Abstract
d-Serine, a co-agonist of N-methyl d-aspartate (NMDA) receptors, has been implicated in neurological and psychiatric disorders such as cerebral ischemia, lateral amyotrophic sclerosis, or schizophrenia. d-Serine signaling represents an important pharmacological target for treating these diseases; however, the biochemical mechanisms controlling extracellular d-serine levels in vivo are still unclear. d-Serine heteroexchange through small neutral amino acid transporters has been shown in cell cultures and brain slices and could provide a biochemical mechanism for the control of d-serine extracellular concentration in vivo. Alternatively, exocytotic d-serine release has also been proposed. In this study, the dynamics of d-serine release and clearance were explored in vivo on a second-by-second time scale using microelectrode biosensors. The rate of d-serine clearance in the rat frontal cortex after a microionophoretic injection revealed a transporter-mediated uptake mechanism. d-Serine uptake was blocked by small neutral l-amino acids, implicating alanine-serine-cysteine (ASC) transporters, in particular high affinity Asc-1 and low affinity ASCT2 transporters. Interestingly, changes in alanine, serine, or threonine levels resulted in d-serine release through ASC transporters. Asc-1, but not ASCT2, appeared to release d-serine in response to changes in amino acid concentrations. Finally, neuronal silencing by tetrodotoxin increased d-serine extracellular concentration by an ASC-transporter-dependent mechanism. Together, these results indicate that d-serine heteroexchange through ASC transporters is present in vivo and may constitute a key component in the regulation of d-serine extracellular concentration.
Keywords: electrochemical detection, amperometry, rat, extracellular concentration, interstitial fluid
d-Serine, an endogenous co-agonist of glutamatergic N-methyl d-aspartate (NMDA) receptors, has been implicated in human diseases related to NMDA receptor dysfunction such as schizophrenia, amyotrophic lateral sclerosis, and cerebral ischemia.1−4 The pharmacological modulation of extracellular d-serine levels is therefore an exciting avenue of research for the treatment of these pathologies. For example, an increase in d-serine may compensate for the hypoactivation of NMDA receptors observed in schizophrenia,5−7 whereas a reduction of d-serine levels could reduce NMDA-mediated excitotoxicity in ischemia or neurodegenerative diseases.4,8−10
The biochemical mechanisms controlling extracellular d-serine levels in vivo are still a matter of debate. Concerning d-serine clearance, in vitro studies have shown that d-serine is taken up from the extracellular space by transporter-mediated uptake.11 At least two small neutral amino acid transporters have been suggested to mediate d-serine uptake. The neuronal, Na+ independent alanine-serine-cysteine 1 (Asc-1) transporter12,13 has a high affinity for serine, alanine, cysteine, and threonine and is selective for small neutral d- and l-amino acids.14 Asc-1 is responsible for most of the d-serine uptake by synaptosomes.15 Another candidate for d-serine uptake is the Na+-dependent alanine-serine-cysteine-threonine 2 transporter (ASCT2),16 which has a high affinity for l-alanine, l-serine, l-threonine, l-glutamine, and l-asparagine, and has a comparatively lower affinity for d-serine.17 ASCT2 is predominantly expressed in glia, and, despite its low affinity for d-serine, it has been implicated in d-serine uptake in cell culture studies.11,18−20
The mechanisms of d-serine release are also a matter of debate. d-Serine may be released via Ca2+-dependent exocytosis21−23 or via amino acid heteroexchange through ASC transporters in neurons and/or astrocytes.24 For example, experiments on astrocyte cultures and brain slices showed d-serine release in response to small neutral amino acids, suggesting the existence of an amino acid heteroexchange mechanism involving d-serine release coupled to the transport of another amino acid in the opposite direction.11,25
In this study, we used an enzymatic microelectrode biosensor that allows selective d-serine detection on a second-by-second time scale26,27 to directly test the existence of a d-serine heteroexchange mechanism in vivo. We used local delivery of d-serine, l-amino acids, or S-methyl-l-cysteine (SMLC, an Asc-1 inhibitor)28,29 to the vicinity of our microelectrode biosensor. Using this approach, we identified the function of ASC transporters in d-serine uptake and release in the cortex of anesthetized rats, and proposed specific roles for Asc-1 and ASCT2 transporters in the regulation of d-serine levels.
Results and Discussion
To obtain direct in vivo information on the rapid dynamics of d-serine release and clearance, we used a specific d-serine biosensor made with DAAO purified from yeast. This biosensor minimizes potential tissue lesions and preserves the integrity of the blood-brain barrier.26,27 The DAAO enzyme (it preferentially oxidizes neutral d-amino acids like d-alanine, d-proline, or d-serine30), but the DAAO-based microelectrode biosensors are selective for d-serine in the CNS in vivo, as it is highly enriched in d-serine compared to other neutral d-amino acids.31 We have previously demonstrated a negligible difference (6%) between d-serine concentrations estimated by our biosensors and those measured using HPLC in rat brain homogenates.26 Finally, d-serine electrochemical signals are undetectable in the cerebellum, a brain structure known to be devoid of d-serine in adulthood,32,33 further confirming the specificity of our measurements.27
Identification of a d-Serine Uptake Mechanism in Vivo
To study the uptake of d-serine in vivo, we used a theoretical model designed to study the diffusion of neurotransmitters in the brain.34 The experimental paradigm consists of releasing a molecule within the brain parenchyma and detecting its diffusion with a biosensor placed at a small distance from the release point. The detected concentration profile is then fitted with a mathematical equation that takes into account diffusion, the tortuosity of the extracellular space, and uptake. In the current study, we used an ionophoretic source to release d-serine and measured its concentration with a d-serine microelectrode biosensor placed a few hundred micrometers away from the pipet (Figure 1A). d-Serine is a neutral molecule at physiological pH and can be ejected using the electro-osmotic flow created by the applied potential in the microionophoresis pipet.35,36 Following this protocol, the microelectrode biosensor detected an increase in d-serine oxidation current followed by a return to baseline. This pattern was reproducible over multiple injections (Figure 1A). In such conditions, the concentration of d-serine, at a distance r from the releasing point can be described with the equation:37
| 1 |
where D is the diffusion coefficient of d-serine, λ is the tortuosity, and α is the extracellular fraction of the brain (i.e., the ratio of the volume of the extracellular space by the total volume of the tissue, estimated at 0.2 in the cerebral cortex37). Q is defined as the source term, which describes the release of d-serine during the injection. For ionophoretic ejection of a neutral molecule, Q is directly proportional to the applied current in the glass pipet.36,38 The last term represents the uptake component which, for simplification, is assumed to be linear with d-serine concentration (k′C, where k′ is the uptake constant). Under these conditions, this equation has an analytical solution described by the following equation:37
| 2 |
with
| 3 |
with
| 4 |
and where T0 is the duration of the injection, D* = D/λ2 is the apparent diffusion coefficient in the brain (corrected for tortuosity) and θ = (k′/D*)1/2.
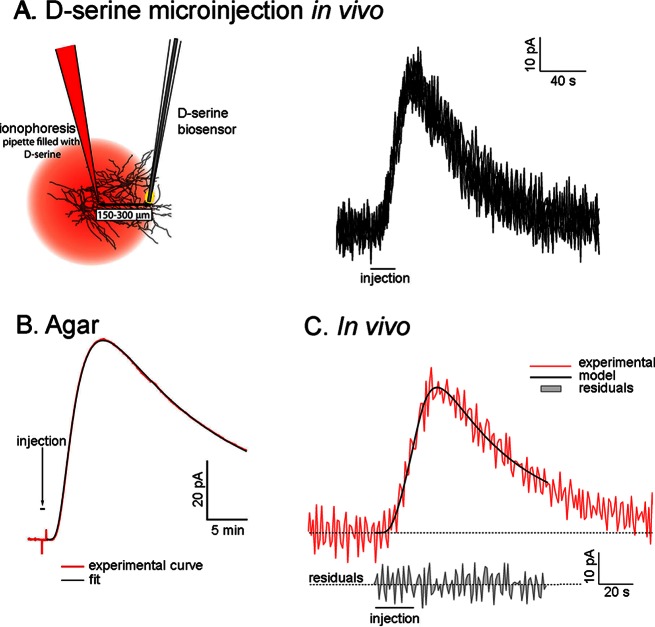
Figure 1.
Identification of a d-serine reuptake mechanism in vivo. (A) Experimental design in which d-serine is injected by microionophoresis near a biosensor in the rat frontal cortex. Overlay of six successive d-serine microinjections showing a reproductible d-serine signal detected by the biosensor. (B) d-Serine clearance in 0.3% agarose can be modeled with free diffusion and almost no uptake. (C) The kinetics of d-serine clearance in vivo reveals an uptake mechanism that limits d-serine lifetime in the extracellular space.
We first validated this model in a gel of 0.3% agar, which has been found to be a good model for the diffusion of molecules in the brain extracellular fluid.37 In this model, the current recorded at our biosensors increased shortly after the initiation of d-serine ionophoresis, and decayed when the injection was stopped. These experimental curves were then fitted with eq 2 to yield a computed value of D and k′. In the agar medium, we determined a value of D = 8.0 ± 0.8 × 10–10 m2·s–1 (n = 15) that is in good agreement with the chemical constants published in the literature,39 and a very low value of k′ = 1.5 ± 0.08 × 10–5 s–1, which reflects the absence of d-serine reuptake in agar (Figure 1B). We then fitted the pattern of d-serine extracellular concentrations following the ionophoretic application of d-serine to the frontal cortex of anesthetized rats with our theoretical model. The distance between the ionophoresis pipet and the biosensor ranged from 150 to 300 μm (as determined at the end of each experiment), and each ionophoretic d-serine injection lasted between 5 and 100 s at 200–1000 nA. For each experiment, we chose a different T0 because the distance between the pipet and the biosensor varied (sensitivity of the biosensor and number of d-serine molecules ejected by unit of current also varied). When a measurable d-serine signal was detected, T0 (always between 5 and 100s) was then fixed throughout the experiment and used for modeling the d-serine curve.
We fitted eq 2 to our amperometric recordings using a set of five coefficients that could be varied during the fitting computation. Initial values had to be provided to the model: the distance between the sensor and the injection pipet (r), and the duration of the injection (T0) were set equal to their actual experimental values; D* was set to 3 × 10–10 m2·s–1 which corresponded to D = 8 × 10–10 m2·s–1 (according to our results obtained using 0.3% agar) and λ = 1.6 (according to tortuosity values determined by Nicholson and Sykova37); θ was set initially at 104, and Q, representing the number of injected d-serine molecules, was set to 10–11. This initial value was set empirically, but could vary freely during the fitting process to end with very different values that fitted the experimental curve. The five parameters r, T0, D*, θ and Q, were then allowed to vary freely until the fit converged. The fit was estimated to be correct if the residuals were evenly distributed around zero, and if the fitted values of r (distance) and T0 (duration) stayed within 20% of their real experimental value (Figure 1C).
When d-serine was injected by ionophoresis into the cortex, its concentration near the biosensor increased rapidly, and returned to baseline values much more quickly than did d-serine in our experiments in agar (Figure 1). Basal levels of d-serine could not be determined using the current approach but have been measured around 2.3 μM in the frontal cortex using similar biosensors.27 The fact that the d-serine signal returned to baseline was a good indication (1) that all of the d-serine that had been injected was cleared from the extracellular space, and (2) that the sensitivity of the biosensor was not affected by the procedure. When we fitted the theoretical equation to our in vivo recordings, we obtained an uptake constant of k′ = 0.128 ± 0.01 s–1 (n = 28), which represents the rate of d-serine uptake in the cortical parenchyma.
Small Neutral Amino Acids Compete with d-Serine Uptake
No specific d-serine transporter has been identified so far; however, the Asc and ASCT families of amino acid exchangers can transport d-serine.15,16d-Serine is reported to be taken up by nonspecific small neutral amino acid transporters of the alanine-serine-cysteine (ASC) family, such as the ASCT2 or Asc-1 transporters.11,14,15 To determine the role of ASC transporters in d-serine uptake in vivo, we examined d-serine uptake in the presence of interfering small neutral amino acids, which would compete with d-serine for transporter-mediated uptake. This was accomplished by infusing l-threonine, l-alanine, and l-serine near the microelectrode biosensor by reverse dialysis (Figure 2A).
Figure 2.
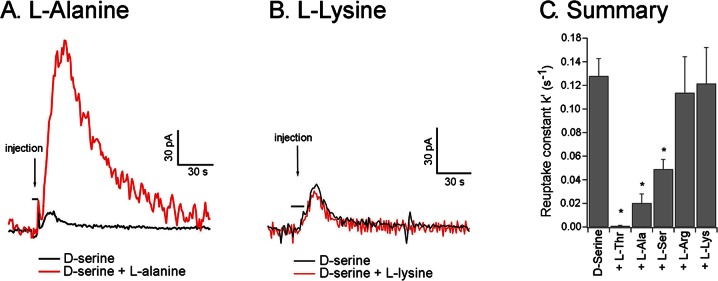
Small neutral amino acids interfere with d-serine reuptake. (A) Infusion of l-alanine by reverse microdialysis near the biosensor dramatically decreased the efficiency of d-serine reuptake. (B) l-Lysine infusion did not modify d-serine uptake. (C) Summary results of the transporter competition experiments using infusion of l-amino acids: d-serine uptake is reduced by the application of l-alanine, l-serine, and l-threonine, but not l-lysine or l-arginine, suggesting that d-serine uptake is mediated by ASC transporters. *p < 0.01.
The infusion of l-threonine, l-alanine, and l-serine (100 mM in the microdialysis probe) strongly reduced d-serine uptake (Figure 2A). In the presence of these amino acids, d-serine ionophoresis yielded much higher d-serine peak concentrations at the biosensor, and the d-serine decay time increased. The d-serine uptake coefficient (k′) was considerably diminished by the presence of the small neutral amino acids, reaching 8.2 ± 5 × 10–4 s–1 in the presence of l-threonine (2.6% of the control value, n = 6, p < 0.01), 2.0 ± 0.8 × 10–2 s–1 for l-alanine (10.5% of the control value, n = 6, p < 0.01), and 4.9 ± 0.8 × 10–2 s–1 for l-serine (28% of the control value, n = 6, p < 0.01; Figure 2C), indicating that d-serine reuptake was at least partially blocked by these amino acids. It should be noted that l-amino acids are neither substrates nor inhibitors of the DAAO enzyme,40,41 and thus do not affect biosensor response.42 We also tested two basic amino acids that are not substrates for the ASCT2 or Asc-1 transporters: l-arginine and l-lysine.11,14 These two amino acids did not interfere significantly with d-serine uptake in vivo (Figure 2B). The value of the uptake coefficient (k′) was 1.1 ± 0.3 × 10–1 s–1 in the presence of l-arginine (89% of the control value, n = 6, p = 0.75) and 1.2 ± 0.3 × 10–1 s–1 in the presence of l-lysine (95% of the control value, n = 6, p = 0.9; Figure 2C). These results indicate that d-serine reuptake in vivo is inhibited by competition with small neutral amino acids, suggesting that ASC transporters such as ASCT2 or Asc-1 are involved in d-serine uptake.
Distinguishing Asc-1- and ASCT2-Mediated Transport Using S-Methyl-l-cysteine or l-Asparagine
Of the Asc family, only the Asc-1 subtype is present in the brain, and is predominantly expressed in neurons. The high affinity of Asc-1 for d-serine (KM = 20 μM)15 has made it the primary candidate for d-serine transport in vivo.11,24,43 ASCT transporters are Na+-dependent, and both ASCT1 and ASCT2 are present in the brain (16) However, ASCT1 does not appear to transport d-serine.44 As the affinity of ASCT2 for d-serine is lower than that of Asc-1 (about 1 mM),20 its role in d-serine regulation has been underappreciated. However, in synaptosomes prepared from Asc-1 knockout mice, a residual d-serine uptake could be detected that was likely reliant upon ASCT2 transporters. Therefore, significant d-serine uptake through ASCT2 transporters could not be excluded a priori.
To identify the role for each of these subtypes, we infused rat cortex with l-asparagine, a specific substrate of ASCT2 transporters with very low affinity for Asc-116,17 and one of the most efficient inhibitors of ASCT2-mediated d-alanine transport.12,17 We also administered SMLC, an Asc-1 transporter blocker that is inactive on system A transporters or serotonin, norepinephrine, and glutamate reuptake.28,29 Benzyl-serine, another pharmacological compound that blocks ASCT2 transporters,45 could not be used in this study because it produced a nonspecific electrochemical signal that was detected by our biosensor. We found that l-asparagine (100 mM) infused into the vicinity of the d-serine biosensor successfully competed with d-serine. Under these conditions, the d-serine reuptake constant decreased from 5.6 ± 1.6 × 10–2 s–1 to 4.3 ± 1.5 × 10–2 s–1 (−24.7%, n = 6, p = 0.011, Figure 3A, C). Similarly, SMLC (1 mM) infusion reduced the d-serine uptake coefficient from 5.1 ± 1.3 × 10–2 s–1 to 3.3 ± 1 × 10–2 s–1 (−35.6%, n = 6, p = 0.015, Figure 3B, C). These results therefore indicate that d-serine reuptake in vivo is mediated by both ASCT2 and Asc-1.
Figure 3.
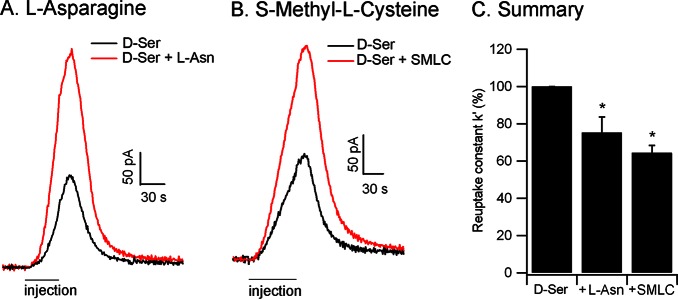
Asc-1 and ASCT2 contribute to d-serine reuptake. (A) Infusion of l-asparagine, a specific substrate of ASCT2 (n = 6) or (B) SMLC, a specific Asc-1 inhibitor (n = 6), both decreased d-serine reuptake. (C) Summary results of d-serine uptake inhibition by l-asparagine or SMLC, implicating both Asc-1 and ASCT2 transporters in d-serine reuptake. The uptake coefficient k′ is expressed as the percentage of its value when d-serine alone is injected. *p < 0.05.
Interestingly, 1 mM SMLC infusion did not produce any significant change in d-serine basal level. However, in a recent microdialysis study, Ishiwata et al. detected an increase in extracellular d-serine in response to 1 mM, but not 100 μM SMLC.29 This apparent discrepancy probably results from the fact that our electrochemical recordings were performed at a distance of about 500 μm from the microdialysis probe, at which the effective SMLC concentration was much lower. The fact that SMLC did not change basal d-serine levels at the biosensor suggests that its concentration was probably in the order of 100 μM or below, at which no change in other l-amino acids have been detected.29
d-Serine Release Evoked by Small Neutral Amino Acids
In our reverse dialysis experiments, we rapidly delivered large amounts of l-amino acids into the vicinity of the d-serine microelectrode biosensor. Because ASC transporters are known to mediate amino acid hetero-exchange in vitro,11,12,25 we were interested in determining the effects that increased small neutral amino acid concentrations may have upon extracellular d-serine levels. The infusion of l-alanine, l-threonine, or l-serine into the rat cortex induced a rapid, transient increase in the oxidation current measured by the d-serine microelectrode biosensor. This transient increase in d-serine level was followed by a return to a slightly elevated baseline (Figure 4A). For example, l-threonine infusion induced an increase in the d-serine extracellular concentration peaking at 16 ± 5 μM (Figure 4A). By contrast, infusion of basic amino acids such as l-lysine or l-arginine, that are not substrate for ASCT2 or Asc-1 transporters, produced only a small shift in the baseline d-serine oxidation current recorded by the microelectrode biosensor (Figure 4B). There was no difference between the shift in the baseline current at the biosensor after l-arginine or l-lysine infusion and that observed 15–20 min after l-serine, l-alanine, or l-threonine infusion (well after the peak in d-serine concentration). Therefore, there was no evidence that the d-serine extracellular concentration remained significantly elevated after its transient increase induced by small neutral amino acids. The slight shift in the baseline electrochemical current was more likely due to a small nonspecific oxidation current due to the large amounts of l-amino acids delivered to the tissue.
Figure 4.
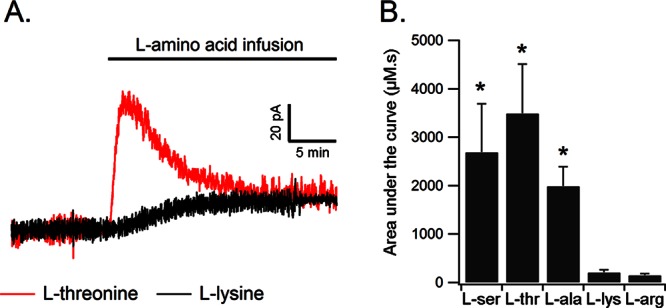
d-Serine release evoked by local infusion of small neutral amino acids. (A) l-Threonine infusion near the biosensor evokes an initial transient increase in extracellular d-serine concentrations that lasts approximately 5 min. In contrast, l-lysine infusion produces only a small nonspecific shift in the baseline current. (B) d-Serine release is evoked by the infusion of small neutral amino acids like l-alanine, l-serine, and l-threonine, but not by l-lysine or l-arginine. This amino acid profile suggests that d-serine release is mediated by ASC transporters.
As l-alanine, l-threonine, and l-serine can compete with d-serine reuptake at the ASCT2 and Asc-1 transporters, it could be argued that the increase in d-serine concentration evoked by infusion of these amino acids may result from an inhibition of d-serine reuptake, followed by d-serine accumulation in the extracellular space. It is unlikely, however, that reuptake blockade alone could explain the kinetics of d-serine concentration changes that we observed. Transporter blockade usually produces a slow, long lasting increase in neurotransmitter levels (see, for example Oldenziel et al.46 for blockade of glutamate transporters, or Martina et al.47 for blockade of glycine transporters), which is not compatible with the biphasic changes produced by the infusion of small neutral amino acids. Here, d-serine release probably occurred through an amino acid heteroexchange that quickly depleted the intracytoplasmic pool of free d-serine, resulting in a transient increase in d-serine extracellular concentration lasting only a few minutes. The d-serine released locally by heteroexchange was probably cleared by free diffusion into the brain parenchyma within a few minutes.
We then sought to discriminate the roles of ASCT2 and Asc-1 in d-serine release. By infusing l-asparagine near the biosensor, we tested whether ASCT2 transporters would be specifically challenged to release endogenous d-serine by a heteroexchange mechanism. However, l-asparagine produced only modest changes in d-serine-mediated oxidation currents that were smaller than those produced by l-alanine, l-threonine, or l-serine, and were not significantly different from the effects of l-arginine or l-lysine, which are not substrates for ASC transporters (area under the curve = 1031.66 ± 626 μM·s for l-asparagine, 3490.51 ± 1022 for l-threonine, and 146.03 ± 35.33 for l-arginine, n = 6). An ANOVA followed by an LSD posthoc test revealed a significant difference between these amino acids (F(2,17) = 5.62, p = 0.013) with a significant difference between l-threonine and l-asparagine (p = 0.02) but not between l-asparagine and l-arginine (p = 0.34).This result indicates that l-asparagine, that specifically triggers amino acid heteroexchange through ASCT2 transporters, does not induce significant d-serine release. In separate experiments, we tested the ability of l-serine to induce d-serine release in the presence of the Asc-1 blocker SMLC. We again detected small deflections in d-serine-mediated oxidation currents that were significantly smaller than those produced by l-serine alone (2689.44 ± 1002.41 for l-serine, 382.43 ± 170.38 μM·s for l-serine + SMLC, 146.03 ± 35.33 for arginine, n = 6). ANOVA followed by LSD posthoc test (F(2,17) = 3.624, p = 0.048) indicated that l-serine in the presence of SMLC was significantly different from l-serine alone (p = 0.048) but not from l-arginine (p = 0.83, Figure 5C, D). These data demonstrate that d-serine release was impaired when Asc-1 transporters were blocked by SMLC.
Figure 5.
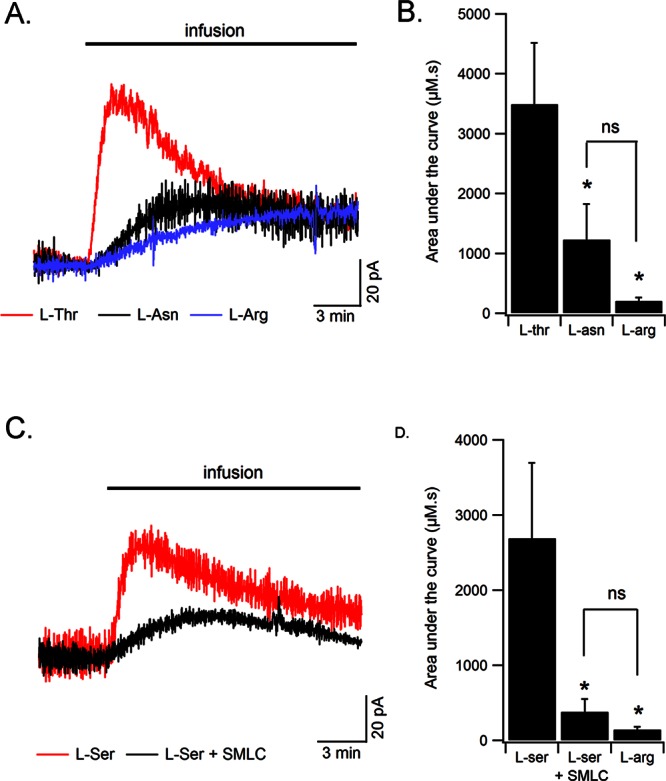
Asc-1 but not ASCT2 contribute to d-serine release. (A) l-Threonine infusion near the biosensor produced a transient increase in d-serine extracellular concentration that was much larger than that produced by l-asparagine or l-arginine. (B) Summary results of the overall amount of released d-serine (area under the curve in μM·s) by l-threonine, l-asparagine, or l-arginine (n = 9). (C) SMLC significantly decreases d-serine release evoked by l-serine (n = 6). (D) Summary results of the overall amount of d-serine detected by the biosensor in response to the infusion of l-serine, l-serine in the presence of SMLC, or l-arginine. A significant proportion of d-serine is released through Asc-1 transporters.
d-Serine release therefore appeared to be mediated by Asc-1 but not ASCT2 transporters. The implication of ASCT2 transporters in d-serine uptake but not release is supported by their predominant localization in astrocytes (see however ref (16)), which possess high intracellular l-glutamine and l-serine concentration. Therefore, as ASCT2 transporters have a much higher affinity for l-glutamine and l-serine, they should preferentially release these molecules when challenged by extracellular small neutral amino acids.48−50 Previous work has shown that astrocytes likely release d-serine through other pathways, such as volume-regulated anion channels or exocytosis.21,23,24
Effects of Neuronal Silencing by Tetrodotoxin (TTX) on d-Serine Extracellular Concentration
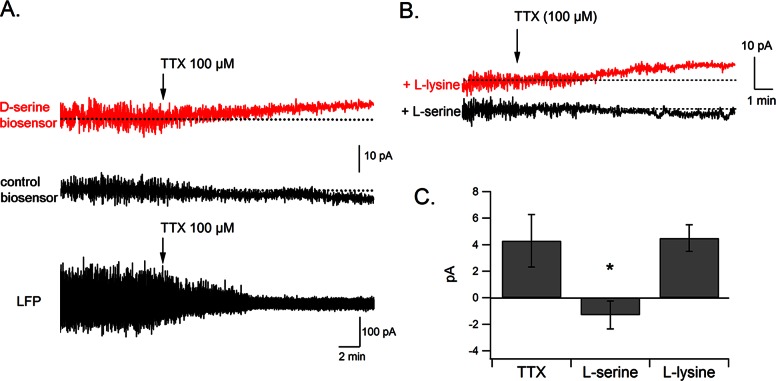
We then determined whether such heteroexchange mechanisms could be engaged during more physiological functioning of the CNS. In this regard, several studies have reported that d-serine levels can be modified by changes in neuronal activity.43,51−55 In the current experiments, we were unable to observe any obvious change in d-serine level in response to electrical stimulation in the cortex or in the hippocampus (data not shown). However, we clearly detected an increase in d-serine levels in response to the blockade of action potentials with TTX (+10.7 ± 5% in d-serine oxidation current, n = 6, p = 0.04, Figure 6A). Local field potentials (LFPs) were apparent on the raw amperometric trace, before we averaged the signal to measure oxidation currents.56,57 LFPs rapidly diminished following TTX microinjection (100 μM), confirming a decrease in neuronal activity around the biosensor. This effect was accompanied by a slow increase in the d-serine signal. This increase was not detected by control biosensors (Figure 6A), and corresponded to an elevation of extracellular d-serine levels of 0.4 ± 0.09 μM. This effect is consistent with a previous study by Hashimoto et al.58 Given that NMDA receptor activation can trigger nitric oxide (NO) release that inhibits SR,54 it is possible that TTX, by blocking neuronal transmission and NMDA receptor activation might have relieved this block and induced an increase in d-serine synthesis and release at least in part through this pathway.
Figure 6.
TTX increases d-serine concentration via an ASC transporter-dependent mechanism. (A) TTX microinjection near the biosensor causes a progressive block of neuronal activity, evidenced by the disappearance of local field potentials, together with an increase in d-serine extracellular concentration. TTX administered near a control biosensor does not produce any noticeable change in oxidation current. (B) Systemic administration of l-serine, but not l-lysine (1 g/kg i.p.), inhibits the effect of TTX on d-serine levels, suggesting the involvement of ASC transporters in this process. (C) Summary results showing a significant inhibition of the TTX effect by l-serine.
To determine the involvement of ASC transporters in the TTX-induced increase in d-serine, we injected rats systemically with a large dose of l-serine or l-lysine (1 g/kg i.p.). We reasoned that, as these amino acids cross the blood brain barrier, these systemic injections would raise the intracerebral concentrations of l-serine or l-lysine. Elevated l-serine (but not l-lysine) levels would thus interfere with the normal functioning of ASC transporters. In preliminary experiments, we quantified l-serine levels in rat brain homogenates using HPLC and determined that, 1 h following l-serine administration (1 g/kg i.p.), the intracerebral level of l-serine had more than doubled (441 ± 26 pmol/mg wet tissue in control animals, 1225 ± 276 pmol/mg 1 h after l-serine injection, n = 6, p = 0.036), confirming an earlier report by Pernot et al.27 When TTX was applied in vivo in l-serine injected rats, the increase in d-serine level was completely blocked. The d-serine signal remained stable or slightly decreased, similar to control recordings (−1.3 ± 1 pA, n = 6, p < 0.01, Figure 6B, C). However, after l-lysine injection, TTX produced an increase in the d-serine signal (+ 4.7 ± 1 pA, n = 6, Figure 6B, C) of similar amplitude to that produced by TTX alone (+4.3 ± 2.1 pA). These results indicate that the effects of TTX on d-serine extracellular concentration are blocked via competition by small neutral amino acids, suggesting an involvement of ASC transporters in activity-dependent changes in d-serine levels.
d-Serine Release: Heteroexchange versus Exocytosis
In addition to transporter-mediated d-serine release, some studies using astrocyte cultures have suggested that d-serine may also be released via Ca2+-dependent exocytosis.21−23 By analyzing synaptic currents in hippocampal slices, Henneberger et al.55 also suggested that d-serine release could be exocytotic. In contrast, a recent study found that d-serine can be released by neurons in primary culture and acute brain slices, and that this release is essentially Ca2+-insensitive, which is incompatible with exocytosis.24 In addition, neuronal d-serine release evoked by heteroexchange with exogenous d-isoleucine can modulate long-term potentiation in hippocampal slices.25 Although our results clearly implicate ASC transporters in extracellular d-serine regulation, we cannot rule out the possibility of an alternative exocytotic release pathway. Exocytotic d-serine release could potentially be specific to the synaptic cleft, and therefore not detectable by our microelectrode biosensors, which sample the extrasynaptic compartment. d-Serine stores have been identified in intracellular organelles present in astrocytes,22,33 and represent more than 99% of total d-serine present in the brain.27 In our experimental conditions, electrical stimulation did not produce detectable d-serine release in the extrasynaptic space, suggesting that if d-serine was released in response to neuronal stimulation, it would not significantly diffuse outside the synaptic cleft. Therefore, it is possible that d-serine is differentially regulated by hetero-exchange versus exocytosis in the extra- and intrasynaptic compartments, respectively. These two mechanisms could thus mediate different kinetics of d-serine fluctuation, whereby fast increases rely on exocytosis, and slower bidirectional adaptations might require mechanisms of hetero-exchange.
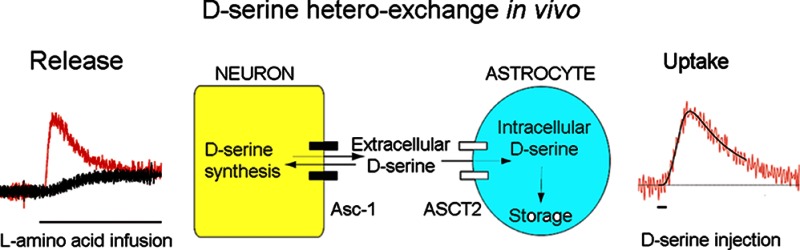
Up to now, the identification of d-serine exocytosis has been almost exclusively restricted to astrocytes and Bergman glia,21,23,55,59 whereas Asc-1 transporters susceptible to release d-serine are preferentially neuronal. It is therefore possible that exocytosis and heteroexchange are d-serine release mechanisms utilized by glial cells and neurons, respectively. In support of this concept, an interesting model suggesting the existence of a serine shuttle between astrocytes and neurons has recently been proposed.49 Because SR expression is predominantly neuronal, d-serine synthesis would occur primarily in neurons, from which it would be released into the extracellular space to be taken up and stored in astrocytes. Our current study provides in vivo evidence that supports this model by placing Asc-1 and ASCT2 transporters in key positions to mediate such serine shuttle (Figure 7).
Figure 7.
Schematic representation of d-serine transport mechanisms through Asc-1 and ASCT2 transporters. d-Serine is synthesized in neurons by SR, an enzyme whose activity can be up- or downregulated by multiple cofactors. Newly synthesized d-serine is rapidly released into the extracellular space via Asc-1 transporters, before reuptake by neurons via Asc-1, or by astrocytes, via ASCT2. d-Serine transported into astrocytes could be stored for subsequent release (forebrain) or degraded by endogenous DAAO (cerebellum). ATP, adenosine triphosphate; PICK1, protein interacting with C Kinase 1; GRIP, glutamate receptor interacting protein; NO, nitric oxide; PIP2, phosphatidylinositol biphosphate.
Conclusion
The present results indicate that ASC transporters are important players in the regulation of extracellular d-serine concentrations in vivo by mediating both d-serine release and uptake. As d-serine is a potential new target for therapeutic intervention in schizophrenia, amyotrophic lateral sclerosis, and ischemia, it is likely that such hetero-exchange mechanisms will need to be taken into account in the development of new drugs targeting NMDA receptor activity. In particular, the development of specific inhibitors of Asc-1 or ASCT2 transporters administered alone, or in combination with exogenous d-serine, appears to be a promising avenue for the pharmacological modulation of d-serine levels in the brain.
Materials and Methods
d-Serine Microelectrode Biosensors
d-Serine microelectrode biosensors were prepared as previously described.26,60 Briefly, the biosensors employed here consisted of a 25 μm 90% Pt/10% Ir wire (Goodfellow, Huntington, U.K.), whose tip (50 or 100 μm long, depending on the experiment) extrudes from a pulled glass micropipet. The platinum wire was covered with an electropolymerized layer of poly-m-phenylenediamine and a manually deposited layer of Rhodotorula gracilisd-amino acid oxidase (DAAO, EC 1.4.3.3.41,61), immobilized by cross-linking with poly(ethylene glycol) diglycidyl ether (PEGDE) at 55 °C during 2 h. This temperature is low enough to preserve the activity of the DAAO enzyme. The biosensors made using PEGDE at 55 °C showed a better sensitivity than those prepared using glutaraldehyde, indicating that denaturation, if present at all, is not a major problem for the functioning of the biosensors.62
In Vivo Experiments
All in vivo experiments were performed on male Wistar rats (Elevage Janvier, Le Genest Saint Isle, France) weighing 250–350 g. Experimental protocols were approved by the local committee on animals in research at the University Claude Bernard Lyon I (protocol BH2010-19) and were performed in accordance with European directive 86/609/CEE. Rats were anesthetized by an intraperitoneal injection of urethane (Sigma, Saint Quentin Fallavier, France, 1.5–2 g/kg) and were immobilized in a stereotactic apparatus (Stoelting Corporation, Wood Dale, IL). Body temperature was maintained at 37 °C using a homeothermic blanket (LSI Letica, Barcelona, Spain). An Ag/AgCl reference electrode was placed on top of the skull and regularly wetted with PBS to maintain electrical contact. Biosensors were implanted in the frontal cortex (anteroposterior +3 mm from bregma, lateral +1 mm, depth +1.5 mm). Amperometric recordings, at a potential fixed at 500 mV vs Ag/AgCl, began between 60 and 90 min after implantation, when the electrochemical current recorded by the biosensor had stabilized. To estimate in vivo extracellular d-serine concentrations, the oxidation current recorded in vivo was divided by the sensitivity of the biosensor in standard solutions at the conclusion of the experiment and corrected for temperature.
d-Serine Microinjections in Vitro
For d-serine administration by microionophoresis in vitro, a 50-μm long d-serine microelectrode biosensor was inserted into a gel made from 0.3% agar diluted in 10 mM PBS, An ionophoresis pipet, with a tip diameter of ∼5 μm and filled with 1 M d-serine diluted in Ringer’s solution (147 mM NaCl, 4 mM KCl, 1.3 mM CaCl2) was placed at 150–800 μm from the tip of the d-serine sensor. Following a stabilization period of 10–20 min, ionophoretic injections of d-serine were performed during 5–100 s at currents of 200–1000 nA with a MVCS-02C ionophoresis amplifier (NPI electronics, Tamm, Germany).
d-Serine Microinjections in Vivo
For d-serine administration by microionophoresis in vivo, a 100 μm long d-serine microelectrode biosensor was inserted into the frontal cortex of anesthetized rats. An ionophoresis pipet, with a tip diameter of ∼5 μm and filled with 1 M d-serine dissolved in Ringer’s solution, was placed at 150–800 μm from the tip of the d-serine sensor. Following a stabilization period of 60–90 min, ionophoretic injections of d-serine were performed during 5–100 s at currents of 200–1000 nA with a MVCS-02C ionophoresis amplifier (NPI electronics, Tamm, Germany). The distance between the ionophoretic pipet and the biosensor was determined at the end of the experiment, after removing the rat from the stereotactic apparatus, by placing the biosensor and the pipet at the same coordinates as in vivo and measuring the distance with a stereomicroscope. No blank biosensor was used to control the specificity of the d-serine signal because the signal was always time-locked with the d-serine injection. Moreover, d-serine injection experiments have been performed and controlled with blank biosensors by Pernot et al.27
For TTX (citrate salt, Tocris, Bristol, U.K.) pressure microinjections, a micropipet was implanted at a distance of 150–300 μm from the biosensor. TTX was ejected continuously using constant pressure between 12 and 20 psi delivered by a PV820 pneumatic picopump (WPI, Stevenage, U.K.).
Reverse Microdialysis
The infusion of l-amino acids or SMLC (Sigma, Saint Quentin Fallavier, France) was performed by reverse microdialysis. An Elite 12 microdialysis probe (2 mm long and 0.5 mm diameter, CMA Microdialysis, Solna, Sweden) was inserted into the brain, near the tip (0.3–1 mm) of the biosensor. Ringer’s solution was circulated in the probe at the speed of 3 μL/min by an infusion dual syringe pump (Harvard apparatus), linked to a CMA 110 liquid switch (CMA microdialysis) allowing rapid changes of the perfusion solution from control Ringer’s to a 100 mM l-amino acid solution or a 1 mM SMLC solution.
Recordings
For amperometric recordings, we used a VA-10 electrochemistry amplifier (NPI electronics, Tamm, Germany) with a two electrode potentiostat (500 MΩ feedback resistor). Data acquisition was performed using an ITC-18 acquisition board (Instrutech, Port Washington, NY) driven with homemade software based on Igor Pro 6.0 procedures (Wavemetrics, Eugene, OR). The oxidation current was sampled at 1000 Hz with a 20 Hz low-pass filter and averaged over 1000 points, yielding a final sampling frequency of 1 Hz. The applied potential is 500 mV vs Ag/AgCl.
Analysis of Uptake Experiments, Statistics
Analysis of the uptake experiments was performed with the Igor Pro 6.0 software package. The fit of the injection/uptake curves was performed using the fitting tool of the software. Data are expressed as mean ± standard error of the mean (SEM). Comparisons between two data groups were performed using two-tailed Student’s t test for equal or unequal variances, as determined by the F test (significance level of p < 0.05). For statistical comparisons of three or more data groups, we used an ANOVA followed by Fisher’s LSD posthoc test. The analysis tool-pack of Igor Pro 6.0 and SPSS 12.0 for Windows were used for the statistical analyses.
Acknowledgments
We are grateful to Dr. Alison Mungenast for helpful comments on an earlier version of the manuscript. We thank Aïcha Compard and Animalerie Rockefeller (SCAR) for animal care. The Lyon Neuroscience Research Center is part of SFR Santé Lyon Est (UCBL, UMS 3453 CNRS, US7 Inserm).
Author Contributions
C.M. and P.P. designed and performed in vivo experiments, analyzed the data, and wrote the manuscript. N.V. designed and fabricated biosensors and analyzed data. L.P. expressed and purified enzymes for biosensor fabrication and wrote the manuscript. S.M. supported the project, designed experiments, analyzed data, and wrote the manuscript.
This study was supported by Inserm U1028, Université Claude Bernard Lyon I, and by grants from Agence Nationale pour la Recherche (ANR-09-BLAN-0063 Neurosense) to S.M. L.P. thanks the financial support of from Fondo di Ateneo per la Ricerca. P.P., C.M., and N.V. are recipients of Ph.D. fellowships from Ministère de la Recherche.
The authors declare no competing financial interest.
References
- Katsuki H.; Nonaka M.; Shirakawa H.; Kume T.; Akaike A. (2004) Endogenous d-serine is involved in induction of neuronal death by N-methyl-d-aspartate and simulated ischemia in rat cerebrocortical slices. J. Pharmacol. Exp. Ther. 311, 836–844. [DOI] [PubMed] [Google Scholar]
- Bendikov I.; Nadri C.; Amar S.; Panizzutti R.; De Miranda J.; Wolosker H.; Agam G. (2007) A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr. Res. 90, 41–51. [DOI] [PubMed] [Google Scholar]
- Hashimoto K.; Fukushima T.; Shimizu E.; Komatsu N.; Watanabe H.; Shinoda N.; Nakazato M.; Kumakiri C.; Okada S.; Hasegawa H.; Imai K.; Iyo M. (2003) Decreased serum levels of d-serine in patients with schizophrenia: evidence in support of the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry 60, 572–576. [DOI] [PubMed] [Google Scholar]
- Thompson M.; Marecki J. C.; Marinesco S.; Labrie V.; Roder J. C.; Barger S. W.; Crow J. P. (2012) Paradoxical roles of serine racemase and d-serine in the G93A mSOD1 mouse model of amyotrophic lateral sclerosis. J. Neurochem. 120, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H. J.; Tiihonen J.; Wahlbeck K. (2005) Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 72, 225–234. [DOI] [PubMed] [Google Scholar]
- Pollegioni L.; Sacchi S. (2010) Metabolism of the neuromodulator d-serine. Cell. Mol. Life Sci. 67, 2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia M. A.; Madeira C.; Alheira F. V.; Silva T. C.; Tannos F. M.; Vargas-Lopes C.; Goldenstein N.; Brasil M. A.; Ferreira S. T.; Panizzutti R. (2012) Plasma levels of d-serine in Brazilian individuals with schizophrenia. Schizophr. Res. 142, 83–87. [DOI] [PubMed] [Google Scholar]
- Sasabe J.; Chiba T.; Yamada M.; Okamoto K.; Nishimoto I.; Matsuoka M.; Aiso S. (2007) d-Serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 26, 4149–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E.; Shleper M.; Balan L.; Dumin E.; Wolosker H. (2006) Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J. Biol. Chem. 281, 14151–14162. [DOI] [PubMed] [Google Scholar]
- Kirschner D. L.; Wilson A. L.; Drew K. L.; Green T. K. (2009) Simultaneous efflux of endogenous d-ser and l-glu from single acute hippocampus slices during oxygen glucose deprivation. J Neurosci Res. 87, 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C. S.; Reis M.; Panizzutti R.; de Miranda J.; Wolosker H. (2002) Glial transport of the neuromodulator d-serine. Brain Res. 929, 202–209. [DOI] [PubMed] [Google Scholar]
- Fukasawa Y.; Segawa H.; Kim J. Y.; Chairoungdua A.; Kim D. K.; Matsuo H.; Cha S. H.; Endou H.; Kanai Y. (2000) Identification and characterization of a Na+-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d- and L-amino acids. J. Biol. Chem. 275, 9690–9698. [DOI] [PubMed] [Google Scholar]
- Nakauchi J.; Matsuo H.; Kim D. K.; Goto A.; Chairoungdua A.; Cha S. H.; Inatomi J.; Shiokawa Y.; Yamaguchi K.; Saito I.; Endou H.; Kanai Y. (2000) Cloning and characterization of a human brain Na(+)-independent transporter for small neutral amino acids that transports d-serine with high affinity. Neurosci. Lett. 287, 231–235. [DOI] [PubMed] [Google Scholar]
- Helboe L.; Egebjerg J.; Moller M.; Thomsen C. (2003) Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur. J. Neurosci. 18, 2227–2238. [DOI] [PubMed] [Google Scholar]
- Rutter A. R.; Fradley R. L.; Garrett E. M.; Chapman K. L.; Lawrence J. M.; Rosahl T. W.; Patel S. (2007) Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating d-serine reuptake in the mouse CNS. Eur. J. Neurosci. 25, 1757–1766. [DOI] [PubMed] [Google Scholar]
- Gliddon C. M.; Shao Z.; LeMaistre J. L.; Anderson C. M. (2009) Cellular distribution of the neutral amino acid transporter subtype ASCT2 in mouse brain. J. Neurochem. 108, 372–383. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N.; Endou H.; Kanai Y. (1996) Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 271, 14883–14890. [DOI] [PubMed] [Google Scholar]
- Hayashi F.; Takahashi K.; Nishikawa T. (1997) Uptake of d- and l-serine in C6 glioma cells. Neurosci. Lett. 239, 85–88. [DOI] [PubMed] [Google Scholar]
- Dun Y.; Mysona B.; Itagaki S.; Martin-Studdard A.; Ganapathy V.; Smith S. B. (2007) Functional and molecular analysis of d-serine transport in retinal Muller cells. Exp. Eye Res. 84, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z.; Kamboj A.; Anderson C. M. (2009) Functional and immunocytochemical characterization of d-serine transporters in cortical neuron and astrocyte cultures. J. Neurosci. Res. 87, 2520–2530. [DOI] [PubMed] [Google Scholar]
- Mothet J. P.; Pollegioni L.; Ouanounou G.; Martineau M.; Fossier P.; Baux G. (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc. Natl. Acad. Sci. U.S.A. 102, 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M.; Galli T.; Baux G.; Mothet J. P. (2008) Confocal imaging and tracking of the exocytotic routes for d-serine-mediated gliotransmission. Glia 56, 1271–1284. [DOI] [PubMed] [Google Scholar]
- Martineau M.; Shi T.; Puyal J.; Knolhoff A. M.; Dulong J.; Gasnier B.; Klingauf J.; Sweedler J. V.; Jahn R.; Mothet J. P. (2013) Storage and Uptake of d-Serine into Astrocytic Synaptic-Like Vesicles Specify Gliotransmission. J. Neurosci. 33, 3413–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D.; Kartvelishvily E.; Shleper M.; Klinker C. M.; Bowser M. T.; Wolosker H. (2010) Neuronal release of d-serine: a physiological pathway controlling extracellular d-serine concentration. FASEB J. 24, 2951–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D.; Artoul S.; Segal A. C.; Kolodney G.; Radzishevsky I.; Dikopoltsev E.; Foltyn V. N.; Inoue R.; Mori H.; Billard J. M.; Wolosker H. (2013) Neuronal d-Serine and Glycine Release Via the Asc-1 Transporter Regulates NMDA Receptor-Dependent Synaptic Activity. J. Neurosci. 33, 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernot P.; Mothet J. P.; Schuvailo O.; Soldatkin A.; Pollegioni L.; Pilone M.; Adeline M. T.; Cespuglio R.; Marinesco S. (2008) Characterization of a yeast d-amino acid oxidase microbiosensor for d-serine detection in the central nervous system. Anal. Chem. 80, 1589–1597. [DOI] [PubMed] [Google Scholar]
- Pernot P.; Maucler C.; Tholance Y.; Vasylieva N.; Debilly G.; Pollegioni L.; Cespuglio R.; Marinesco S. (2012) d-Serine diffusion through the blood-brain barrier: Effect on d-serine compartmentalization and storage. Neurochem. Int. 60, 837–845. [DOI] [PubMed] [Google Scholar]
- Thomsen C., Helboe L., and Egebjerg J. (2003) Use of ASC-1 inhibitors to treat neurological and psychiatric disorders. US Patent 20050176826 .
- Ishiwata S.; Ogata S.; Umino A.; Shiraku H.; Ohashi Y.; Kajii Y.; Nishikawa T.. Increasing effects of S-methyl-l-cysteine on the extracellular d-serine concentrations in the rat medial frontal cortex. Amino Acids 2013, in press. [DOI] [PubMed]
- Pollegioni L.; Falbo A.; Pilone M. S. (1992) Specificity and kinetics of Rhodotorula gracilis d-amino acid oxidase. Biochim. Biophys. Acta 1120, 11–16. [DOI] [PubMed] [Google Scholar]
- Hamase K.; Konno R.; Morikawa A.; Zaitsu K. (2005) Sensitive determination of d-amino acids in mammals and the effect of d-amino-acid oxidase activity on their amounts. Biol. Pharm. Bull. 28, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Schell M. J.; Brady R. O. Jr.; Molliver M. E.; Snyder S. H. (1997) d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 17, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. M.; Diaz C. M.; Macnab L. T.; Sullivan R. K.; Pow D. V. (2006) Immunocytochemical analysis of d-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia 53, 401–411. [DOI] [PubMed] [Google Scholar]
- Rice M., and Nicholson C. (1995) Diffusion and ion shifts in the brain extracellualr microenvironnement and their relevance for voltametric measurements (Boulton A., Baker G., and Adams R., Eds.), Humana Press, Totowa, NJ. [Google Scholar]
- Sieg A.; Jeanneret F.; Fathi M.; Hochstrasser D.; Rudaz S.; Veuthey J. L.; Guy R. H.; Delgado-Charro M. B. (2008) Extraction of amino acids by reverse iontophoresis: simulation of therapeutic monitoring in vitro. Eur. J. Pharm. Biopharm. 70, 908–913. [DOI] [PubMed] [Google Scholar]
- Pikal M. (1992) The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Delivery Rev. 46, 281–305. [DOI] [PubMed] [Google Scholar]
- Nicholson C.; Sykova E. (1998) Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215. [DOI] [PubMed] [Google Scholar]
- Sieg A.; Guy R. H.; Delgado-Charro M. B. (2004) Electroosmosis in transdermal iontophoresis: implications for noninvasive and calibration-free glucose monitoring. Biophys. J. 87, 3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Li C. G.; Ye C. H.; Liu M. L. (2001) Determination of molecular self-diffusion coefficient using multiple spin-echo NMR spectroscopy with removal of convection and background gradient artifacts. Anal. Chem. 73, 3528–3534. [DOI] [PubMed] [Google Scholar]
- Pollegioni L.; Falbo A.; Pilone M. S. (1992) Specificity and kinetics of Rhodotorula gracilis d-amino acid oxidase. Biochim. Biophys. Acta 1120, 11–16. [DOI] [PubMed] [Google Scholar]
- Pollegioni L.; Molla G.; Sacchi S.; Rosini E.; Verga R.; Pilone M. S. (2008) Properties and applications of microbial d-amino acid oxidases: current state and perspectives. Appl. Microbiol. Biotechnol. 78, 1–16. [DOI] [PubMed] [Google Scholar]
- Pernot P.; Mothet J. P.; Schuvailo O.; Soldatkin A.; Pollegioni L.; Pilone M.; Adeline M. T.; Cespuglio R.; Marinesco S. (2008) Characterization of a Yeast d-Amino Acid Oxidase Microbiosensor for d-Serine Detection in the Central Nervous System. Anal. Chem. 80, 9. [DOI] [PubMed] [Google Scholar]
- O’Brien K. B.; Bowser M. T. (2006) Measuring d-serine efflux from mouse cortical brain slices using online microdialysis-capillary electrophoresis. Electrophoresis 27, 1949–1956. [DOI] [PubMed] [Google Scholar]
- Shafqat S.; Tamarappoo B. K.; Kilberg M. S.; Puranam R. S.; McNamara J. O.; Guadano-Ferraz A.; Fremeau R. T. Jr. (1993) Cloning and expression of a novel Na(+)-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J. Biol. Chem. 268, 15351–15355. [PubMed] [Google Scholar]
- Grewer C.; Grabsch E. (2004) New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. 557, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenziel W. H.; Dijkstra G.; Cremers T. I.; Westerink B. H. (2006) In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res. 1118, 34–42. [DOI] [PubMed] [Google Scholar]
- Martina M.; Gorfinkel Y.; Halman S.; Lowe J. A.; Periyalwar P.; Schmidt C. J.; Bergeron R. (2004) Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J. Physiol. 557, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer A.; Brookes N.; Ganapathy V.; Dimmer K. S.; Wagner C. A.; Lang F.; Broer S. (1999) The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. J. Neurochem. 73, 2184–2194. [PubMed] [Google Scholar]
- Wolosker H. (2011) Serine racemase and the serine shuttle between neurons and astrocytes. Biochim. Biophys. Acta 1814, 1558–1566. [DOI] [PubMed] [Google Scholar]
- Yang J. H.; Wada A.; Yoshida K.; Miyoshi Y.; Sayano T.; Esaki K.; Kinoshita M. O.; Tomonaga S.; Azuma N.; Watanabe M.; Hamase K.; Zaitsu K.; Machida T.; Messing A.; Itohara S.; Hirabayashi Y.; Furuya S. (2010) Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of d-serine, an N-methyl-d-aspartate receptor co-agonist, in adult brain. J. Biol. Chem. 285, 41380–41390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A.; Kanda J.; Oka T. (2000) Effects of N-methyl-d-aspartate, kainate or veratridine on extracellular concentrations of free d-serine and L-glutamate in rat striatum: an in vivo microdialysis study. Brain Res. Bull. 53, 347–351. [DOI] [PubMed] [Google Scholar]
- Mustafa A. K.; Kim P. M.; Snyder S. H. (2004) d-Serine as a putative glial neurotransmitter. Neuron Glia Biol. 1, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. M.; Aizawa H.; Kim P. S.; Huang A. S.; Wickramasinghe S. R.; Kashani A. H.; Barrow R. K.; Huganir R. L.; Ghosh A.; Snyder S. H. (2005) Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc. Natl. Acad. Sci. U.S.A. 102, 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. K.; Kumar M.; Selvakumar B.; Ho G. P.; Ehmsen J. T.; Barrow R. K.; Amzel L. M.; Snyder S. H. (2007) Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of d-serine formation. Proc. Natl. Acad. Sci. U.S.A. 104, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C.; Papouin T.; Oliet S. H.; Rusakov D. A. (2010) Long-term potentiation depends on release of d-serine from astrocytes. Nature 463, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Lin S. C.; Nicolelis M. A. (2009) Acquiring local field potential information from amperometric neurochemical recordings. J. Neurosci .Methods 179, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano A.; Marinesco S.; Pain F.; Meiller A.; Gurden H. (2012) Reconstruction of field excitatory post-synaptic potentials in the dentate gyrus from amperometric biosensor signals. J. Neurosci. Methods 206, 1–6. [DOI] [PubMed] [Google Scholar]
- Hashimoto A.; Oka T.; Nishikawa T. (1995) Extracellular concentration of endogenous free d-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience 66, 635–643. [DOI] [PubMed] [Google Scholar]
- Kakegawa W.; Miyoshi Y.; Hamase K.; Matsuda S.; Matsuda K.; Kohda K.; Emi K.; Motohashi J.; Konno R.; Zaitsu K.; Yuzaki M. (2011) d-Serine regulates cerebellar LTD and motor coordination through the delta2 glutamate receptor. Nat. Neurosci. 14, 603–611. [DOI] [PubMed] [Google Scholar]
- Vasylieva N.; Barnych B.; Meiller A.; Maucler C.; Pollegioni L.; Lin J. S.; Barbier D.; Marinesco S. (2011) Covalent enzyme immobilization by poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 26, 3993–4000. [DOI] [PubMed] [Google Scholar]
- Pollegioni L.; Molla G.; Campaner S.; Martegani E.; Pilone M. S. (1997) Cloning, sequencing and expression in E. coli of a d-amino acid oxidase cDNA from Rhodotorula gracilis active on cephalosporin C. J. Biotechnol. 58, 115–123. [DOI] [PubMed] [Google Scholar]
- Vasylieva N.; Barnych B.; Meiller A.; Maucler C.; Pollegioni L.; Lin J. S.; Barbier D.; Marinesco S. (2011) Covalent enzyme immobilization by poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 26, 3993–4000. [DOI] [PubMed] [Google Scholar]