Abstract
Recent evidence has shown that the dorsal striatum of the rat is arranged as a patchwork of domains that exhibit distinct dopamine kinetics and concentrations. This raises the pressing question of how these distinct domains are maintained, especially if dopamine is able to diffuse through the extracellular space. Diffusion between the domains would eliminate the concentration differences and, thereby, the domains themselves. The present study is a closer examination of dopamine’s ability to diffuse in the extracellular space. We used voltammetry to record dopamine overflow in dorsal striatum while stimulating the medial forebrain bundle over a range of stimulus currents and frequencies. We also examined the effects of drugs that modulated the dopamine release (raclopride and quinpirole) and uptake (nomifensine). Examining the details of the temporal features of the evoked profiles reveals no clear evidence for long-distance diffusion of dopamine between fast and slow domains, even though uptake inhibition by nomifensine clearly prolongs the time that dopamine resides in the extracellular space. Our observations support the conclusion that striatal tissue has the capacity to retain dopamine molecules, thereby limiting its tendency to diffuse through the extracellular space.
Keywords: Dopamine, dopamine transporter, diffusion, evoked release, voltammetry, dorsal striatum
Central dopamine (DA) systems participate in numerous aspects of brain function,1,2 and their dysfunction contributes to a broad array of disorders and diseases including Parkinson’s disease,3 schizophrenia,4 and attention deficit hyperactivity disorder.5 Broadly speaking, the physiological function of the DA molecules themselves is to bind to post- and presynaptic receptors to modulate the activity of the postsynaptic targets6 and to self-regulate DAergic activity,7 respectively. Consequently, numerous drugs act by modulating extracellular DA concentrations (e.g., L-DOPA, MAO inhibitors, and inhibitors of the dopamine transporter (DAT)8−11) or by modulating or mimicking the binding of DA to its receptors (DA agonists and antagonists).12,13 Some of the drugs that target DA systems have important therapeutic applications14,15 while others have high potential for illicit abuse:16−18 some therapeutic drugs are also abused.19 Thus, it is significant to know the extracellular DA concentration per se, to know the kinetics of DA release and clearance that determine the concentration, and to know the actions of drugs that target DA systems.
Recently, we have demonstrated that the DA terminal field in the rat dorsal striatum contains a patchwork of kinetic spatial domains. The fast and slow domains were brought to light by recordings of extracellular DA with fast-scan cyclic voltammetry (FSCV) at carbon fiber microelectrodes during electrical stimulation of the medial forebrain bundle (MFB). The extracellular concentration of DA, the kinetics of DA release and clearance, the short-term plasticity of DA release, and the actions of DA-targeting drugs are each domain-dependent.20−23 The patchwork phenomenon brings a new perspective to the often mentioned heterogeneity of striatal DA,20,24−27 because DA is notably homogeneous within the fast and slow domains.23 Although the patchwork has only recently been described, there is precedence for the phenomenon as DA is known to function on multiple time courses28 and local differences in short-term plasticity of DA have been reported before.29−33
Our prior findings indicate that the slow domains exist under a state of tonic autoinhibition derived from a tonic basal extracellular DA concentration sufficient to activate presynaptic D2 autoreceptors.20,21 In contrast, such an autoinhibitory tone is absent in the fast domains.20,21 This implies the presence of a persistent DA concentration gradient between the extracellular spaces of fast and slow domains. At present, however, it is unclear why DA extracellular diffusion34−38 would not eliminate the concentration gradient and, hence, the domain-dependent autoinhibitory tone. Thus, the goal of the present study was to examine evoked DA responses in fast and slow domains of the dorsal striatum under a broader range of experimental conditions than used in our previous studies. The evoked responses reported herein support the novel conclusion that DA’s ability to diffuse between the fast and slow domains is severely limited. We discuss this conclusion in the context of the restrictions on extracellular diffusion as described by Nicholson and co-workers.37,39−41
Results and Discussion
Domain-Dependent Effects of Stimulus Intensity: 60 Hz, 180 Pulses
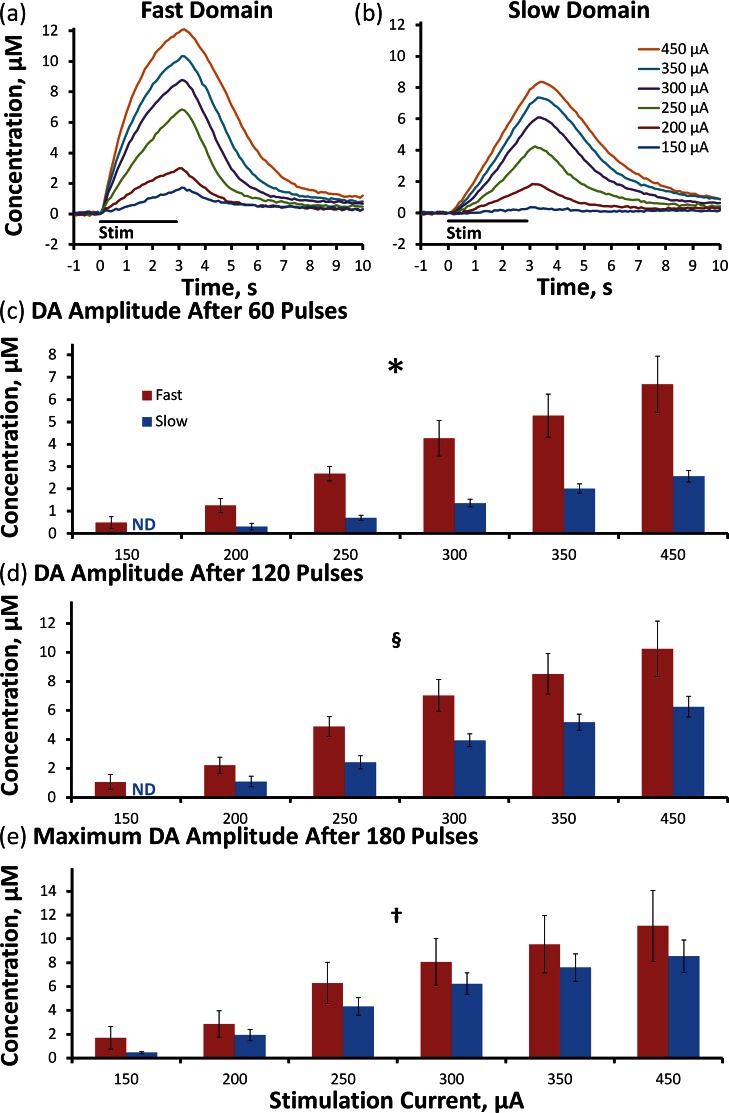
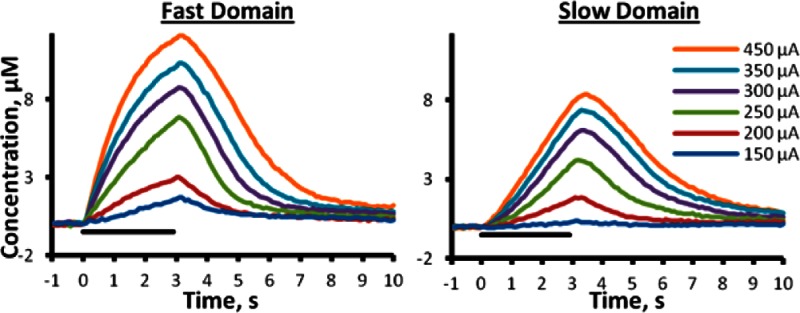
We recorded evoked DA release in objectively identified (see Methods) fast (Figure 1a, n = 5) and slow (Figure 1b, n = 8) domains of individual rats over a range of stimulus current intensities (150–450 μA, 180 pulses, 60 Hz: the solid lines in Figure 1a,b are the averages (error bars omitted for clarity) of responses recorded in different rats; error bars and statistics are reported in Figure 1c–e (see also Supporting Information Figure 1). The responses recorded in fast and slow domains are different in both amplitude and temporal profile. The amplitudes at three time points were subjected to a two-way ANOVA with repeated measures: the factors were the stimulus intensity and domain type (ANOVA details in the figure legend). The stimulus intensity was a significant factor at 60 (Figure 1c, p < 0.00002), 120 (Figure 1d, p < 0.00002), and 180 stimulus pulses (Figure 1e, p < 0.0001). The domain type (fast and slow) was a significant factor at 60 and 120 stimulus pulses (Figure 1c, p < 0.0005; Figure 1d, p < 0.005) but not at 180 stimulus pulses (Figure 1e, p > 0.05); this latter effect is due to the time course of the two profiles, as fast responses begin rapidly and slow down whereas slow responses begin slowly and speed up.
Figure 1.
The intensity of a 180-pulse stimulation significantly affects the average evoked DA overflow in the fast (a) and slow (b) domains of n = 5 and n = 8 individual rats, respectively. The average (±SEM) DA amplitude after 60 (c) and 120 (d) stimulus pulses is significantly different between fast and slow domains, but not at maximum amplitude (e). (Two-way ANOVA with repeated measures: *, stimulation intensity F(1.1,11.9) = 44.758, p < 0.00002, domain (fast vs slow) F(1.1,11.9) = 28.818, p < 0.0005, interactions F(1,11) = 10.956, p < 0.02; §, stimulation intensity F(1.1,12.5) = 43.122, p < 0.00002, domain F(1,11) = 15.267, p < 0.005; †, stimulation intensity F(1.2,13.5) = 27.614, p < 0.0001).
The responses in Figure 1 exhibit overshoot; that is, the DA signal continues to increase after the end of the stimulus. The amplitude and duration of the overshoot depend on both the stimulus intensity and the type of domain (see Supporting Information Figure 2). The overshoots observed here are more obvious than in our previous studies20,23 that involved shorter stimulus durations at a stimulus intensity of 240 or 270 μA. The less obvious overshoot associated with milder stimulus conditions is consistent with the dependence of the overshoot on the magnitude of evoked release observed here (Supporting Information Figure 2).
At stimulus intensities ≥ 250 μA, the responses in both fast and slow domains exhibit a constant rate of linear DA clearance after the stimulus ends (Figure 1a,b), indicating that the extracellular DA concentration is sufficient to saturate the dopamine transporter (DAT). When the DAT is saturated, the slope of the linear clearance profile is the apparent Vmax of DA uptake.22,24,42 The apparent Vmax in fast domains, 3.91 ± 0.40 μM/s, is significantly larger than that in slow domains, 2.46 ± 0.14 μM/s (t test of independent samples: p < 0.0002).
The results in Figure 1 (and Supporting Information Figures 1 and 2) extend our prior studies, which were conducted at a single stimulus intensity,20,23 by demonstrating that objectively identified domains exhibit distinct rates of evoked DA release and clearance (apparent Vmax) over a broad range of stimulus intensities. This confirms that domain-dependent responses are not restricted to a narrow set of stimulus parameters and (vide infra) establishes a starting point for a more detailed examination of the properties of the domains themselves.
Domain-dependent Effects of Stimulus Intensity: 60 Hz, 12 Pulses
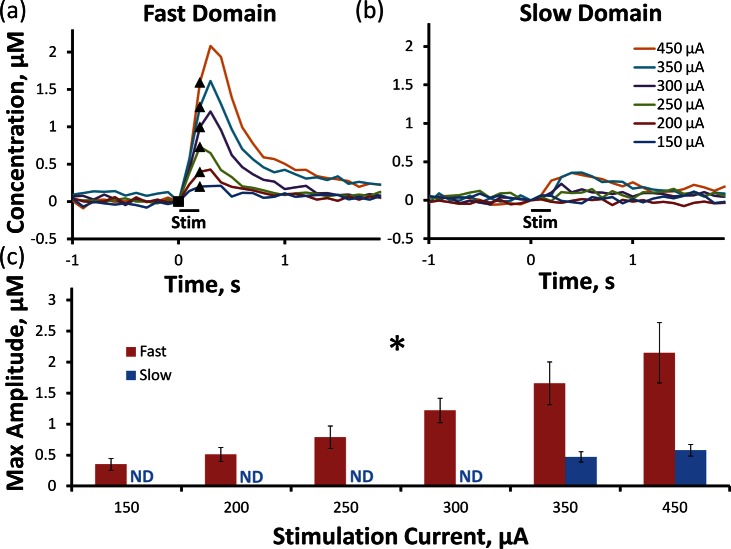
Brief stimuli (12 pulses, 150–450 μA, 60 Hz) evoke robust DA responses in fast (Figure 2a) but not slow (Figure 2b) domains (the lines in Figure 2 are the average of responses recorded at the same sites used to obtain Figure 1; error bars are reported in Figure 2c). In fast domains, the stimulus intensity significantly affects the response amplitude (Figure 2c, one-way ANOVA with repeated measures, details provided in the figure legend). The expanded time scale of Figure 2 shows the details of DA release when the stimulus begins. In fast domains (Figure 2a), DA release is detected during the first FSCV scan performed 100 ms (6 stimulus pulses) after the stimulus begins. There is little or no delay in DA clearance at stimulus intensities ≤ 250 μA, but a 100 ms delay appears at intensities ≥ 300 μA (the clearance delay is discussed in the section below entitled Response Overshoot). In slow domains (Figure 2b), the brief stimuli evoked no quantifiable responses at intensities below 350 μA; higher intensities evoked delayed responses with small amplitudes. Under the conditions of this experiment, Figure 2 shows that any contribution to the slow domain response derived from diffusion of DA from the fast domain is minimal.
Figure 2.
12-pulse stimulation reveals the difference between fast and slow domains. Evoked DA overflow is observed in the fast domain (a) following each stimulus intensity ranging from 150 to 450 μA, whereas DA is only present in the slow domain (b) during 350 and 450 μA 12-pulse stimulations. The average (±SEM) maximum DA amplitude (c) is significantly increased by stimulus intensity in the fast domain. (One-way ANOVA with repeated measures: *, stimulation intensity F(1.1,4.6) = 11.972, p < 0.05). The solid symbols mark the beginning (square) and ending (triangle) of each stimulus.
Domain-Dependent Effects of Stimulus Frequency: 250 μA, 180 Pulses
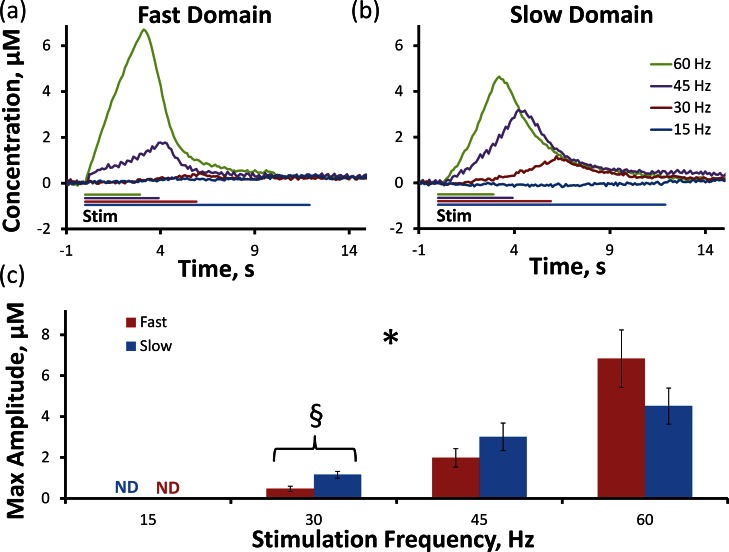
Varying the stimulus frequency (15–60 Hz, 180 stimulus pulses, 250 μA) has domain-dependent effects on evoked responses (the solid lines in Figure 3a,b are the average responses with error bars omitted for clarity; see also Supporting Information Figure 3). Responses were nondetectable at 15 Hz. The stimulus frequency significantly affected the response amplitude (Figure 3c, p < 0.000001, two-way ANOVA with repeated measures, details provided in the figure legend, 15 Hz data omitted from the ANOVA). Although the domain type was not a significant factor, both the 30 and 45 Hz stimuli evoked larger maximum amplitudes in the slow domains (the difference at 30 Hz was significant, t test of independent samples, p < 0.05). As far as we are aware, this amplitude reversal (i.e., sites exhibiting larger amplitude at 60 Hz exhibiting lower amplitudes at 45 and 30 Hz) has not been described before. Even so, the observed dependence of the amplitude on stimulus frequency is consistent with the difference in DA clearance from fast and slow domains22,23 (see discussion of the apparent Vmax values in Figure 1, above). Lower stimulus frequencies provide more time between the stimulus pulses for DA clearance, so the response amplitude decreases with frequency. However, the amplitude decreases more rapidly in the fast domain because DA clearance is faster there. So, this is the first report showing that the amplitudes of fast and slow responses exhibit a differential dependence on stimulus frequency.
Figure 3.
Fast (a) and slow (b) domains are significantly altered by the frequency of the stimulus (two-way ANOVA with repeated measures: *, stimulation frequency F(1.4,15.3) = 38.022, p < 0.000001). 180-pulse stimulation lasts for 12 s at 15 Hz, 6 s at 30 Hz, 4 s at 45 Hz, and 3 s at 60 Hz. 30 Hz stimulation produces significantly higher maximum evoked DA overflow (c) in the slow domain (t test of independent samples: §, p < 0.05).
The responses recorded at 30 and 45 Hz show no sign of DA diffusing from fast to slow domains. First, the responses show that DA is more rapidly cleared from the fast domains (see previous paragraph), in which case it is not available to diffuse to the slow domains. Second, since the evoked concentration is higher in the slow domains (see also Supporting Information Figure 3 for a direct comparison), there is no concentration gradient to drive diffusion from fast to slow domains. So, under the conditions of this experiment, diffusion of DA from fast to slow domains is not evident.
Diffusion after Uptake Inhibition
Uptake inhibition prolongs DA’s lifetime in the extracellular space.8,23,43,44 Thus, an important question is whether uptake inhibition also increases DA’s diffusion distance. Without uptake inhibition, evoked DA in slow domains is barely detectable during 200 ms stimuli (Figure 2b). However, robust responses are detected in fast and slow domains after rats are treated with nomifensine, a DA uptake inhibitor.23 Nevertheless, our previous results did not indicate diffusion between the fast and slow domains. The temporal profiles of the fast and slow responses after nomifensine administration were identical (see Figure 9 of ref (23)). If the slow domain response were due to diffusion, then it should have risen slower, peaked later, and lasted longer than the fast domain response; these features were not observed. Thus, we are interested now to investigate further whether uptake inhibition promotes diffusion of DA between fast and slow domains.
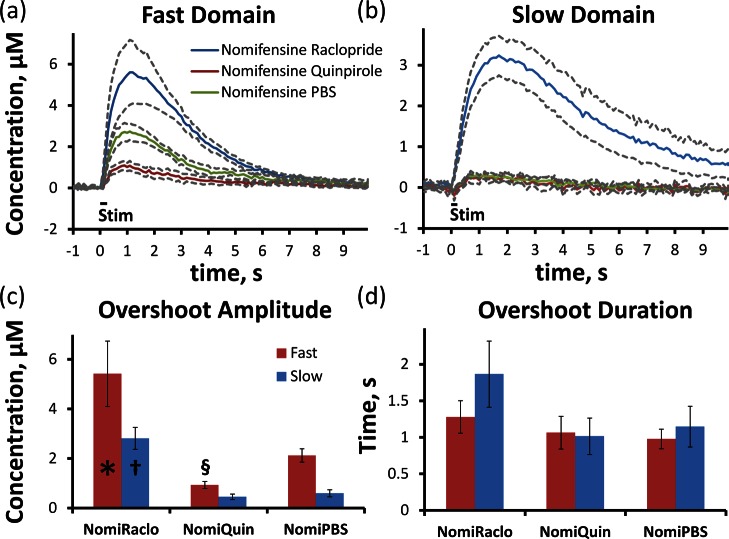
We have extended this line of investigation by modulating evoked DA release with a D2 receptor antagonist, raclopride (2 mg/kg i.p.), and a D2 receptor agonist, quinpirole (1 mg/kg i.p.),20,21 in nomifensine-treated rats. In fast domains (Figure 4a), raclopride significantly (p < 0.05) increased (Figure 4c), while quinpirole significantly (p < 0.005) decreased (Figure 4c), the response amplitude (ANOVA details provided in the figure legend). In slow domains (Figure 4b), evoked DA responses were barely detectable after vehicle (PBS) or quinpirole administration (the post-nomifensine responses in Figure 4 are smaller than those in Figure 9 of ref (23) due to the extra 30 min interval after the vehicle injection). Even though DA in the fast domains was elevated for ∼6–7 s (Figure 4a, red and green), diffusion of DA to the slow domains was not detected (Figure 4b, red and green). Raclopride significantly (p < 0.0005) increased the response amplitude in the slow domain without an initial delay (Figure 4b, blue), confirming the presence of autoinhibited DA terminals in slow domains. Figure 4 confirms that even after uptake inhibition voltammetry records the DA concentration in the immediate proximity of the recording electrode but does not “pick up” DA diffusing between domains.
Figure 4.
D2 targeting drugs alter the average amplitude of the nomifensine induced DA overshoot in fast (a) and slow (b) domains. In the fast domain, raclopride (n = 5) significantly increases the amplitude of DA overshoot (c), and quinpirole (n = 6) significantly decreases the amplitude of DA overshoot compared to PBS (n = 5) control. In the slow domain, raclopride (n = 6) significantly increases the overshoot duration, while quinpirole (n = 6) has no significant effect compared to PBS (n = 6) control. The D2 induced changes in DA amplitude do not significantly alter the duration (d) of the nomifensine induced overshoot. (t test of independent samples: *, p < 0.05; §, p < 0.005; †, p < 0.0005.)
Response Overshoot
The responses in Figures 1–4 exhibit overshoot; that is, the DA signal continues to increase after the stimulus ends (see also Supporting Information Figures 1–3). Overshoot is usually attributed to a diffusion gap between the recording electrode and DA terminals but our results show that this cannot be so. The amplitude and duration of overshoot is highly sensitive to the magnitude of DA overflow, that is, the net rate of DA release and clearance. There is a systematic increase in overshoot duration and amplitude, in both domains, with increasing stimulus intensity (Supporting Information Figure 2). Likewise, overshoot increases with stimulus frequency (Figure 3 and Supporting Information Figure 3) and after uptake inhibition (Figure 4 and ref (23)), both of which increase DA overflow. Finally, our results show that all recording sites exhibit overshoot if overflow is high enough. If overshoot were due to a physical diffusion gap, then overflow would be a permanent feature of the responses recorded at any given site: it would not be sensitive to the stimulus or pharmacological conditions.
Inspection of our results shows that overshoot is strongly impacted by DAT kinetics. Overshoot is smaller in fast domains compared to slow domains (Supporting Information Figure 2), which coincides with the larger apparent Vmax of clearance in the fast domains (above), and is largest after uptake inhibition (Figure 4). Thus, we report here for the first time that overshoot is a function of DAT kinetics rather than a diffusion gap.
DA Diffusion after Uptake Inhibition: Low Frequency Stimulation
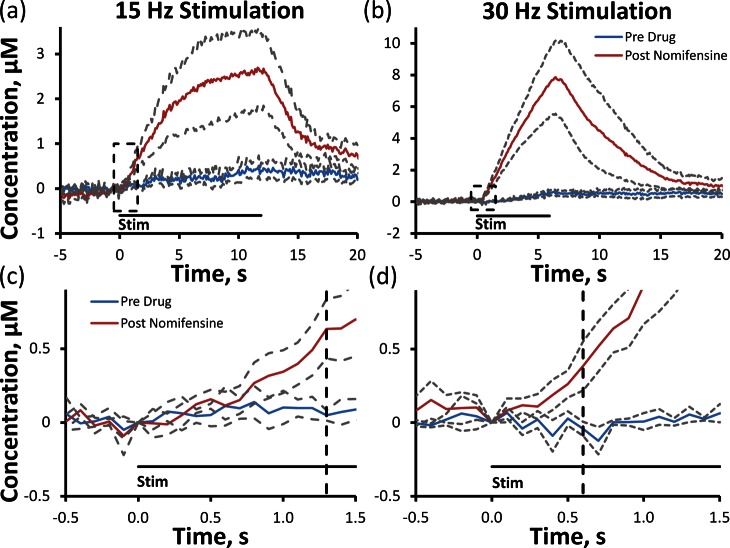
In slow domains, evoked DA release is non- or barely detectable at stimulus frequencies of 15 or 30 Hz, respectively (250 μA, 180 stimulus pulses, Figure 5a,b). This might be because uptake prevents DA from reaching the electrode. However, there is an obvious problem with this explanation because the intervals between the 15 and 30 Hz stimulus pulses (67 and 33 ms, respectively) are substantially less than the time needed for DA clearance. For example, under mild stimulus conditions, pseudo-first-order DA clearance from slow domains requires several seconds (e.g., Figure 1b, 200–300 μA). In that case, complete DA clearance in 67 ms should not be possible. So, there is a timing mismatch between these observations.
Figure 5.
Nomifensine increases the average (±SEM) evoked DA overflow in the slow domain in response to low frequency 15 (a) and 30 Hz (b) stimulations in n = 4 rats. After treatment with nomifensine, DA arrives after 1.3 s of 15 Hz (c, enlargement of the dashed box in (a)) stimulation (vertical dashed line, 19 stimulus pulses) and after 0.6 s of 30 Hz (d, enlargement of the dashed box in (b)) stimulation (vertical dashed line, 18 stimulus pulses).
Nomifensine (20 mg/kg i.p.) dramatically increased the response amplitude in slow domains at 15 and 30 Hz (Figure 5a,b: solid lines are the averages from n = 4 rats, and dashed lines are the confidence intervals based on the SEM). The first 1.5 s of the responses are displayed on an expanded scale in Figure 5c and d to emphasize that nomifensine did not abolish the initial delay in evoked release. At 15 Hz, the DA signal increased significantly 1.3 s after the stimulus began (t test of paired samples, p < 0.05 compared to pre drug response), that is, after 19 stimulus pulses. At 30 Hz, the DA signal reached significance 600 ms after the stimulus began (t test of paired samples, p < 0.05 compared to pre drug response), that is, after 18 stimulus pulses. Thus, regardless of the stimulus frequency, the same number of stimulus pulses, that is, the same amount of DA release, was required before the DA signal appeared at the electrode.
Restricted Diffusion of DA in the Extracellular Space of the Rat Striatum
In a previous report,23 we suggested that Nicholson’s model of restricted diffusion37,39−41 explained several unexpected features of evoked DA responses in the dorsal striatum. This same mechanism explains the new findings of the present study. A major new finding is that DA’s ability to diffuse between fast and slow domains is severely limited. Obviously, DA diffuses between DA terminals and the recording electrode in both domains: DA would not be detected otherwise. However, we find no clear evidence that DA makes its way from the fast domains to the slow domains under any conditions we have examined. The simplest explanation for this observation is that pathways through the extracellular space are obstructed, as described by Nicholson and co-workers.37,39−41
A second new finding is the dependence of DA overshoot on the magnitude of evoked overflow. Our working hypothesis is that restricted diffusion causes DA to be “held up” in the extracellular space and then either make its way to the electrode or return to DA terminals according to the balance between the rate of release and clearance. Thus, there is less overshoot if release is small (mild stimulation conditions) or if uptake is more efficient (DA is cleared faster in the fast domain than from the slow domain22). There is more overshoot if uptake cannot efficiently remove DA from the extracellular space, allowing more DA to “leak” over to the electrode. This happens when release is large (longer, more intense, or higher frequency stimulation) or when uptake is slowed (observed in the slow domain or following uptake inhibition).
A third major finding is in regard to the response to low frequency stimulation (15 Hz, Figure 5). The absence of a detectable response highlights the timing mismatch issue explained above. Again, our working hypothesis is that DA is “held up” in the extracellular space, but in this case, because the stimulus frequency is so low, there is not enough DA to overcome the capacity of the restrictions upon diffusion and DA is cleared by DAT without “leaking” over to the electrode. Thus, restricted diffusion accounts for the timing mismatch in these low-frequency responses.
Conclusion
Numerous investigators, ourselves included, have long adopted a by-now conventional model to describe DA’s diffusion in the extracellular space. In the conventional model, the diffusion coefficient is affected by tissue tortuosity and the DA’s lifetime for diffusion is constrained by DA uptake. While the conventional model aptly describes some evoked responses, it does not capture the several new features of the domain-dependent responses recorded from the dorsal striatum. A central conclusion stemming from this finding is that restricted diffusion, the subject of in-depth investigations by Nicholson and co-workers,37,39−41 plays a dominant role in determining DA’s spatiotemporal dynamics in the extracellular space. Thus, we conclude that restricted diffusion enables DA terminal field to maintain a domain-dependent autoinhibitory tone, a demonstrated feature of the dorsal striatum’s domain patchwork.
Methods
Carbon Fiber Electrodes
A single carbon fiber (7 μm diameter, T650; Cytec Carbon Fibers LLC, Piedmont, SC) was threaded into a borosilicate capillary (0.4 mm ID, 0.6 mm OD; A-M systems Inc., Sequim, WA) and pulled to a fine tip using a vertical puller (Narishige, Los Angeles, CA). The tip was sealed with a low-viscosity epoxy (Spurr Embedding Kit; Polysciences Inc., Warrington, PA), and the exposed fiber was trimmed to a length of 200 μm. A drop of mercury established the electrical connection between the fiber and a nichrome wire (Nichrome; Goodfellow, Oakdale, PA). Electrodes were soaked for 30 min in isopropyl alcohol prior to use.45 Postcalibration was carried out using freshly prepared, nitrogen-purged dopamine HCl (Sigma Aldrich, St. Louis, MO) standard solutions in artificial cerebrospinal fluid (144 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 1.0 mM Mg2+, 149.1 mM Cl–, and 2.0 mM phosphate, pH 7.4). Concentrations in vivo were obtained using postcalibration results.
Fast-Scan Cyclic Voltammetry
Voltammetry was performed with an EI 400 potentiostat (Ensman Instruments; Bloomington, IN) under software control (CV Tar Heels v4.3, courtesy of Dr. Michael Heien, University of Arizona, Tucson, AZ). The voltammetric waveform consisted of three linear potential ramps starting at the rest potential of 0 V (vs Ag/AgCl) first to +1.0 V, then to −0.5 V, and back to 0 V at a scan rate of 400 V/s: the waveform was applied at a frequency of 10 Hz. DA was identified by inspection of background-subtracted voltammograms and quantified with the oxidation current between +0.5 and +0.7 V on the initial ascending potential ramp.
Surgical and Stimulation Procedures
The University of Pittsburgh’s Institutional Animal Care and Use Committee reviewed and approved all procedures involving animals. Male Sprague–Dawley rats (250–350 g; Hilltop, Scottsdale, PA) were anesthetized with isoflurane (2.5% by volume O2) and maintained at a body temperature of 37 °C (Harvard Apparatus; Holliston, MA). Anesthetized rats were placed in a stereotaxic frame (David Kopf, Tujunga, CA) with the incisor bar raised to 5 mm above the interaural line.46 Dura mater was exposed by craniotomies and removed to allow insertion of the reference, working, and stimulating electrodes. Contact between the brain surface and a Ag/AgCl reference electrode was established via a salt bridge. A carbon fiber electrode was implanted into the dorsal striatum (2.5 mm anterior to bregma, 2.5 mm lateral from bregma, and 5–6 mm below the cortical surface). A stainless steel, twisted bipolar stimulating electrode (MS303/a; Plastics One, Roanoke, VA) was aimed at the medial forebrain bundle (MFB, 2.2 mm posterior to bregma, 1.6 mm lateral from bregma, and 7–9 mm below the cortical surface) and lowered until evoked DA release was observed in the striatum.47−49 The MFB stimulation was a biphasic, constant-current, square-wave waveform delivered by a pair of optical stimulus isolators (Neurolog 800, Digitimer; Letchworth Garden City, U.K.). The stimulus pulse width was held constant at 4 ms throughout this study.
Objective Identification of Fast and Slow Domains in Dorsal Striatum
All experiments described herein began with an initial procedure to position the recording carbon fiber electrode in an objectively identified fast or slow domain. For this initial procedure, MFB stimulation was performed with a frequency of 60 Hz and a current intensity of 250 μA. Fast and slow domains were identified according to our previously described classification criteria:20,22,23 fast domains were identified by robust evoked DA release during the first 100 ms of the stimulus and subsequent short-term depression of evoked release, whereas slow domains were identified by an initial delay and subsequent short-term facilitation of evoked release. This classification scheme is objective because responses exhibiting short-term facilitation versus depression are easily discriminated. The recording electrode was lowered from its initial vertical coordinate (5 mm below dura) in small increments (50–100 μm), and the stimulus repeated until a fast domain was identified; to restrict these experiments to the dorsal striatum, lowering was stopped after 1 mm (6 mm below dura) if no fast domain was identified and responses from the slow domain were recorded.
Experimental Design
Whereas our previous studies of the domain phenomenon were conducted with a single stimulus frequency and current intensity, herein we report evoked responses in objectively identified fast and slow domains (see previous paragraph) over a broader range of stimulus durations (12–180 pulses), frequencies (15–60 Hz), and current intensities (150–450 μA).
We also investigated the effects of raclopride and quinpirole on evoked responses in nomifensine-treated rats. First, recording carbon fiber electrodes were implanted in rats using the procedure, described above, for objective identification of fast and slow domains in dorsal striatum. An initial predrug stimulus response was recorded (200 ms, 60 Hz, 250 μA). Nomifensine (20 mg/kg i.p.), was administered and a second stimulus response was recorded 30 min later (the initial and postnomifensine-only responses are not shown here; such responses are reported elsewhere (ref (23) (Taylor 2012)). Then, raclopride (2 mg/kg i.p.), quinpirole (1 mg/kg i.p.), or vehicle (PBS) was administered, and a final stimulus response was recorded 30 min later (see Figure 4).
In a separate experiment in four additional rats (see Figure 5), we examined the effects of nomifensine on low-frequency stimulus responses (15 and 30 Hz, 180 stimulus pulses, 250 μA).
Davidson et al.50 report that prolonged exposure to nomifensine decreases the sensitivity of carbon fiber electrodes to DA. However, in our hands, the impact of nomifensine on evoked responses is robust so any major effect of nomifensine on the electrode sensitivity seems unlikely. Moreover, in this study, we compare the effects of raclopride and quinpirole under the same nomifensine pretreatment conditions, so we assume any effect of nomifensine would be equivalent and not affect the comparisons of major interest in this work.
Data Analysis
Current versus time graphs were generated using the peak oxidation potential for DA. Linear DA clearance rate was assessed by determining the slope of the descending phase of the response with r2 > 0.96. The overshoot duration is the time period from the end of stimulation that DA continues to increase in amplitude, and the overshoot amplitude is the concentration of DA increase during that duration. Statistical analyses were by one- and two-way ANOVA with a repeated measures design as well as post hoc pairwise comparison of main effects using a 95% confidence interval (IBM SPSS Statistics 20 software) and t tests of independent (Figures 1, 3, and 4) or paired (Figure 5) samples.
Glossary
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- PBS
phosphate buffered saline
- FSCV
fast scan cyclic voltammetry
- CFE
carbon fiber electrode
Supporting Information Available
Supplementary Figure 1 is a rearrangement of Figure 1, directly comparing fast and slow domains at each stimulus intensity. Supplementary Figure 2 shows the average (±SEM) overshoot duration and amplitude observed in Figure 1 in response to altering stimulus intensity. Supplementary Figure 3 is a rearrangement of Figure 3 to directly compare the fast and slow domain responses to 30 and 45 Hz stimulation. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
Mitch Taylor designed, conducted, and analyzed all experiments, designed images, and co-wrote the manuscript. Alex Ilitchev contributed to the nomifensine-raclopride data set. Adrian Michael secured funding, contributed to the experimental design, and co-wrote the manuscript.
This work was financially supported by the NIH (Grant MH 075989).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Goto Y.; Otani S.; Grace A. A. (2007) The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53, 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J. A.; Rodriguez-Oroz M. C.; Benitez-Temino B.; Blesa F. J.; Guridi J.; Marin C.; Rodriguez M. (2008) Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. 23, S548–S559. [DOI] [PubMed] [Google Scholar]
- Pavese N.; Rivero-Bosch M.; Lewis S. J.; Whone A. L.; Brooks D. J. (2011) Progression of monoaminergic dysfunction in Parkinson’s disease: A longitudinal (18)F-dopa PET study. NeuroImage 56, 1463–1468. [DOI] [PubMed] [Google Scholar]
- Mizuno Y.; Bies R. R.; Remington G.; Mamo D. C.; Suzuki T.; Pollock B. G.; Tsuboi T.; Watanabe K.; Mimura M.; Uchida H. (2012) Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: A cross-sectional study. Prog. Neuropsychopharmacol. Biol. Psychiatry 37, 182–187. [DOI] [PubMed] [Google Scholar]
- Ludolph A. G.; Kassubek J.; Schmeck K.; Glaser C.; Wunderlich A.; Buck A. K.; Reske S. N.; Fegert J. M.; Mottaghy F. M. (2008) Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: A 3,4-dihdroxy-6- F-18 fluorophenyl-l-alanine PET study. NeuroImage 41, 718–727. [DOI] [PubMed] [Google Scholar]
- Korchounov A.; Meyer M. F.; Krasnianski M. (2010) Postsynaptic nigrostriatal dopamine receptors and their role in movement regulation. J. Neural Transm. 117, 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K.; Gothert M.; Kilbinger H. (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 69, 864–989. [DOI] [PubMed] [Google Scholar]
- Church W. H.; Justice J. B.; Byrd L. D. (1987) Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur. J. Pharmacol. 139, 345–348. [DOI] [PubMed] [Google Scholar]
- Brannan T.; Prikhojan A.; MartinezTica J.; Yahr M. D. (1995) In vivo comparison of the effects of inhibition of MAO-A versus MAO-B on striatal L-DOPA and dopamine metabolism. J. Neural Transm.: Parkinson's Dis. Dementia Sect. 10, 79–89. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. (2002) L-DOPA: From a biologically inactive amino acid to a successful therapeutic agent. Amino Acids 23, 65–70. [DOI] [PubMed] [Google Scholar]
- Schiffer W. K.; Volkow N. D.; Fowler J. S.; Alexoff D. L.; Logan J.; Dewey S. L. (2006) Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse 59, 243–251. [DOI] [PubMed] [Google Scholar]
- Seeman P.; Lee T.; Chauwong M.; Wong K. (1976) Antipsychotic drug doses and neuroleptic dopamine receptors. Nature 261, 717–719. [DOI] [PubMed] [Google Scholar]
- Kapur S.; Zipursky R. B.; Remington G. (1999) Clinical and theoretical implications of 5-HT(2) and D(2) receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am. J. Psychiatry 156, 286–293. [DOI] [PubMed] [Google Scholar]
- Gottwald M. D.; Aminoff M. J. (2011) Therapies for dopaminergic-induced dyskinesias in parkinson disease. Ann. Neurol. 69, 919–927. [DOI] [PubMed] [Google Scholar]
- Guo X. F.; Zhang Z. C.; Zhai J. G.; Fang M. S.; Hu M. R.; Wu R. R.; Liu Z. N.; Zhao J. P.; Early-Stage Schizophrenia O. (2012) Effects of antipsychotic medications on quality of life and psychosocial functioning in patients with early-stage schizophrenia: 1-year follow-up naturalistic study. Compr. Psychiatry 53, 1006–1012. [DOI] [PubMed] [Google Scholar]
- Sulzer D.; Chen T. K.; Lau Y. Y.; Kristensen H.; Rayport S.; Ewing A. (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 15, 4102–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J. A.; Carelli R. M. (2007) Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J. Neurosci. 27, 3535–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E. M.; Stuber G. D.; Heien M.; Wightman R. M.; Carelli R. M. (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618. [DOI] [PubMed] [Google Scholar]
- White B. P.; Becker-Blease K. A.; Grace-Bishor K. (2006) Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. J. Am. Coll. Health 54, 261–268. [DOI] [PubMed] [Google Scholar]
- Moquin K. F.; Michael A. C. (2009) Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J. Neurochem. 110, 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. X.; Moquin K. F.; Michael A. C. (2010) Evidence for coupling between steady-state and dynamic extracellular dopamine concentrations in the rat striatum. J. Neurochem. 114, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin K. F.; Michael A. C. (2011) An inverse correlation between the apparent rate of dopamine clearance and tonic autoinhibition in subdomains of the rat striatum: a possible role of transporter-mediated dopamine efflux. J. Neurochem. 117, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. M.; Jaquins-Gerstl A.; Sesack S. R.; Michael A. C. (2012) Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J. Neurochem. 122, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R. M.; Amatore C.; Engstrom R. C.; Hale P. D.; Kristensen E. W.; Kuhr W. G.; May L. J. (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25, 513–523. [DOI] [PubMed] [Google Scholar]
- May L. J.; Wightman R. M. (1989) Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain Res. 487, 311–320. [DOI] [PubMed] [Google Scholar]
- Kawagoe K. T.; Garris P. A.; Wiedemann D. J.; Wightman R. M. (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51, 55–64. [DOI] [PubMed] [Google Scholar]
- Garris P. A.; Wightman R. M. (1995) Distinct pharmacological regulation of evoked dopamine efflux in the amygdala and striatum of the rat in vivo. Synapse 20, 269–279. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2007) Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288. [DOI] [PubMed] [Google Scholar]
- Cragg S. J.; Hille C. J.; Greenfield S. A. (2002) Functional domains in dorsal striatum of the nonhuman primate are defined by the dynamic behavior of dopamine. J. Neurosci. 22, 5705–5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S. J. (2003) Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J. Neurosci. 23, 4378–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague P. R.; McClure S. M.; Baldwin P. R.; Phillips P. E. M.; Budygin E. A.; Stuber G. D.; Kilpatrick M. R.; Wightman R. M. (2004) Dynamic gain control of dopamine delivery in freely moving animals. J. Neurosci. 24, 1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita J. M.; Parker L. E.; Phillips P. E. M.; Garris P. A.; Wightman R. M. (2007) Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J. Neurochem. 102, 1115–1124. [DOI] [PubMed] [Google Scholar]
- Chadchankar H.; Yavich L. (2011) Sub-regional differences and mechanisms of the short-term plasticity of dopamine overflow in striatum in mice lacking alpha-synuclein. Brain Res. 1423, 67–76. [DOI] [PubMed] [Google Scholar]
- Engstrom R. C.; Wightman R. M.; Kristensen E. W. (1988) Diffusional distortion in the monitoring of dynamic events. Anal. Chem. 60, 652–656. [Google Scholar]
- Nicholson C. (1995) Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophys. J. 68, 1699–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. L.; Michael A. C. (2000) Changes in the kinetics of dopamine release and uptake have differential effects on the spatial distribution of extracellular dopamine concentration in rat striatum. J. Neurochem. 74, 1563–1573. [DOI] [PubMed] [Google Scholar]
- Sykova E.; Nicholson C. (2008) Diffusion in brain extracellular space. Physiol. Rev. 88, 1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. E.; Cragg S. J. (2008) Dopamine spillover after quantal release: Rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev. 58, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S.; Hrabe J.; Nicholson C. (2003) Dead-space microdomains hinder extracellular diffusion in rat neocortex during ischemia. J. Neurosci. 23, 8351–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S.; Nicholson C. (2004) Contribution of dead-space microdomains to tortuosity of brain extracellular space. Neurochem. Int. 45, 467–477. [DOI] [PubMed] [Google Scholar]
- Tao A.; Tao L.; Nicholson C. (2005) Cell cavities increase tortuosity in brain extracellular space. J. Theor. Biol. 234, 525–536. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Reith M. E. A.; Wightman R. M.; Kawagoe K. T.; Garris P. A. (2001) Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J. Neurosci. Methods 112, 119–133. [DOI] [PubMed] [Google Scholar]
- Floresco S. B.; West A. R.; Ash B.; Moore H.; Grace A. A. (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973. [DOI] [PubMed] [Google Scholar]
- Cragg S. J.; Rice M. E. (2004) DAncing past the DAT at a DA synapse. Trends Neurosci. 27, 270–277. [DOI] [PubMed] [Google Scholar]
- Bath B. D.; Michael D. J.; Trafton B. J.; Joseph J. D.; Runnels P. L.; Wightman R. M. (2000) Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal. Chem. 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- Pellegrino L. J., Pellegrino A. S., and Cushman A. J. (1979) A Stereotaxic Atlas of the Rat Brain, Plenum Press, New York, NY. [Google Scholar]
- Ewing A. G.; Bigelow J. C.; Wightman R. M. (1983) Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science 221, 169–171. [DOI] [PubMed] [Google Scholar]
- Kuhr W. G.; Ewing A. G.; Caudill W. L.; Wightman R. M. (1984) Monitoring the stimulated release of dopamine with in vivo voltammetry. I: Characterization of the response observed in the caudate nucleus of the rat. J. Neurochem. 43, 560–569. [DOI] [PubMed] [Google Scholar]
- Stamford J. A.; Kruk Z. L.; Millar J. (1988) Stimulated limbic and striatal dopamine release measured by fast cyclic voltammetry: anatomical, electrochemical and pharmacological characterisation. Brain Res. 454, 282–288. [DOI] [PubMed] [Google Scholar]
- Davidson C.; Ellinwood E. H.; Douglas S. B.; Lee T. H. (2000) Effect of cocaine, nomifensine, GBR 12909 and WIN 35428 on carbon fiber microelectrode sensitivity for voltammetric recording of dopamine. J. Neurosci. Methods 101, 75–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.