Abstract
Targeted genetic studies can facilitate phenotypic analyses and provide important insights into development and other complex processes. The SWI2/SNF2 DNA-dependent ATPase Domino (Dom) of Drosophila melanogaster, a component of the Tip60 acetyltransferase complex, has been associated with a wide spectrum of cellular processes at multiple developmental stages. These include hematopoiesis, cell proliferation, homeotic gene regulation, histone exchange during DNA repair, and Notch signaling. To explore the wider gene network associated with Dom action, we used RNAi directed against domino (dom) to mediate loss-of-function at the wing margin, a tissue that is readily scored for phenotypic changes. Dom RNAi driven through GAL4-UAS elicited dominant wing nicking that responded phenotypically to the dose of dom and other loci known to function with dom. We screened for phenotypic modifiers of this wing phenotype among 2500 transpositions of the EP P element and found both enhancers and suppressors. Several classes of modifier were obtained, including those encoding transcription factors, RNA regulatory proteins, and factors that regulate cell growth, proliferation and autophagy, a lysosomal degradation pathway that affects cell growth under conditions of starvation and stress. Our analysis is consistent with prior studies, suggesting that Dom acts pleiotropically as a positive effector of Notch signaling and a repressor of proliferation. This genetic system should facilitate screens for additional loci associated with Dom function, and complement biochemical approaches to their regulatory activity.
Keywords: chromatin, gene regulation, cell proliferation, autophagy, Notch signaling
Genetic interaction screening is a powerful tool for the analysis of complex processes. In cases where a gene acts throughout development, targeting loss of function to a later stage can abrogate early lethality and allow application of effective genetic strategies. In Drosophila, targeted genetic screening has been used to study the genetics of key developmental pathways operating in specific tissues at particular times (Zhong and Yedvobnick 2009). Such screening often uses the yeast GAL4-UAS system to express high levels of normal or mutated versions of genes in a defined fashion. Phenotypes derived from such constructs allow screens for modifiers, thereby expanding the known set of genes that contribute to a pathway (Brand and Perrimon 1993; Rorth 1996). For example, by targeting the wing margin and eye, these methods were used to identify novel genes that contribute to the Notch pathway, a major signaling system of metazoans (Hall et al. 2004; Alexander et al. 2006; Kankel et al. 2007; Shalaby et al. 2009). These screens, among others (Mummery-Widmer et al. 2009, Yatim et al. 2012), have revealed a wide network of loci that impinge on Notch signaling at numerous levels. One identified locus named domino (dom), originally linked to hematopoiesis and homeotic gene repression (Braun et al. 1997, Ruhf et al. 2001), also interacts genetically with Notch during wing margin formation (Hall et al. 2004). Subsequent studies further elaborated dom’s role in Notch pathway regulation (Eissenberg et al. 2005; Gause et al. 2006) and other processes including exchange of phosphorylated histone H2Av as part of the Tip60 acetyltransferase complex (Kusch et al. 2004), germline and somatic stem cell self-renewal (Xi and Xie 2005), and repression of E2F responsive loci (Lu et al. 2007). The dom gene sequence predicts two major proteins of the SWI2/SNF2 class of DNA-dependent ATPases, implicating dom in gene regulation at the level of chromatin modification/nucleosome remodeling (Ruhf et al. 2001). Dom proteins are widely expressed in embryos and imaginal discs, and the sequence is highly conserved (Ruhf et al. 2001; Kusch et al. 2004; Eissenberg et al. 2005). Moreover, alleles of dom result in larval or pupal lethality (Ruhf et al. 2001). We reasoned that a multifunctional chromatin remodeling protein that acts at the wing margin would be a practical choice for a targeted genetic modifier screen. Genetic changes that interact with a dom-associated wing phenotype should allow a sensitive screen for genes functioning with, or regulated by Dom. Therefore, we constructed a strain that expresses RNAi directed against dom transcripts at the wing margin. This strain shows a dominant wing phenotype that can be modified through changes in expression of loci known to interact with dom. Using this strain in a transposon-based genetic screen we obtained several classes of modifier, including those encoding transcription factors, RNA-binding proteins, and several proteins associated with growth regulation and autophagy. These modifiers link Dom function to cell proliferation, as suggested by others (Braun et al. 1997; Ruhf et al. 2001; Lu et al. 2007). Thus, this preliminary screen for modifiers of a dom wing phenotype indicates that it is a reliable method to further dissect dom function.
Materials and Methods
Drosophila strains
Strains were obtained from the following laboratories: C96-GAL4 (G. Boulianne, University of Toronto, Toronto, ON, Canada), blk-GAL4 l (M. Hoffman, University of Wisconsin, Madison, WI), vg-GAL4, pnr-GAL4, nd1, UAS-N (activated Notch, weakly expressing strain, S. Artavanis-Tsakonas, Harvard Medical School, Boston, MA), UAS-Wdb (J. Jia, University of Kentucky, Lexington, Kentucky), pumilio01688 (H. Lin, Yale University, New Haven, CT), atg1 = Unc513 and UAS-Unc51(8) (T. Tomoda, Beckman Research Institute, Duarte, CA), ptc-GAL4, DeltaBX9 (M. Muskavitch, Boston College, Chestnut Hill, MA), mamN2G (J. Campos-Ortega, deceased), dom RNAi (3) and UAS-DomB (M. Ruhf, University of Cincinnati, Cincinnati, OH), domEP2371 (Exelixis, San Francisco, CA), cyclin EAR95/Cy and UAS-Rbf-280 (K. Moberg, Emory University, Atlanta, GA). C96-MamH was described previously (Helms et al. 1999).
The following strains were obtained from the Bloomington (BL) Stock Center: P{EP}pebEP55 (5358), y1 w1; Ki1 P{Δ2-3}99B (4368), y1 w67c23; P{lacW}dom1/CyO (10767), y1 w*; dom3/SM6a (9260), y1 w*; dom9/CyO, y+ (9261), snoe1/FM7a (8745), y1 v1; P{TRiP.JF01502}attP2 dom (31054), y1 w67c23; P{EPgy2}lilli11976 (20719), EcRM554fs/SM6b (4894), lilli[A17-2] cn1 bw1/CyO (5726), y1 w67c23; P{EPgy2}emcEY01657/TM3, Sb1 Ser1 (20124); emc[D] rho[ve-1] rs[2] st[1] bul[D]/TM1(1032), w[*]; lola[ORE119]/CyO (28284), y1 w*; P{UAST-YFP.RabX1.T19N}Pabp201/SM5 (9838), y1 w67c23; P{EPgy2}Tudor-SNEY07875 (17412), w1118; Lk62/TM6B, Tb1 (8707), y1 w*; P{UASp-YFP.Rab30.T21N}PP2A wdb07 (9813), y1 w*; P{UASp-YFP.Rab9Fb.D66L}PP2A wdb19/TM3, Sb1 (9844), y1 v1; P{TRiP.JF02897}attP2 atg6 (28060), y1 v1; P{TRiP.JF02787}attP2 atg7 (27707), y1 v1; P{TRiP.JF02895}attP2 e* atg8A /TM3, Sb1 (28989), y1 v1; P{TRiP.JF02706}attP2 atg8B (27554), y1 v1; P{TRiP.JF02704}attP2 atg12 (27552), y1 v1; P{TRiP.JF02891}attP2 atg9 (28055), y1 v1; P{TRiP.JF02703}attP2 atg5 (27551), y1 v1; P{TRiP.JF03003}attP2 atg4 (28367), y1 v1; P{TRiP.JF02786}attP2 atg2 (27706), y1 v1; P{TRiP.JF02898}attP2 atg18 (28061), y1 v1; P{TRiP.HM05150}attP2 PP2A wdb (28939), tara1/TM3 Sb (6403), y1 v1; P{TRiP.JF03141}attP2/TM3, Sb1 PP2A tws (28714), y1 sc* v1; P{TRiP.HM05256}attP2 PP2A wrd (30512), y1 v1; P{TRiP.JF02805}attP2 PP2A mts (27723), y1 v1; P{TRiP.JF03316}attP2 PP2A 29B (29384), y1 v1; P{TRiP.HM04075}attP2 CK1 (31763), and w*; P{UAS-dco.K}4 (26274). cn1 E(Pc)1 bw1/SM5 (3056), y1 w1118; P{lacW}MRG15j6A3/TM3, Sb1 (10290), y1; P{SUPor-P}Nipped-AKG10162/CyO (16514), y1 w67c23; P{EPgy2}reptEY12756/TM3, Sb1 Ser1 (21384), y1 v1; P{TRiP.HM05049}attP2 Tip60 (28563).
Construction of UAS-domR plasmid
The forward primer GGGAGTCCGATGGTGAGTTA and reverse primer ACTTGCGCTCATTCATTGTG were used to amplify a 1057-bp segment of genomic DNA from the dom locus (genomic position 17215713–17216769). This segment spanned the 3′ end of exon 5, intron 5, and most of exon 6 (Ruhf et al. 2001) and was chosen to minimize nucleotide similarities to other genomic regions. The PCR product was cloned into pCR2.1 (Invitrogen) and then subcloned into SympUAST-w (Giordano et al. 2002). The resulting plasmid UAS-domR was transformed into the germline by Genetic Services, Inc. Chromosome 3 inserts of UAS-domR and C96-GAL4 were recombined to create the C96-domR/TM3 Sb strain. The C96-GAL4 driver expresses across the dorsal-ventral wing margin of third-instar larvae (Gustafson and Boulianne 1996; Helms et al. 1999). The C96-domR chromosome is associated with a dominant but partially penetrant wing nicking phenotype described in Results.
Generation of EP transpositions and scoring for modifiers
Mobilization of the X chromosome-linked w+EP55 element via the transposase P[ry+ Δ2-3] Ki1 (99B) was described in detail previously (Kazemi-Esfarjani and Benzer 2000; Alexander et al. 2006). After introduction of the transposase into w+ EP males, 3 males were mated in a vial with 5 white-eyed (w1118) females, which are homozygous for w− on the X chromosomes. Any male offspring from this cross exhibiting red eyes must have a transposition of the element from the X chromosome to an autosome or rarely to the Y chromosome. Non Ki flies with nonmottled red eyes were selected to eliminate the transposase and stabilize the insertion. Only a single transposition male from each vial was analyzed. Each male carrying a transposition was mated to C96-domR/TM3 Sb females, and progeny were scored for enhancement or suppression of the wing nicking phenotype. Modifiers were retested by crossing once again to C96-domR, then outcrossed to a balancer strain, and made homozygous or balanced in the case of recessive lethals. A subset of modifiers was mated to candidate mutations, including the Notch pathway loci mastermind and Delta. In some cases, modifiers were also mated with deficiencies covering P element hotspots to eliminate multiple alleles. Modifiers were also crossed with the C96-GAL4 strain to rule out wing effects that were independent of dom RNAi. All modifiers described here exhibited no phenotypes with this control cross. Likewise, we did not observe significant interaction of the modifiers with a strain exhibiting a GAL4-driven eye phenotype, suggesting that the modifiers were not selected based on a strictly GAL4-dependent effect.
EP modifiers were scored as enhancers or suppressors of C96-domR based on the penetrance of wing nicking relative to that of the w1118 control crosses, rather than the severity of wing blade loss or nicking. Crosses of C96-domR to w1118 produce a 57% penetrant phenotype (57% of wings exhibit at least one anterior margin nick). Each EP test against C96-domR included w1118 controls, and wing nicking percentages were normalized to the 57% control value between experiments. For the twelve EP lines described here, the differences in penetrance between C96-domR and that of w1118 were highly significant (P < 0.001, chi-square test) (Table 2).
Table 2. Characterization of modifier alleles.
| EP# | Gene | Insertion Site (position) | Mutation | C96-domR | N Wings Scored | C96-MamH | N Wings Scored |
|---|---|---|---|---|---|---|---|
| 425 | tara | Intron (12075314) | LOF | S (5%) | 414 | S+ | 92 |
| 558 | pabp2 | Exon (4019484) | LOF | E (88%) | 312 | E | 100 |
| 573 | Lk6 | Intron (7585856) | LOF | E (68%) | 1023 | S | 206 |
| 593 | Tudor-SN | Upstream (264378) | GOF | E (74%) | 426 | ne | 288 |
| 939 | EcR | Intron (2007989) | LOF | E (88%) | 336 | E | 110 |
| 1000 | lola | Not Determined | LOF | E (91%) | 251 | E+ | 100 |
| 1037 | wdb | Intron (23402526) | GOF | S (20%) | 368 | S+ | 98 |
| 1202 | atg1 | Exon (12798085) | LOF | S (0%) | 338 | S+ | 90 |
| 1538 | lola | Intron (6421948) | LOF | E (91%) | 330 | E+ | 86 |
| 1561 | emc | Upstream (749363) | GOF | E (85%) | 240 | S+ | 130 |
| 1630 | lilli | Intron (2900668) | GOF | E (81%) | 378 | E+ | 194 |
| 1646 | pum | Intron (4983814) | LOF | E (70%) | 250 | E+ | 130 |
The percentage of wing nicking is shown for crosses of C96-domR to EP modifiers in column labeled C96-domR. All wing nicking differences were highly significant (P < 0.001, chi-square test relative to w1118 control; 57%, Table 1). All EP tests included w1118 controls, and wing nicking percentages were normalized to 57% control value between experiments. EP 1000 was determined to be an allele of lola through a complementation test. The C96-MamH genotype produces 100% wing nicking when outcrossed to w1118 control. Among this control class we determined the percent of wings with weak, moderate, and strong effects including extent of nicks and blade loss. We then compared the distribution of severity in the EP cross progeny to determine if there was suppression (S), strong suppression (S+), enhancement (E), strong enhancement (E+) or no effect (ne); these data are presented in column labeled C96-MamH. GOF, gain of function; LOF, loss of function.
Generation of domEP2371 revertant
w+domEP2371 is a recessive lethal, P element-induced allele of dom that does not complement the dom1 or dom3 allele. w+domEP2371 was mobilized with transposase and w− revertants isolated. Each revertant was assayed for restoration of dom function through crosses to dom1 and dom3. w−domEP2371Rev complemented both alleles.
Identification of genomic sequence flanking EPs through inverse PCR
Procedures for the isolation of genomic DNA, restriction digestion, ligation, and inverse PCR were derived from the BDGP Website (http://www.fruitfly.org/about/methods/inverse.pcr.html). PCR products were purified with a QIAquick column (Qiagen), quantified on gels, and sequenced commercially by Macrogen. Sequence data were analyzed via FlyBlast (http://flybase.net/blast), and the EP UAS-driver oriented to genomic sequences by using the FlyBase Genome Browser.
Antibody staining of third instar larval wing discs
The following protocol (Brennan et al. 1998) was followed for the fixation, staining, and washing of imaginal discs. Discs were dissected in 0.1 M sodium phosphate buffer (pH 7.4), fixed (2% paraformaldehyde, 75 mM lysine, 0.25% sodium periodate, 50 mM sodium phosphate pH 7.4) for 45 min, and washed (0.1 M sodium phosphate, 0.1% Triton X-100, pH 7.4). They were blocked in wash buffer containing 10% normal goat serum (NGS) and incubated with primary antiserum for 60 min at 37°. The discs were then washed twice, blocked, and incubated with secondary antibodies overnight at 4°. After three final washes, the discs were mounted in Slowfade (Molecular Probes). Images were obtained using an MRC 1024 model confocal microscope (Bio-Rad) and assembled with Photoshop software (Adobe). The following antibodies and dilutions were used: Wg (1:20 dilution, mouse monoclonal; Developmental Studies Hybridoma Bank), Cut (1:20 dilution, mouse monoclonal; Developmental Studies Hybridoma Bank). Alexa 488 secondary antibodies were obtained from Invitrogen and used at a dilution of 1:200). Discs derived from dom9 and dom3 larvae were selected opposite a green fluorescent protein (GFP)-marked balancer.
Mounting of wings
Wings were dehydrated in isopropanol, mounted in Euparol, and photographed under a dissecting microscope (Hall et al. 2004).
Results
Generation of a domino RNAi-based dominant wing phenotype
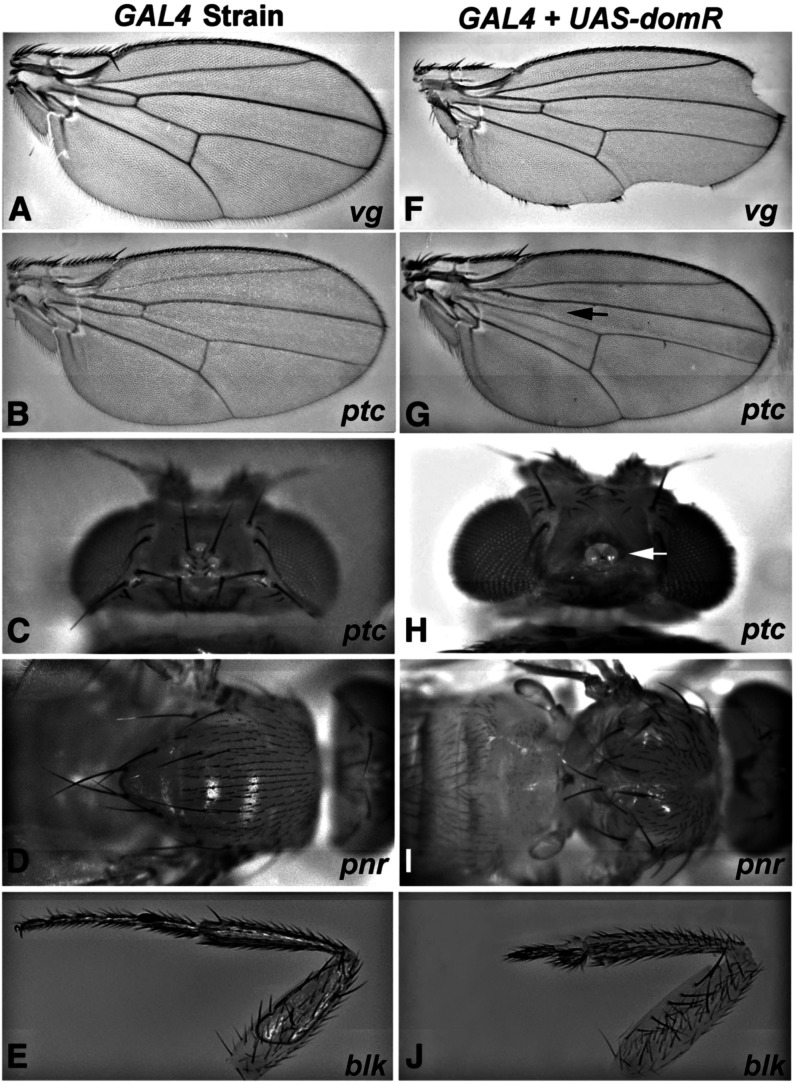
We cloned a segment of dom (Ruhf et al. 2001) into a modified pUAST vector (sympUAST) to allow symmetrical transcription of sequences cloned in between two sets of UAS (Giordano et al. 2002). The targeted dom sequence (see Materials and Methods ) is common to both major transcripts (Ruhf et al. 2001) and was selected to minimize matches to other genomic sites, as well as CAN repeats (Ma et al. 2006). We crossed transformants of the sympUAST construct (domR) with a panel of GAL4 lines that drive expression in various imaginal tissues. As shown in Figure 1, the domR construct elicits prominent phenotypic effects when driven in the wing, head, notum, and legs (Figure 1, F–J) compared to the controls (Figure 1, A–E) expressing GAL4 alone. Wing nicking via vg-GAL4 is consistent with our identification of dom as a Notch modifier (Hall et al. 2004). These data are also consistent with the reported expression of dom in imaginal discs (Ruhf et al. 2001).
Figure 1.
GAL4-driven dom-RNAi construct produces adult phenotypes. (A–E) Control tissues derived from crosses of GAL4 lines to w1118; (F–J) results when the same GAL4 lines were crossed with the UAS-domR RNAi construct. (A and F) vg-GAL4 wing nicks; (B and G) ptc-GAL4 loss of anterior crossvein (arrow); (C and H) ptc-GAL4 loss of head bristles and fusion of ocelli (arrow); (D and I) pnr-GAL4 notum fusion incomplete; (E and J) blk-GAL4 loss of distal leg segments.
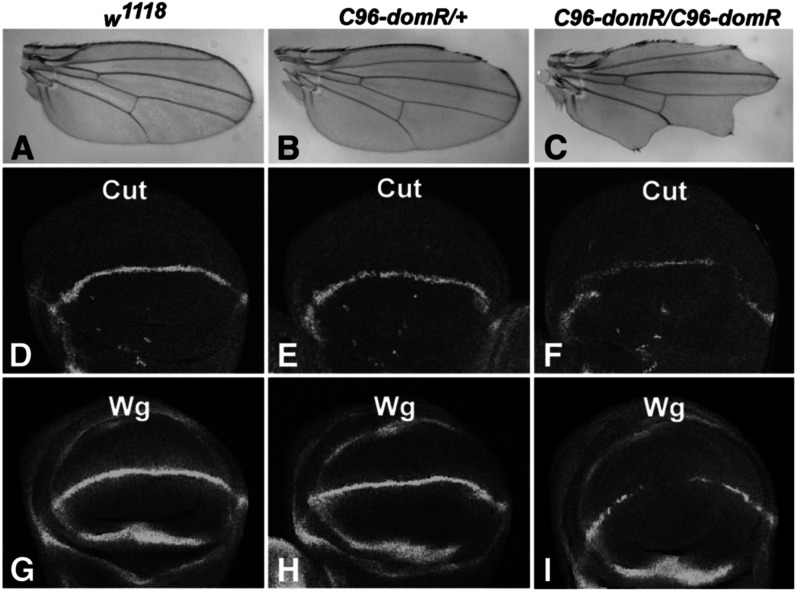
We also observed domR wing effects when driven with C96-GAL4 (Helms et al. 1999), though to a lesser extent than with vg-GAL4. C96-GAL4 and domR transgenes were recombined on chromosome 3 (C96-domR) to perform genetic tests (Figure 2). The predominant effects in C96-domR heterozygotes occur along the anterior margin, whereas effects are widespread in homozygotes (Figure 2, B and C). We assayed the wing margin protein markers Cut and Wg (Helms et al. 1999) in wing discs and observed minor stain depressions in heterozygotes (Figure 2, E and H) and substantial loss of staining in homozygotes (Figure 2, F and I).
Figure 2.
dom RNAi expression affects wing margin formation. Panels show wings or wing imaginal discs stained for Cut or Wg from w1118 (A, D, and G); C96-domR heterozygotes (B, E, and H); and C96-domR homozygotes (C, F, and I). C96-domR is a recombinant chromosome containing both C96-GAL4 and UAS-domR RNAi transgenes.
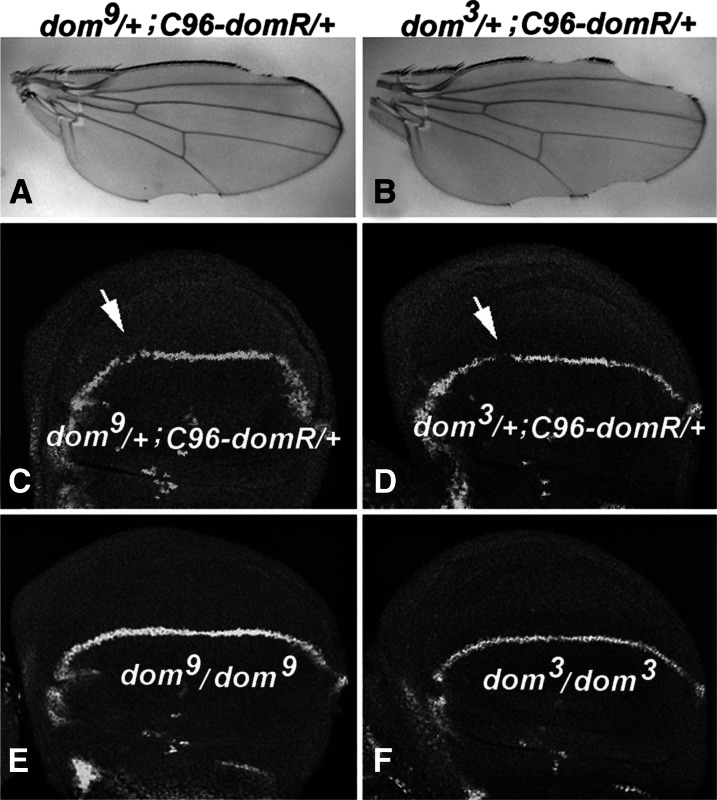
We also measured the effectiveness of domR relative to that of canonical loss-of-function (LOF) dom alleles in down-regulating the Cut protein. The weak dom9 allele is less severe than the moderate allele dom (Ruhf et al. 2001; and see Table 1), and neither allele produces a wing phenotype as a heterozygote after outcross to w1118 (data not shown). As expected, transheterozygotes of C96-domR with dom9 show a weaker phenotype than transheterozygotes of C96-domR with dom3 (Figure 3, A and B). Staining of wing discs for these transheterozygotes reveals depressions in Cut expression, especially in sections of the future anterior margin (Figure 3, C and D). Homozygotes for dom9 and dom3 survive as larvae (Figure 3, E and F) and show wing margin levels of Cut that are higher than the transheterozygotes of C96-domR. Thus, one copy of the C96-domR construct appears to diminish dom function to a greater extent than these canonical alleles of dom, using Cut expression as the measure.
Table 1. Validation C96-domR RNAi phenotype.
| Tester Genotype | % of Nicked Wings | N Wings Scored |
|---|---|---|
| w1118 | 57% | 1756 |
| dom1 | 84% | 492 |
| dom3 | 98% | 140 |
| dom9 | 72% | 116 |
| dom2371 | 81% | 296 |
| dom2371rev | 59% | 318 |
| UAS-DomB | 20% | 644 |
| dom RNAi 3 | 79% | 1139 |
| dom RNAi TRiP | 76% | 939 |
Tester genotypes are transheterozygous for C96-domR chromosome. The dom2371rev revertant combined with C96-domR does not show wing nicking at a significantly higher frequency (59%) than w1118 control combined with C96-domR (57%; P = 0.63, chi-square test). All remaining genotypes were highly significant (P < 0.001).
Figure 3.
Effects of domR relative to canonical dom alleles. (A and C) Adult wing and wing disc derived from dom9/+; C96-domR/+ heterozygotes. (B and D) Same, derived from dom3/+; C96-domR/+ heterozygotes. Arrows show future anterior wing margin with gaps in staining. (E and F) Wing disc tissues from dom9/dom9 homozygotes and dom3/dom3 homozygotes, respectively. Wing discs were stained for Cut expression.
Validation of dom RNAi Effects
To validate domR RNAi effects (Ma et al. 2006; Perrimon and Mathey-Prevot 2007) we performed a series of genetic tests (Table 1). The table shows the percent nicked wings in genotypes heterozygous for the C96-domR construct and various test chromosomes, including a w1118 control. C96-domR/w1118 heterozygotes exhibit a 57% penetrant wing nicking effect (57% of wings show at least one anterior nick). When transheterozygous with strong or moderate LOF alleles for dom the penetrance rises, for example, dom1 (84%) and dom3 (98%), whereas less enhancement is evident with a weak allele, for example, dom9 (72%), that derives from the identical genetic background as dom1 and dom3. The P element insertion allele dom2371 also exhibits strong enhancement (81%), whereas a dom+ revertant chromosome (dom2371Rev) eliminates the enhancement (59%). Moreover, when the normal Dom B (UAS-DomB) protein, which is highly expressed in imaginal discs (Ruhf et al. 2001), is driven along with C96-domR, significant rescue is observed, as the penetrance of wing nicking drops to 20%. We also examined two independent dom RNAi constructs for enhancement of the C96-domR phenotype (dom RNAi 3 and dom RNAi TRiP), and both constructs produced significant enhancement. These data demonstrate that the C96-domR RNAi wing phenotype responds as expected to loss- or gain-of-function (GOF) for dom, verifying its effect on dom function.
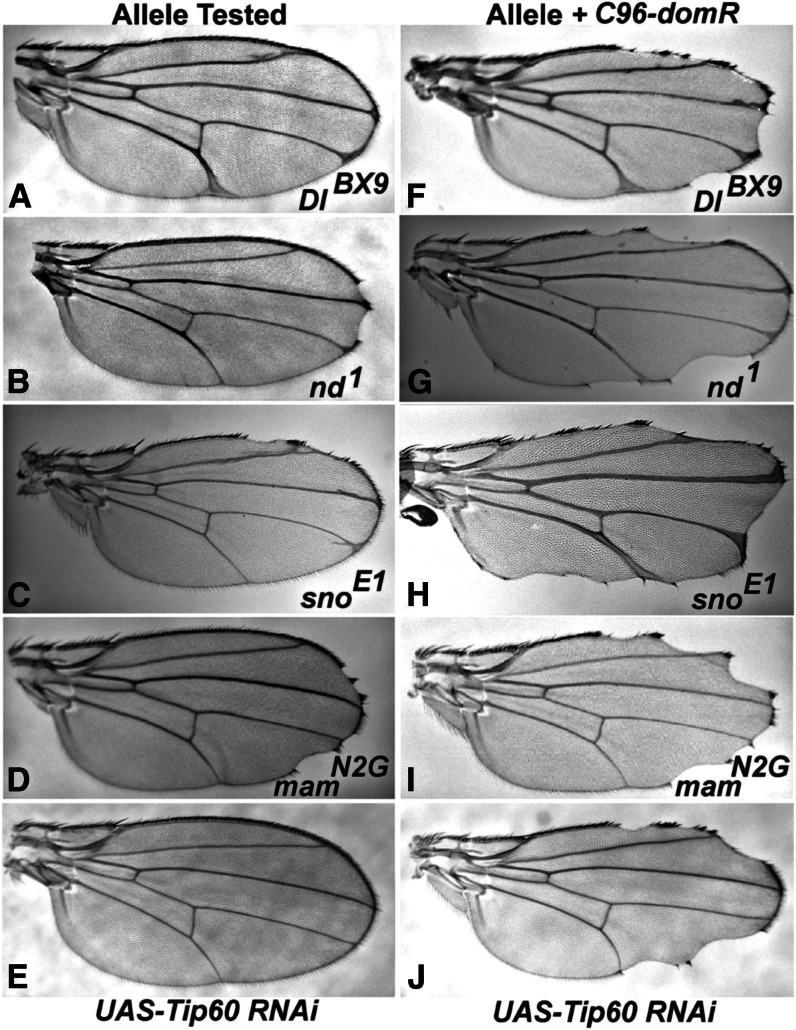
We also tested C96-domR for other predicted genetic interactions. Prior studies (Hall et al. 2004; Eissenberg et al. 2005) and Cut and Wg expression data (Figures 2 and 3) predict an effect on Notch. Likewise, an interaction is predicted for LOF in loci encoding components of the Tip60 acetyltransferase complex, because Dom is a member of that complex (Kusch et al. 2004). Alleles of canonical Notch pathway loci (Delta, Notch, and mastermind) and strawberry notch (Figure 4, F–I) exhibit strong phenotypic enhancement of C96-domR relative to that of wings from outcrosses to w1118 (Figure 4, A–D). Similarly, LOF for Tip60 generated through RNAi exhibits no phenotype (Figure 4E). However, Tip60 RNAi coexpression leads to strong enhancement of the C96-domR wing margin phenotype (Figure 4J). We tested mutations in several other loci that encode Tip60 complex components (Kusch et al. 2004) and observed a significant increase in wing nicking penetrance (P < 0.01) for alleles of E(Pc), MRG15, TRA1, and rept. Although the effects were not as strong as for Tip60, these data further validate the domR phenotype and also demonstrate that Tip60 complex components function at the wing margin.
Figure 4.
dom RNAi expression modifies Notch pathway and Tip60 mutation phenotypes. (A–D) Wings from heterozygotes or hemizygotes for alleles of the Notch pathway loci. (A) Delta (DlBX9/+); (B) notchoid (nd1/Y); (C) strawberry notch (snoE1/Y); and (D) mastermind (mamN2G/+), each exhibits minor wing phenotypes. (F–I) When these genotypes were combined with C96-domR, the phenotypes exhibited significant enhancement: (F) DlBX9/C96-domR; (G) nd1/Y; C96-domR/+; (H) snoE1 /Y; C96-domR/+; and (I) mamN2G/+; C96-domR/+. (E) Wings from flies expressing UAS-Tip60 RNAi across the margin under C96-GAL4 regulation exhibit a wild-type phenotype. (J) When the UAS-Tip60 RNAi construct is combined with C96-domR, very strong enhancement of the C96-domR wing phenotype is apparent.
Screen for dom modifiers
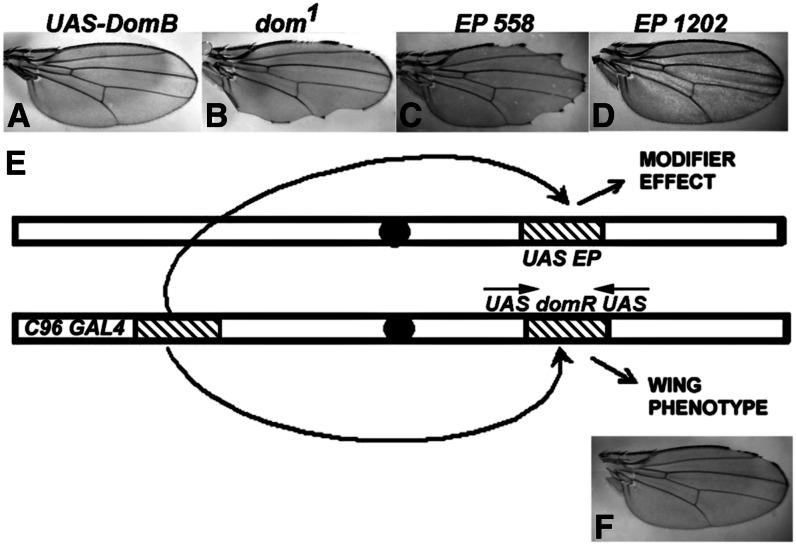
C96-domR heterozygosity creates a dominant but hypomorphic condition appropriate for genetic screening (Figure 2). We performed a screen for modifiers of this phenotype through mobilization of EP, a P transposon that creates both overexpression and LOF alleles (Rorth 1996; Thibault et al. 2004). EP elements were mobilized, and each insertion was tested for phenotypic effects when combined with C96-domR. The C96-GAL4 element within C96-domR drives both dom RNAi and the sequence downstream of the EP insertion, potentially creating a modifier effect (Figure 5). For example, the wing phenotype of C96-domR/w1118 (Figure 5F) can be either suppressed by overexpression of a normal Dom product (Figure 5A) or enhanced by a dom LOF allele (Figure 5B). Thus, the C96-domR wing phenotype allows detection of enhancers and suppressors. Figure 5, C and D, shows two modifiers from the screen, an enhancer (EP558) and a suppressor (EP1202).
Figure 5.
dom modifier screen. (E) shows the recombinant chromosome C96-domR containing the C96-GAL4 and UAS-domR transgenes. It produces the weak wing nicking phenotype shown in (F; penetrance of ∼57%). (A and B) Controls demonstrating that the wing phenotype is affected by changes in the level of wild-type Dom expression, either through GAL4 coexpression of a wild-type Dom construct [UAS-DomB (A)] or through a LOF allele [dom1 (B)], as described in the text. The EP screen involves testing individual EP insertion chromosomes as transheterozygotes with C96-domR (E). In these genotypes, C96-GAL4 can drive both UAS-domR and a random sequence if the EP insertion is oriented appropriately. The EP element also generates LOF alleles. Wing modifications, such as enhancement [EP558 (C), 88% penetrance and increased severity] or suppression [EP1202 (D), 0% penetrance, total suppression] are scored.
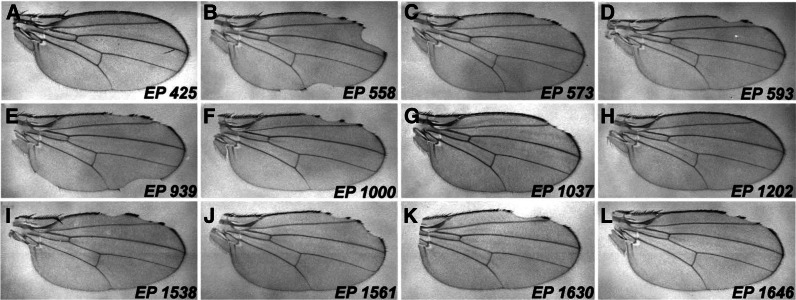
We tested 2500 transpositions and focused on 12, 9 enhancers and 3 suppressors (Table 2). Several additional modifiers were identified through complementation tests as canonical Notch pathway components and not characterized further. None of the EP strains exhibits a wing phenotype alone or when crossed with C96-GAL4. This demonstrates that phenotypes derive from a synergistic interaction due to expression of dom RNAi and consequent loss of dom function. Inverse PCR and DNA sequencing were performed to identify the most proximal locus and determine the EP orientation relative to the coding region. Predicted overexpression (GOF) or insertional knockout (LOF) effects are shown in Table 2. One suppressor and three enhancers are oriented for a GOF effect. Table 2 lists these modifiers along with the affected loci, sites of insertion, and effects on the penetrance of the C96-domR phenotype. We validated the genetic interactions of C96-domR with independent alleles of the targeted loci. These alleles were either GOF or LOF, matching the predicted nature of the EP modifier. As shown in Table 3, C96-domR exhibits parallel genetic interactions with these strains and the original EPs. Figure 6 shows representative wings from C96-domR as transheterozygotes with the 12 EP insertions.
Table 3. Corroboration of EP insertion alleles.
| EP# | Corroborating Allele | C96-domR (% of Nicking) | N Wings Scored |
|---|---|---|---|
| 425 | tara1 | S (6%) | 300 |
| 558 | pabp201 | E (96%) | 361 |
| 573 | LK62 | E (95%) | 594 |
| 593 | P(EPg42)Tudor-SNEy07875 | E (79%) | 736 |
| 939 | EcRM554fs | E (81%) | 144 |
| 1000 | lolaORE119 | E (97%) | 114 |
| 1037 | UAS-Wdb | S (48%) | 620 |
| 1202 | unc513 (atg1) | S (36%) | 1126 |
| 1538 | lolaORE119 | E (97%) | 114 |
| 1561 | P{EPgy2}emcEY01657 | E (83%) | 262 |
| 1630 | P{EPgy2}lilliEY11976 | E (100%) | 283 |
| 1646 | 1(3)pum01688 | E (90%) | 392 |
The interaction between the corroborating allele for each EP and C96-domR is as described for Table 2. All wing nicking differences vs. w1118 control (57%) were highly significant (P < 0.001, chi-square test). For the GOF alleles EPs 1630, 1561, 593, and 1037, we corroborated with overexpression strains. E, enhancement; S, suppression.
Figure 6.
EP modifier effects on C96-domR wing phenotypes. Wing mounts were prepared from crosses of the C96-domR strain with the following EP modifiers: (A) EP 425 (tara); (B) EP 558 (pabp2); (C) EP 573 (Lk6); (D) EP 593 (Tudor-SN); (E) EP 939 (EcR); (F) EP 1000 (lola); (G) EP 1037 (wdb); (H) EP 1202 (atg1); (I) EP 1538 (lola); (J) EP 1561 (emc); (K) EP 1630 (lilli); (L) EP 1646 (pum). Note that the EPs are classified as enhancers or suppressors based upon the penetrance of wing nicking relative to that of w1118 control crosses to C96-domR, rather than the severity of wing blade loss or nicking (see Materials and Methods). These data are summarized in Table 2.
The 12 modifiers encode three classes of functions. The first two classes include transcription factors [taranis (tara), lilliputian (lilli), longitudinals lacking (lola), Ecdysone Receptor (EcR), and extramacrochaete (emc)] and proteins that regulate RNA function, including pumilio (pum), polyA binding protein 2 (pabp2), and Tudor-SN. Members of both classes have been associated with wing formation and/or Notch signaling previously (Kankel et al. 2007; Shalaby et al. 2009). The third class encodes proteins linked to cell growth and autophagy pathways, and includes the Ser/Thr protein phosphatase (PP2A) regulator widerborst (wdb; Vereshchagina et al. 2008), the protein kinase Lk6 (Arquier et al. 2005), and atg1, a regulator of autophagy, which is a lysosomal degradation pathway that affects cell growth (Zirin and Perrimon 2010).
Table 2 also summarizes interactions between the EP modifiers and a strain that drives truncated Mastermind across the wing margin (C96-MamH), which creates a Notch pathway LOF phenotype. Expression of truncated Mastermind has been shown to depress Notch signaling in multiple contexts including the wing margin (Helms et al. 1999), where it leads to a 100% penetrant nicking phenotype. Most of the EP strains exhibit similar interactions between C96-domR and C96-MamH as enhancers or suppressors, as expected. However, for the case of the C96-domR enhancer EP 1561 (emc) there was strong suppression of C96-MamH. This was validated with the canonical GOF allele (emcD), which also suppressed, and two LOF emc alleles, which enhanced (data not shown). The C96-domR enhancer EP 573 (Lk6) slightly suppressed C96-MamH. However, the Lk62 LOF allele enhanced C96-MamH, matching its effect on C96-domR. The C96-domR enhancer strain EP 593 (Tudor-SN) did not affect C96-MamH, possibly reflecting activity directed at RNAi processing (see Discussion).
dom wing phenotype is sensitive to changes in growth and autophagy loci
The atg1 (unc51) gene regulates autophagy and growth pathways in numerous organisms including Drosophila (Zirin and Perrimon 2010). The EP 1202 (atg1) modifier allele, as well as its corroborating unc513 allele (Toda et al. 2008), each behave as strong suppressors of C96-domR (Tables 2 and 3), indicating a link of dom function to these processes. Likewise, the EP 573 allele of Lk6 and the corroborating Lk62 allele (Tables 2 and 3) are both strong enhancers of the C96-domR phenotype. Lk6 is related to mammalian kinases that regulate cell growth and division (Arquier et al. 2005). Finally, EP 1037 and the corroborating GOF UAS-Wdb strain are suppressors of C96-domR (Tables 2 and 3). Wdb, a regulatory subunit of PP2A has been associated with both cell growth regulation and autophagy (Arquier et al. 2005, Vereshchagina et al. 2008; Banreti et al. 2012). The isolation of three modifiers associated with these processes suggested that loss of dom function may enrich for this class of loci.
Table 4 shows data from crosses of C96-domR with strains expressing RNAi directed against 10 different autophagy pathway loci, as well as one strain that overexpresses the normal atg1 product. Seven of the 10 assayed atg genes enhance the C96-domR wing phenotype when their function is depressed, indicating that normal autophagy activity can limit wing margin loss derived from depressed dom function (atg6, atg7, atg8A, atg8B, atg12, atg9, and atg5). Three RNAi strains, atg2, atg4 , and atg18 , do not show a significant effect, and these loci encode various functions within the autophagy pathway (Chang and Neufeld 2010). It is possible that these strains do not effectively down-regulate their target loci or that, alternatively, there may be genetic redundancy for certain loci. The enhancement effect of multiple atg RNAi strains contrasts with the atg1 effect, where LOF was observed to suppress C96-domR (Tables 2 and 3). Moreover, overexpression of atg1 across the wing margin strongly enhances the wing phenotype (Table 4), consistent with the LOF suppression effect. The differential effects of atg1 vs. other atg genes likely reflect the additional roles of atg1 in translational efficiency and growth regulation (Lee et al. 2007), beyond its role in autophagy regulation (see Discussion).
Table 4. Additional genetic interactions of dom.
| Genotype | C96-domR (% of Nicking) | N Wings Scored |
|---|---|---|
| UAS- atg1 (Unc51) | E (86%) | 322 |
| atg6, TRiP | E (82%) | 2074 |
| atg7, TRiP | E (75%) | 1724 |
| atg8A, TRiP | E (73%) | 1645 |
| atg8B, TRiP | E (78%) | 2227 |
| atg12, TRiP | E (73%) | 2147 |
| atg9, TRiP | E (72%) | 1520 |
| atg5, TRiP | E (63%) | 1773 |
| atg4, TRiP | ne (58%) | 2049 |
| atg2, TRiP | ne (58%) | 1736 |
| atg18, TRiP | ne (55%) | 1845 |
| PP2A wdb7 Regulatory | E (70%) | 638 |
| PP2A wdb, TRiP Regulatory | E (91%) | 236 |
| PP2A tws, TRiP Regulatory | E (83%) | 1021 |
| PP2A wrd, TRiP Regulatory | E (68%) | 1748 |
| PP2A mts, TRiP Catalytica | E (100%) | 478 |
| PP2A 29B, TRiP Scaffolda | E (100%) | 350 |
| CK1, UAS-dcoK4 | E (90%) | 1028 |
| CK1, dco TRiP | S (40%) | 1390 |
Column labeled C96-domR shows the percentage of wing nicking from crosses of C96-domR to listed genotypes. All genotypes are UAS-regulated except for wdb7. The interaction between these genotypes and C96-domR is as described for Table 2. All wing nicking differences were highly significant (P < 0.001, chi-square test), except for those labeled ne (no effect). Regulatory, catalytic and scaffold indicate the encoded function within the PP2A phosphatase complex. E, enhancer; S, suppressor.
These strains exhibited wing nicking at high penetrance with control crosses to C96-GAL4; all other UAS strains showed normal wings in the control cross.
The EP 1037 and corroborating UAS-Wdb strains appear to suppress C96-domR through overexpression of Wdb, a regulatory subunit of the PP2A phosphatase (Tables 2 and 3). We examined other strains with LOF for either wdb or other regulatory, scaffold and catalytic subunits of the PP2A complex, and observed enhancement of C96-domR (Table 4). This is consistent with the suppression derived from Wdb overexpression. These data predict that a class of Ser/Thr kinases may act antagonistically to PP2A phosphatase. Casein kinase 1 (Ck1) overexpression strongly enhances the C96-domR wing phenotype, whereas LOF suppresses (Table 4). These effects are opposite to those derived from alterations in PP2A phosphatase. PP2A and CK1 have been shown to act antagonistically in other developmental contexts (Jia et al. 2009).
dom and its modifiers interact genetically with a proliferation-defective genotype
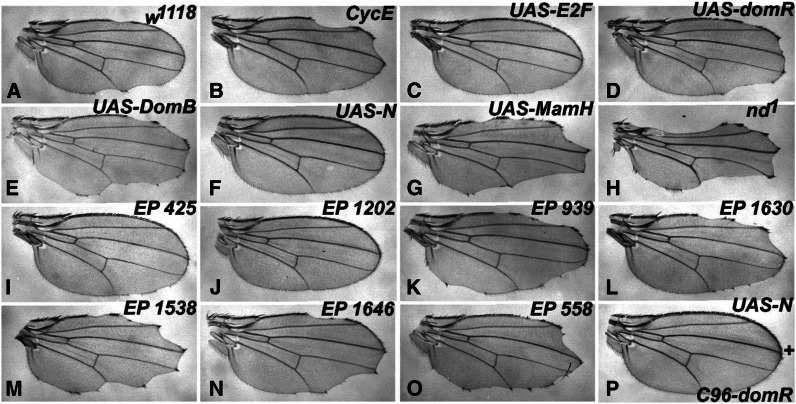
Dom has been implicated in the regulation of cell proliferation (Braun et al. 1997; Lu et al. 2007). Therefore, we generated a strain with a dominant proliferation-defective phenotype at the wing margin, and tested for genetic interaction with C96-domR and its modifiers. The test utilizes a mutated version of the Rbf protein (Rbf-280), where four Cdk phosphorylation sites have been inactivated. This results in a constitutively active form of Rbf that blocks growth and proliferation in wing tissue (Xin et al. 2002). We found that when UAS-Rbf280 is driven by C96-GAL4 at the margin (C96+Rbf280), it elicits a dominant, partially penetrant wing nicking phenotype and loss of a subset of anterior margin bristles (Figure 7A). As predicted for a proliferation defect, this phenotype is enhanced by a mutation in cycE and suppressed by overexpression of E2F (Figure 7, B and C). The combination of C96+Rbf280 with UAS-domR leads to enhanced penetrance and more severe loss of wing material (Figure 7D); enhancement was also observed with the canonical dom1 and dom3 alleles to a significant but lesser extent (data not shown).
Figure 7.
Interactions of the C96+Rbf280 proliferation-defective genotype. In the descriptions below, numbers in parentheses indicate percentage of nicked wings and number of wings scored (N). (A) (30%, N = 440) shows that a constitutively active form of Rbf (Rbf-280) driven across the wing margin via C96-GAL4 (UAS-Rbf-280 + C96-GAL4/+, referred to here as C96+Rbf280) creates a partially penetrant, dominant wing nicking phenotype as a heterozygote with w1118. (B–O) Wings transheterozygous with C96+Rbf280. (B) (64%, N = 120) shows an enhanced wing phenotype via combination with cycEAR95; (C) (18%, N = 86) shows partial rescue in combination with UAS-E2F. Both the UAS-domR RNAi transgene (D) (77%, N = 164) and the UAS-DomB transgene (E) (68%, N = 166) elicit strong enhancement. UAS-DomB did not elicit wing nicking in control crosses to C96-GAL4, data not shown (0%, N = 120). (F) (2%, N = 360) Nearly complete suppression of the wing phenotype by coexpression of an activated Notch construct, UAS-N, whereas depressed Notch signaling via UAS-MamH (G) (100%, N = 174) or the hemizygous viable nd1 allele (H) (100%, N = 274) strongly enhance. (I–O) C96+Rbf280 wings transheterozygous with EP modifiers from the screen: EP 425 tara (I) (10%, N = 124), EP 1202 atg1 (J) (2%, N = 56), EP 939 EcR (K) (92%, N = 52), EP 1630 lilli (L) (70%, N = 146), EP 1538 lola (M) (98%, N = 50), EP 1646 pum (N) (72%, N= 116), EP 558 pabp2 (O) (80%, N = 54). (P) (0%, N = 253) Complete suppression of the C96-domR wing nicking by coexpression of the activated Notch construct UAS-N. See Figure 2B legend for C96-domR wing phenotype. The penetrance of wing nicking in panels B–P is significantly different than w1118 control crosses (P < 0.01, chi-square test).
Paradoxically, we also observed C96+Rbf280 enhancement when Dom protein was overexpressed across the wing margin via the UAS-DomB transgene (Figure 7E). These results likely reflect pleiotropy of Dom function, because it is necessary for both Notch target expression (Figure 2) and repression of proliferation (Lu et al. 2007). C96-domR RNAi-mediated down regulation of Notch signaling at the wing margin is predicted to depress proliferation (Baonza and Garcia-Bellido 2000; Ferres-Marco et al. 2006) and thereby enhance the C96+Rbf280 phenotype. Consistent with this prediction, coexpression of activated Notch via UAS-N rescued nearly completely the C96+Rbf280 proliferation defect (Figure 7F); whereas depressions in Notch signaling strongly enhanced it (Figure 7, G and H). In contrast, Dom can also act as an inhibitor of cell proliferation through repression of E2F target genes (Lu et al. 2007), and overexpression via UAS-DomB may act primarily by enhancing the cell proliferation-defective phenotype of C96+Rbf280. The effect of dom LOF on Notch signaling is also evident by complete rescue of the C96-domR phenotype through simultaneous expression of UAS-N (Figure 7P).
Two of the C96-domR suppressors, EPs 425 (tara) and 1202 (atg1), were found to be strong suppressors of C96+Rbf280 (Figure 7, I and J). Likewise, several of the C96-domR enhancers were also found to strongly enhance C96+Rbf280, including EPs 939 (EcR), 1630 (lilli), 1538 (lola), 1646 (pum), and 558 (pabp2) (Figure 7, K–O). The remaining modifiers showed much weaker interactions (data not shown).
Discussion
We have described a screen for loci that interact with dom at the wing margin. Based on the intersection of dom with Notch signaling, we expected to identify a broad array of loci, and most of the dom modifiers interact similarly with a strain defective in Notch signaling. Additionally, based on the association of both Notch and dom with cell proliferation (Braun et al. 1997; Baonza and Garcia-Bellido 2000; Ferres-Marco et al. 2006; Lu et al. 2007), it is not surprising that most of the modifiers have been linked to cell growth and division, and exhibit genetic interaction with a proliferation-defective genotype. Moreover, as LOF for dom has also been associated with cell death (Braun et al. 1997), it is possible that this process also contributes to the phenotypes we describe here. Wing margin staining for key markers associated with cell death vs. cell cycling will be necessary to establish the basis for these effects. Nevertheless, the C96-domR phenotype appears pleiotropic, derived from effects on Notch signaling, growth, proliferation and likely other factors operating at the wing margin.
Several modifiers encode transcription factors, such as lilli, tara, and emc, which were identified in a prior screen targeted to the wing (Kankel et al. 2007). Lilli, a protein of the fragile X/Burkitt’s lymphoma class (Su et al. 2001), was also linked to wing margin formation by Bejarano et al. (2008). Tara, a member of the trithorax group, was isolated in a screen for modifiers of a homeotic phenotype (Calgaro et al. 2002). Tara functions opposite to dom with homeotic loci (Ruhf et al. 2001; Calgaro et al. 2002). Antagonism between tara and dom is consistent with our observation that loss of tara suppresses the wing nicking derived from loss of dom. The Tara protein shows sequence similarity to transcriptional regulators of cell cycle proteins (Calgaro et al. 2002), and it is noteworthy that tara mutation also suppresses wing nicking associated with the proliferation-defective C96+Rbf280 strain (Figure 7C). Emc, a negative regulator of HLH transcription factors, has complex functions at the wing margin, affecting both cell proliferation and sensory organ formation (Baonza et al. 2000). Thus, GOF for emc through EP 1561 may enhance margin effects through suppression of sensory bristle formation.
Lola, related to the broad complex class of transcription factors is required for central nervous system development (Giniger et al. 1994) and wing margin patterning through an interaction with cut (Krupp et al. 2005). Lola has also been implicated in cell proliferation and oncogenesis through Notch-mediated repression of Rbf (Ferres-Marco et al. 2006). Therefore, LOF alleles may derepress Rbf expression and inhibit cell proliferation. This is consistent with our observation that lola alleles enhance the C96-domR and C96+Rbf280 strains (Figures 6 and 7). Finally, ecdysone receptor (EcR) function is associated with sensory organ differentiation (Schubiger et al. 2005), and Dom was identified as an EcR cofactor in cultured cells (Davis et al. 2011).
A second class of dom modifier encodes RNA regulatory proteins. Pabp2 regulates polyA tail length, and LOF of pabp2 is associated with aberrant levels of Cyclin B (Benoit et al. 2005). Pumilio is an RNA-binding protein that mediates translational repression (Wharton et al. 1998). Additionally, loss of Pumilio function has been associated with improper regulation of Cyclin B (Sonoda and Wharton 2001). Consistent with a cell cycle defect, EPs 558 (pabp2) and 1646 (pum) strongly enhance the C96+Rbf280 wing phenotype (Figure 7). Tudor-SN has been implicated in transcription, processing, and RNA interference as a subunit of the RNA-induced silencing complex (Friberg et al. 2009). The Tudor-SN overexpression alleles EP 593 and P(EPg42)Tudor-SNEy07875 (Tables 2 and 3) could act through enhanced production of dom RNAi. The observation that EP 593 does not modify C96-MamH or C96+Rbf280 phenotypes, which are both produced independently of RNAi, supports this idea.
The third class of modifier links dom to antagonistic growth and autophagy pathways. During growth, the degradation of organelles and long-lived proteins associated with autophagy is suppressed through inactivation of Atg1, a serine/threonine kinase (Zirin and Perrimon 2010). In contrast, during conditions of cellular starvation or stress Atg1 is not suppressed. It is required for autophagy induction, thereby providing raw materials for cell survival. However, Atg1 has additional functions, including the down-regulation of growth through inactivation of S6 kinase. The S6 kinase normally phosphorylates ribosomal protein S6, and this activity is a hallmark of cell growth (Lee et al. 2007). Thus, Atg1 functions at a key juncture, to both induce autophagy and prevent cell growth under conditions inappropriate for growth. Our data demonstrates that depressed atg1 function suppresses the C96-domR phenotype, whereas overexpression enhances it. Mutation of atg1 should lead to elevations in S6 kinase activity (Lee et al. 2007) and increases in translation and cell division, and this could mediate the wing margin rescue we observe.
Concomitantly, rescue derived from atg1 mutation should be associated with depressed autophagy induction. This atg1 effect would contradict our data on seven other atg loci, where LOF enhances the wing phenotype, rather than suppresses. The data from these seven loci suggest that normal levels of autophagy act to limit wing margin loss associated with C96-domR. This could occur, for example, if cells interpret dom loss as stress and launch autophagy as a response to provide the raw materials for repair. These conflicting data can be reconciled if, in the case of atg1 mutation, the resultant growth elevation is epistatic to effects of autophagy depression. During conditions favoring growth and cell division, autophagy may no longer be required for wing margin rescue. Our observation that EP 1202 (LOF atg1) suppresses the wing defects derived from both C96-domR and C96+Rbf280 favors this idea (Figures 6 and 7). The association of dom with atg mutations is consistent with an earlier report linking autophagy to Notch signaling in the wing (Thumm and Kadowaki 2001).
Additional effects of dom loss on autophagy may contribute to the wing margin phenotype. Mammalian Tip60 protein, upon phosphorylation and activation by the GSK3 kinase, acetylates and activates Atg1 (ULK1) during autophagy induction (Lin et al. 2012). This reveals a role for Tip60 acetyltransferase directly in autophagy regulation, rather than through genetic regulation. However, it is not known if this mechanism operates in Drosophila and, if it does, whether Dom or the remainder of the Tip60 complex also plays a role.
A second link to growth and autophagy pathways derived from our screen was wdb, which encodes a protein phosphatase (PP2A) regulatory subunit. Wdb regulates many functions, including protein kinase activity and growth (Vereshchagina et al. 2008). Wdb was also implicated as a positive regulator of autophagy in Drosophila, targeting several Atg proteins (Banreti et al. 2012). One of the postulated targets is Atg1, which has been shown to be a PP2A target in C. elegans (Ogura et al. 2010). Alternatively, Wdb could covalently modify Dom protein and alter its activity. Wdb has been associated with Hedgehog signaling through dephosphorylation and down regulation of the cubitus interruptus protein (Jia et al. 2009). That study showed opposite effects mediated by PP2A phosphatase vs. CK1 kinase, similar to our observations with the C96-domR phenotype (Table 4). Although we have no evidence supporting such modifications, the predicted Dom sequence contains consensus sites for CK1 phosphorylation (data not shown).
Finally, we found that mutations in the Lk6 locus affect the C96-domR phenotype (Tables 2 and 3). The Drosophila Lk6 protein is the functional homolog of mammalian Mnk kinases, which regulate the activity of translational initiation factor eIF4E and growth through phosphorylation. Mutation of Lk6 has been associated with organismal growth depression and reduced wing size through reduced cell number (Arquier et al. 2005). Contrasting effects of Lk6 on growth in Drosophila have also been reported, dependent on nutrient levels (Reiling et al. 2005), indicating that regulation of Lk6 is sensitive to culture conditions.
These links of dom to growth and autophagy are likely related to its effects on Notch signaling and cell proliferation, with some contribution due to a cell death effect also plausible. Our results (Figures 2 and 7), in conjunction with prior reports, indicate a Dom requirement for both Notch target expression and repression of cell proliferation (Hall et al. 2004; Eissenberg et al. 2005; Gause et al. 2006; Lu et al. 2007). Together, these results suggest a model where Dom contributes to positive regulation of Notch signaling, which in turn stimulates cell proliferation. Dom is subsequently involved in negative regulation of proliferation through repression of E2F-dependent loci. Although this model is consistent with most data, other work has implicated Dom as a positive effector during proliferation. Larvae homozygous for the most severe dom alleles were observed to be lacking imaginal discs and exhibited a reduction in brain neuroblasts (Ruhf et al. 2001), and recently a study of larval tissues showed that Dom is required for expression of several cell cycle loci, including the cyclins E, B, B3, A, CDC2, and string (Walker et al. 2011). Additionally, Lu et al. (2007) observed that Dom is resident at the promoter of numerous E2F target loci required for cell proliferation. However, contrary to its role in proliferation inhibition, they found that reduction of Dom levels is not associated with elevated expression of various S-phase loci, including cyclin E. An interesting possibility is that Dom functions at both inactive and active E2F target promoters, potentially contributing to a switch between negative and positive regulation of transcription and cell division. Notch signaling could contribute to the switch, in the same manner that the canonical pathway regulates targets such as E(spl) loci, through the displacement of a repression complex and recruitment of transcriptional activators (Lubman et al. 2007). Under such circumstances, the phenotypic consequences of altered Dom levels could vary significantly, as previously observed (Braun et al. 1997; Ruhf et al. 2001; Lu et al. 2007; Walker et al. 2011). Additional biochemical assays of Dom and Notch function will be necessary to address such possibilities.
In conclusion, we found that targeting LOF for dom to the wing margin created a sensitized genotype associated with a partially penetrant, dominant phenotype. This phenotype was scored for dosage-sensitive modifiers, allowing an efficient scan for other loci that function with dom. Our analysis demonstrated that dom modifiers are enriched for loci that contribute to the regulation of cell growth and proliferation, which is consistent with prior studies of dom function. This genetic system will facilitate screening for novel loci involved with growth regulatory mechanisms, and complement biochemical approaches to the same questions.
Acknowledgments
We thank Shreya Sane for assistance with the screen, Joyce Jones for iPCR assays, and Wooly Pierre for wing disc stains. This research was supported by funds from the National Science Foundation and Ammerman Foundation (B.Y.).
Footnotes
Communicating editor: H. Salz
Literature Cited
- Alexander S. J., Woodling N. S., Yedvobnick B., 2006. Insertional inactivation of the L13a ribosomal protein gene of Drosophila melanogaster identifies a new Minute locus. Gene 368: 46–52 [DOI] [PubMed] [Google Scholar]
- Arquier N., Bourouis M., Colombani J., Leopold P., 2005. Drosophila Lk6 kinase controls phosphorylation of eukaryotic translation initiation factor 4E and promotes normal growth and development. Curr. Biol. 15: 19–23 [DOI] [PubMed] [Google Scholar]
- Bánréti A., Lukácsovich T., Csikós G., Erdélyi M., Sass M., 2012. PP2A regulates autophagy in two alternative ways in Drosophila. Autophagy 8: 1–14 [DOI] [PubMed] [Google Scholar]
- Baonza A., Garcia-Bellido A., 2000. Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc. Natl. Acad. Sci. U S A 97: 2609–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano F., Luque C. M., Herranz H., Sorrosal G., Rafel N., et al. , 2008. A gain-of-function suppressor screen for genes involved in dorsal-ventral boundary formation in the Drosophila wing. Genetics 178: 307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit B., Mitou G., Chartier A., Temme C., Zaessinger S., et al. , 2005. An essential cytoplasmic function for the nuclear poly(A) binding protein, PABP2, in poly(A) tail length control and early development in Drosophila. Dev. Cell 9: 511–522 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Braun A., Lemaitre B., Lanot A., Zachary D., Meister M., 1997. Drosophila immunity: Analysis of larval hemocytes by P element mediated enhancer trap. Genetics 147: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Ashburner M., Moses K., 1998. Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development 125: 2653–2664 [DOI] [PubMed] [Google Scholar]
- Calgaro S., Boube M., Cribbs D. L., Bourbon H. M., 2002. The Drosophila gene taranis encodes a novel trithorax group member potentially linked to the cell cycle regulatory apparatus. Genetics 160: 547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Neufeld T. P., 2010. Autophagy takes flight in Drosophila. FEBS Lett. 584: 1342–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. B., Sangil I., Berry G., Olayokun R., Neves L. H., 2011. Identification of common and cell type specific LXXLL motif EcR cofactors using a bioinformatics refined candidate RNAi screen in Drosophila melanogaster cell lines. BMC Dev. Biol. 11: 66–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Wong M., Chrivia J. C., 2005. Human SRCAP and Drosophila melanogaster Dom are homologs that function in the Notch signaling pathway. Mol. Cell. Biol. 25: 6559–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferres-Marco D., Gutierrez-Garcia I., Vallejo D. M., Bolivar J., Gutierrez-Aviño F. J., et al. , 2006. Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature 439: 430–436 [DOI] [PubMed] [Google Scholar]
- Friberg A., Corsini L., Mourão A., Sattler M., 2009. Structure and ligand binding of the extended Tudor domain of D. melanogaster Tudor-SN. J. Mol. Biol. 387: 921–934 [DOI] [PubMed] [Google Scholar]
- Gause M., Eissenberg J. C., Macrae A. F., Dorsett M., Misulovin Z., et al. , 2006. Nipped-A, the Tra1/TRRAP subunit of the Drosophila SAGA and Tip60 complexes, has multiple roles in Notch signaling during wing development. Mol. Cell. Biol. 26: 2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Tietje K., Jan L. Y., Jan Y. N., 1994. lola encodes a putative transcription factor required for axon growth and guidance in Drosophila. Development 120: 1385–1398 [DOI] [PubMed] [Google Scholar]
- Giordano G., Rendina R., Peluso I., Furia M., 2002. RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics 160: 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K., Boulianne G. L., 1996. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39: 174–182 [DOI] [PubMed] [Google Scholar]
- Hall L. E., Alexander S. J., Chang M., Woodling N. S., Yedvobnick B., 2004. An EP Over Expression Screen for Genetic Modifiers of Notch Pathway Function in Drosophila. Genet. Res. 83: 71–82 [DOI] [PubMed] [Google Scholar]
- Helms W., Lee H., Ammerman M., Parks A., Muskavitch M., et al. , 1999. Engineered truncations in the Drosophila Mastermind protein disrupt Notch pathway function. Dev. Biol. 215: 358–374 [DOI] [PubMed] [Google Scholar]
- Jia H., Liu Y., Yan W., Jia J., 2009. PP4 and PP2A regulate Hedgehog signaling by controlling Smo and Ci phosphorylation. Development 136: 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel M., Hurlbut G., Upadhyay G., Yajnik V., Yedvobnick B., et al. , 2007. Investigating the genetic circuitry of Mastermind in Drosophila, a Notch signal effector. Genetics 177: 2493–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P., Benzer S., 2000. Genetic suppression of polyglutamine toxicity in Drosophila. Science 287: 1837–1840 [DOI] [PubMed] [Google Scholar]
- Krupp J. J., Yaich L. E., Wessells R. J., Bodmer R., 2005. Identification of genetic loci that interact with cut during Drosophila wing-margin development. Genetics 170: 1775–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch T., Florens L., Macdonald W. H., Swanson S. K., Glaser R. L., et al. , 2004. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306: 2084–2087 [DOI] [PubMed] [Google Scholar]
- Lee S. B., Kim S., Lee J., Park J., Lee G., et al. , 2007. ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 8: 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Li T. Y., Liu Q., Zhang C., Li X., et al. , 2012. GSK3–TIP60–ULK1 signaling pathway links growth factor deprivation to autophagy. Science 336: 477–481 [DOI] [PubMed] [Google Scholar]
- Lu J., Ruhf M., Perrimon N., Leder P., 2007. A genome wide RNA interference screen identifies putative chromatin regulators essential for E2F repression. Proc. Natl. Acad. Sci. U S A 104: 9381–9386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman O. Y., Ilagan M. X., Kopan R., Barrick D., 2007. Quantitative dissection of the Notch:CSL interaction: insights into the Notch-mediated transcriptional switch. J. Mol. Biol. 365: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Creanga A., Lum L., Beachy P. A., 2006. Prevalence of off-target effects in Drosophila RNA interference screens. Nature 443: 359–363 [DOI] [PubMed] [Google Scholar]
- Mummery-Widmer J. L., Yamazaki M., Stoeger T., Novatchkova M., Bhalerao S., et al. , 2009. Genome-wide analysis of Notch signaling in Drosophila by transgenic RNAi. Nature 458: 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K., Okada T., Mitani S., Gengyo-Ando K., Baillie D. L., et al. , 2010. Protein phosphatase 2A cooperates with the autophagy-related kinase UNC-51 to regulate axon guidance in Caenorhabditis elegans. Development 137: 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Mathey-Prevot B., 2007. Matter arising: off-targets and genome-scale RNAi screens in Drosophila. Fly (Austin) 1: 1–5 [DOI] [PubMed] [Google Scholar]
- Reiling J. H., Doepfner K. T., Hafen E., Stocker H., 2005. Diet-dependent effects of the Drosophila Mnk1/Mnk2 homolog Lk6 on growth via eIF4E. Curr. Biol. 15: 24–30 [DOI] [PubMed] [Google Scholar]
- Rorth P., 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. U S A 93: 12418–12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhf M. L., Braun A., Papoulas O., Tamkun J. W., Randsholt N., et al. , 2001. The domino gene of Drosophila encodes novel members of the SWI2/SNF2 family of DNA-dependent ATPases, which contribute to the silencing of homeotic genes. Development 128: 1429–1441 [DOI] [PubMed] [Google Scholar]
- Schubiger M., Carré C., Antoniewski C., Truman J. W., 2005. Ligand-dependent de-repression via EcR/USP acts as a gate to coordinate the differentiation of sensory neurons in the Drosophila wing. Development 132: 5239–5248 [DOI] [PubMed] [Google Scholar]
- Shalaby N. A., Parks A. L., Morreale E. J., Osswalt M. C., Pfau K. M., et al. , 2009. A screen for modifiers of Notch signaling uncovers Amun, a protein with a critical role in sensory organ development. Genetics 182: 1061–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J., Wharton R. P., 2001. Drosophila brain tumor is a translational repressor. Genes Dev. 15: 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M. A., Wisotzkey R. G., Newfeld S. J., 2001. A screen for modifiers of decapentaplegic mutant phenotypes identifies lilliputian, the only member of the fragile-X/Burkitt’s lymphoma family of transcription factors in Drosophila melanogaster. Genetics 157: 717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., et al. , 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyback. Nat. Genet. 3: 283–287 [DOI] [PubMed] [Google Scholar]
- Thumm M., Kadowaki T., 2001. The loss of Drosophila APG4/AUT2 function modifies the phenotypes of cut and Notch signaling pathway mutants. Mol. Genet. Genomics 266: 657–663 [DOI] [PubMed] [Google Scholar]
- Toda H., Mochizuki H., Flores R., III, Josowitz R., Krasieva T. B., et al. , 2008. UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev. 22: 3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereshchagina N., Ramel M. C., Bitoun E., Wilson C., 2008. The protein phosphatase PP2A-B’ subunit Widerborst is a negative regulator of cytoplasmic activated Akt and lipid metabolism in Drosophila. J. Cell Sci. 121: 3383–3392 [DOI] [PubMed] [Google Scholar]
- Walker J., Kwon S. Y., Badenhorst P., East P., McNeill H., et al. , 2011. Role of elongator subunit Elp3 in Drosophila melanogaster larval development and immunity. Genetics 187: 1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton R. P., Sonoda J., Lee T., Patterson M., Murata Y., 1998. The Pumilio RNA-binding domain is also a translational regulator. Mol. Cell 6: 863–872 [DOI] [PubMed] [Google Scholar]
- Xi R., Xie T., 2005. Stem cell self-renewal mediated by chromatin remodeling factors. Science 310: 1487–1489 [DOI] [PubMed] [Google Scholar]
- Xin S., Weng L., Xu J., Du W., 2002. The role of RBF in developmentally regulated cell proliferation in the eye disc and in cyclin D/Cdk4 induced cellular growth. Development 129: 1345–1356 [DOI] [PubMed] [Google Scholar]
- Yatim A., Benne C., Sobhian B., Laurent-Chabalier S., Deas O., et al. , 2012. NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol. Cell 48: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Yedvobnick B., 2009. Targeted gain-of-function screening in Drosophila using GAL4-UAS and random transposon insertions. Genet. Res 91: 243–258 [DOI] [PubMed] [Google Scholar]
- Zirin J., Perrimon N., 2010. Drosophila as a model system to study autophagy. Semin. Immunopathol. 32: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]