Interneuron excitation might be mediated by astrocytic glutamate release.
For most of the last century, one of the major dogmas of neuroscience has been that the brain is composed of two major cell types: neurons, which perform all of the interesting functions required for cognition, and glia, which perform the essential but humble job of keeping the neurons healthy. However, the last decade has seen a slow but steady erosion of this neuron-centric view. First, it was found that neuronal activity can generate glial calcium responses (1–3); more recently, glia have been found to reciprocate and affect neuronal activity (4–6) through mechanisms that are poorly understood. Much of the available evidence indicates that glial activation leads to calcium-dependent glutamate release (4, 6–8), raising the intriguing possibility that glia can drive neuronal activity through some kind of glutamatergic transmission. In this issue of PNAS, Liu et al. (9) show that astrocytes in the hippocampus can cause γ-aminobutyric acid (GABA) release from interneurons through activation of the kainate subtype of ionotropic glutamate receptor.
One of the major obstacles in examining glia–neuron interactions is that few methods can be used to selectively activate glia without also activating neurons. To circumvent this problem, Liu et al. loaded astrocytes in area CA1 of the hippocampus with caged calcium. They then used focal photolysis to uncage and selectively increase astrocytic calcium levels, which they monitored by using calcium imaging. They simultaneously recorded the responses of neighboring GABAergic interneurons by conventional patch–clamp recording. After photolysis, astrocytic calcium levels were increased, and a coincident flurry of GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in nearby interneurons.
How does astrocytic calcium lead to an increased frequency of sIPSCs? sIPSCs can be either action potential-dependent or -independent so, in principle, astrocytes could cause the effect by increasing interneuronal spiking or by increasing the release of action potential-independent, miniature IPSCs (mIPSCs). However, an analysis of mIPSCs studied in isolation revealed that the frequency of mIPSCs is actually reduced by calcium uncaging, indicating that the observed increase in sIPSCs is caused by an excitation of interneurons that synapse onto the recorded interneuron (Fig. 1). Liu et al. reasoned that this excitation might be mediated by astrocytic glutamate release, followed by interneuronal glutamate receptor activation. Consistent with this idea, pharmacological analysis showed that the effect of uncaging on sIPSCs could be blocked by antagonism of kainate receptors containing the GluR5 subunit but was resistant to antagonism of other ionotropic glutamate receptor subtypes. In contrast, the effect of uncaging on mIPSCs was independent of ionotropic glutamate receptors.
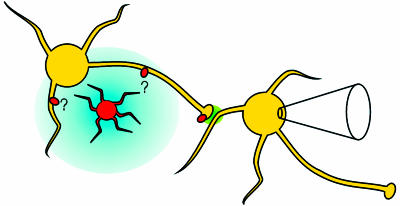
Fig. 1.
A model showing the circuit involved in the experiments of Liu et al. (9). An astrocyte (orange cell) is loaded with caged calcium. Upon photolysis, the astrocyte releases glutamate (blue), which spreads to activate GluR5-containing kainate receptors (red ovals) on nearby hippocampal interneurons (yellow cells). The activation of kainate receptors leads to increased firing of the interneuron, causing enhanced spontaneous GABA release (green). This enhanced release is detected as an increase in the frequency of sIPSCs on a nearby interneuron, monitored by patch–clamp recording. The range of glutamate after release from the astrocyte is unknown, as is the subcellular location of the interneuronal kainate receptors that sense it.
Kainate receptors are perhaps the most poorly understood members of the glutamate receptor family, but, by fortuitous coincidence, the role of kainate receptors on hippocampal interneurons has been the subject of much recent study. Kainate receptors regulate the activity of interneurons in the hippocampus by a variety of different mechanisms. Kainate receptors have been found to drive interneuronal firing by a direct depolarization of the somato–dendritic region of the cell (10–12), where they contribute to postsynaptic responses via conventional glutamatergic transmission (10, 11). However, kainate receptor activation can cause ectopic spiking even when the soma is held in voltage-clamp, indicating an excitatory action of kainate receptors at an electrotonically distant compartment of the cell where the clamp is poor, probably the axon (13). To add to the complexity, it has been reported that kainate receptors also are located in the presynaptic terminal of interneurons, where they are thought to enhance GABA release onto other interneurons (14, 15); however, this finding is controversial (13).
With so many subpopulations of kainate receptors on interneurons, which ones are activated by astrocytes (Fig. 1)? It seems unlikely that the astrocytic effect is mediated by kainate receptors in the presynaptic terminal, because these receptors are reported to increase the frequency of mIPSCs (14, 15), which was not observed. Because astrocytic activation does not generate an excitatory current in the recorded interneuron, Liu et al. argue that somato–dendritic kainate receptors are also unlikely to be the target of astrocytic glutamate, favoring instead that astrocytes cause the increase in sIPSCs through activation of axonal kainate receptors. One caveat, however, is that the spatial reach of glutamate released from the astrocyte is unknown. This means that the interneurons that are actually driven by the astrocytic glutamate to produce the increased frequency of sIPSCs may have stronger responses to the astrocyte than the recorded interneuron in which those sIPSCs are detected. It will be of interest to determine whether kainate receptor-mediated ectopic spiking can be directly observed in response to astrocytic stimulation.
Regardless of the specific subcellular location of the kainate receptors involved, it is tempting to speculate on the functional consequences of this astrocytic effect. The current study (9) indicates that astrocytic activation increases the inhibitory drive onto interneurons but does not indicate whether inhibitory drive onto pyramidal cells also is enhanced; it is noteworthy, however, that a previous study with some of the same coauthors (6) has reported that elevating calcium in astrocytes can increase both the amplitude and frequency of mIPSCs onto pyramidal cells through mechanisms that depend on ionotropic glutamate receptors. Taking the two studies together, it seems plausible that the astrocyte–interneuron connection has the effect of globally increasing inhibition. However, it remains unclear whether the glutamate released by astrocytes is detected only by interneurons, or by pyramidal cells, as well. In this context, it is of interest to note that kainate receptors also have been found to regulate glutamate release in area CA1 (16, 17) and also are present on the CA1 pyramidal cells themselves (12); in neither case has an endogenous trigger for activating these receptors been identified.
Much remains to be learned about the extent to which astrocytic activity can shape or drive neuronal responses and circuit function, but, for several reasons, it seems likely that astrocyte-driven effects will be dramatic. First, this study provides evidence that astrocytes drive interneurons, which are themselves powerful regulators of circuit activity; the firing of a single interneuron can synchronize the activity of >1,000 pyramidal cells (18). In addition, estimates based on anatomical data suggest that a single astrocyte can contact >100,000 synapses (19). Finally, astrocytes are extensively coupled, allowing cross-cell propagation of calcium waves that travel over long distances (1, 5, 7); however, it should be noted that, for unknown reasons, the present study did not observe these calcium waves in response to calcium uncaging. Collectively, these observations make it seem unlikely that glial signaling will be involved in highly localized processing but may indicate a role in large-scale neuromodulation or in switching between different modes of circuit behavior.
In conclusion, the finding that astrocytes can drive interneuronal activity provides a mechanism by which glia can communicate with neurons, and the observation that kainate receptors mediate this form of communication suggests a previously undescribed function for these poorly understood receptors. We are still a long way, to put it mildly, from redefining glia as the center of cognition. However, it is increasingly apparent that glia play a more influential role in the brain than previously imagined, and that some of our most central assumptions about the division of labor in the brain are due for revision.
See companion article on page 3172.
References
- 1.Dani, J. W., Chernjavsky, A. & Smith, S. J. (1992) Neuron 8, 429-440. [DOI] [PubMed] [Google Scholar]
- 2.Porter, J. T. & McCarthy, K. D. (1996) J. Neurosci. 16, 5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosche, J., Matyash, V., Moller, T., Verkhratsky, A., Reichenbach, A. & Kettenmann, H. (1999) Nat. Neurosci. 2, 139-143. [DOI] [PubMed] [Google Scholar]
- 4.Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S. & Haydon, P. G. (1994) Nature 369, 744-747. [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard, M. (1994) Science 263, 1768-1771. [DOI] [PubMed] [Google Scholar]
- 6.Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. (1998) Nat. Neurosci. 1, 683-692. [DOI] [PubMed] [Google Scholar]
- 7.Hassinger, T. D., Atkinson, P. B., Strecker, G. J., Whalen, L. R., Dudek, F. E., Kossel, A. H. & Kater, S. B. (1995) J. Neurobiol. 28, 159-170. [DOI] [PubMed] [Google Scholar]
- 8.Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. (1997) J. Neurosci. 17, 7817-7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, Q.-s., Xu, Q., Arcuino, G., Kang, J. & Nedergaard, M. (2004) Proc. Natl. Acad. Sci. USA 101, 3172-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C. & Ben-Ari, Y. (1998) Nat. Neurosci. 1, 470-478. [DOI] [PubMed] [Google Scholar]
- 11.Frerking, M., Malenka, R. C. & Nicoll, R. A. (1998) Nat. Neurosci. 1, 479-486. [DOI] [PubMed] [Google Scholar]
- 12.Bureau, I., Bischoff, S., Heinemann, S. F. & Mulle, C. (1999) J. Neurosci. 19, 653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semyanov, A. & Kullmann, D. M. (2001) Nat. Neurosci. 4, 718-723. [DOI] [PubMed] [Google Scholar]
- 14.Mulle, C., Sailer, A., Swanson, G. T., Brana, C., O'Gorman, S., Bettler, B. & Heinemann, S. F. (2000) Neuron 28, 475-484. [DOI] [PubMed] [Google Scholar]
- 15.Cossart, R., Tyzio, R., Dinocourt, C., Esclapez, M., Hirsch, J. C., Ben-Ari, Y. & Bernard, C. (2001) Neuron 29, 497-508. [DOI] [PubMed] [Google Scholar]
- 16.Chittajallu, R., Vignes, M., Dev, K. K., Barnes, J. M., Collingridge, G. L. & Henley, J. M. (1996) Nature 379, 78-81. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya, H. & Ozawa, S. (1998) J. Physiol. 509, 833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobb, S. R., Buhl, E. H., Halasy, K., Paulsen, O. & Somogyi, P. (1995) Nature 378, 75-78. [DOI] [PubMed] [Google Scholar]
- 19.Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. (2002) J. Neurosci. 22, 183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]