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 53.Secchiero P, Corallini F, Gonelli A, Dell’Eva R, Vitale M, Capitani S, Albini A, Zauli G. Antiangiogenic activity of the mdm2 antagonist nutlin-3. Circ Res. 2007;100:61–69. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- 54.Bermudez B, Lopez S, Pacheco YM, Villar J, Muriana FJ, Hoheisel JD, Bauer A, Abia R. Influence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary artery. Cardiovasc Res. 2008;79:294–303. doi: 10.1093/cvr/cvn082. [DOI] [PubMed] [Google Scholar]
- 55.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150:1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 56.Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: A nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 57.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the prospective investigation of the vasculature in uppsala seniors (pivus) study. Eur Heart J. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 58.Hochholzer W, Morrow DA, Giugliano RP. Novel biomarkers in cardiovascular disease: Update 2010. Am Heart J. 2010;160:583–594. doi: 10.1016/j.ahj.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Sakhuja R, Green S, Oestreicher EM, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB, Januzzi JL., Jr Amino-terminal pro-brain natriuretic peptide, brain natriuretic peptide, and troponin t for prediction of mortality in acute heart failure. Clin Chem. 2007;53:412–420. doi: 10.1373/clinchem.2006.074047. [DOI] [PubMed] [Google Scholar]
- 60.Wang TJ. Significance of circulating troponins in heart failure: If these walls could talk. Circulation. 2007;116:1217–1220. doi: 10.1161/CIRCULATIONAHA.107.721845. [DOI] [PubMed] [Google Scholar]
- 61.Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature. 2008;451:949–952. doi: 10.1038/nature06802. [DOI] [PubMed] [Google Scholar]
- 62.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the american college of chest physicians and the international society for heart and lung transplantation: Endorsed by the heart rhythm society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.