Abstract
The treatment of depression with selective serotonin reuptake inhibitors, SSRIs, is important to study on a neurochemical level because of the therapeutic variability experienced by many depressed patients. We employed the rapid temporal capabilities of fast scan cyclic voltammetry at carbon fiber microelectrodes to study the effects of a popular SSRI, escitalopram (ESCIT), marketed as Lexapro, on serotonin in mice. We report novel, dynamic serotonin behavior after acute ESCIT doses, characterized by a rapid increase in stimulated serotonin release and a gradual rise in serotonin clearance over 120 min. Dynamic changes after acute SSRI doses may be clinically relevant to the pathology of increased depression or suicidality after onset of antidepressant treatment. Due to the short-term variability of serotonin responses after acute ESCIT, we outline difficulties in creating dose response curves and we suggest effective means to visualize dynamic serotonin changes after SSRIs. Correlating chemical serotonin patterns to clinical findings will allow a finer understanding of SSRI mechanisms, ultimately providing a platform for reducing therapeutic variability.
Keywords: 5-HT, FSCV, carbon fiber microelectrode, SSRI, mice
Depression is a highly prevalent neuropsychiatric disorder characterized by low mood or self-esteem. Fatigue, migraines, anxiety, and changes in weight are among the many additional symptoms of depression.1 More than 9% of adult Americans are diagnosed with this disorder,2 and in 2011 over $11 billion was spent on pharmacological treatments.3 Selective serotonin-reuptake inhibitors (SSRIs) are the most frequently prescribed antidepressants and their usage appears to be rising.4 SSRIs carry side effects,5 and while their acute pharmacodynamic effects are rapid, several weeks of chronic administration may be required before clinical effectiveness can be achieved.6 During the initial dosing period, highly vulnerable patients are encouraged to seek psychological therapy to counteract increased suicide risks.7,8 Therefore, it is valuable to understand serotonin neurochemistry in experimental models in vivo after acute and chronic SSRI doses to elucidate the underlying mechanisms of SSRI action.
In this Letter, as a first step toward this goal, we seek to understand serotonin responses to escitalopram (ESCIT), one of the most popular SSRIs commercially known as Lexapro. While responses to ESCIT have previously been studied behaviorally9 and neurochemically with microdialysis,10 we are interested in characterizing the rapid serotonin responses afforded by fast-scan cyclic voltammetry (FSCV). FSCV at carbon fiber microelectrodes dynamically measures serotonin in the rat substantia nigra, pars reticulata (SNr) upon electrical stimulation of the dorsal raphe nucleus (DRN) or the medial forebrain bundle (MFB).11,12 Here we find the same to be true in mice. Additionally, we find that an acute dose of ESCIT dramatically alters both stimulated serotonin release and reuptake. This effect is both dose and time dependent in the short term (0–120 min). Interestingly, we find that there is a complex relationship between serotonin amplitude and clearance time after drug administration (amplitude and clearance vary differently and independently with time for each dose). We discuss these important findings in the context of the short-term effects of SSRIs and describe difficulties in constructing dose response curves.
Results and Discussion
SSRIs inhibit serotonin reuptake via the serotonin transporter (SERT). Researchers have traditionally studied the neurochemical mechanisms of acute SSRI uptake inhibition using microdialysis. Many studies show that acute SSRIs, independent of administration mode, augment basal serotonin levels.13−16 In the brain region studied here, the SNr, citalopram (CIT) was reported to increase serotonin levels by 660%.17 Because SSRIs prolong serotonin’s lifetime in the extracellular space,18 an increase in microdialysate serotonin supports a straightforward mechanism of uptake inhibition. However, because traditional microdialysis cannot distinguish dynamic events, a more rapid chemical analysis may increase our understanding of in vivo SSRI mechanisms.
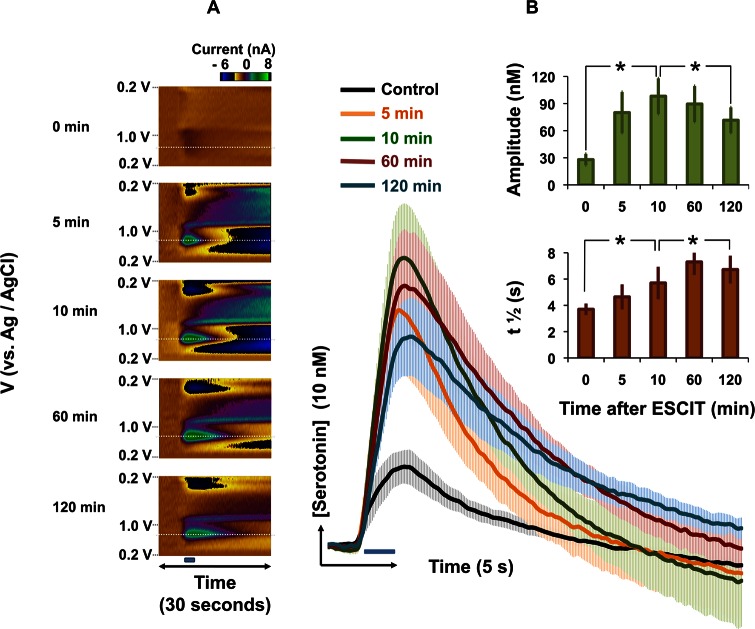
FSCV can differentiate between changes in serotonin release and uptake. FSCV signals typically constitute an initial rapid amplitude increase due to electrical stimulation, which reaches a maximum at the end of the stimulation. At the cessation of stimulation, the signal is dominated by a decay curve, the t1/2 of which is an index of serotonin clearance. As such, we will refer to the components of this signal as amplitude and t1/2. We explored the effect of an acute dose of ESCIT (10 mg kg–1) on serotonin release and uptake in SNr in mice in vivo. Figure 1A displays a typical color plot for serotonin release in the SNr evoked by electrical stimulation of the MFB (the stimulation is denoted by the horizontal blue bar between and the vertical gray box underlying the color plots). The amplitude (13.7 nM) and t1/2 (3.0 s) agree well with values reported for rats in vivo.19 Figure 1B shows the same response 60 min after ESCIT (10 mg kg–1) administration. The t1/2 of the signal increased to 5.8 s, and was accompanied by a 10-fold increase in the amplitude (132 nM) of the signal. Figure 1C is a superposition of [serotonin] versus time taken at the voltage indicated by the horizontal white dashed lines in Figure 1A and B. The inset in Figure 1C displays the cyclic voltammograms obtained at the vertical white dashed lines from both color plots. These cyclic voltammograms identify serotonin based on the positions of the oxidation and reduction peaks.11
Figure 1.
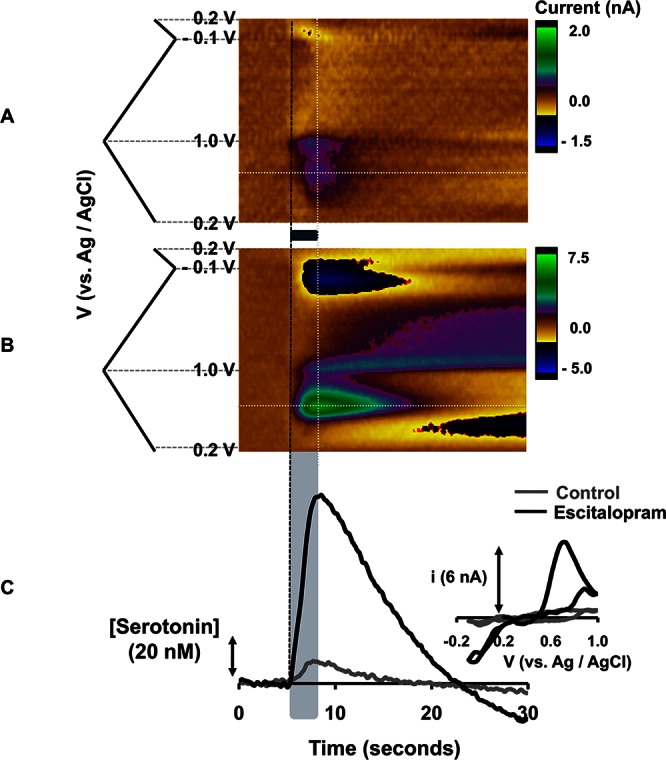
(A,B) Color plots with potential on the y-axis plotted against time on the x-axis and the current response represented in false color. These plots represent (A) the signal obtained in the SNr of an anesthetized mouse upon MFB stimulation (black bar under the color plot denotes the stimulation time and duration) (B) The same signal 1 h after ESCIT (10 mg kg–1) administration. (C) [Serotonin] vs time extrapolated from the horizontal dashed lines in (A) and (B) with inset cyclic voltammograms taken from the vertical white dashed lines.
We chose to study ESCIT because it is one of the most clinically useful SSRIs.3,20 However, other SSRIs such as fluoxetine and sertraline exhibit similar modes of action (selective SERT inhibition), and we have observed similar increases in serotonin amplitude in vivo with these two agents (unpublished observations).
A comparable potentiation of serotonin amplitude has previously been observed in vivo in anesthetized rats with CIT.11,19 Specifically, serotonin was found to increase by 380%11 and 480%.19 Current and previous findings in the intact brain of rats and mice are not in agreement with the data from tissue slice preparations. For example, Bunin et al. reported on the effects of fluoxetine on serotonin in slice preparations in the rat SNr.21,22 A modest increase in serotonin release (∼10%) was attributed to delayed clearance (increased t1/2). John and Jones reported similar findings in mouse SNr slices.23 It is not likely that the affinity of SERT for serotonin is different between in vivo and slice preparations. Therefore, the increase in stimulated serotonin release in response to ESCIT administration, of up to 700% of pretreatment values, appears to be specific to intact brain studies. These findings suggest that synaptic processes in intact brains additionally modulate SSRI mechanisms.
Figure 1 displays the serotonin response 60 min after ESCIT administration. We initially chose this time based on the pharmacodynamics of ESCIT (maximum transporter occupancy at 60 min33). However, if measurements are taken at 5–10 min intervals from 0 to 120 min, a more complete profile emerges. Figure 2A displays color plots taken at different intervals after ESCIT administration (10 mg kg–1) in a representative experiment. Figure 2B shows averaged [serotonin] versus time traces taken at the voltage indicated by the horizontal white dashed lines in each color plot in Figure 2A. The stimulation is denoted by the blue bar directly below the traces. Averaged serotonin release before drug administration (t = 0) was 28.0 ± 6.4 nM, and t1/2 was 3.7 ± 0.4 s. Ten minutes after drug administration, there was a significant increase in serotonin amplitude (98.1 ± 19.7 nM, p < 0.001) and a significant increase in t1/2 (5.7 ± 1.2 s, p < 0.001). Over the short-term, the t1/2 further significantly increased from the 10 min value, reaching 7.1 ± 1.1 s (p < 0.001) at 120 min. A significant decrease in amplitude occurred from 10 to 120 min (to 71.5 ± 14.2 nM, p = 0.011). These changes in serotonin amplitude and t1/2 over time are displayed in the histograms (inset Figure 2B).
Figure 2.
(A) Color plots with potential on the y-axis plotted against time on the x-axis and the current response represented in false color. These plots represent the time course of the serotonin signal after ESCIT administration in a representative animal. (B) [Serotonin] vs time, averaged for five animals (±SEM) taken from the maximum current value from the horizontal white dashed lines from each time course. The blue bar denotes stimulation onset and duration. Inset: Histograms showing maximum serotonin amplitude (green) and t1/2 (orange) with time after ESCIT administration. Stars represent statistically different significance of comparisons between data sets (p = 0.05 is used as a two-tailed cutoff for statistical significance).
The underlying cause of our primary finding (increased stimulated serotonin release after SSRIs in vivo) could be synaptic processes occurring in the intact brain that additionally modulate SSRI mechanisms. The data in Figure 2 show that these processes are rapid. Since evoked serotonin release is not expected to saturate the SERT,34 then a rapid increase in amplitude supports independence between release and uptake. Additionally, despite the rapid pharmacodynamics of ESCIT,33 the t1/2 of serotonin clearance continues to increase up to 120 min after administration. If we assume that ESCIT does not change its structure or function, then it is possible that SSRIs cause dynamic physiological changes outside of their accepted mode of action.
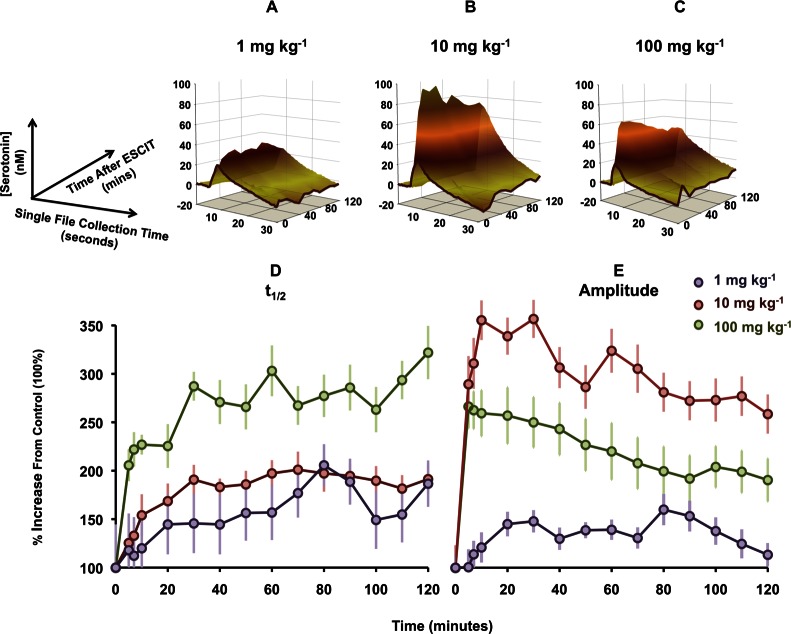
The data we have presented in Figures 1 and 2 were after a single dose of ESCIT (10 mg kg–1). To gain a better understanding of ESCIT using FSCV, it is useful to create dose response curves. There are two dynamic variables in our data that we could potentially utilize for a dose–response: serotonin amplitude and t1/2. It is difficult to assess which of the two components of our signal is more physiologically relevant for a dose–response. Additionally, the signal is not stable with time. It would be desirable to create a standardized method for dose response curves (i.e., to take the amplitude at a particular time after ESCIT for all doses). For this to hold, it is critical that the amplitude/clearance patterns we observed for 10 mg kg–1 change in a similar manner for other doses. In Figure 3A–C we compare the effects of 3 doses of ESCIT (1, 10, and 100 mg kg–1). We display this in 3-dimensions allowing amplitude (y-axis) and t1/2 (x-axis) to be observed with time after ESCIT (z-axis) (measurements were taken at 5–10 min intervals after ESCIT administration). The control (no-drug) response is highlighted in dark brown. The amplitude and clearance profiles varied across doses. The maximum amplitude and t1/2 at each time point are plotted separately, as a percentage from baseline, for ease of interpretation (Figure 3D and E).
Figure 3.
Dose responses to varying doses of ESCIT (A–C). The three-dimensional plots are [serotonin] vs time vs time after ESCIT administration. (D,E) Maximal values of amplitude and t1/2, respectively, taken from (A)–(C) at each time course for each dose. Values are presented as a percent change from baseline.
For t1/2, there was a clear response order with progressive doses. The maximum t1/2 was 202 ± 32.1% for 1 mg kg–1, 211 ± 14.6% for 10 mg kg–1, and 478 ± 205% for 100 mg. Over 120 min, all three doses displayed a continued t1/2 increase, but the time to reach a significant increase again also followed the dose order. Significance was achieved after 20 min for 1 mg kg–1 (147 ± 8.60% (p = 0.025)), 7 min for 10 mg kg–1 (131 ± 16.0% (p = 0.02)), and 5 min for 100 mg kg–1 (277 ± 101% (p = 0.026)).
The dose response order for t1/2 did not hold for amplitude. Here, the highest response was elicited by the middle dose, a phenomenon previously observed with DAT inhibition.35 For 1 mg kg–1, the maximum amplitude was 165 ± 19.7%, for 10 mg kg–1 this was 441 ± 145%, and for 100 mg kg–1 this was 257 ± 26.0%. These maxima were reached at 80, 30, and 7 min, respectively. The time taken to reach significantly increased amplitude was 20 min for 1 mg kg–1 and 5 min for both 10 and 100 mg kg–1 (all p < 0.001). For all three doses, there was a significant decrease at 120 min compared to the maximum amplitude (p < 0.01 for 1 mg kg–1, p = 0.01 for 10 mg kg–1, and p = 0.007 for 100 mg kg–1).
We are unable to find a consistent relationship in the serotonin profile after ESCIT with different doses. While this is physiologically interesting, it creates difficulties in reporting standard dose responses. This challenge highlights a necessity for multivariate modeling of the data to mathematically describe the serotonin profile in three dimensions with each dose. In the absence of multivariate models, dose response data is best visualized in the 3-D formats shown in Figure 3.
Understanding the clinical variability of SSRIs is further confounded by our poor understanding of their in vivo mechanisms. We studied the effects of acute doses of ESCIT on serotonin with FSCV at carbon fiber microelectrodes in mice. We showed that dynamic neurochemical changes accompanied ESCIT; notably that, over the short-term, serotonin release was greatly and rapidly augmented while its clearance only gradually increased. Finding that dynamic changes accompany acute SSRI doses are clinically interesting, since there is little clinical evidence for therapeutic relief after an acute dose. Our results underline the depth of information that can be offered by FSCV, and with relevant mathematical modeling of SSRI responses we can start to compare neurochemical profiles to clinical profiles. Ultimately, key chemical characteristics of SSRIs may be identified and correlated to specific clinical effects.
Methods
Animals and Surgery
Handling and surgery on male C57BL/6J mice weighing 20–25 g (Jackson Laboratory, Bar Harbor, ME) were in agreement with The Guide for the Care and Use of Laboratory Animals, approved by the Institutional Animal Care and Use Committees (IACUC) of Wayne State University. Food and water were offered ad libitum to the mice housed with a 12 h light/dark cycle. After urethane (25% dissolved in 0.9% NaCl solution, Hospira, Lake Forest, IL) was injected intraperitoneally, stereotaxic surgery (David Kopf Instruments, Tujunga, CA) was performed. A heating pad sustained mouse body temperature around 37 °C (Braintree Scientfic, Braintree, MA). Stereotaxic coordinates were taken in reference to bregma.36 A Nafion modified carbon fiber microelectrode was lowered into the SNr (AP: −1.58, ML: +1.40, DV: −4.2 to −4.8). The procedure for nafion electrodeposition is described in ref (11). This electrode was adjusted in the dorsal/ventral plane until a desired serotonin signal was observed. A stainless steel stimulating electrode (diameter: 0.2 mm, Plastics One, Roanoke, VA) was positioned into the MFB (AP: −3.28, ML: +1.10, DV: −5.0). Biphasic pulse trains applied through a linear constant current stimulus isolator (NL800A, Neurolog, Medical Systems Corp., Great Neck, NY) provoked serotonin efflux. The 60 Hz trains were 350 μA each phase, 2 ms in width, and 2 s in length. An Ag/AgCl reference electrode was implanted into the brain’s opposite hemisphere. Chloride was electroplated onto silver wire using 0.1 M HCl (13 V vs tungsten). After the experiment, 13 V (vs AgAgCl) was applied to the electrode surface in order to lesion tissue directly surrounding the carbon fiber. This lesion aids in verifying electrode placement using histology.
FSCV Procedures
T-650 carbon fibers (diameter: 7 μm, Goodfellow, Oakdale, PA) were aspirated into glass capillaries (internal diameter: 0.4 mm, external diameter: 0.6 mm, AM Systems, Carlsborg, WA). A vertical pipet puller (Narishige Group, Tokyo, Japan) was used to taper the filled capillaries under gravity. The protruding carbon fibers were cut to approximately 150 μm, and Nafion was electrodeposited as described previously.11 The serotonin or “5-HT waveform” was used:37 the electrode was scanned at 1000 V s–1 from 0.1 to 1.0 V at 10 Hz and was held at 0.2 V. Electrodes with this waveform have a precalibration sensitivity of 49.5 nA ± 10.2 μM–1 to serotonin,11 and this value was used as a calibration factor. Post calibrations were not performed because the working electrode was utilized to create a lesion by applying 13 V to the electrode surface. Custom software, written using LabVIEW 2009 (National Instruments, Austin, TX), and hardware were used to collect data. A PCIe-6341 DAC/ADC Card (National Instruments) generated the waveform and collected the data. Potentials were measured against an Ag/AgCl reference electrode.
Data Analysis
Custom built software, written in LabVIEW 2009, was used for background subtraction, data analysis, and signaling processing including digital filtering (zero phase, Butterworth, fourth order, 5 kHz). The Butterworth filter is a maximally flat magnitude filter that applies an algorithm to remove noise with a cutoff of 5 kHz (based on serotonin waveform scan rate of 1000 V s–1). Two-dimensional moving average smoothing was performed on nine points with zero-phase correction. Using all temporal data points for amplitude and t1/2, one way repeated measures ANOVAs were conducted. Post hoc tests of the significance of linear combinations of ANOVA parameter estimates were used to determine the statistical significance of changes between different time points. This analysis was performed on Stata (StataCorp, version 12.1, College Station, TX) and p = 0.05 was used as a two-tailed cutoff for statistical significance. When baselines were subject to drift, a manual baseline correction was performed. Three-dimensional plots were created using SigmaPlot (Systat Software, San Jose, CA). The data in Figure 3 were analyzed using eDAQ Chart Software (Denistone East, Australia). Pooled data is presented as n = 5 ± standard error of the mean (SEM).
Drugs
Escitalopram oxalate was obtained from Sigma-Aldrich (St. Louis, MO). It was dissolved in 0.9% NaCl Hospira, (Lake Forest, IL) and injected into the intraperitoneal space at a volume of 0.1 mL 20 g–1 body weight (based on the molecular weight of the escitalopram salt). ESCIT (10 mg kg–1) was chosen as a high to intermediate dose based on previous studies in mice and rats.10,38 The low dose, 1 mg kg–1, was chosen because doses lower than this do not typically have an effect on the FSCV signal. The high dose, 100 mg kg–1, was chosen to have the same order of magnitude difference as the other two doses.
Acknowledgments
The authors acknowledge Kristin Gallik, Anisa Zeqja, and Calvin Austin for experimental assistance.
Author Contributions
P.H. and K.M.W. designed the experiments. K.M.W. performed the experiments. P.H. and K.M.W. analyzed data and wrote the manuscript.
This research was supported by Wayne State University Start-up Funds (P.H., PI).
The authors declare no competing financial interest.
References
- Hamilton M. (1960) A rating scale for depression. J. Neurol., Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez O., Berry J. T., Mcknight-Eily L. R., Strine T., Edwards K. W., and Croft J. B. (2010) Current Depression Among Adults - United States, 2006 and 2008. In Morbidity and Mortality Weekly Report, Center for Disease Control and Prevention, Hyattsvilles, MD. [Google Scholar]
- Lindsley C. W. (2012) The top prescription drugs of 2011 in the United States: antipsychotics and antidepressants once again lead CNS therapeutics. ACS Chem. Neurosci. 3, 630–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L., Brody D., and Gu Q. (2011) Antidepressant use in persons aged 12 and over: United States, 2005–2008. In NCHS data brief, National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Ferguson J. M. (2001) SSRI Antidepressant Medications: Adverse Effects and Tolerability. J. Clin. Psychiatry 3, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg A. J.; Chesen C. L. (2000) How fast are antidepressants?. J. Clin. Psychiatry 61, 712–721. [DOI] [PubMed] [Google Scholar]
- Stone M.; Laughren T.; Jones M. L.; Levenson M.; Holland P. C.; Hughes A.; Hammad T. A.; Temple R.; Rochester G. (2009) Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 339, b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D.; Doucette S.; Glass K. C.; Shapiro S.; Healy D.; Hebert P.; Hutton B. (2005) Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 330, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C.; Bergqvist P. B.; Brennum L. T.; Gupta S.; Hogg S.; Larsen A.; Wiborg O. (2003) Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activitie. Psychopharmacology (Berlin, Ger.) 167, 353–362. [DOI] [PubMed] [Google Scholar]
- Ceglia I.; Acconcia S.; Fracasso C.; Colovic M.; Caccia S.; Invernizzi R. W. (2004) Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br. J. Pharmacol. 142, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P.; Dankoski E. C.; Petrovic J.; Keithley R. B.; Wightman R. M. (2009) Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal. Chem. 81, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P.; Dankoski E. C.; Wood K. M.; Ambrose R. E.; Wightman R. M. (2011) In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J. Neurochem. 118, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. J.; Gundlah C.; Auerbach S. B. (1995) Systemic uptake inhibition decreases serotonin release via somatodendritic autoreceptor activation. Synapse 20, 225–233. [DOI] [PubMed] [Google Scholar]
- Invernizzi R.; Bramante M.; Samanin R. (1995) Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 696, 62–66. [DOI] [PubMed] [Google Scholar]
- Moret C.; Briley M. (1996) Effects of acute and repeated administration of citalopram on extracellular levels of serotonin in rat brain. Eur. J. Pharmacol. 295, 189–197. [DOI] [PubMed] [Google Scholar]
- Bosker F. J.; Klompmakers A. A.; Westenberg H. G. (1995) Effects of single and repeated oral administration of fluvoxamine on extracellular serotonin in the median raphe nucleus and dorsal hippocampus of the rat. Neuropharmacology 34, 501–508. [DOI] [PubMed] [Google Scholar]
- Thorre K.; Ebinger G.; Michotte Y. (1998) 5-HT4 receptor involvement in the serotonin-enhanced dopamine efflux from the substantia nigra of the freely moving rat: a microdialysis study. Brain Res. 796, 117–124. [DOI] [PubMed] [Google Scholar]
- Baumann P. (1996) Pharmacology and pharmacokinetics of citalopram and other SSRIs. Int. Clin. Psychopharmacol. 11(Suppl 1), 5–11. [DOI] [PubMed] [Google Scholar]
- Hashemi P.; Dankoski E. C.; Lama R.; Wood K. M.; Takmakov P.; Wightman R. M. (2012) Brain dopamine and serotonin differ in regulation and its consequences. Proc. Natl. Acad. Sci. U.S.A. 109, 11510–11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A.; Furukawa T. A.; Salanti G.; Geddes J. R.; Higgins J. P.; Churchill R.; Watanabe N.; Nakagawa A.; Omori I. M.; McGuire H.; Tansella M.; Barbui C. (2009) Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373, 746–758. [DOI] [PubMed] [Google Scholar]
- Bunin M. A.; Prioleau C.; Mailman R. B.; Wightman R. M. (1998) Release and uptake rates of 5-hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. J. Neurochem. 70, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Bunin M. A.; Wightman R. M. (1998) Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmissio. J. Neurosci. 18, 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John C. E.; Jones S. R. (2007) Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology 52, 1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R. M.; Kuhr W. G.; Ewing A. G. (1986) Voltammetric detection of dopamine release in the rat corpus striatum. Ann. N.Y. Acad. Sci. 473, 92–105. [DOI] [PubMed] [Google Scholar]
- Ewing A. G.; Wightman R. M. (1984) Monitoring the stimulated release of dopamine with in vivo voltammetry. II: Clearance of released dopamine from extracellular fluid. J. Neurochem. 43, 570–577. [DOI] [PubMed] [Google Scholar]
- Addy N. A.; Daberkow D. P.; Ford J. N.; Garris P. A.; Wightman R. M. (2010) Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J. Neurophysiol. 104, 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr N. R.; Daniel K. B.; Belle A. M.; Carelli R. M.; Wightman R. M. (2010) Probing presynaptic regulation of extracellular dopamine with iontophoresis. ACS Chem. Neurosci. 1, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft J. C.; Osterhaus G. L.; Ortiz A. N.; Garris P. A.; Johnson M. A. (2009) In vivo dopamine release and uptake impairments in rats treated with 3-nitropropionic acid. Neuroscience 161, 940–949. [DOI] [PubMed] [Google Scholar]
- Mitch Taylor I.; Jaquins-Gerstl A.; Sesack S. R.; Michael A. C. (2012) Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J. Neurochem. 122, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott Z. A.; Fox M. E.; Walsh P. L.; Urban D. J.; Ferrel M. S.; Roth B. L.; Wightman R. M. (2013) Noradrenergic Synaptic Function in the Bed Nucleus of the Stria Terminalis Varies in Animal Models of Anxiety and Addiction. Neuropsychopharmacology 10.1038/npp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Kile B. M.; Wightman R. M. (2009) In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur. J. Neurosci. 30, 2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L.; Jakala P.; Tanila H. (2005) Noradrenaline overflow in mouse dentate gyrus following locus coeruleus and natural stimulation: real-time monitoring by in vivo voltammetry. J. Neurochem. 95, 641–650. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M.; Smith D. G.; Brennum L. T.; Sanchez C. (2008) Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice. Br. J. Pharmacol. 155, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez X. A., Bressler A. J., and Andrews A. M. (2007) Determining Serotonin and Dopamine Uptake Rates in Synaptosomes Using High-Speed Chronoamperometry. In Electrochemical Methods for Neuroscience (Michael A. C., and Borland L. M., Eds.), CRC Press: Boca Raton, FL. [PubMed] [Google Scholar]
- Song R.; Zhang H. Y.; Li X.; Bi G. H.; Gardner E. L.; Xi Z. X. (2012) Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc. Natl. Acad. Sci. U.S.A. 109, 17675–17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., and Watson C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th ed., Elsevier, Amsterdam. [Google Scholar]
- Jackson B. P.; Dietz S. M.; Wightman R. M. (1995) Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 67, 1115–1120. [DOI] [PubMed] [Google Scholar]
- Fish E. W.; Faccidomo S.; Gupta S.; Miczek K. A. (2004) Anxiolytic-like effects of escitalopram, citalopram, and R-citalopram in maternally separated mouse pups. J. Pharmacol. Exp. Ther. 308, 474–480. [DOI] [PubMed] [Google Scholar]