Abstract
In vivo calibration of microdialysis probes is required for interpreting measured concentrations. The most popular method of in vivo calibration is no-net-flux (NNF), which requires infusing several concentrations of neurotransmitters to determine in vivo recoveries (extraction fraction or Ed) and extracellular concentrations. A new method for in vivo calibration of microdialysis of neurotransmitters using glutamate (GLU) and dopamine (DA) as model analytes is reported. 13C6-DA and 13C5-GLU were perfused through microdialysis probes as internal calibrators. Using liquid chromatography with mass spectrometry, it was possible to distinguish the 13C-forms from the endogenous forms of each neurotransmitter. Ed was directly calculated by measuring the loss of the 13C-forms during infusion. The measured endogenous 12C forms of the neurotransmitters could be corrected for Ed to give calibrated extracellular concentrations in vivo. Retrodialysis of stable-isotope-labeled (SIL) neurotransmitters gave Ed and extracellular concentrations of 13C5-GLU and 13C6-DA that matched no-net-flux measurements; however, the values were obtained in a fraction of time because no added measurements were required to obtain the calibration. Ed was reduced during uptake inhibition for GLU and DA when measured by SIL retrodialysis. Because Ed is directly measured at each microdialysis fraction, it was possible to monitor changes in Ed under transient conditions created by systemic injection of uptake inhibitors. The results show that DA and GLU concentrations are underestimated by as much as 50% if not corrected for Ed during uptake inhibition. SIL retrodialysis provides equivalent information to NNF at much reduced time and animal use.
Keywords: No-net-flux, dopamine, glutamate, retrodialysis, cocaine, nucleus accumbens
Neurotransmitter concentrations in the extracellular space represent the balance between release (e.g., by exocytosis and reverse transport) and removal (e.g., by reuptake and enzymatic degradation). Measurement of concentration dynamics in this space is valuable for understanding neuronal communication. Microdialysis is a popular approach for such measurements, but its use is hampered by the difficulty of quantifying neurotransmitter concentrations in vivo. In this study, we report a novel approach to measuring in vivo recovery and quantification.
The recovered concentration of an analyte from a microdialysis probe (Cout) is a complex function of concentration external to the probe (Cext) and transport into the probe. Relative recovery (RR), defined as Cout/Cext, can be directly measured in vitro by fixing Cext and measuring Cout. However, it has long been recognized that in vitro recovery, which is determined primarily by the probe, dialysis flow rate, analyte, and temperature, is not necessarily accurate in vivo.1−3 In particular, factors such as tissue permeability, reuptake, and metabolism will affect recovery in vivo.
For many experiments, only the relative change of neurotransmitter is of interest, and impact of probe recovery is not considered; however, measurement of in vivo recovery and quantification, defined as determining the apparent extracellular concentration (Capp),4,5 can be crucial to obtaining meaningful results.3,6−10 Several methods for in vivo calibration have been developed including low flow,11,12 no-net-flux (NNF or Lönnroth method),9,13 and retrodialysis methods.14−16
In the low flow method, the dialysis flow rate is reduced so that recovery is ∼100%, making it, in principle, independent of external processes. A related alternative is to measure recovery at different flow rates and extrapolate to zero flow.17 These methods are rarely used because they require long times (either to collect enough sample or to measure at different flow rates), but a recent innovation of using low flow to recover, then higher flow to pass sample to the analytical system overcomes this obstacle.18
The most common in vivo calibration method is NNF. In this method, several concentrations of analyte are infused into the dialysis probe (Cin) while recording Cout.9,13 The difference (Cin – Cout), which corresponds to flux across the probe membrane, is plotted against Cin, so that the x-intercept is the point where flux is zero. The point of NNF corresponds to Capp. The slope of the NNF line, (Cin – Cout)/(Cin-Capp), is extraction fraction (Ed), a measure of in vivo probe recovery. Providing a value for Ed is a strong advantage of the NNF method. For example, it has been shown that the Ed of dopamine (EdDA) is relatively insensitive to inhibition of release, synthesis, and metabolism but strongly affected by uptake inhibition.9 This insight allows Ed to be an indirect measure of DA uptake in vivo. Knowledge of Ed can also be helpful in interpreting changes in Capp. If a drug or genetic manipulation evokes a change in Capp, it is not clear if this is related to a change in release or reuptake; however, knowledge of Ed can aid interpretation. For example, acute ethanol was shown to increase DA concentration recovered in the nucleus accumbens. After showing no change in EdDA, the increase in Capp could be attributed to increased DA release.3
Despite the appeal of NNF, it suffers from several disadvantages. By requiring infusion of several concentrations, it is time-consuming. Further, it assumes that recovery is constant over the course of the experiment; however, this is not always true. For example, if uptake is inhibited by a systemic drug injection, then uptake and recovery will change as the drug concentration changes.6 This effect can be accounted for by using dynamic NNF (dNNF) in which one concentration of a neurotransmitter is perfused through the probe for one animal.3,6 By infusing different animals with different concentrations and then pooling animals at different concentrations, a NNF trace can be calculated for each time point. dNNF allows for the measurement of Ed and Capp under transient conditions; however, a large number of animals are required. Further, it assumes minimal probe variability and precludes observation of individual differences.
In vivo calibration can also be achieved by using “retrodialysis”. In this case, calibration is achieved before (or after) an experiment by infusing the target compound through the probe in vivo while Capp is known to be zero. Measuring Cout allows a direct measurement of the loss or extraction of analyte by the sample (e.g., brain) and calculation of Ed. This value can be used to quantify results in that subject during an experiment. The method is readily used for exogenous chemicals, such as drugs; however, it cannot be used for neurotransmitters which cannot be removed from the brain to give a Capp = 0. This method also assumes that recovery is constant over the course of the experiment.
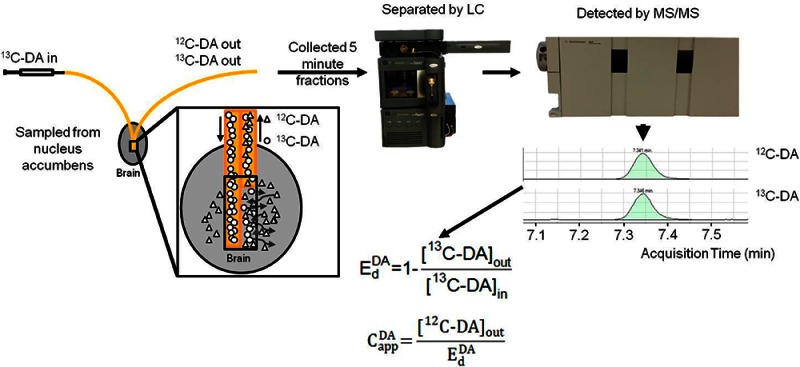
A variation of this method is to continuously infuse a similar but distinguishable form of the compound through the probe. Every fraction collected then allows determination of Ed of the infused compound (which has Capp = 0) which can then be used to quantify the Capp for the target analyte. For example, ropivacaine was infused to measure Ed and then quantify bupivacaine, which differs by 1 methyl group from ropivacaine.19 This approach assumes that the modified form behaves identically to the actual analyte. A better standard to infuse is a stable-isotope labeled (SIL) form. In a SIL retrodialysis experiment, the concentration of the labeled analyte in the brain (CappSIL) = 0 so that Ed can be calculated directly:
| 1 |
Because Ed of the analyte is equal to EdSIL, the apparent concentration can be calculated by
| 2 |
where Cmeasuredendogenous is the concentration of endogenous analyte. This approach has been previously used with drugs. For example, d3-morphine and 13C4-cortisone have been infused to monitor Ed of these drugs and quantify them in vivo.15,16
Retrodialysis of SIL neurotransmitters has not been reported, but it would be expected to be especially useful because neurotransmitters are the most frequent target of microdialysis measurements. Retrodialysis of SIL neurotransmitters would also allow dynamic changes in Ed to be recorded. Here we describe the use of this method for DA and glutamate (GLU). 13C forms of these compounds were infused constantly during experiments, allowing calculation of Ed at every time point. At the same time, the endogenous 12C form of each neurotransmitter was collected. The different forms of each compound were measured and distinguished using liquid chromatography–mass spectrometry. Comparison to NNF revealed that the SIL retrodialysis method yields equivalent results. An important advantage of the SIL method is that it allows in vivo correction for in vivo extraction of every dialysate sample with no additional measurements in contrast to NNF.
Results and Discussion
In Vitro SIL Retrodialysis
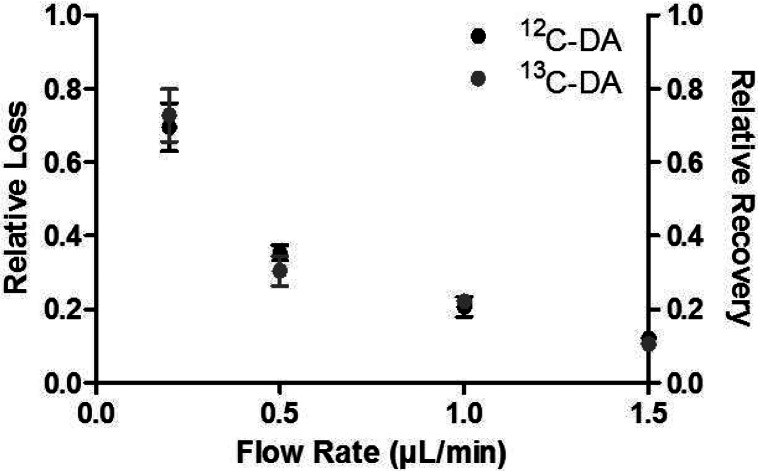
In principle, relative loss of 13C6-DA should be the same as relative recovery of 12C-DA that is being sampled. To test this idea, 200 nM 13C6-DA was perfused through a probe sampling from a well-stirred solution of 200 nM 12C-DA. The relative loss of 13C6-DA was within 10% of the recovery of 12C-DA across a range of flow rates showing that transport to and from the probe in vitro was equivalent (Figure 1).
Figure 1.
200 nM 13C6-DA was perfused through the probe sitting in a well stirred vial of 200 nM 12C-DA. The loss of the 13C6-DA was compared to the recovery of the 12C-DA across four flow rates (0.2, 0.5, 1.0, and 1.5 μL/min). The recovery matched the loss at each flow rate, showing diffusion rates in vitro are equal.
Effect of Infused Transmitter on Endogenous Neurotransmitter
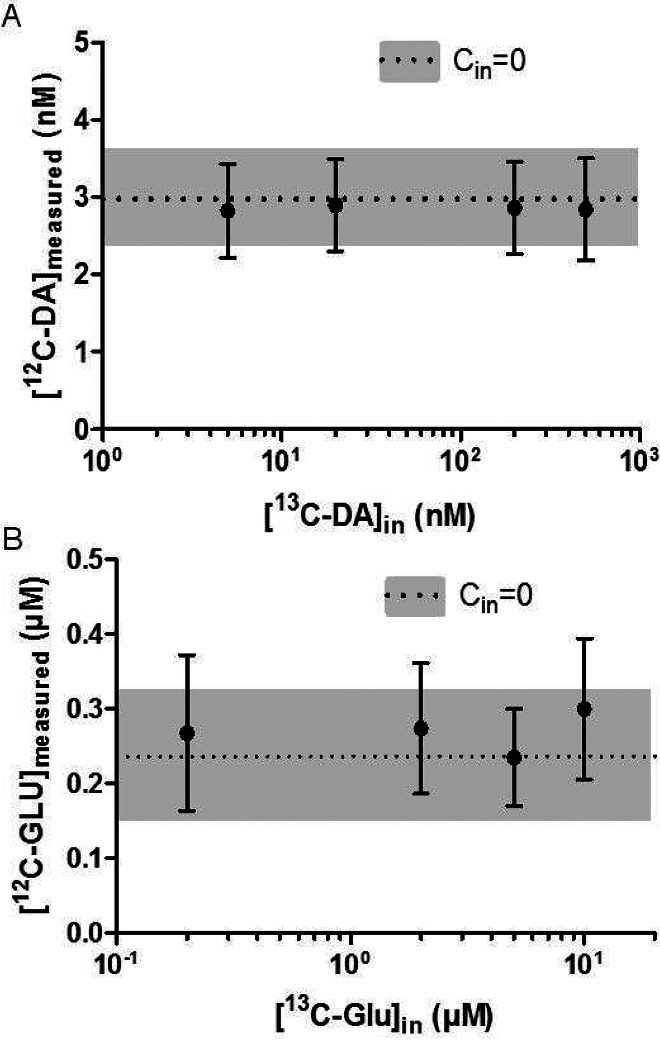
Both NNF and SIL retrodialysis rely on infusing a neurotransmitter through the dialysis probe. Such compounds may affect the process being measured by activating receptors (e.g., autoreceptors) or by affecting uptake (e.g., saturating uptake). To determine if infusion of neurotransmitter affected endogenous levels, we infused SIL neurotransmitter while monitoring the endogenous form being collected from the nucleus accumbens. For concentrations typically used for NNF, the infused SIL form had no effect on the concentration of the 12C form (i.e., endogenous form) collected for both GLU and DA (see Figure 2). This result confirms the conclusion previously reached for lack of an effect by infused DA on DA during NNF and extends it to GLU.9
Figure 2.
(A) When varying concentrations of 13C6-DA perfused through the probe, 12C-DA measured remained constant. The dotted line indicates the CoutDA when Cin = 0, and the gray bars represent the SEM with no 13C6-DA perfused (N = 5, error bars are SEM). (B) Perfusing up to 10 μM 13C5-GLU had no effect on the endogenous concentration of GLU. The dotted line and gray bar indicate CoutGLU when Cin = 0. N = 5, error bars show SEM.
In Vivo SIL Retrodialysis
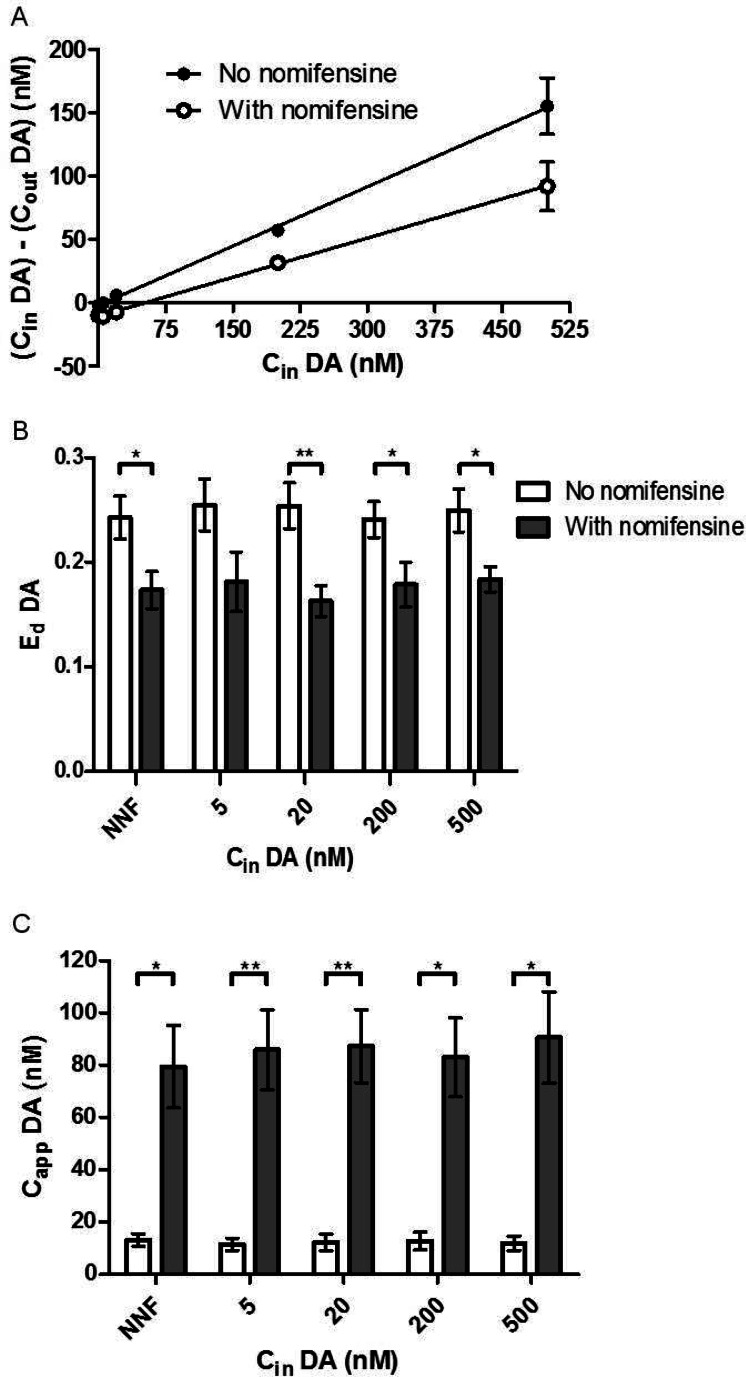
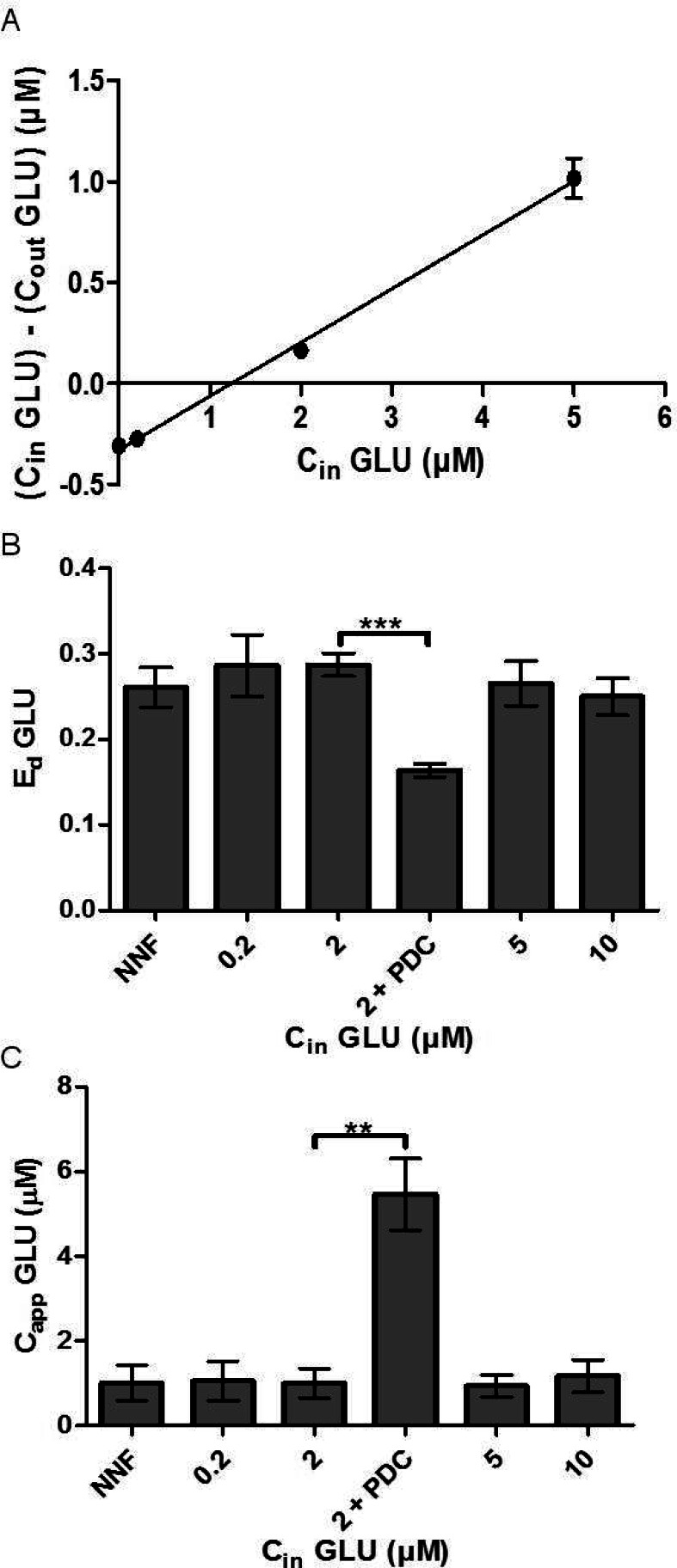
We next sought to determine if Ed and Capp measured by SIL retrodialysis of 13C labeled neurotransmitters would be equivalent to that measured by NNF. For these experiments, the 13C labeled neurotransmitter was infused at different concentrations and Ed was calculated using eq 1 for each concentration infused. For NNF, Cin was the concentration of 13C labeled neurotransmitter perfused through the probe and Cout was calculated by summing [13C6-DA] and [12C-DA]. The same method was used for GLU. By NNF EdDA = 0.24 ± 0.02 (Figure 3A) and Ed = 0.26 ± 0.02 (Figure 4A). The average EdDA measured by SIL retrodialysis was 0.26 ± 0.03, 0.25 ± 0.02, 0.24 ± 0.02, and 0.25 ± 0.02 with 5, 20, 200, and 500 nM 13C6-DA infused, respectively (Figure 3B). In principle, the highest possible Ed value is that for a well-stirred solution in vitro.4 It has previously been found that in the nucleus accumbens Ed approaches the maximal values, likely because of the effects of high uptake rates.7 In agreement with these previous observations, our in vivo values were not statistically different from the in vitro value of 0.22 ± 0.003 at the same flow rate shown in Figure 1. The slight differences are most likely due to the variability inherent in probes. The average EdGLU measured by SIL retrodialysis was 0.28 ± 0.04, 0.29 ± 0.01, 0.27 ± 0.03, and 0.25 ± 0.02 with 0.2, 2, 5, and 10 μM 13C5-GLU infused, respectively (Figure 4B). The Ed values measured by NNF and SIL retrodialysis (at all concentrations) were not statistically different and were on average within 5% of each other. These results show that Ed was not affected by the concentration infused and SIL retrodialysis was equivalent to NNF under these conditions.
Figure 3.
(A) A representative NNF curve from one rat shows how perfusing nomifensine through the probe (black open circles), EdDA (the slope) reduces and Capp increases (x-intercept) compared to no nomifensine (black solid circles). Cin consisted of varying concentrations of 13C6-DA. Cout was measured by summing the total DA measured (the 12C and 13C form). (B) EdDA was compared between SIL retrodialysis calibration and NNF calibration. Nomifensine, a DA uptake inhibitor, was perfused through the probe to demonstrate the reduction of Ed. (C) CappDA was also calculated using eq 2. At all concentrations of 13C6-DA perfused, Capp and EdDA matched the NNF values. Error bars show SEM (n = 5 for each group). Paired t tests were used for comparison. ** indicates p-value < 0.01, and * indicates p-value < 0.05.
Figure 4.
(A) Sample NNF curve is shown when perfusing varying concentrations of 13C5-GLU through the probe. Cout was calculated by summing [13C5-GLU measured] and [12C-GLU measured]. (B) SIL retrodialysis was compared to NNF to measure EdGLU. Ed SIL retrodialysis values matched that of the NNF values. 750 μM PDC was perfused with 2 μM 13C6-DA, which decreased EdGLU (p < 0.001, unpaired t test). (C) Ed and [12C-GLU measured] were used to calculate CappGLU. Capp for SIL retrodialysis matched CappGLU measured by NNF. After perfusion of 750 μM PDC, Capp increased 550% (p-value < 0.001). Error bars show SEM (n = 5 except for PDC infusion, n = 4).
With Ed calculated and the dialysate concentration of neurotransmitter measured, Capp could be calculated. By NNF, CappDA in the nucleus accumbens was calculated to be 13 ± 2 nM. Using SIL retrodialysis (eq 2), Capp was measured to be 11 ± 2, 12 ± 3, 13 ± 3, and 12 ± 3 nM for 5, 20, 200, and 500 nM 13C6-DA infused, respectively (Figure 3C). These concentrations match well with previously reported results for NNF measurements of DA in the nucleus accumbens of awake rats, for example, 8.8,3 11.4,7 10,9 and 5.2 nM.20 The values also match well the concentration determined by signal averaging DA transients over several minutes in the nucleus accumbens with fast-scan cyclic voltammetry. By averaging the transients, the extracellular DA concentration was estimated to be ∼20 nM.21
CappGLU was calculated to be 1.0 ± 0.4 μM using NNF. Using SIL retrodialysis, Capp was calculated to be 1.1 ± 0.5, 1.0 ± 0.3, 1.0 ± 0.3, and 1.2 ± 0.4 μM with 0.2, 2, 5, and 10 μM CinGLU perfused through the probe, respectively (Figure 4C). Capp and CappGLU measured with NNF were within 8% that measured by SIL retrodialysis and were not statistically different, showing that these methods give equivalent results for both neurotransmitters. Our values fell within acceptable ranges compared to previous studies which used uncalibrated microdialysis.22,23 Little work has been performed with quantitative microdialysis of GLU in the nucleus accumbens for direct comparison.
It has previously been reported that EdDA is largely governed by reuptake so that uptake inhibition causes Ed to decrease.9 As a result, if EdDA is measured prior to uptake inhibition then the concentration would be underestimated either in absolute concentration or by percent of baseline during uptake inhibition. The effect of uptake inhibition is rationalized by considering that DA is primarily removed from the extracellular space via uptake so that decreasing the uptake will lower the concentration gradient between the extracellular space and the probe. To determine if SIL retrodialysis and NNF offered equivalent responses to this perturbation, we blocked uptake by perfusing 5 μM nomifensine through the probe at all 13C6-DA concentrations infused. As shown in Figure 3, nomifensine decreased Ed by 30% as measured by both NNF and SIL retrodialysis. With nomifensine in the probe, EdDA was calculated to be 0.17 ± 0.2 using NNF. With SIL retrodialysis, Ed was calculated to be 0.18 ± 0.03, 0.16 ± 0.02, 0.18 ± 0.02, and 0.18 ± 0.01 with 5, 20, 200, and 500 nM 13C6-DA perfused through the probe. As expected, CappDA increased with nomifensine. Using NNF, Capp was calculated to be 80 ± 20 nM. Using SIL retrodialysis, CappDA was calculated to be 86 ± 16, 87 ± 14, 83 ± 15, 91 ± 18 nM for 5, 20, 200, and 500 nM 13C6-DA perfused through the probe, respectively.
Similar to EdDA, Ed has also been shown to be controlled by reuptake.24 To determine if a change in EdGLU could be measured by SIL retrodialysis during uptake inhibition, we perfused 750 μM of the GLU uptake inhibitor l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC) through the probe and performed SIL retrodialysis using 2 μM 13C5-GLU. As shown in Figure 4B and C, Ed was decreased by 40% as measured by SIL retrodialysis (p < 0.001) while CappGLU increased 550% (p < 0.005). These results show that (1) SIL retrodialysis and NNF provide equivalent results during uptake inhibition and (2) uptake inhibition decreases Ed similar to EdDA.
Ed Changes under Transient Conditions
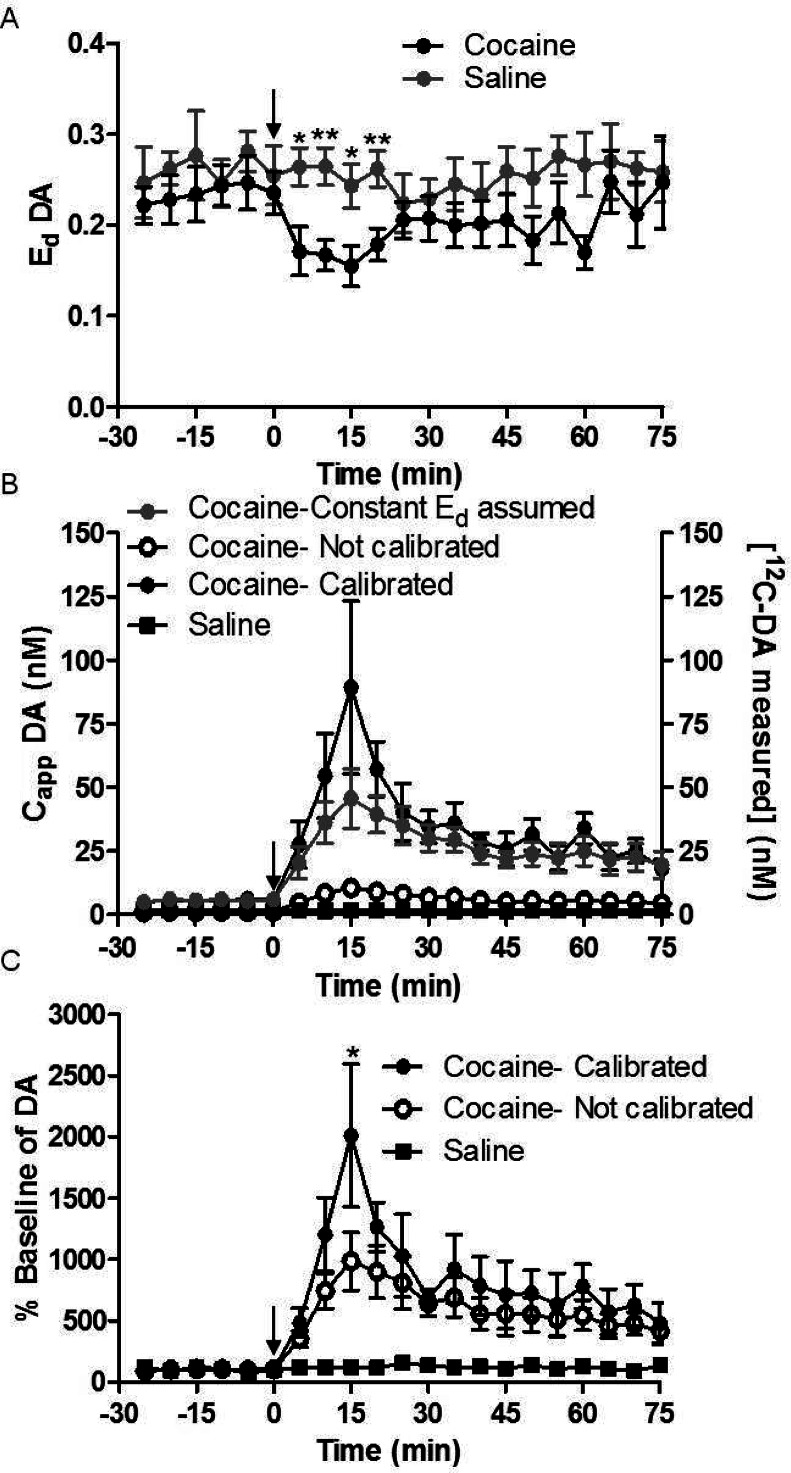
The above results show that uptake inhibition affects Ed and therefore interpretation of the magnitude of dialysate concentrations that are detected. It is reasonable to expect that uptake inhibition is steady during drug infusion through a probe; however, it will change during a systemic injection due to pharmacokinetics. To demonstrate that SIL retrodialysis could monitor dynamic changes in Ed, we used the method to follow CappDA and Ed during systemic cocaine injection (20 mg/kg, i.p.) while infusing 200 nM 13C6-DA through the probe for the duration of the experiment. As shown in Figure 5, EdDA decreased by an average of 35% within 20 min of cocaine injection (p-value < 0.005 using the mixed model regression) compared to saline injection, similar to previous reports using dNNF.6 For the first 20 min after cocaine injection Ed was lower than after saline injection (p-values <0.05, 0.005, 0.05, and 0.005 for the fractions collected at 5, 10, 15, and 20 min after injection using unpaired t test).
Figure 5.
(A) EdDA was measured during a cocaine challenge. At t = 0, cocaine (20 mg/kg i.p., black solid circles) or saline (gray solid circles) was administered. Ed was reduced by 35% (p-value < 0.005) in the presence of cocaine compared to saline. (B) [12C-DA measured] (right axis, black open circles), CappDA with a constant Ed assumed (EdDA measured prior to cocaine injection, gray solid circles), and Capp measured with SIL retrodialysis calibration (black solid circles) are shown when cocaine was administered. [12C-DA measured] was also measured after saline injection (right axis, black solid squares). N = 7 for each group, saline and cocaine. CappDA was an average of 160% greater than Capp with constant EdDA for the first 20 min (p-value < 0.001). (C) Calibrated (black solid circles) and conventional (black open circles) microdialysis was measured in % basal of 12C-DA after cocaine injection. Using the mixed model regression, there was an average 200% increase in % basal of calibrated vs not-calibrated (p-value < 0.001). Paired t tests show significance of individual fractions. Saline produced no change in DA (black solid squares). N = 7 and error bars show SEM. ** indicates p-value < 0.01, and * indicates p-value < 0.05.
Because EdDA was changing following cocaine injection, the SIL retrodialysis method reported a higher Capp than if it were measured assuming a constant EdDA (where Ed was measured prior to cocaine injection) as shown in Figure 5. Using mixed model regression, the SIL retrodialysis CappDA was measured to be 160% greater on average compared to a constant Ed over the first 20 min of sampling following cocaine injection (p < 0.001). In addition, the relative increase in CappDA was 160% higher when calibrated by SIL retrodialysis compared to a constant Ed over the first 20 min of drug (p < 0.001). The peak increase as a percent of basal with calibrated DA was measured to be 190% higher than the noncalibrated measurement (p-value < 0.05).
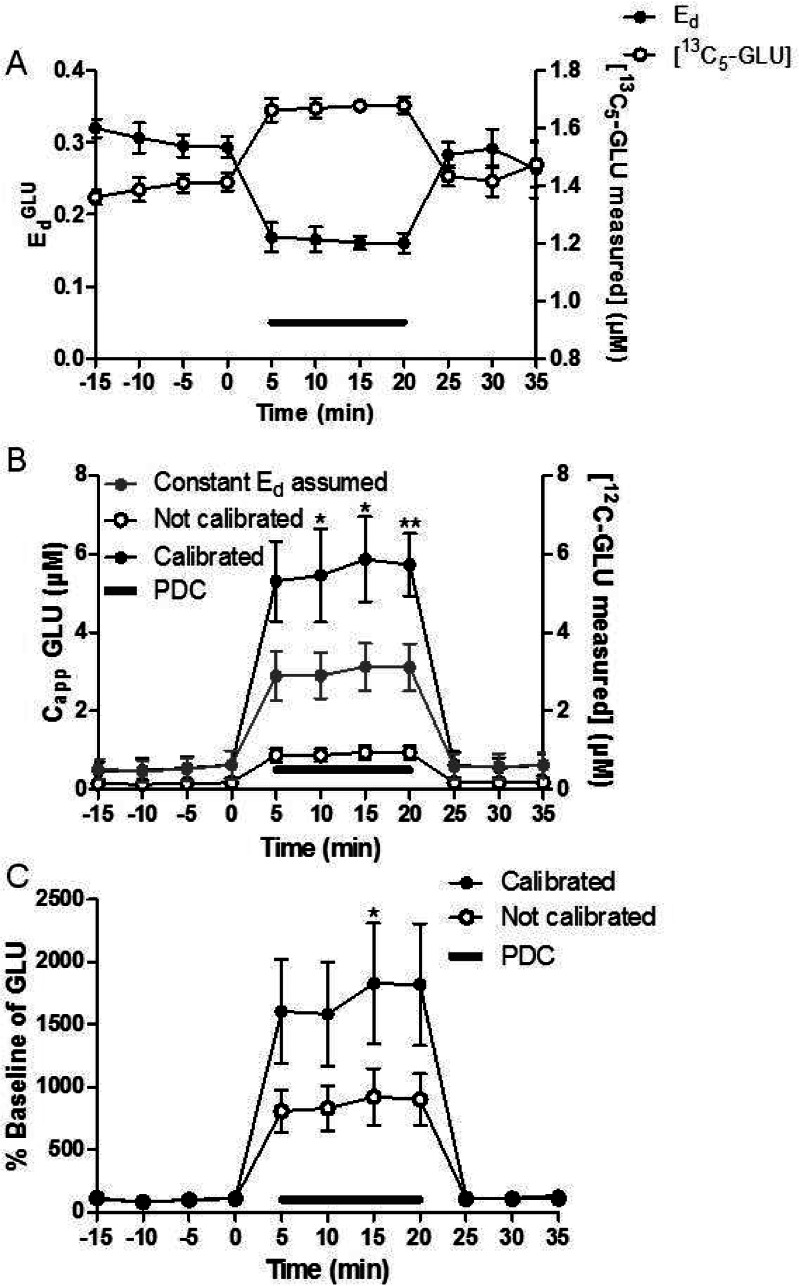
While quantitative microdialysis has been used previously to measure CappDA under transient conditions, no previous reports have demonstrated such measurements for Capp or EdGLU. To show how SIL retrodialysis can measure Ed and CappGLU under transient conditions, we monitored both as PDC was perfused through the probe. Mixed model regression showed a 45% decrease of Ed (p-value < 0.001) after PDC was perfused through the probe (Figure 6). With calibration at each point by SIL retrodialysis, CappGLU was measured to be 190% higher than if assuming a constant Ed (EdGLU measured with SIL retrodialysis prior to PDC perfusion, p-value < 0.001 using the mixed model regression). Using a paired t test, three of the four calibrated microdialysis fractions had statistically higher Capp (p-value < 0.05, 0.05, and 0.005 for 10, 15, and 20 min after PDC infusion, respectively). This effect was extended to relative changes as well. Calibrated by SIL retrodialysis, the percent basal change was 2-fold larger compared to noncalibrated measurements (p-value < 0.001 using mixed model regression model, refer to Figure 6C). Thus, even relative changes are in error when not using calibration.
Figure 6.
(A) EdGLU was measured as 750 μM PDC was perfused (gray line) through the probe. Ed (black solid circles) values reached the minimum value within 5 min (p-value < 0.001 compared to aCSF). With 2 μM 13C5-GLU perfused through the probe, the addition of PDC to the perfusate increased [13C5-GLU measured] (right axis, black open circles). (B) [12C-GLU measured] (right axis, black open circles), CappGLU with constant Ed assumed (EdGLU measured prior to PDC, gray solid circles), and Capp (black solid circles) were measured as PDC was perfused. CappGLU was on average 190% higher compared to Capp with a constant EdGLU assumed (p-value < 0.001). (C) % baseline of GLU for calibrated (black solid circles) vs conventional (black open circles) microdialysis is shown. The calibrated measurements were 200% larger compared to the conventional microdialysis (p-value < 0.001). Paired t tests were used for individual fractions to test significance as well. Error bars show SEM (n = 4). ** indicates p-value < 0.01, and * indicates p-value < 0.05.
The experiments show that SIL retrodialysis and NNF provide equivalent Capp and Ed for two different neurotransmitters across a wide range of conditions. This result is in good agreement with expectation, since the techniques both rely on the principle of measuring the extraction or loss of a molecule of interest from the probe in the in vivo environment. Because of this equivalency, the methods not only provide the same information but are subject to the same limitations. In particular, tissue factors that might influence NNF results would also influence SIL retrodialysis.4,25,26
SIL Retrodialysis Is an Efficient Method
The largest disadvantage to performing NNF is the added time and expense needed to complete a study. For example, a previous report showing that PDC lowers EdGLU required 7 h of fraction collection time when using NNF.24 With SIL retrodialysis of 13C5-GLU, the same measurement with the same number of replicates required 30 min. If dNNF were to be used to capture the dynamics of Ed, a minimum of 3 concentrations of perfused GLU would be needed requiring 12 animals assuming 4 replicates for each [GLU]in. In contrast, the SIL retrodialysis method required 4 animals for an equal number of replicates. Using SIL retrodialysis is more efficient in terms of time and animal usage than NNF. In addition to time saved for a single analyte, multiple analytes could be used for SIL retrodialysis simultaneously, saving further time, because the HPLC-MS method described herein allows for quantifying multiple SIL neurotransmitters in a single sample.22 The ease of use with SIL retrodialysis is compromised by the need for a mass spectrometer to perform these measurements; however, as mass spectrometers become more available, this disadvantage will wane.
Apart from the time and work saved, SIL retrodialysis with 13C labeled neurotransmitters also allows monitoring for artifacts that may be caused by perfusing neurotransmitter through the probe. By measuring endogenous 12C-DA and 12C-GLU across multiple perfused concentrations of 13C6-DA and 13C5-GLU, we determined that the concentrations perfused had no measurable effects on endogenous DA and GLU. Thus, it is possible to directly show that the calibration method does not perturb the system being sampled.
Use of Ed to Aid in Data Interpretation
The availability of a more efficient method for measuring Ed in vivo may enable quantitative microdialysis studies to be more routinely performed. Such quantification may be significant for many studies. The use of Ed can help discern the source of a concentration change. For example, one study showed that EdDA was constant for 2 days, allowing concentration changes to be related to endogenous changes rather than artifacts of recovery change.7 In another study, ethanol was shown to increase DA and GLU in the nucleus accumbens and the hippocampus.3,24 By showing that Ed did not change (requiring 24 animals, 2 h for each animal for DA and 13 animals, 7 h for each animal for GLU), it was possible to demonstrate the change in concentration is induced by release and not a decrease in reuptake. In principle, similar studies by SIL retrodialysis could significantly save on animals and time required. Quantification may also be important for determining effects of genetic manipulation. For example, distinguishing the dose-dependent effects of SERT gene on extracellular serotonin concentration required calibrated microdialysis measurements.10 The heterozygous SERT knockout showed no difference in basal serotonin concentrations in the striatum using uncalibrated microdialysis compared to the wild type; however, when using NNF, the heterozygous SERT knockout was shown to have an increased Capp compared to the wild type. We may expect that other genetic modifications may also result in more subtle changes in neurotransmitter concentration that are best detected by quantification. The availability of SIL retrodialysis could aid in such studies.
Quantification may also be important when comparing techniques. The relative DA increase measured in the nucleus accumbens by microdialysis following a 3 mg/kg dose of cocaine i.v. was an order of magnitude lower than that observed by voltammetry.27,28 While some of the difference may be attributed to differences in temporal resolution (peak changes may happen quickly and be recorded by voltammetry but are averaged resulting in lower values by microdialysis), some difference must also be due to use of noncalibrated microdialysis probes in these studies. As shown here and elsewhere, lack of calibration can result in substantial underestimation of extracellular concentrations and relative increases by microdialysis.9 It is clearly desirable to use in vivo calibration if comparing different methods. The use of SIL retrodialysis in such studies will reduce the time and animal cost associated with the measurements and therefore enhance the feasibility of such comparisons.
Conclusions
Quantification by in vivo calibration is an important tool for microdialysis measurements. A robust and efficient new method for measuring Ed and Capp of neurotransmitters has been demonstrated using SIL retrodialysis. Using SIL retrodialysis provides a more accurate neurochemical profile compared to conventional microdialysis, which can aid in interpreting results.
Methods
Chemicals and Reagents
All chemicals and reagents were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Artificial cerebral spinal fluid (aCSF) comprised of 145 mM NaCl, 2.68 mM KCl, 1.01 mM MgSO4, 1.22 mM CaCl2, 1.55 mM Na2HPO4, and 0.45 mM NaH2PO4 (Fisher Scientific, Pittsburgh, PA). 13C6-DA was purchased from CDN isotopes (Quebec, Canada), and 13C5-GLU was purchased from Cambridge Isotopes (Andover, MA). Mobile phase of the LC column included 10 mM ammonium formate and 0.15% formic acid. Cocaine hydrochloride was purchased from the University of Michigan hospital (Ann Arbor, MI).
Microdialysis Probes
Probes were constructed as previously described.29 In summary, 40/100 μm (i.d./o.d.) fused silica capillaries (Polymicro Technologies, Phoenix, AZ) were glued side-by-side with a 2 mm offset. The capillaries were ensheathed in a regenerated cellulose membrane with both ends sealed by polyimide sealing resin (Grace, Deerfield, IL). Flow rate of perfusion fluid through the probe was 1 μL/min unless stated otherwise.
Sample Derivatization and Analysis
Each sample was derivatized with benzoyl chloride and analyzed as described previously.22 Briefly, 5 μL samples were sequentially mixed with 2.5 μL of 100 mM sodium tetraborate, 2.5 μL of benzoyl chloride (2% in acetonitrile, v/v), and 2.5 μL of internal standard. The internal standard comprised 10 μM GLU and DA reacted with 13C6-benzoyl chloride (CDN Isotopes) in 100 mM sodium tetraborate. The internal standard was then diluted 1:100 in DMSO and 1% formic acid (v/v). Between each reagent addition, the samples were vortexed. The samples were analyzed on a Waters UPLC system with a Waters HSS T3 column (1 mm × 100 mm, 1.8 μm). A Waters/Micromass Quattro Ultimatriple quadrupole or an Agilent 6410 triple quadrople mass spectrometer was used for detection. Mobile phase A was 10 mM ammonium formate and 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The peak areas of each analyte were divided by the area of the internal standard. The limits of detection for DA and GLU were 0.03 and 5 nM, respectively.22 These limits of detection for this method are sufficient for in vivo measurements and are comparable to that of electrochemical detection.10,30,31
Measuring in Vitro Loss and Recovery
For in vitro studies, 200 nM 13C6-DA was perfused through probes at flow rates of 0.2, 0.5, 1.0, and 1.5 μL/min. The probe sampled from a well-stirred vial of 200 nM 12C-DA. The recovery of the 12C-DA was compared to the loss of the 13C6-DA at each flow rate. Samples were collected every 5 min with three replicates of each flow rate, and three probes were tested.
Surgery
Male Sprague–Dawley rats were anesthetized with ketamine (65 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and dexdomitor (0.25 mg/kg, Pfizer Animal Health, New York, NY) and placed in a stereotaxic frame. A burr hole was drilled where the cannula (Plastics One, Inc., Roanoke, VA) was being implanted (+1.6 A/P, ± 1.1 L, cannula aimed to nucleus accumbens).32 The rats were unilaterally cannulated. Cannulae were implanted alternately between the left and right side of the brain. Additional burr holes were drilled for skull screws to hold the cap in place. The cannula was lowered 4 mm from the top of the skull and dental cement (A-M Systems, Inc., Sequim, WA) was used to hold the cannula in place. A stylet (Plastics One, Inc.) was inserted into the cannula and the rat was allowed to recover for 5–10 days prior to the experiment. All animal procedures were approved by the University Committee for the Use and Care of Animals at the University of Michigan.
The day before the experiment, a microdialysis probe was perfused with aCSF at a flow rate of 1 μL/min. The rat was lightly anesthetized in an isofluorane drop box. The probe was inserted into the cannula with the active area extending 2–4 mm past the cannula to sample from the nucleus accumbens. Probe placements were checked with histology (data not shown). The rat was tethered to a Raturn (Bioanalytical Systems, Inc., West Lafayette, IN). Overnight, the flow rate was lowered to 0.2 μL/min.
Measuring Extraction Fraction of DA and GLU
On the day of the experiment, the flow rate of the probe was increased to 1 μL/min. Varying concentrations of 13C6-DA (0, 5, 20, 200, 500 nM) were perfused through the probe with and without 5 μM nomifensine. Solutions were switched by hand by disconnecting the fluid line and reattaching another syringe. The time required for the liquid to travel from the syringe to the probe was approximately 10 min. Each concentration of DA was allowed to equilibrate for 10 min prior to sampling, as was performed in previous NNF calibrations.9 Each fraction was 5 μL, and at each concentration three samples were collected. The same procedure for DA was performed with GLU. 13C5-GLU at varying concentrations (0, 0.2, 2, 5, and 10 μM) was perfused through the probe. Although a Raturn was used for all experiments, liquid swivels could be used as well. The internal volume of liquid swivels may be a concern for fast temporal resolution of retrodialysis, but no more than that of conventional microdialysis.
An injection of cocaine (20 mg/kg, i.p.) was administered to the rat to show the effect of a DA uptake inhibitor under transient conditions. The probe was perfused with 200 nM 13C6-DA for the duration of the experiment. A concentration of 200 nM 13C6-DA was chosen because it produced a large signal so that smaller changes in EdDA could be measured. Capp and EdDA were measured on every sample, which were collected every 5 min. As a control, saline (i.p.) was administered to the rat rather than cocaine.
To show the effect of an uptake inhibitor on EdGLU under transient conditions, 750 μM PDC (Tocris, Bristol, U.K.) was perfused through the probe along with 2 μM 13C5-GLU. Ed and CappGLU were measured during the PDC infusion. The doses and modes of delivery for cocaine and PDC were chosen to replicate doses and modes of delivery of previous studies to show how retrodialysis can be used practically.6,24,33
Statistical Analysis
For comparison of NNF to SIL retrodialysis, t tests were performed. Paired t tests were used for the DA measurements because rats were used as their own control between vehicle and uptake inhibitor perfusion. For GLU studies, unpaired t tests were used because not all rats were matched with the PDC perfusion. When comparing the noncalibrated versus calibrated GLU/DA concentrations, a linear mixed model regression was used to test significance. Analysis of variance (ANOVA) could not be used because, for most of the experiments described in this study, the same rat was used in multiple groups (i.e., calibrated and not calibrated). The multiple measurements on a single rat violate the assumption of independence between groups; however, a repeated measures ANOVA may have sufficed. The mixed model regression was used because it has less strict assumptions than a repeated measures ANOVA, but provides similar results.34 SPSS (IBM, Armonk, NY) was used for the mixed model regression. The rat ID was used as the subject, calibration was used as a factor, time was used as a covariate, and the measurement (i.e., Ed, % basal, concentration) was tested as the dependent variable.
Acknowledgments
We thank Melissa Plegue at the Center for Statistical Consultation and Research at the University of Michigan for help with the statistical analysis.
Author Contributions
N.D.H. and R.T.K. conceived the concept, designed experiments, and wrote the manuscript. N.D.H. performed all experiments.
This research was funded by NIH R37 EB003320.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Chefer V. I.; Zapata A.; Shippenberg T. S.; Bungay P. M. (2006) Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J. Neurosci. Methods 155, 187–193. [DOI] [PubMed] [Google Scholar]
- Bungay P. M.; Morrison P. F.; Dedrick R. L. (1990) Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 46, 105–119. [DOI] [PubMed] [Google Scholar]
- Yim H. J.; Gonzales R. A. (2000) Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 22, 107–115. [DOI] [PubMed] [Google Scholar]
- Bungay P. M.; Newton-Vinson P.; Isele W.; Garris P. A.; Justice J. B. (2003) Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J. Neurochem. 86, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins-Gerstl A.; Shu Z.; Zhang J.; Liu Y.; Weber S. G.; Michael A. C. (2011) Effect of Dexamethasone on Gliosis, Ischemia, and Dopamine Extraction during Microdialysis Sampling in Brain Tissue. Anal. Chem. 83, 7662–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R. J.; Justice J. B. (1993) Quantitative microdialysis under transient conditions. Anal. Chem. 65, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Tang A.; Bungay P. M.; Gonzales R. A. (2003) Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J. Neurosci. Methods 126, 1–11. [DOI] [PubMed] [Google Scholar]
- Szapacs M. E.; Mathews T. A.; Tessarollo L.; Ernest Lyons W.; Mamounas L. A.; Andrews A. M. (2004) Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J. Neurosci. Methods 140, 81–92. [DOI] [PubMed] [Google Scholar]
- Smith A. D.; Justice J. B. (1994) The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J. Neurosci. Methods 54, 75–82. [DOI] [PubMed] [Google Scholar]
- Mathews T. A.; Fedele D. E.; Coppelli F. M.; Avila A. M.; Murphy D. L.; Andrews A. M. (2004) Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods 140, 169–181. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E.; Enoksson S.; Moberg E.; Bolinder J.; Arner P. (1997) Absolute concentrations of glycerol and lactate in human skeletal muscle, adipose tissue, and blood. Am. J. Physiol.: Endocrinol. Metab. 273, E584–E592. [DOI] [PubMed] [Google Scholar]
- Rosdahl H.; Hamrin K.; Ungerstedt U.; Henriksson J. (1998) Metabolite levels in human skeletal muscle and adipose tissue studied with microdialysis at low perfusion flow. Am. J. Physiol.: Endocrinol. Metab. 274, E936–E945. [DOI] [PubMed] [Google Scholar]
- Lonnroth P.; Jansson P. A.; Smith U. (1987) A microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol.: Endocrinol. Metab. 253, E228–E231. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wong S. L.; Sawchuk R. J. (1993) Microdialysis Calibration Using Retrodialysis and Zero-Net Flux: Application to a Study of the Distribution of Zidovudine to Rabbit Cerebrospinal Fluid and Thalamus. Pharm. Res. 10, 1411–1419. [DOI] [PubMed] [Google Scholar]
- Bengtsson J.; Boström E.; Hammarlund-Udenaes M. (2008) The use of a deuterated calibrator for in vivo recovery estimations in microdialysis studies. J. Pharm. Sci. 97, 3433–3441. [DOI] [PubMed] [Google Scholar]
- Sun L.; Stenken J. A.; Brunner J. E.; Michel K. B.; Adelsberger J. K.; Yang A. Y.; Zhao J. J.; Musson D. G. (2008) An in vivo microdialysis coupled with liquid chromatography/tandem mass spectrometry study of cortisol metabolism in monkey adipose tissue. Anal. Biochem. 381, 214–223. [DOI] [PubMed] [Google Scholar]
- Jacobson I.; Sandberg M.; Hamberger A. (1985) Mass transfer in brain dialysis devices -- a new method for the estimation of extracellular amino acids concentration. J. Neurosci. Methods 15, 263–268. [DOI] [PubMed] [Google Scholar]
- Cremers T. I. F. H.; de Vries M. G.; Huinink K. D.; Loon J. P. v.; Hart M. v. d.; Ebert B.; Westerink B. H. C.; De Lange E. C. M. (2009) Quantitative microdialysis using modified ultraslow microdialysis: Direct rapid and reliable determination of free brain concentrations with the MetaQuant technique. J. Neurosci. Methods 178, 249–254. [DOI] [PubMed] [Google Scholar]
- Clément R.; Malinovsky J.-M.; Dollo G.; Le Corre P.; Chevanne F. o.; Le Verge R. (1998) In vitro and in vivo microdialysis calibration using retrodialysis for the study of the cerebrospinal distribution of bupivacaine. J. Pharm. Biomed. Anal. 17, 665–670. [DOI] [PubMed] [Google Scholar]
- Parsons L. H.; Justice J. B. (1992) Extracellular Concentration and In Vivo Recovery of Dopamine in the Nucleus Accumbens Using Microdialysis. J. Neurochem. 58, 212–218. [DOI] [PubMed] [Google Scholar]
- Owesson-White C. A.; Roitman M. F.; Sombers L. A.; Belle A. M.; Keithley R. B.; Peele J. L.; Carelli R. M.; Wightman R. M. (2012) Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J. Neurochem. 121, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P.; Mabrouk O. S.; Hershey N. D.; Kennedy R. T. (2012) In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography-Mass Spectrometry. Anal. Chem. 84, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z.-B.; Tzschentke T. M.; Brodin E.; Wise R. A. (1998) Electrical Stimulation of the Prefrontal Cortex Increases Cholecystokinin, Glutamate, and Dopamine Release in the Nucleus Accumbens: an In Vivo Microdialysis Study in Freely Moving Rats. J. Neurosci. 18, 6492–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer V.; Meis J.; Wang G.; Kuzmin A.; Bakalkin G.; Shippenberg T. (2011) Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict. Biol. 16, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C. (2006) Effects of tissue trauma on the characteristics of microdialysis zero-net-flux method sampling neurotransmitters. J. Theor. Biol. 238, 863–881. [DOI] [PubMed] [Google Scholar]
- Peters J. L.; Michael A. C. (1998) Modeling Voltammetry and Microdialysis of Striatal Extracellular Dopamine: The Impact of Dopamine Uptake on Extraction and Recovery Ratios. J. Neurochem. 70, 594–603. [DOI] [PubMed] [Google Scholar]
- Wise R.; Leeb K.; Pocock D.; Newton P.; Burnette B.; Justice J. (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berlin, Ger.) 120, 10–20. [DOI] [PubMed] [Google Scholar]
- Heien M. L. A. V.; Khan A. S.; Ariansen J. L.; Cheer J. F.; Phillips P. E. M.; Wassum K. M.; Wightman R. M. (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. U.S.A. 102, 10023–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church W. H.; Justice J. B. (1987) Rapid sampling and analysis of extracellular dopamine in vivo. Anal. Chem. 59, 712–716. [DOI] [PubMed] [Google Scholar]
- Rutherford E. C.; Pomerleau F.; Huettl P.; Strömberg I.; Gerhardt G. A. (2007) Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J. Neurochem. 102, 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. L.; Venton B. J.; Heien M. L. A. V.; Wightman R. M. (2003) Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem. 49, 1763–1773. [DOI] [PubMed] [Google Scholar]
- Paxinos G., and Watson C. (2007) The rat brain in stereotaxic coordinates, Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- Montiel T.; Camacho A.; Estrada-Sánchez A. M.; Massieu L. (2005) Differential effects of the substrate inhibitor l-trans-pyrrolidine 2,4-dicarboxylate (PDC) and the non-substrate inhibitor dl-threo-β-benzyloxyaspartate (dl-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience 133, 667–678. [DOI] [PubMed] [Google Scholar]
- Shin J. H. (2009) Application of Repeated-Measures Analysis of Variance and Hierarchical Linear Model in Nursing Research. Nurs. Res. 58, 211–217. [DOI] [PubMed] [Google Scholar]