Abstract
Background
Pregnancy is associated with marked maternal cardiovascular/hemodynamic changes. A greater number of pregnancies may be associated with long-term subclinical changes in left ventricular (LV) remodeling.
Methods
Among 2,234 white, black, Hispanic, and Chinese women (mean age 62 years) in the MESA, we used linear regression to relate live births and cardiac magnetic resonance imaging LV measures. Covariates included age, ethnicity, height, income, education, birth country, smoking, menopause, and oral contraceptive duration. Models were additionally adjusted for potential mediators: systolic blood pressure, antihypertensive use, total/high-density lipoprotein cholesterol, triglycerides, diabetes, and body mass index. We performed sensitivity analyses excluding 763 women in the lowest socioeconomic group: annual income <$25,000 and lower high school level of education.
Results
With each live birth, LV mass increased 1.26 g; LV end-diastolic volume, 0.74 mL; and LV end-systolic volume, 0.45 mL; LV ejection fraction decreased 0.18% (P trend <0.05). Changes were most notable for the category of women with ≥5 pregnancies. Upon adjustment for potential biologic mediators, live births remained positively associated with LV mass and end-systolic volume. Live births remained significantly associated with LV end-systolic, end-diastolic volumes, and LV mass (P trend ≤0.02) after excluding women in the lowest socioeconomic group.
Conclusions
Number of live births is associated with key LV structural and functional measures in middle to older ages, even after adjustment for sociodemographic factors and cardiovascular disease risk factors. Hemodynamic changes during pregnancy may be associated with cardiac structure/function beyond childbearing years.
Number of pregnancies is associated with later-life cardiovascular disease (CVD)1–3 events and with incident congestive heart failure.2,4 The exact mechanisms of these associations remain unclear. Pregnancy and the peripartum period are both associated with marked maternal cardiovascular hemodynamic changes including alterations in heart rate, systolic and diastolic blood pressure, stroke volume, cardiac output, systemic vascular resistance, left ventricular (LV) ejection fraction (EF), and left LV mass.5 An evaluation of cardiovascular physiology up to 1 year postpartum suggested that increases in LV volumes and cardiac output may persist in the near-term postpartum period and are accentuated by having subsequent pregnancies as compared with no prior pregnancies.6 Furthermore, in a prospective cohort study of peripartum cardiomyopathy (for which having ≥4 pregnancies is an independent risk factor),7 investigators demonstrated that a subclinical form of peripartum cardiomyopathy exists that does not necessarily progress to overt heart failure.8 It is unclear whether pregnancy-related cardiovascular physiologic changes are cumulative over successive pregnancies or are associated with differences in LV structure or function.
In the present study, we sought to determine whether the number of pregnancies was associated with changes in key LV structural and functional measures in women free of CVD. The availability of data on the number of pregnancies and cardiac magnetic resonance imaging (MRI) in the MESA allowed us to study whether changes in LV function and structure associated with increasing number of pregnancies resulting in live births.
Methods
Study population
The MESA, begun in July 2000, was designed to investigate the prevalence, correlates, and progression of CVD in individuals without clinically manifest or symptomatic CVD.9 This prospective cohort study enrolled 6,814 participants (including 3,601 women) aged 45 to 84 years from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and St Paul, MN). The recruitment of participants has been previously described.9 Cohort participants were 38% white (n = 2,624), 28% black (n = 1,895), 22% Hispanic (n = 1,492), and 12% Chinese (n = 803). Institutional review boards at all study centers approved the study protocol, and informed consent was obtained from participants. Cardiac MRI was performed in 4,869 participants at their baseline examination. Among 2,622 women in the MESA study with cardiac MRI information seen at examination 1, we excluded women with missing (n = 344) or unusable (n = 3) information regarding number of pregnancies and with a severely depressed EF of 15% (n = 1). We further excluded women with a number of live births that exceeded number of pregnancies (for ease of interpretation, we wished to exclude women having multiple pregnancies, and this approach was our best approximation of singleton live births) (n = 40). We decided a priori to limit this investigation to “singleton” live births because we hypothesized that having multiple births may disproportionately change maternal cardiovascular hemodynamics and could have potentially affected our results in a spurious fashion. However, because our estimate of singleton birth comes solely from 2 questions regarding (1) number of pregnancies and (2) number of live births, we realize that a small amount of potential misclassification with regard to capturing true singleton births could have occurred. Our final study sample upon exclusions consisted of 2,234 women.
Cardiac MRI
Magnetic resonance imaging examinations were performed using scanners with 1.5-T magnets as previously described.10 Functional parameters and LV mass were determined by volumetric imaging. Imaging data were read using MASS software (version 4.2; Medis, Leiden, the Netherlands) at a single reading center by readers trained in the MESA protocol and without knowledge of risk factor information. Papillary muscles were included in the assessment of LV chamber volumes and excluded from LV mass estimates. Cardiac output was calculated as stroke volume (end-diastolic volume minus end-systolic volume) × heart rate. Ejection fraction was calculated as stroke volume divided by end-diastolic volume. An intraclass reliability estimate of 0.95 means that 5% of the total variability is attributed to reader measurement error. For LV mass, intraclass correlation for a set of 155 duplicate readings was 0.97 (95% CI 0.96–0.98); for end-diastolic volume, 0.98 (CI 0.97–0.99); and for end-systolic volume, 0.95 (CI 0.93–0.96).
Sociodemographic and CVD risk factor ascertainment
Sociodemographic information, medical history, anthropometric measurements, and laboratory data were collected starting at the first participant examination of the MESA cohort (July 2000 to August 2002). Information about age, gender, ethnicity, and medical and pregnancy history were obtained by questionnaires. Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL or use of hypoglycemic medications. Use of antihypertensive and other medications was based on clinical staff entry of prescribed medications verified by the staff. Annual household income was categorized as follows: (<$20,000, $20,000–49,999, or >$50,000). Education was categorized by highest attained level (no high school diploma, completed high school diploma, and completed college/graduate school). Place or birth was considered as either United States or non–United States.
Statistical analysis
Descriptive analyses were performed to examine the associations between cardiovascular factors and sociodemographic variables and number of pregnancies. The primary exposure variable, number of live births, was considered as 0, 1, 2, 3, 4, or ≥5. For the purposes of linear analyses, the highest live birth category was given the value of 6 because this was the median number of births within that category. The 6 MRI variables of LV structure/function were treated as continuous measures. Linear regression analysis was performed to relate number of live births and dependent variables (LV end-systolic volume [LVESV], LV end-diastolic volume [LVEDV], LV mass, LV stroke volume [LVSV], LVEF, and mass/volume ratio). Models were adjusted for age and race (model 1): model 1 covariates plus height (to account for possible confounding effects of body size) and potential sociodemographic confounders including income, education, place of birth, cigarette use, and menopausal status, past or present oral contraceptive use (model 2). Model 2 was considered our primary model. Model 3 included model 2 covariates plus possible intermediate factors between exposure and dependent variables. Thus, model 3 included model 2 covariates plus systolic blood pressure, antihypertensive medication use, total/high-density lipoprotein (HDL) cholesterol, tri-glycerides, diabetes, and body mass index. Least square means for the LV measures are presented, adjusting for model 2 covariates.
Secondary models
Effect modification by ethnicity was tested by placing an interaction term in model 2 (for each dependent variable). We additionally studied which biologic mediators additionally included in model 3 most changed parameter estimates and changed statistical significance by running model 3 without each mediator (ie, model 3 minus diabetes, model 3 without body mass index, etc) for all dependent variables. We considered a potential nonlinear association between number of live births and LV remodeling by fitting a model with parity as a quadratic term for each of the LV-dependent variables (adjusting for model 2 covariates). We created a dichotomous variable for having any singleton live births vs no singleton live births and considered this in models 1 and 2. To better determine the potential effects of extreme levels of socioeconomic status and the lower prevalence of live births ≥5 on our results, we performed a sensitivity analysis removing those women in the lowest income and education categories (sensitivity analysis n = 1,471).
All analyses were performed using SAS version 9.2 (SAS, Cary, NC). Two-tailed 95% CIs and P values are given, with 2-sided P values <.05 regarded as significant.
Dr Parikh and this work were supported through a grant from the National Heart, Lung, and Blood Institute (F32 HL096390-01). This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The sponsors had no role in the study design, analyses, writing, or decision to publish the manuscript. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
The characteristics of the 2,234 women (mean age 62 years) in our study sample are displayed in Table I, stratified by number of live births. The number of live births ranged from 0 to 18. The mean age was greater for women with increasing number of live births. Hispanic and Chinese women had more live births, whereas black and white women had fewer live births. Systolic and diastolic blood pressure and triglycerides were all higher with increasing numbers of live births. Prevalence of current or former smoking and HDL cholesterol was lower with increasing numbers of live births.
Table I.
Baseline characteristics by number of live births among women in the MESA (n = 2,234)
| No. of live births
|
||||||
|---|---|---|---|---|---|---|
| 0 (n = 144) | 1 (n = 371) | 2 (n = 646) | 3 (n = 477) | 4 (n = 279) | ≥5 (n = 317) | |
| Age (y) | 57.5 ± 10.3 | 59.5 ± 9.8 | 60.1 ± 9.7 | 62.2 ± 9.6 | 64 ± 10.1 | 66.9 ± 8.6 |
| Race, n (%) | ||||||
| White | 70 (48.6) | 119 (32.1) | 273 (42.3) | 188 (39.4) | 98 (35.1) | 74 (23.3) |
| Chinese | 6 (4.2) | 49 (13.2) | 103 (15.9) | 67 (14.1) | 46 (16.5) | 34 (10.7) |
| African American | 54 (37.5) | 139 (37.5) | 164 (25.4) | 115 (24.1) | 63 (22.6) | 77 (24.3) |
| Hispanic | 14 (9.7) | 64 (17.3) | 106 (16.4) | 107 (22.4) | 72 (25.8) | 132 (41.6) |
| Income | ||||||
| <$20,000 | 24 (16.7) | 100 (27) | 142 (22) | 138 (28.9) | 98 (35.1) | 174 (54.9) |
| $20,000–$49,999 | 64 (44.4) | 141 (38) | 237 (36.7) | 180 (37.7) | 107 (38.4) | 108 (34.1) |
| ≥$50,000 | 56 (38.9) | 130 (35) | 267 (41.3) | 159 (33.3) | 74 (26.5) | 35 (11) |
| Education | ||||||
| No high school diploma | 3 (2.1) | 24 (6.5) | 37 (5.7) | 47 (9.9) | 32 (11.5) | 36 (11.4) |
| Completed high school | 4 (2.8) | 22 (5.9) | 46 (7.1) | 50 (10.5) | 35 (12.5) | 108 (34.1) |
| Completed college or graduate school or trade school | 137 (95.1) | 325 (87.6) | 563 (87.2) | 380 (79.7) | 212 (76) | 173 (54.6) |
| Born in the United States | 113 (78.5) | 258 (69.7) | 426 (66.1) | 310 (65.3) | 180 (64.8) | 167 (53) |
| Systolic blood pressure (mm Hg) | 120.3 ± 21.1 | 123.8 ± 22.8 | 124.5 ± 22.3 | 126.3 ± 22.6 | 128.2 ± 22.9 | 132.2 ± 23.9 |
| Diastolic blood pressure (mm Hg) | 68.6 ± 10.1 | 69.3 ± 10 | 69 ± 10.5 | 69.1 ± 10.8 | 68.9 ± 10.1 | 69.4 ± 10.3 |
| Total cholesterol (mg/dL) | 196.4 ± 36.3 | 196.5 ± 37 | 202.1 ± 36.3 | 201.5 ± 35.5 | 199.7 ± 35.2 | 198.5 ± 37.2 |
| HDL cholesterol (mg/dL) | 61.6 ± 18 | 58.1 ± 15.7 | 56.9 ± 15.8 | 56.4 ± 15.5 | 54.8 ± 13 | 53.2 ± 13.6 |
| Triglycerides (mg/dL)* | 91 (70–123) | 101 (73–139) | 111 (76–158) | 109 (80–156) | 119 (86–167) | 120 (85–186) |
| Lipid-lower medication, n (%) | 18 (12.5) | 77 (20.8) | 103 (16) | 73 (15.3) | 50 (18) | 62 (19.6) |
| Hypertension, n (%) | 51 (35.4) | 156 (42.1) | 273 (42.3) | 223 (46.8) | 135 (48.4) | 172 (54.3) |
| Antihypertensive therapy, n (%) | 40 (27.8) | 125 (33.7) | 208 (32.2) | 166 (34.8) | 103 (36.9) | 134 (42.3) |
| Diabetes†, n (%) | 13 (9) | 54 (14.6) | 70 (10.8) | 48 (10.1) | 27 (9.7) | 56 (17.7) |
| Cigarette smoking, n (%) | ||||||
| Former | 60 (41.7) | 110 (29.7) | 194 (30.1) | 125 (26.3) | 71 (25.5) | 68 (21.6) |
| Current | 28 (19.4) | 64 (17.3) | 68 (10.5) | 46 (9.7) | 19 (6.8) | 28 (8.9) |
| Menopause, n (%) | 94 (65.3) | 289 (77.9) | 508 (78.6) | 395 (82.8) | 245 (88.1) | 294 (92.7) |
| Hormone replacement therapy (current user), n (%) | 42 (33.9) | 98 (30.3) | 211 (37.3) | 140 (31.9) | 78 (30.5) | 70 (23) |
| Oral contraceptive pills: total no. of years taken | 6.8 ± 5.7 | 6.3 ± 5.8 | 6.2 ± 5.8 | 6 ± 6.3 | 5.9 ± 6.1 | 4.6 ± 5 |
| BMI (kg/m2) | 28 ± 6.3 | 28 ± 5.7 | 27.6 ± 5.5 | 28.1 ± 5.5 | 28.3 ± 5.4 | 29.5 ± 5.7 |
| Height (cm) | 162.21 ± 6.88 | 160.69 ± 7.18 | 160.51 ± 6.94 | 159.57 ± 6.51 | 158.8 ± 6.66 | 157.21 ± 7.05 |
BMI indicates Body mass index.
Median and interquartile range.
Diabetes defined as fasting serum glucose >126 mg/dL or on hypoglycemic medications.
Primary analysis
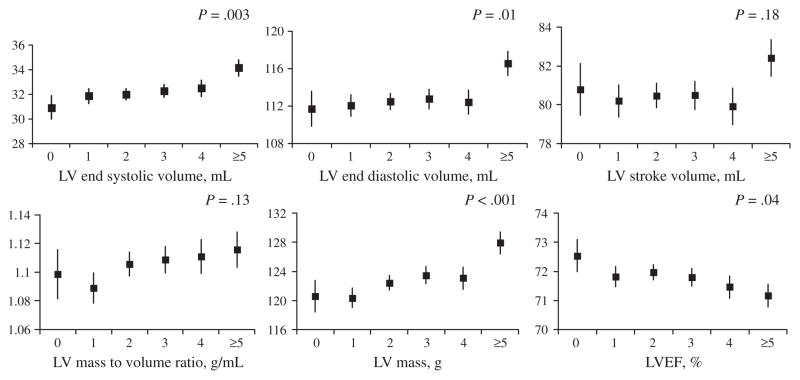
Number of live births was positively associated with LV mass (1.26 g per live birth), end-diastolic volume (0.74 mL per live birth), end-systolic volume (0.45 mL per live birth), and inversely associated with LVEF (−0.18% per live birth) after adjustment for age, race, and socio-demographic variables (models 1 and 2) (Table II). With additional adjustment for potential biologic mediators (model 3), results were attenuated and number of live births remained significantly associated with LV mass and LVESV. However, number of live births was no longer significantly associated with LV end-diastolic volume, and live births was borderline associated with LVEF (P = .05) (Table II). Number of live births was not associated with LV mass-to-volume ratio or stroke volume (Table II). Figure 1 shows the adjusted means for LV measures and the P values for trend across categories of live births. Changes were most marked in the category of women with 5 or more live births.
Table II.
Average difference (β) in LV structure and function measures by MRI per 1 greater category of number of live births (0, 1, 2, 3, 4, ≥5*) in the MESA
| LV mass (g), β ± SE (P value) | LVEDV (mL), β ± SE (P value) | LVESV (mL), β ± SE (P value) | Stroke volume (mL), β ± SE (P value) | LV mass/volume (g/mL), β ± SE (P value) | LVEF (%),β ± SE (P value) | |
|---|---|---|---|---|---|---|
| Model 1 | 1.06 ± 0.34 (.002) | 0.56 ± 0.3 (.06) | 0.4 ± 0.15 (.008) | 0.16 ± 0.22 (.45) | 0.004 ± 0.003 (.09) | −0.18 ± 0.08 (.04) |
| Model 2 | 1.26 ± 0.34 (.0002) | 0.74 ± 0.29 (.01) | 0.45 ± 0.15 (.003) | 0.29 ± 0.21 (.18) | 0.004 ± 0.003 (.13) | −0.18 ± 0.09 (.04) |
| Model 3 | 0.6 ± 0.28 (.03) | 0.27 ± 0.27 (.31) | 0.31 ± 0.15 (.04) | −0.04 ± 0.19 (.86) | 0.003 ± 0.003 (.31) | −0.17 ± 0.09 (.05) |
| Model 2 sensitivity analysis (excluding lowest socioeconomic strata†) | 1.78 ± 4.04 (<.0001) | 1.02 ± 2.6 (.01) | 0.49 ± 2.42 (.02) | 0.52 ± 1.84 (.07) | 0.01 ± 1.58 (.11) | −0.16 ± −1.41 (.16) |
Model 1 covariates: age and race; model 2 covariates: age, race, height, income category, education, place of birth, cigarette use, menopausal status, and oral contraceptive use; model 3 covariates: model 2 covariates + systolic blood pressure, antihypertensive medication use, total/HDL cholesterol, triglycerides, diabetes, and body mass index.
The highest parity category (ie, 6) represents the median number in the ≥5 births category.
Subset of n =1,471 women with at least high school educational level and with annual household income ≥$20,000.
Figure 1.
Number of live births and LV structure and functional measures by cardiac MRI among 2,234 women in the MESA. Least square mean and standard errors are adjusted for age, race, height, income category, education, place of birth, cigarette use, menopausal status, and oral contraceptive use.
Secondary analyses
There was no evidence of effect modification by ethnicity upon associations of single live birth number with LV measures (all P values for interaction term >0.1).
In additional analysis, we found that body mass index was the single covariate that most materially changed the parameter estimates and P values between models 2 and 3 for all dependent variables (data not shown).
Upon fitting a quadratic term, we found no evidence of nonlinear association between parity and LV function measures (all P values >.05, data not shown). We found no significant association between ≥1 vs 0 singleton live births and LV remodeling variables (all P values for model 1s and 2 >0.15).
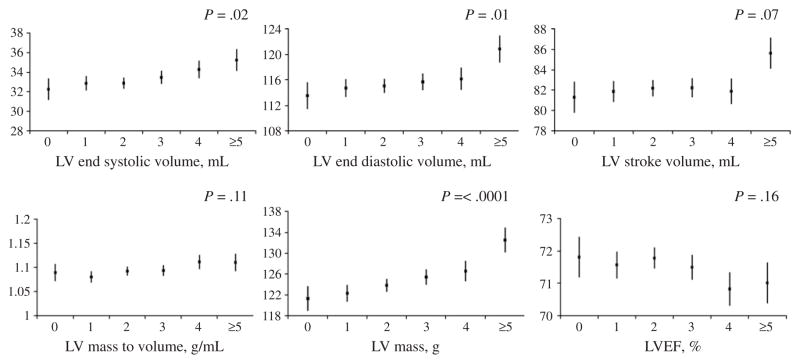
In sensitivity analysis removing approximately one third of women from our total sample who were in the lowest sociodemographic strata, we found that in our primary model, similar results between number of live births and LV remodeling were seen as compared with the total sample (Table II; Figure 2). The association between number of live births and EF was no longer statistically significant.
Figure 2.
Number of live births and LV structure and functional measures by cardiac MRI among a subset of 1,471 women with annual household income >$25,000 and who have completed high school level of education in the MESA. Least square mean and standard errors are adjusted for age, race, height, income category, education, place of birth, cigarette use, menopausal status, and oral contraceptive use.
Discussion
Summary of findings
Among 2,234 women free of CVD (mean age 62 years), number of live births was positively associated with subsequent LVEDV, LVESV, and LV mass and inversely associated with LVEF. Changes were most notable for women with 5 or more live births. After adjustment for potential biologic mediators, number of live births remained significantly associated with LV mass and LVESV and inversely associated with LVEF at a borderline level of statistical significance. The effects were similar across categories of ethnicity. Changes in LV remodeling were most marked for the category of women with 5 or greater pregnancies. In sensitivity analyses removing the lowest socioeconomic strata, results were largely similar (although less statistically significant likely due to smaller sample size).
Underlying mechanisms
The cardiovascular structural and functional changes during normal pregnancy are due to a combination of increased preload, reduced afterload,11 and shunting of blood to the uteroplacental circulation and to hormonal changes during pregnancy.12 For instance, there are estrogen-mediated changes in vascular tone and up-regulation of the renin-angiotensin-aldosterone axis.12 In general, cardiac structural and functional changes of pregnancy are considered “physiologic”13 rather than pathologic. Whereas some prior physiologic studies in humans support the notion that hemodynamic changes in pregnancies may not entirely revert back to normal,6,14 others suggest a complete postpartum reversion to cardiovascular homeostasis.15,16 However, prior studies are limited by relatively small numbers and relatively short follow-up.15,16 One prior evaluation of 15 nulliparous and 15 parous women during the first year postpartum suggested that increases in LV volumes and cardiac output may persist postpartum and are accentuated by having 1 prior pregnancy as compared with no prior pregnancies.6 An echocardiographic evaluation of 36 women throughout pregnancy and 8 weeks postpartum found normal LV systolic function parameters in all patients 1 week postpartum but persistence of LV hypertrophy and LV diastolic dysfunction for nearly 2 months postpartum.14
A recent observational cohort study in a health maintenance population identified 4 or more pregnancies (as compared with 1 pregnancy) as a risk factor for peripartum cardiomyopathy.7 This study did not find a trend in incidence of peripartum cardiomyopathy across parity categories, suggesting a possible threshold at 4 or more pregnancies.7 This is consistent with our findings that having 5 or more pregnancies had the most notable changes in LV remodeling.
The fact that associations were attenuated in models adjusting for possible pathophysiologic intermediaries in the pathway between live births and LV changes suggests that factors known to increase with increasing number of pregnancies, such as increased adiposity,17,18 low HDL cholesterol,19 and insulin resistance20—and which are related to LV remodeling21–23—may partially underlie some of the associations that we observed. Indeed, accounting for body mass index above and beyond sociodemographic factors most altered the association between live births and LVEDV or LVEF. Perinatal weight gain has been demonstrably related to incident heart disease.24 Given that we do not have measures of adiposity around the time of pregnancy and in the interim before middle age, we could not fully assess the role of adiposity in this study.
Strengths and limitations
Prior studies of pregnancy associated changes have used echocardiographic data, which less accurately estimates LV mass as compared with the criterion standard, cardiac MRI.25,26 Thus, our study benefited from use of cardiac MRI measures of LV structure and function, which likely decreased potential misclassification and represents a notable strength of our study design. Likewise, our study benefited from the extensive phenotypic data available on participants of the MESA cohort.
There are, however, several limitations that we should acknowledge. Our study has inherent within it a survival bias, given that our participants survived into middle age. We did not have reproductive history information aside from number of pregnancies and number of live births. Thus, we could not confirm details such as multiple pregnancies and spontaneous abortions, and misclassification with respect to births being truly singleton could have occurred. However, the degree of misclassification would likely have been small. In addition, adverse pregnancy outcomes such as intrauterine growth retardation, preeclampsia, and gestational diabetes have been linked to both CVD risk factors and CVD events.27–29 History of preeclampsia during pregnancy may be associated with decreased parity,30 whereas intrauterine growth restriction31 and gestational diabetes32,33 are associated with greater number of pregnancies. We were unable to account for these potential confounding factors. We accounted for several socioeconomic confounders but may not have fully accounted for potential confounding effects of socioeconomic factors with incident heart failure.34 However, our sensitivity analysis removing the lowest socioeconomic strata did not substantially change associations between number of live births and LV structural/functional measures. Prior studies have assessed the association between number of pregnancies and CVD outcomes in men to investigate possible unmeasured psychosocial confounders.35 We did not have information on number of children among male participants in MESA; however, prior studies in men have not shown a positive relationship between number of children and coronary disease34 or carotid intima media thickness36 and have even demonstrated an inverse association between number of children and CVD death in men.37 Finally, we did not have interim measures of LV function proximate to the last pregnancy and before the cardiac MRI measures at the first MESA examination cycle.
Implications
Ranges of LVEDV, LVESV, LV mass, and LVEF are well within the “normal” range.
However, even asymptomatic LV dysfunction is associated with heart failure and death,38 and increases in LV mass by as little as 1 g as measured by cardiac MRI in the multiethnic study of atherosclerosis have been related to a 1.8-fold increase in hazard of incident congestive heart failure.39 Thus, although seemingly small, when considered at the population level, even modest changes in LV remodeling may have public health relevance.
Conclusions
Number of live births is associated with modest changes in key LV structural and functional measures in middle- to older-aged women, most notably among women reporting 5 or more live births during their lifetime. It is uncertain whether these changes are fully mediated through traditional CVD risk factors or reflect subclinical peripartum LV remodeling that persists postpartum.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Author Contributions
Drs Parikh and Lloyd-Jones had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosures
None.
References
- 1.Colditz GA, Willett WC, Stampfer MJ, et al. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. 1987;126:861–70. doi: 10.1093/oxfordjournals.aje.a114723. [DOI] [PubMed] [Google Scholar]
- 2.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328:1528–33. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 3.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7:641–3. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159:215–221.e6. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Elkayam U, Braunwald E. Heart Disease. W.B. Saunders Co; 1992. Pregnancy and cardiovascular disease; pp. 1790–809. [Google Scholar]
- 6.Clapp JF, III, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–73. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP, Croen LA, Chiang V, et al. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118:583–91. doi: 10.1097/AOG.0b013e318229e6de. [DOI] [PubMed] [Google Scholar]
- 8.Fett JD, Christie LG, Carraway RD, et al. Unrecognized peripartum cardiomyopathy in Haitian women. Int J Gynaecol Obstet. 2005;90:161–6. doi: 10.1016/j.ijgo.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 11.Mone SM, Sanders SP, Colan SD. Control mechanisms for physiological hypertrophy of pregnancy. Circulation. 1996;94:667–72. doi: 10.1161/01.cir.94.4.667. [DOI] [PubMed] [Google Scholar]
- 12.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol. 1983;245(5 Pt 1):R720–9. doi: 10.1152/ajpregu.1983.245.5.R720. [DOI] [PubMed] [Google Scholar]
- 13.Dorn GW., 2nd The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–70. doi: 10.1161/HYPERTENSIONAHA.106.079426. [DOI] [PubMed] [Google Scholar]
- 14.Schannwell CM, Schoebel FC, Zimmermann T, et al. Left ventricular diastolic function in normal pregnancy. A prospective study using M-mode echocardiography and Doppler echocardiography. Dtsch Med Wochenschr. 2000;125:1069–73. doi: 10.1055/s-2000-7356. [DOI] [PubMed] [Google Scholar]
- 15.Schannwell CM, Zimmermann T, Schneppenheim M, et al. Left ventricular hypertrophy and diastolic dysfunction in healthy pregnant women. Cardiology. 2002;97:73–8. doi: 10.1159/000057675. [DOI] [PubMed] [Google Scholar]
- 16.Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv. 1994;49(12 Suppl):S1–14. doi: 10.1097/00006254-199412011-00001. [DOI] [PubMed] [Google Scholar]
- 17.Koch E, Bogado M, Araya F, et al. Impact of parity on anthropometric measures of obesity controlling by multiple confounders: a cross-sectional study in Chilean women. J Epidemiol Community Health. 2008;62:461–70. doi: 10.1136/jech.2007.062240. [DOI] [PubMed] [Google Scholar]
- 18.Smith DE, Lewis CE, Caveny JL, et al. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–51. [PubMed] [Google Scholar]
- 19.Lewis CE, Funkhouser E, Raczynski JM, et al. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144:247–54. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson WK, Asao K, Brancati F, et al. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2006;29:2349–54. doi: 10.2337/dc06-0825. [DOI] [PubMed] [Google Scholar]
- 21.Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 22.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 23.Ingelsson E, Sundstrom J, Arnlov J, et al. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–56. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 25.Germain P, Roul G, Kastler B, et al. Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J. 1992;13:1011–9. doi: 10.1093/oxfordjournals.eurheartj.a060307. [DOI] [PubMed] [Google Scholar]
- 26.Keenan NG, Pennell DJ. CMR of ventricular function. Echocardiography. 2007;24:185–93. doi: 10.1111/j.1540-8175.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 27.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29:2078–83. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 28.Irgens HU, Reisaeter L, Irgens LM, et al. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–7. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodie VA, Freeman DJ, Sattar N, et al. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ego A, Subtil D, Grange G, et al. Should parity be included in customised fetal weight standards for identifying small-for-gestational-age babies? Results from a French multicentre study. BJOG. 2008;115:1256–64. doi: 10.1111/j.1471-0528.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21:103–13. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz GS, Lapinski RH, Wein R, et al. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992;135:965–73. doi: 10.1093/oxfordjournals.aje.a116408. [DOI] [PubMed] [Google Scholar]
- 34.Ingelsson E, Lind L, Arnlov J, et al. Socioeconomic factors as predictors of incident heart failure. J Card Fail. 2006;12:540–5. doi: 10.1016/j.cardfail.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Ness RB, Cobb J, Harris T, et al. Does number of children increase the rate of coronary heart disease in men? Epidemiology. 1995;6:442–5. doi: 10.1097/00001648-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Skilton MR, Serusclat A, Begg LM, et al. Parity and carotid atherosclerosis in men and women: insights into the roles of childbearing and child-rearing. Stroke. 2009;40:1152–7. doi: 10.1161/STROKEAHA.108.535807. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg ML, Park Y, Hollenbeck AR, et al. Fatherhood and the risk of cardiovascular mortality in the NIH-AARP Diet and Health Study. Hum Reprod. 2011;26:3479–85. doi: 10.1093/humrep/der305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 39.Jain A, McClelland RL, Polak JF, et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the Multi-Ethnic Study of Atherosclerosis (MESA) Circ Cardiovasc Imaging. 2011;4:8–15. doi: 10.1161/CIRCIMAGING.110.959403. [DOI] [PMC free article] [PubMed] [Google Scholar]