Abstract
Context
The Women’s Health Initiative Estrogen-alone Trial was stopped early after 7.1 years (mean) follow-up. Postintervention health outcomes have not been reported.
Objective
To examine health outcomes associated with randomization to conjugated equine estrogen (CEE) treatment in women with prior hysterectomy after 10.7 (mean) years follow-up through August 2009.
Design, Setting, and Participants
The intervention phase was a double-blind, placebo-controlled, randomized trial of CEE, 0.625 mg/day or placebo in 10,739 US postmenopausal women aged 50–79 years with prior hysterectomy. Follow-up continued after the planned trial completion date among 7645 (78%) surviving participants who provided written consent.
Main Outcome Measures
The primary outcomes were CHD and invasive breast cancer. A global index of risks and benefits included these 2 endpoints plus stroke, pulmonary embolism, colorectal cancer, hip fracture, and death.
Results
Postintervention risks for women assigned to CEE vs. placebo were similar to the intervention period for CHD (annualized rates 0.64% in CEE vs. 0.67% in placebo; hazard ratio (HR)=0.97, 95% CI 0.75–1.25), breast cancer (0.26% vs. 0.34%; HR=0.75, 0.51–1.09), and total mortality (1.47% vs. 1.48%; HR=1.00, CI 0.84–1.18). Postintervention risks changed for stroke (0.36% vs. 0.41%; HR=0.89, 0.64–1.24), deep vein thrombosis (0.17% vs. 0.27%; HR=0.63, 0.41–0.98), and hip fracture (0.36% vs. 0.28%; HR=1.27, 0.88–1.82). Over the entire follow-up, lower breast cancer incidence in the CEE group persisted (0.27% vs. 0.35%; HR=0.77, 0.62–0.95). Health outcomes were more favorable for younger compared to older women for CHD (p for age-interaction=0.049), total MI (p-interaction=0.007), colorectal cancer (p-interaction=0.04), total mortality (p-interaction =0.04), and global index (p-interaction=0.009).
Conclusions
Among postmenopausal women with prior hysterectomy followed for 10.7 years, CEE use for a median of 5.9 years was not associated with an increased or decreased risk of CHD, deep vein thrombosis, stroke, hip fracture, colorectal cancer, or total mortality. A decreased risk of breast cancer persisted.
Keywords: estrogen, coronary heart disease, breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, total mortality, Women’s Health Initiative
INTRODUCTION
The Women’s Health Initiative (WHI) Estrogen alone Trial is a double-blind, placebo controlled, randomized clinical trial evaluating effects of conjugated equine estrogens (CEE) on chronic disease incidence among postmenopausal women with prior hysterectomy. The trial intervention was stopped one year early after 7.1 (mean) years of follow-up because of an increased stroke risk and little likelihood of altering the balance of risk to benefit by the planned termination date. Analyses of the intervention period suggested treatment effects differed by age with younger women on CEE having lower risk of coronary heart disease (CHD), colorectal cancer, total death, and global index compared to older women.1 However, the tests for interaction were statistically significant only for colorectal cancer.1
All previous reports of this trial were limited to outcomes occurring during the intervention phase. We now report information on postintervention outcomes through 10.7 years mean follow-up. This pre-planned analysis has three objectives: 1) to assess the long-term effects of CEE intervention on health outcomes; 2) to determine whether effects of CEE on health outcomes differed between the intervention and post-intervention periods; and 3) to determine if previously identified suggestions of age-specific differences in effects of CEE on health outcomes persisted after stopping intervention.
METHODS
Intervention Phase
Details of the WHI Estrogen-alone Trial have been published previously.1, 2 Briefly, postmenopausal women aged 50–79 were recruited at 40 US clinical centers between 1993 and 1998. Women were eligible if they had prior hysterectomy, were not taking hormone therapy and had anticipated 3-year survival. Women were excluded for prior breast cancer or other cancer within ten years except non-melanoma skin cancer, or prior venous thromboembolism if screened after 1997. The study protocol was approved by institutional review boards at participating institutions and all participants provided written informed consent. This trial is registered with clinicaltrials.gov (NCT00000611).
A total of 10,739 women were randomly assigned to oral 0.625 mg per day of CEE (Premarin®, Wyeth Ayerst, Philadelphia, Pennsylvania) or matching placebo. Randomization was implemented at the WHI Clinical Coordinating Center using a permuted block algorithm, stratified by clinical center and age group.1 The clinical trial target size of 12,375 was calculated to provide 81% power to detect a 21% reduction in CHD at 9 years follow-up. With the actual randomized sample size, the power estimate was 72% for a 21% reduction in CHD.
When the active intervention phase ended after 7.1 years (mean) on February 29, 2004, vital status was known for 95% of participants with 5.4% deceased. By this time 54% of participants had stopped taking study medication. Median time on treatment was 5.9 or 5.8 years in the CEE vs. placebo groups, respectively (interquartile range 2.5–7.3 years). The median adherent time on treatment (taking > 80% of study pills), was 3.5 years in both groups (interquartile range 1.5–6.5 years).
Clinical outcomes were collected through semi-annual mailed questionnaires and annual clinic visits. Outcomes were verified,3 initially by trained physician adjudicators at the local clinical centers by medical record review, followed by final adjudication at the WHI Clinical Coordinating Center. All adjudicators were blinded to treatment assignment. This report examines all outcomes presented in the initial report.1
Demographic characteristics and medical history were collected by self-report using standardized questionnaires. Race/ethnicity was reported by participants within pre-defined categories matching the US Census. This information was required by the funding agency to monitor minority representation is the trial.
Postintervention Period and Extension
The postintervention period began on March 1, 2004 when participants were instructed to discontinue study pills. The current report reflects a mean (SD) duration of postintervention follow-up of 47.2 (20.7) months through August 14, 2009. After the protocol-specified termination date of March 31, 2005, subsequent follow-up required written reconsent which was obtained from 77.9% (n=3778) and 78.4% (n=3867) of surviving CEE and placebo group participants, respectively. Outcomes identified from annual mailed questionnaires were verified by medical record review as previously described.3 Annual mammograms were encouraged and tracked by annual mammography report review. During postintervention, 3.6–4.7% of women from the CEE group and 2.7–3.0% of placebo women reported estrogen-alone use (any route of administration) on annual questionnaires.
Statistical Analyses
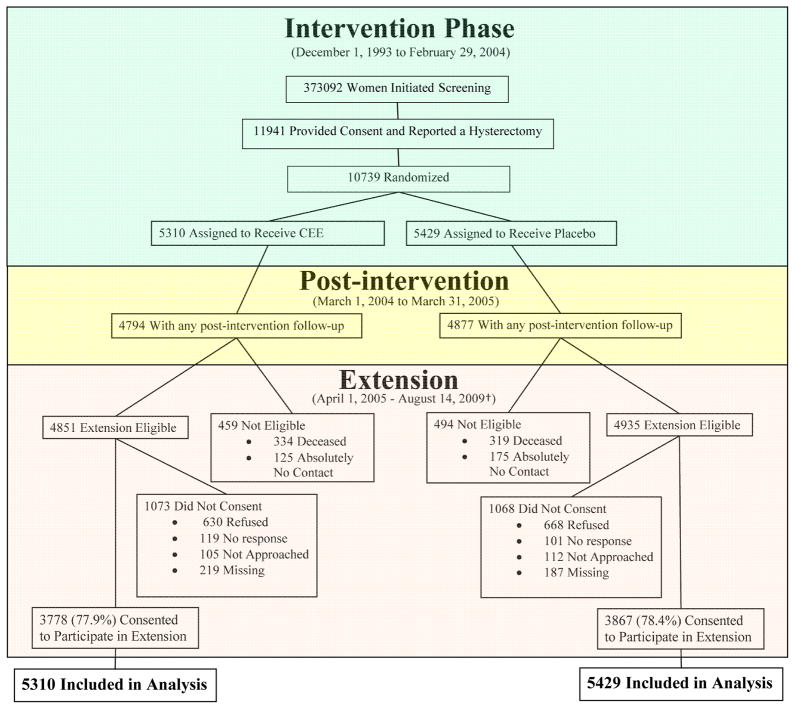
Primary analyses included all randomized participants using time-to-event methods and are based on the intention-to-treat principle as described previously.4 Thus, all randomized participants were included in the analyses according to their randomized group assignment until they last provided follow-up information (Figure 1). Entry baseline characteristics for women who reconsented were compared by randomization group using Chi-square and t-test statistics.
Figure 1.
Consort diagram of the WHI Hormone Therapy Estrogen-Alone Trial through extended follow-up
* Data as of August 14, 2009.
†Consent status as of August 14, 2009.
Annualized rates of clinical events were estimated for the intervention period, the postintervention period, and the entire follow-up period by dividing the number of events by the corresponding person-time in each phase. Cumulative incidence curves are shown for each trial phase with shading used to represent quintiles of intended duration of intervention (elapsed time from randomization until the intervention ended on February 29, 2004). Hazard ratios were estimated using Cox proportional hazards models5 stratified by age, prior disease (if appropriate), and randomization status in the WHI Dietary Modification Trial.6 Models were constructed for each clinical endpoint where women contributed follow-up time until the end of the interval, the date of their first relevant clinical event, or the date of death or withdrawal from the study whichever came first. Formal tests of the differences between hazard ratios in the intervention vs. postintervention phases were calculated by inclusion of a binary term for trial phase as a time-dependent variable as previously described.4 Absolute rates and attributable risks (rate differences between CEE and placebo groups) were also calculated. All statistical tests were two-sided. Nominal P-values are reported without adjustment for multiple outcomes or sequential looks during the clinical trial follow-up period. Age-stratified subgroup analyses are reported for 10 outcomes; at the 0.05 level of significance, 0–1 interaction p-values could be statistically significant based on chance alone.
To determine whether non-consent to postintervention follow-up importantly influenced risk estimates, inverse probability weighting analyses were conducted using methods described previously.4 Adherence sensitivity analyses were also conducted by censoring follow-up six months after participants became non-adherent (taking < 80% of study pills or starting non-protocol hormone therapy). For these analyses, participants who reconsented or were adherent, respectively, were included in analyses that used the inverse of the participant’s estimated reconsent/adherence probability as a weighting factor.
Statistical software SAS, version 9.2 and R, version 2.11 were used for these analyses.
RESULTS
Baseline Characteristics
The participant flow throughout the study is outlined in Figure 1. Among the women who reconsented, baseline characteristics remained similar to those published at entry1 and were evenly distributed by randomized treatment assignment (Table 1). Small differences were observed for parity and bilateral oophorectomy between randomization groups.
Table 1.
Baseline characteristics of Women’s Health Initiative Participants who consented to extended follow-up after enrollment in the Hormone Therapy Estrogen-Alone Trial (April 2005)
| Active | Placebo | P-Value1 | |||
|---|---|---|---|---|---|
| N=3778 | % | N=3867 | % | ||
| Age group at screening | 0.88 | ||||
| 50–59 | 1223 | 32.4 | 1232 | 31.9 | |
| 60–69 | 1740 | 46.1 | 1799 | 46.5 | |
| 70–79 | 815 | 21.6 | 836 | 21.6 | |
|
| |||||
| Race/ethnicity | 0.27 | ||||
| White | 2945 | 78.0 | 3001 | 77.6 | |
| Black | 514 | 13.6 | 565 | 14.6 | |
| Hispanic | 189 | 5.0 | 181 | 4.7 | |
| American Indian | 31 | 0.8 | 18 | 0.5 | |
| Asian/Pacific Islander | 54 | 1.4 | 49 | 1.3 | |
| Unknown | 45 | 1.2 | 53 | 1.4 | |
|
| |||||
| HRT use status | 0.43 | ||||
| Never used | 1929 | 51.1 | 1916 | 49.6 | |
| Past user | 1304 | 34.5 | 1373 | 35.5 | |
| Current user | 544 | 14.4 | 575 | 14.9 | |
|
| |||||
| HT Duration | 0.52 | ||||
| < 5 years | 960 | 51.9 | 1036 | 53.1 | |
| 5 – 10 years | 348 | 18.8 | 377 | 19.3 | |
| ≥ 10 years | 541 | 29.3 | 538 | 27.6 | |
|
| |||||
| Body-mass index (kg/m2), baseline | 0.21 | ||||
| < 25 | 785 | 20.9 | 771 | 20.1 | |
| 25 – < 30 | 1289 | 34.3 | 1391 | 36.2 | |
| ≥ 30 | 1687 | 44.9 | 1683 | 43.8 | |
|
| |||||
| Smoking status | 0.30 | ||||
| Never | 1988 | 53.1 | 1972 | 51.5 | |
| Past | 1417 | 37.9 | 1489 | 38.9 | |
| Current | 336 | 9.0 | 370 | 9.7 | |
|
| |||||
| Parity | 0.04 | ||||
| Never pregnant/Never had term pregnancy | 350 | 9.3 | 307 | 8.0 | |
| ≥1 term pregnancy | 3400 | 90.7 | 3539 | 92.0 | |
|
| |||||
| Age at first birth, y | 0.53 | ||||
| <20 | 822 | 27.0 | 872 | 27.3 | |
| 20 – 29 | 2060 | 67.7 | 2128 | 66.7 | |
| 30+ | 163 | 5.4 | 190 | 6.0 | |
|
| |||||
| Hysterectomy age group | 0.17 | ||||
| <40 | 1495 | 39.8 | 1501 | 39.0 | |
| 40–49 | 1643 | 43.7 | 1662 | 43.2 | |
| 50–54 | 345 | 9.2 | 412 | 10.7 | |
| 55+ | 275 | 7.3 | 271 | 7.0 | |
|
| |||||
| Bilateral oophorectomy | 1370 | 39.0 | 1507 | 41.8 | 0.01 |
|
| |||||
| Treated diabetes (pills or shots) | 243 | 6.4 | 250 | 6.5 | 0.95 |
|
| |||||
| Hypertensive (Self-report or high BP) | 1806 | 51.1 | 1844 | 51.2 | 0.92 |
|
| |||||
| History of high cholesterol requiring pills | 490 | 14.3 | 536 | 15.5 | 0.16 |
|
| |||||
| Statin group | 288 | 7.6 | 302 | 7.8 | 0.76 |
|
| |||||
| Aspirin use >=80 mg for at least 30 days | 712 | 18.8 | 784 | 20.3 | 0.12 |
|
| |||||
| History of angina | 243 | 6.5 | 253 | 6.6 | 0.82 |
|
| |||||
| History of CABG/PTCA | 69 | 1.9 | 70 | 1.8 | 0.96 |
|
| |||||
| Stroke ever | 51 | 1.3 | 47 | 1.2 | 0.60 |
|
| |||||
| History of DVT or PE | 65 | 1.7 | 60 | 1.6 | 0.56 |
|
| |||||
| History of fracture age 55+ | 455 | 16.5 | 447 | 15.8 | 0.51 |
|
| |||||
| Times fell down last 12 months | 0.16 | ||||
| None | 2368 | 67.5 | 2331 | 65.2 | |
| 1 time | 680 | 19.4 | 722 | 20.2 | |
| 2 times | 296 | 8.4 | 346 | 9.7 | |
| 3 or more times | 164 | 4.7 | 174 | 4.9 | |
Test of association.
Comparison of Intervention and Postintervention Findings
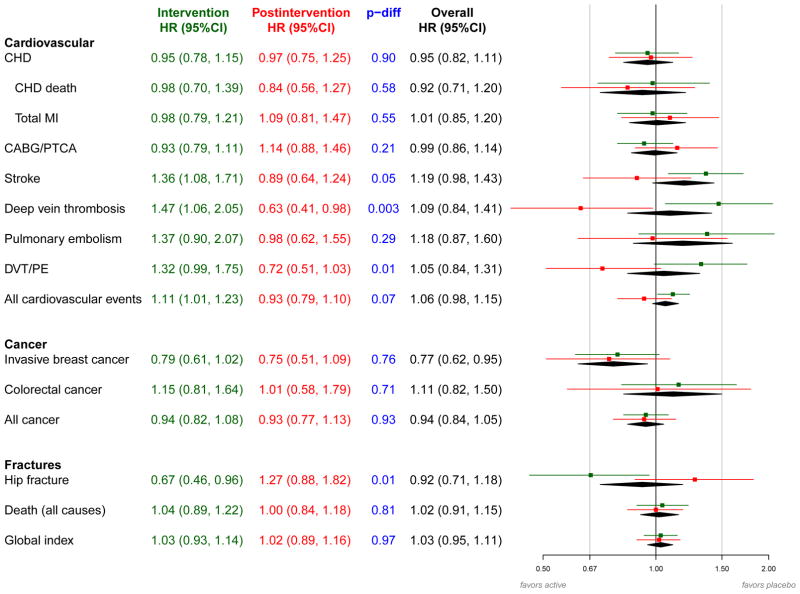
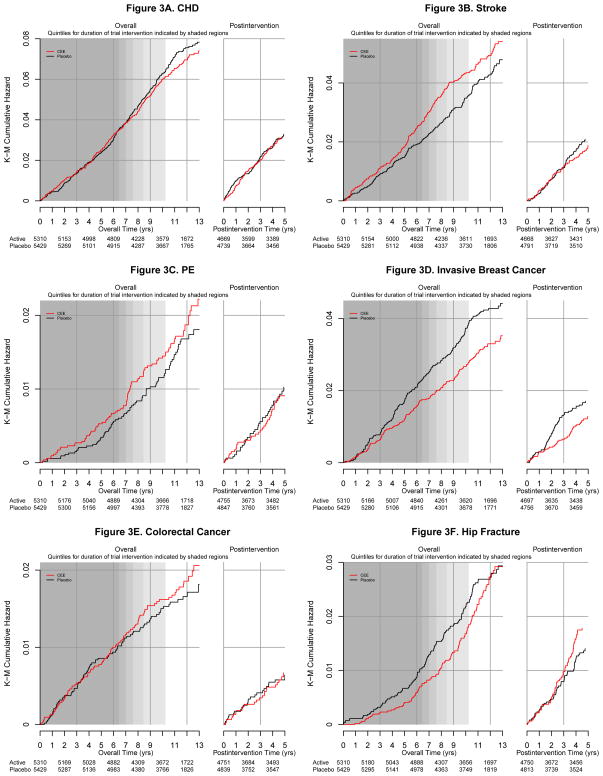
Incident clinical events by randomization assignment and corresponding hazard ratios for the intervention, postintervention, and overall follow-up periods are summarized in Figure 2. Hazard ratios for CHD during the postintervention period were close to unity (Figures 2 and 3a) and similar to those observed during the intervention. The increased stroke risk seen during intervention was not present postintervention (0.36% [n=66] in CEE vs. 0.41% [n=77] in placebo, HR 0.89; 95% CI 0.64–1.24, P-difference 0.05; Figures 2 and 3b). Similarly, the increase in deep vein thrombosis and pulmonary embolism (DVT/PE) with CEE use during intervention was not maintained postintervention (0.28% [n=52] vs 0.39% [n=74], HR 0.72; 95% CI 0.51–1.03, Figures 2 and 3c). For all cardiovascular events, the cumulative hazard ratio associated with CEE was 1.06 (95% CI 0.98–1.15, 2.26% [n=1146] vs. 2.12% [n=1113]).
Figure 2.
Effects of randomized assignment to conjugated equine estrogen vs. placebo on clinical outcomes during intervention phase and post-intervention in the Women’s Health Initiative Estrogen-alone Trial (details in Appendix 2 Table).
aHazard ratios and 95% confidence intervals are shown for the intervention phase in green, the post-intervention period in red, and the overall period in black. The P-difference (shown in blue) tests whether the hazard ratio for the intervention phase equals the hazard ratio for the post-intervention period.
bHazard ratios are derived from proportional hazards models stratified by prior disease (for outcomes where women were eligible for enrollment with and without the prevalent condition), age and dietary modification randomization group. Models for the overall 10.7 mean year follow-up period include a time dependent term for trial phase. For the intervention and overall phases, time to event equals 0 on date of randomization. For the postintervention phase, time to event equals 0 on February 29, 2004.
Abbreviations: CABG, coronary artery bypass graft; CEE, conjugated equine estrogen; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; DVT, deep vein thrombosis; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; PTCA, percutaneous transluminal coronary angioplasty.
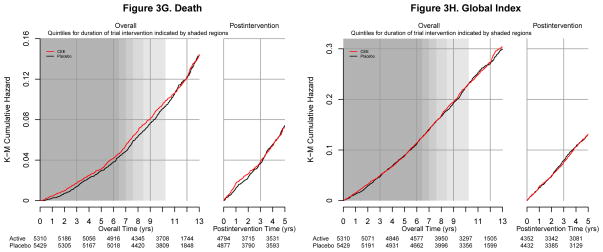
Figure 3.
Cumulative incidence of clinical outcomes by randomized assignment to conjugated equine estrogen or placebo during the intervention phase and post-intervention in the Women’s Health Initiative Estrogen-alone Trial (8 graphs)
aShading represents quintiles of duration of intended intervention and follow-up in the study population (elapsed time from randomization until the intervention ended on February 29, 2004). Darker shading corresponds to a quintile of time in which more women were still being followed; lighter shading corresponds to a quintile of time in which fewer women were still being followed depending on their date of randomization.
During postintervention, 81.2% of women in the CEE group and 81.3% of women in the placebo group had at least one mammogram. Hazard ratios comparing rates of invasive breast cancer in women randomized to CEE vs. placebo were similar during the intervention (HR 0.79; 95% CI 0.61 to1.02) and postintervention phases (HR 0.75; 95% CI 0.51 to1.09) (Figure 2; Figure 3d). Consequently, a statistically significant lower cumulative breast cancer incidence was seen in the CEE compared to the placebo group (0.27% [n=151] vs. 0.35% [n=199], HR 0.77, 95% CI 0.62 to 0.95, P=0.02). Colorectal cancer incidence did not differ for the CEE vs. placebo groups during the intervention or postintervention (Figures 2 and 3e).
The reduced hip fracture risk seen during intervention with CEE was not maintained postintervention (0.36% [n=66] vs. 0.28% [n=53], HR 1.27; 95% CI 0.88 to 1.82; P-difference=0.01; Figure 2) resulting in an overall hazard ratio of 0.92 (95% CI 0.71 to 1.18; 0.20% [n=114] vs. 0.22% [127]). During postintervention, the cumulative incidence curves for the CEE and placebo groups are superimposed for three years and thereafter, hip fracture incidence was slightly higher in the CEE group (Figure 3f).
Randomization to CEE did not influence total mortality or the global index of benefits and harms either during or after intervention (Figures 2, 3g and 3h).
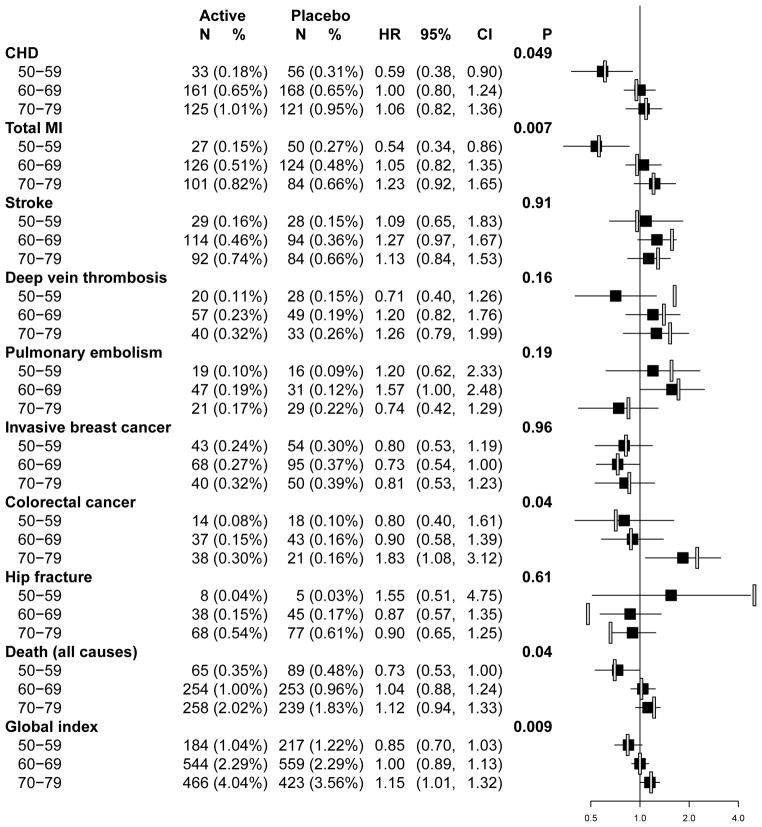
Age-specific Comparisons
The age-specific intervention results are updated in Figure 4 for the overall follow-up period. The overall hazard ratios for CHD differed in women aged 50–59 (HR 0.59, 95% CI 0.38 to 0.90; 0.18% [n=33] vs. 0.31% [n=56]) compared to older women where hazard ratios were near unity (interaction P-value=0.049). For total myocardial infarction, the hazard ratio was 0.54 (95% CI 0.34–0.86, 0.15% [n=27] vs. 0.27% [n=50]) for women aged 50–59; 1.05 (95% CI 0.82–1.35, 0.51% [n=126] vs. 0.48% [n=124] for women aged 60–69; and 1.23 (95% CI 0.92–1.65, 0.82% [n=101] vs. 0.66% [n=84]) for women aged 70–79 (interaction P-value =0.007). A similar pattern was seen when time since menopause (as previously defined7) was examined instead of age for both coronary endpoints (data not shown). Overall, stroke risks were nonsignificantly elevated for all age groups (interaction P-value=0.91). For deep vein thrombosis and pulmonary embolism no age specific differences emerged but the increased risks during intervention subsided postintervention.
Figure 4.
Cumulative annualized incidence ratesa, hazard ratiosb, and 95% confidence intervals for clinical outcomes in the Women’s Health Initiative Estrogen-alone Trial according to ten-year age groupsc at enrollment.
a Annualized incidence rates were estimated for the overall follow-up period by dividing the number of events by the corresponding person-time for participants in each age strata.
b Hazard ratios for the overall follow-up period are shown in the black squares. For comparison, hazard ratios for the intervention phase are shown in the open bars.
cSample sizes at enrollment for each age and randomization group are as follows: age group 50–59, 1637 (CEE), 1673 (placebo); age group 60–69, 2387 (CEE), 2465 (placebo); age group 70–79, 1286 (CEE), 1291 (placebo).
Abbreviations: CEE, conjugated equine estrogen; CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
There were fewer invasive breast cancers in the CEE vs. placebo groups in all three age groups (P-interaction=0.96). The previously observed age interaction for colorectal cancer was still significant considering the entire follow-up period. Women aged 70–79 years at entry experienced nearly two-fold increased risk of colorectal cancer in the CEE vs. placebo groups (HR 1.83, 95% CI 1.08 to 3.12; 0.30% [n=38] vs. 0.16% [n=21], P-interaction=0.04).
Hazard ratios for total mortality and global index differed by age as previously suggested.7 Younger postmenopausal women (age 50–59) randomized to CEE vs. placebo had lower risk of death (HR 0.73; 95% CI 0.53–1.00, 0.35% [n=65] vs. 0.48% [n=89]) compared to no increased risk among women in their 60s (HR 1.04; 95% CI 0.88–1.24, 1.00% [n=254] vs. 0.96% [253]) and a slight increased risk of death among women in their 70s (HR 1.12, 95% CI 0.94–1.33, 2.02% [n=258] vs.1.83% [n=239]; P-interaction=0.04). A similar pattern was observed by age for the global index with possible overall benefit among younger women (HR 0.85; 95% CI 0.70–1.03, 1.04% [n=184] vs. 1.22% [n=217]) and possible harm among the oldest women (HR 1.15, 95% CI 1.01–1.32, 4.04% [n=466] vs. 3.56% [n=423]; P-interaction=0.009).
Expressed as absolute rates per 10,000 women annualized over the average 10.7 year follow-up period, women aged 50–59 using CEE alone had 12 fewer acute myocardial infarctions, 13 fewer deaths, and net 18 fewer adverse events in the global index, compared to women receiving placebo. In contrast, women aged 70–79 using CEE alone had 16 excess myocardial infarctions, 19 excess deaths, and 48 net excess adverse events in the global index, compared to women receiving placebo.
Sensitivity Analyses
The results were similar when using inverse probability weighting to account for censoring due to non-consent for post-intervention follow-up. The hazard ratio for breast cancer for the cumulative follow-up period became 0.81 (95% CI 0.64–1.01). Age-stratified results were virtually identical to those described above with interaction p-values reflecting some loss of precision with the inverse probability weights: CHD (p=0.23); total myocardial infarction (p=0.01); colorectal cancer (p=0.09); death (p=0.13) and global index (p=0.02). In each case, women in their 50s had more favorable hazard ratios than older women.
The results were also similar when women were censored six months after becoming nonadherent during the intervention period. Adherence-adjusted hazard ratios for the overall follow-up period using inverse-probability weights showed an increased risk of stroke (HR=1.50; 95% CI 1.11 to 2.05) and lower breast cancer risk (HR=0.68; 95% CI 0.49 to 0.95) with CEE use. No significant age-interactions emerged for any outcome in the adherence-adjusted analyses; however, power was limited due to substantial censoring.
COMMENT
Among these postmenopausal women with prior hysterectomy who stopped CEE intervention after 5.9 median years of use, several patterns of health risks and benefits seen during the intervention period were not maintained postintervention, while other trends persisted. For CHD, a primary trial endpoint, hazard ratios remained null after stopping intervention and overall. The increase in stroke and venous thromboembolism risk seen among women randomized to CEE during the intervention period rapidly dissipated postintervention as did the hip fracture benefit. The lower breast cancer incidence seen among women randomized to CEE during the intervention period became statistically significant with extended follow-up. Considering the entire follow-up period, rates of total mortality and the global index were essentially the same in the CEE and placebo groups. Statistically significant age interactions for CEE use, suggesting greater safety and possible benefit among women in their 50s and potential harm among older women, were observed for CHD, total myocardial infarction, colorectal cancer, total mortality, and global index.
The statistically significant reduction in breast cancer incidence seen with CEE use continued a trend that emerged during the intervention period.8,9 This finding differs from the preponderance10–12 but not all13,14 observational studies which suggest that CEE use, especially in lean women15,16 and after long duration exposure,17 increases breast cancer incidence. In this randomized trial, we previously reported no significant differences by body mass index (BMI) for CEE effects on breast cancer incidence.8 Investigators from the Million Women Study cohort have suggested,18 based on recent findings,19–21 that time-from-menopause (longer in the WHI vs. shorter in usual clinical practice and observational study cohorts) may account for some of the differences in risk estimates. Alternatively, confounding by differential mammogram use in the observational studies (higher in estrogen users) may explain the finding of higher breast cancer incidence in hormone users.21 Future subgroup analyses in this trial, beyond the scope of the current report, will explore this issue.
A confounding role for diagnostic delay to explain our breast cancer results is unlikely since CEE only modestly influenced breast density22 and mammogram diagnostic performance.23 In terms of biological plausibility, preclinical,24,25 and clinical26 studies suggest adaptive changes that occur in estrogen-exposure gene expression profiles after estrogen deprivation27 may render mammary tumors susceptible to inhibition by estrogen. In contrast to these estrogen-alone results, the WHI combined estrogen plus progestin trial among women with a uterus showed that treatment impeded mammographic accuracy, and significantly increased both breast cancer incidence and breast cancer mortality. 28–30
With extended follow-up, hip fracture cumulative incidence was the same in the CEE and placebo groups. Rates of hip fracture were somewhat higher among CEE vs. placebo participants after stopping intervention. These results are consistent with studies showing accelerated bone loss31 and a short-term increased risk of hip fracture among women who discontinue hormone therapy32 and no fracture risk reduction or elevation in past hormone therapy users.33,34
Our results suggest that women randomized to CEE while in their 50s had fewer CHD events than those randomized to placebo, findings supported by preclinical35 and clinical information36–38 but not applying to older women. In a subset of WHI participants aged 50–59 at study entry, coronary artery calcium measurements, a marker for atherosclerotic plaque burden, were lower following trial completion among women randomized to CEE vs. placebo.36 Other support derives from nonhuman primate models37 and observational studies.39–41 An important caveat is that study participants took unopposed estrogen for a median duration under 6 years.
These new findings emphasize the need to counsel women about hormone therapy differently depending on their age and hysterectomy status. Postmenopausal women with hysterectomy considering initiation of CEE should be counseled about venous thromboembolism and stroke risks during treatment which recede after cessation. Among younger women, no new safety concerns emerged and some risk reductions became apparent. In older women, risks of colorectal cancer, death, and the global index of chronic diseases were elevated over the cumulative follow-up period. The risks and benefits of CEE use for periods longer than 5–6 years cannot be inferred from these data in any age group. Mechanisms underlying the reduced risks of breast cancer in all women, and coronary events in younger but not older women, warrant further study.
Supplementary Material
Acknowledgments
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Role of the Sponsor: The Women’s Health Institute (WHI) Project Office at the National Heart, Lung, and Blood Institute (NHLBI), the Sponsor, reviewed and approved the final manuscript but had no other role in the preparation of this report. Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives.
WHI Investigators
The authors thank the WHI investigators and staff for their outstanding dedication and commitment. A list of key investigators involved in this research follows. A full listing of WHI investigators can be found at the following website: http://www.whi.org.
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Nancy Geller, Leslie Ford.
Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth Patterson, Anne McTiernan, Barbara Cochrane, Julie Hunt, Lesley Tinker, Charles Kooperberg, Martin McIntosh, C. Y. Wang, Chu Chen, Deborah Bowen, Alan Kristal, Janet Stanford, Nicole Urban, Noel Weiss, Emily White; (Medical Research Laboratories, Highland Heights, KY) Evan Stein, Peter Laskarzewski; (San Francisco Coordinating Center, San Francisco, CA) Steven R. Cummings, Michael Nevitt, Lisa Palermo; (University of Minnesota, Minneapolis, MN) Lisa Harnack; (Fisher BioServices, Rockville, MD) Frank Cammarata, Steve Lindenfelser; (University of Washington, Seattle, WA) Bruce Psaty, Susan Heckbert.
Clinical Centers
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller, William Frishman, Judith Wylie-Rosett, David Barad, Ruth Freeman; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic, Jennifer Hays, Ronald Young, Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, Maria Bueche; (Brown University, Providence, RI) Charles B. Eaton, Michele Cyr, Gretchen Sloane, Annlouise Assaf; (Emory University, Atlanta, GA) Lawrence S. Phillips, Vicki Butler, Vivian Porter; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley A.A. Beresford, Vicky M. Taylor, Nancy F. Woods, Maureen Henderson, Robyn Andersen; (George Washington University, Washington, DC) Lisa Martin, Judith Hsia, Nancy Gaba, Richard Katz; (Harbor-UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski, Robert Detrano, Anita Nelson, Michele Geller; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc, Yvonne Michael, Evelyn Whitlock, Victor Stevens, Njeri Karanja; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan, Stephen Sidney, Geri Bailey Jane Hirata; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen, Vanessa Barnabei, Theodore A. Kotchen, Mary Ann C. Gilligan, Joan Neuner; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Linda K Mickel; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn, Philip Greenland, Janardan Khandekar, Liviu Klein, Carol Rosenberg; (Rush University Medical Center, Chicago, IL) Henry Black, Lynda Powell, Ellen Mason; Martha Gulati; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick, Mark A. Hlatky, Bertha Chen, Randall S. Stafford, Sally Mackey; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane, Iris Granek, William Lawson, Catherine Messina, Gabriel San Roman; (The Ohio State University, Columbus, OH) Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, Michael Blumenfeld; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis, Albert Oberman, James M. Shikany, Monika Safford; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, Marcia Ko; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Amy Millen, Michael LaMonte; (University of California at Davis, Sacramento, CA) John Robbins, S. Yasmeen, Lihong Qi; (University of California at Irvine, CA) F. Allan Hubbell, Gail Frank, Nathan Wong, Nancy Greep, Bradley Monk; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan, David Heber, Robert Elashoff, Simin Liu; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer, Michael H. Criqui, Gregory T. Talavera, Cedric F. Garland, Matthew A. Allison; (University of Cincinnati, Cincinnati, OH) Margery Gass, Nelson Watts; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, Yvonne Brinson; (University of Hawaii, Honolulu, HI) J. David Curb, Helen Petrovitch, Beatriz Rodriguez, Kamal Masaki, Patricia Blanchette; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, Jennifer Robinson; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene, Milagros Rosal, Ira Ockene, Robert Yood, Patricia Aronson; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser, Baljinder Singh, Vera Lasser, John Kostis, Peter McGovern; (University of Miami, Miami, FL) Mary Jo O’Sullivan, Linda Parker, JoNell Potter, Diann Fernandez, Pat Caralis; (University of Minnesota, Minneapolis, MN) Karen L. Margolis, Richard H. Grimm, Mary F. Perron, Julia A. Levin; (University of Nevada, Reno, NV) Robert Brunner, William Graettinger, Vicki Oujevolk, Michael Bloch; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss, Pamela Haines, David Ontjes, Carla Sueta, Ellen Wells; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller, Jane Cauley, N. Carole Milas; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, Fran Tylavsky; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski, Robert Schenken, Allison Rodgers, Nicole Budrys; (University of Wisconsin, Madison, WI) Gloria E. Sarto, Douglas Laube, Patrick McBride, Julie Mares, Barbara Loevinger; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins, Greg Burke, Robin Crouse, Scott Washburn; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon.
Women’s Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston- Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
Former Principal Investigators and Project Officers
(Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic, Jennifer Cousins, Jennifer Hays, John Foreyt; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Dallas Hall, Sally McNagny; (Fred Hutchinson Cancer Research Center, Seattle, WA) Maureen Henderson, Ruth Patterson; (George Washington University, Washington, DC) Valery Miller; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael, Evelyn Whitlock, Barbara Valanis; (Kaiser Permanente Division of Research, Oakland, CA) Robert Hiatt; (National Cancer Institute, Bethesda, MD) Carolyn Clifford1; (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Linda Pottern; (Northwestern University, Chicago/Evanston, IL) Philip Greenland; (Rush University Medical Center, Chicago, IL) Henry Black, William Elliott; (The Ohio State University, Columbus, OH) Electra Paskett; (University at Buffalo, Buffalo, NY) Maurizio Trevisan; (University of Alabama at Birmingham, Birmingham, AL) Albert Oberman; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford, Tom Moon, Cheryl Ritenbaugh; (University of California at Irvine, CA) Frank Meyskens, Jr.; (University of California at Los Angeles, CA) Howard Judd1; (University of Cincinnati, Cincinnati, OH) Margery Gass, James Liu, Nelson Watts; (University of Miami, Miami, FL) Marianna Baum, (University of Minnesota, Minneapolis, MN) Richard Grimm; (University of Nevada, Reno, NV) Sandra Daugherty1; (University of North Carolina, Chapel Hill, NC) David Sheps, Barbara Hulka; (University of Tennessee Health Science Center, Memphis, TN) William Applegate; (University of Texas Health Science Center, San Antonio, TX) Robert Schenken; (University of Wisconsin, Madison, WI) Catherine Allen1; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds, Greg Burke; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix. 1deceased
Footnotes
Author information, financial disclosures, and WHI investigators are listed at the end of this article.
Clinical Trials Registration: ClinicalTrials.gov Identifier: NCT00000611
Author Contributions: Dr. LaCroix and Mr. Aragaki had full access to all of the data and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Howard, Manson, Margolis, Stefanick, Wactawski-Wende
Acquisition of data: LaCroix, Brzyski, Chlebowski, Curb, Howard, Johnson, Manson, Margolis, Stefanick, Wactawski-Wende
Analysis and interpretation of the data: LaCroix, Aragaki, Brzyski, Chlebowski, Curb, Johnson, Lewis, Margolis, Martin, Manson, Wactawski-Wende
Drafting of the manuscript: LaCroix, Aragaki
Critical revision of the manuscript for important intellectual content: LaCroix, Brzyski, Chlebowski,
Curb, Howard, Johnson, Lewis, Manson, Margolis, Martin, Stefanick, Wactawski-Wende
Statistical analysis: LaCroix, Aragaki
Obtaining funding: LaCroix, Chlebowski, Curb, Johnson, Lewis, Manson, Stefanick, Wactawski-Wende
Administrative, technical, or material support: Brzyski, Chlebowski, Curb, Howard, Johnson, Lewis,
Manson, Wactawski-Wende
Study supervision: LaCroix, Curb, Johnson, Margolis, Wactawski-Wende
Financial Disclosures: There are no potential conflicts of interest relevant to this article reported by any author. Andrea Z. LaCroix, Ph.D., reports serving on Scientific Advisory Committees for research studies funded by Warner Chilcott and sanofi-aventis, Amgen and Pfizer. Rowan Chlebowski, M.D., has been a consultant for Astra-Zeneca, Novartis, Amgen, and Pfizer; has received funding support from Amgen, and is on speaker’s bureaus for Astra-Zeneca and Novartis. Barbara V. Howard, Ph.D., has received payment for lectures, service on a speakers’ bureau, and consulting services for Merck/Schering-Plough Wyeth Research, and has received research support from donation of drugs from Merck/Schering-Plough. Rowan Chlebowski, MD, has received payment for consulting fees, or honorariums from Novartis, AstraZeneca, Pfizer, and Amgen, and has also received payment for lectures and service on speakers’ bureaus from Novartis and AstraZeneca. None of the other authors have any financial arrangements to disclose. Wyeth Ayerst donated the study drugs as acknowledged in this manuscript.
References
- 1.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopaual women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 2.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 3.Curb D, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 4.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 5.Cox DR. Regression analysis and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 6.Prentice RL, Chlebowski RT, Patterson R, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative randomized controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 8.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 9.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 11.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1998;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 12.Beral V Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 13.Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289(24):3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 14.Kerlikowske K, Miglioretti DL, Ballared-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–4321. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 15.Schairer C, Lubin L, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Feigelson HS, Hildebrand JS, Teras LR, Thun MJ, Rodriguez C. Postmenopausal hormone use and breast cancer associations differ by hormone regimen and histologic subtype. Cancer. 2009;115(5):936–945. doi: 10.1002/cncr.24101. [DOI] [PubMed] [Google Scholar]
- 17.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 18.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167(12):1407–15. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009;27(31):5138–5143. doi: 10.1200/JCO.2008.21.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chlebowski RT, Anderson GL. The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment. J Natl Cancer Inst. 2011;103(4):284–285. doi: 10.1093/jnci/djq561. [DOI] [PubMed] [Google Scholar]
- 22.McTiernan A, Chlebowski RT, Martin C, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a substudy of the Women’s Health Initiative randomized trial. J Clin Oncol. 2009;27(36):6135–6143. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Anderson G, Manson J, et al. Estrogen alone and breast cancer detection by means of mammography and breast biopsy. J Clin Oncol. 2010;28(16):2690–2697. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santen RJ, Song RX, Zhang Z, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95(1–5):155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Jeng MH, Shupnik MA, Bender TP, et al. Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology. 1998;139(10):4164–4174. doi: 10.1210/endo.139.10.6229. [DOI] [PubMed] [Google Scholar]
- 26.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs. high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer. JAMA. 2009;302(7):774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunbier AK, Anderson H, Ghazoui Z, et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010;28(7):1161–1167. doi: 10.1200/JCO.2009.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women’s Health Initiative randomized trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Kuller L, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E PEPI Safety Follow-Up Study (PSFS) Investigators. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162(6):665–672. doi: 10.1001/archinte.162.6.665. [DOI] [PubMed] [Google Scholar]
- 32.Yates J, Barrett-Connor E, Barlas S, Chen YT, Miller PD, Siris ES. Rapid loss of hip fracture protection after estrogen cessation: evidence from the National Osteoporosis Risk Assessment. Obstet Gynecol. 2004;103(3):440–446. doi: 10.1097/01.AOG.0000114986.14806.37. [DOI] [PubMed] [Google Scholar]
- 33.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122(1):9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Banks E, Beral V, Reeves G, Balkwill A, Barnes I Million Women Study Collaborators. Fracture incidence in relation to the pattern of use of hormone therapy in postmenopausal women. JAMA. 2004;291(18):2212–2220. doi: 10.1001/jama.291.18.2212. [DOI] [PubMed] [Google Scholar]
- 35.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 36.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 37.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 38.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348(7):645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 39.Manson JE, Bassuk SS. Invited commentary: Hormone therapy and risk of coronary heart disease – why renew the focus on the early years of menopause? Am J Epidemiol. 2007;166(5):511–517. doi: 10.1093/aje/kwm213. [DOI] [PubMed] [Google Scholar]
- 40.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006;15(1):35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- 41.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.