Abstract
Simultaneous electrochemical and electrophysiological data were recorded to evaluate the effects of controlled local application of dopaminergic agonists and antagonists in awake rats. Measurements were made with a probe consisting of a carbon-fiber microelectrode fused to three iontophoretic barrels used to introduce the drugs of interest. The probe and the manipulator used to position it in the brain of behaving animals were optimized to improve their performance. The effect of the dopamine autoreceptor on electrically stimulated release was demonstrated. Dopamine inhibited the release of endogenous dopamine whereas raclopride, a D2 antagonist, enhanced it, with similar responses in anesthetized and awake animals. We also examined changes in the firing rate of nucleus accumbens (NAc) neurons in awake animals during and after brief (15 s) iontophoretic ejections of SCH 23390 (D1 receptor antagonist) or raclopride. Changes in response to these antagonists were seen both immediately and on a prolonged time scale. Application of raclopride increased the firing rate in 40% of medium spiny neurons (MSNs), of which half responded immediately. Decreases in firing rate were observed in 46% of MSNs after SCH 23390 application. Only 11% of MSNs responded to both antagonists and one MSN (3%) showed no response to either drug. The same prolonged response in firing rate was seen for electrically stimulated and locally applied dopamine in 75% of MSNs. These results are in agreement with previously reported distributions for dopamine receptor subtypes on MSNs and probe the effects of dopamine on these cell populations.
Keywords: Iontophoresis, fast-scan cyclic voltammetry, electrophysiology, dopamine receptors, nucleus accumbens, medium spiny neuron
The modulation of target neurons via neurotransmitter release is central to the regulation of an organism’s behavior. Dopamine plays an extensive role in governing motivated behaviors and our groups have shown that its release coincides with learned associations for rewarding stimuli and drugs of abuse.1−5 Here, we describe a technique that is capable of monitoring extracellular dopamine release and postsynaptic cell activity in awake, behaving animals. At a single carbon-fiber electrode of micrometer dimensions, dopamine release is detected by fast-scan cyclic voltammetry and postsynaptic cell activity is monitored by single-unit recordings. The electrode is coupled to micropipets that with the application of current locally deliver pharmacological agents to investigate the specific receptor(s) activated. This approach was first developed by Millar and co-workers who used fast-scan cyclic voltammetry and single-unit recording at a single carbon-fiber electrode.6 They combined these techniques with iontophoresis to compare the response of striatal medium spiny neurons (MSNs) to locally applied dopamine and endogenous dopamine released by electrical stimulation.7 Despite the utility of this approach, it has been used only rarely in behaving animals.8,9 Here we evaluate this approach in awake animals to probe the effects of dopamine in the nucleus accumbens (NAc), a striatal subregion involved in reward processing, on evoked dopamine release and the electrical activity of MSNs.
Establishing the nature of dopamine responses proved difficult in early studies using iontophoresis combined with single-unit recording for several reasons.10 First, many of those recordings were in anesthetized animals and anesthesia is known to alter neuronal activity.11,12 Second, the amount of drug delivered by iontophoresis was not quantifiable. Too little drug delivered results in a false negative whereas too much leads to nonspecific effects. Applied pump currents were commonly used to compare ejections,13−15 but the same pump current ejected different drug concentrations from barrel to barrel.16 To remedy this, we monitor the iontophoretic coejection of an electroactive molecule with the drug of interest from the same barrel. When the relative mobilities of the coejected substances are known, monitoring the concentration of the electroactive molecule with the carbon-fiber electrode provides an indirect measure of the relative concentration of the coejected nonelectroactive substance.17,18 Third, ionic and electrical artifacts can occur in iontophoresis. Since the chemical and physiological environments are monitored with the combined voltammetry/single-unit recording probe, these problems become apparent during the experiment and can be corrected. Finally, many studies were completed before the recognition of various subtypes of dopamine receptors and before investigators realized that dopamine receptors are coupled to second messenger systems that modulate the effects of classical neurotransmitters such as glutamate. In addition, MSNs have “up” and “down” states during which the cell’s response to dopamine may differ and are not discernible with single-unit recordings.19−21
Interpretation of dopamine responses in prior studies were further complicated by the lack of understanding of the distribution of dopamine receptors. Dopamine neurons in the NAc synapse onto dendrites and spines of MSNs.22 MSNs are GABAergic and comprise 95% of the cell bodies in this region.23 Glutamatergic neurons that project from the cortex also form synapses on MSNs. Recent anatomical discoveries have shown that the predominant dopamine receptors, the D1 and D2 receptors, are segregated between two different MSN populations.24,25 Early studies that focused exclusively on the dorsal striatum showed that some MSNs project axons to the output nuclei of the basal ganglia (substantia nigra and internal capsule of the globus pallidus [GPi]) and are termed the “direct pathway”. Other MSNs project to the external capsule of the globus pallidus (GPe) and are termed the “indirect pathway” because they synapse with neurons that also project back to the output nuclei. Although this circuitry was originally characterized in the dorsal striatum, it is also found in the NAc.26 In the NAc core, 53% of MSNs comprise the direct pathway and have D1 receptors exclusively. Further, 41% of neurons in the NAc core comprise the indirect pathway and have D2 receptors exclusively. Thus, an estimated 6% of MSNs in the NAc have both D1 and D2 receptors.
In this work, we describe the modifications made to the voltammetry/single-unit recording/iontophoresis probes for use in awake animals. These improvements enable examination of the distribution of dopamine receptor subtypes present on MSNs in the NAc. In addition, we have examined the immediate and prolonged cell firing effects of endogenous and exogenous dopamine and dopamine antagonists on the different types of MSNs. Our initial results agree with previously reported distributions of dopamine receptors on MSNs,26 lending credence to our combined technique and supporting previous in vitro and indirect in vivo studies.
Results and Discussion
Voltammetry/Single-Unit/Iontophoresis Measurements
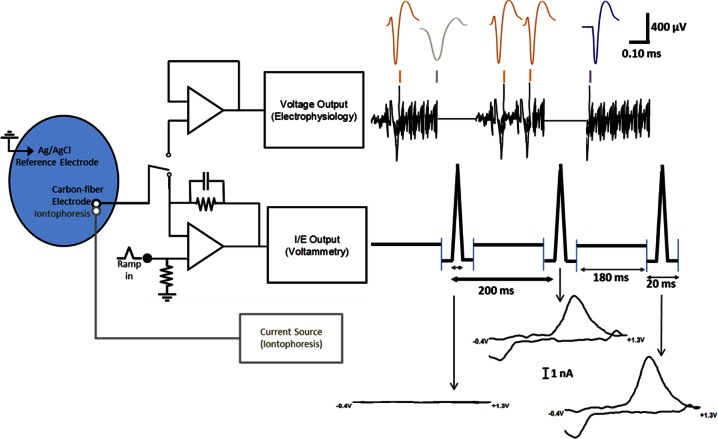
A diagram of the circuitry associated with these experiments is shown in Figure 1.27 In this work, the cyclic voltammograms were collected at 5 Hz (once every 200 ms). With the scan rate employed (400 V/s), a single cyclic voltammogram is recorded in 8.5 ms. The total time with the current transducer connected (20 ms) includes 5.75 ms before the triangular wave to allow for dopamine adsorption and 5.75 ms after the triangular wave to allow the amplifiers to settle. For the remaining 180 ms between scans, the amplifier connected to the carbon-fiber electrode is switched (in <1 μs) from a current transducer to a voltage follower to allow recording of unit activity. Iontophoresis occurs continuously during the switching between voltammetry and single-unit recordings via a separate circuit. The data from each measurement type is collected on separate computers along with time stamps allowing synchronization of the data to iontophoretic ejections or other events.
Figure 1.
Diagram of the electronics and outputs for combined fast-scan cyclic voltammetry/electrophysiology. The same carbon-fiber electrode and reference electrode were used for both circuits and the reference electrode also served as ground for the iontophoresis circuit. The output of the carbon-fiber electrode was connected to a voltage follower to monitor cell firing (upper circuitry). Every 200 ms, the carbon-fiber microelectrode was switched from the electrophysiology circuit to the lower voltammetry circuitry for 20 ms. In this position, the amplifier controlled the electrode potential and the current was monitored. The synchronized outputs for both circuits are shown to the right of the circuitry. When the lower circuit was completed, the electrode was held at −0.4 V then over 8.5 ms scanned from to +1.3 V and back to −0.4 V. The changes in current during each scan are indicated with arrows. During this time, there were no voltage changes seen in the electrophysiology (when circuit was open but program still recorded data). With the upper circuit connected, changes in voltage were monitored. The colored marks above the voltage read out indicate regions of the 180 ms that have been expanded above. Orange spikes are firing of the MSN of interest; purple spikes are firing that looks similar to the MSN of interest but are not included due to the horizontal portion at the start of the spike. The gray spike is an example of an excluded event.
In prior work, we used continuous iontophoresis over multiple minutes to affect cell firing frequency.9 Shorter applications are advantageous because they restrict the drug to the local environment in which chemical changes and single-unit activity are measured. Here, we adopted two methods to monitor ejection effects. With the first method, we measured changes in cell firing or changes in electrically stimulated dopamine release shortly after a 15 s iontophoretic ejection. This approach has the advantage that the electrochemical signal arising from the ejected internal standard does not interfere with endogenous chemical measurements. With the second method we measured changes in cell firing during the 15 s ejections. This method was primarily used with electrophysiological recordings.
Electrode Fabrication
We have made several improvements to the construction of iontophoresis probes from the methods we previously described.17 First, we addressed the electrical coupling that sometimes occurred between the iontophoresis barrels and voltammetric barrel due to the inherent variability in the thickness of the glass pipettes. When the thickness of the glass between the adjacent barrels is thin or there are microfractures in the capillaries, electrical coupling between the iontophoresis barrels is likely to occur. Second, we improved the quality of the glass seal against the carbon fiber to help prevent large background currents during voltammetric recordings. Finally, we modified a commercially available manipulator to facilitate use of the probes in awake animals.
Reduced electrical coupling and improvements to the carbon fiber-glass seal were accomplished using a modified fabrication technique. First, a carbon fiber was inserted into in a single glass capillary, and this assembly was inserted into one barrel of a four-barrel prefused glass assembly (Figure 2A). Heat-shrink tubing was applied to the ends of each multibarrel glass capillary at the location where the chuck of the pipet puller was tightened onto the glass. Tack was placed at the lower end to prevent the inner glass capillary containing the carbon fiber from falling out. The assembly was pulled on a vertical micropipet puller in a two-step process that created two electrodes of the type shown in Figure 2B. The first pull elongated and narrowed the glass while the second pull formed the glass seal around the carbon fiber. The carbon fiber was then trimmed to have an exposed length of 50–75 μm. Before use, the heat-shrink was removed and an electrical connection to the carbon fiber was made with silver epoxy and an inserted silver wire. These procedures minimized the electrical coupling between the electrode and the iontophoresis barrels and resulted in improved carbon-glass seals.
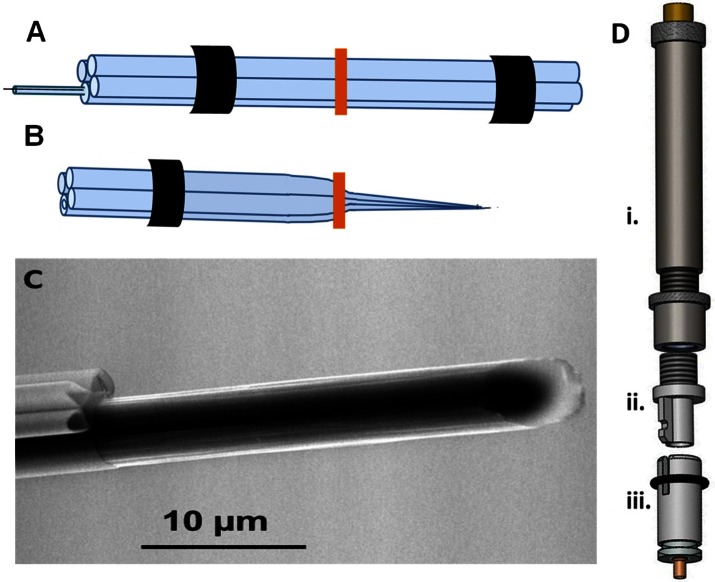
Figure 2.
Modifications to probe construction and hardware for iontophoresis in freely moving animals. (A) Four-barrel prefused pipet ready for pulling with heat shrink on either end where the glass will contact the chucks of the puller. A smaller capillary containing a T-650 carbon fiber is loaded into one barrel. (B) One of the two electrodes created from the two-pull electrode making technique. The vertical bar indicates the midpoint of the pipet before pulling. (C) Environmental scanning electron micrograph of the probe tip showing the glass iontophoresis barrels and carbon fiber. (D) Hardware used to attach and lower iontophoresis probe into the head of a freely moving animal. (i) Commercially available Biela manipulator from Crist Instrument Co. (Part# 3-MMB-3D). (ii) Custom machined adapter that allows use of Biela manipulator with a guide cannula from Bioanalytical Systems, Inc. fabricated by UNC Physics Machine Shop (Chapel Hill, NC). (iii) Commercially available guide cannula from Bioanalytical Systems.
To facilitate use of the voltammetry/single-unit/iontophoresis probe in awake animals and allow for lowering to a precise depth within the brain, the probe was inserted into a commercially available Biela micromanipulator.28 The manipulator contains a metal tube (Figure 2Di) that has four inner grooves to hold a four-barrel pipet. This innermost piece moves up and down as the two outermost parts screw together. The Biela manipulators are designed for connection to the skull with a threaded guide cannula that requires a large diameter (1.5 mm) skull opening. The large hole tends to become blocked with blood clots, which increases the likelihood of pipet breakage. This problem is less frequent with the guide cannula that we use with single-barrel electrodes, which requires a 0.65 mm diameter hole (Figure 2Diii). This guide cannula uses a washer and groove system to attach the micromanipulator to the skull. The polyamide plastic cannula (2 mm long) extending beyond the hub was inserted in a hole drilled in the animal’s brain and permanently affixed with dental cement during surgery. To mate the manipulator with the smaller diameter guide cannula, a custom-machined adapter was fabricated with threads to attach the adapter to the manipulator (UNC Physics Machine Shop, Chapel Hill, NC). The metal ridge of the adapter (Figure 2Dii) mates with the groove in the guide cannula and the washer on the cannula locks the pieces together. This allows for easy attachment on the animal’s head under brief isofluorane anesthesia.
Modulation of Dopamine Evoked Release by Autoreceptors
Because stimulated release is modulated by D2 autoreceptors,29,30 we used the improved probes to examine modulation of electrically stimulated (24 pulses, 60 Hz, 125 μA, repeated at 1 min intervals) dopamine release by agents active at D2 receptors in anesthetized animals. We first used microejections of dopamine (16 ± 1 μM) in an attempt to activate the D2 autoreceptor, but there was no effect on electrically stimulated release (N = 4 animals) with three ejections carried out 5–7 min apart. We then continuously delivered nomifensine (54 ± 2 μM), a dopamine transporter uptake inhibitor, while continuing stimulations at 1 min intervals. Under these conditions, the amplitude of electrically stimulated dopamine release was inhibited 23 ± 6% (p < 0.05, paired t test) by dopamine. This experiment is summarized in Figure 3. A lower concentration of dopamine (1.7 ± 0.5 μM) was also tested in three of the animals and showed the same inhibition of electrically stimulated dopamine release only during nomifensine application. By inhibiting uptake, nomifensine allowed dopamine to diffuse greater distances and remain in the extracellular space longer.31,32 Additionally, continuous inhibition of the transporter may alter autoreceptor function.33
Figure 3.
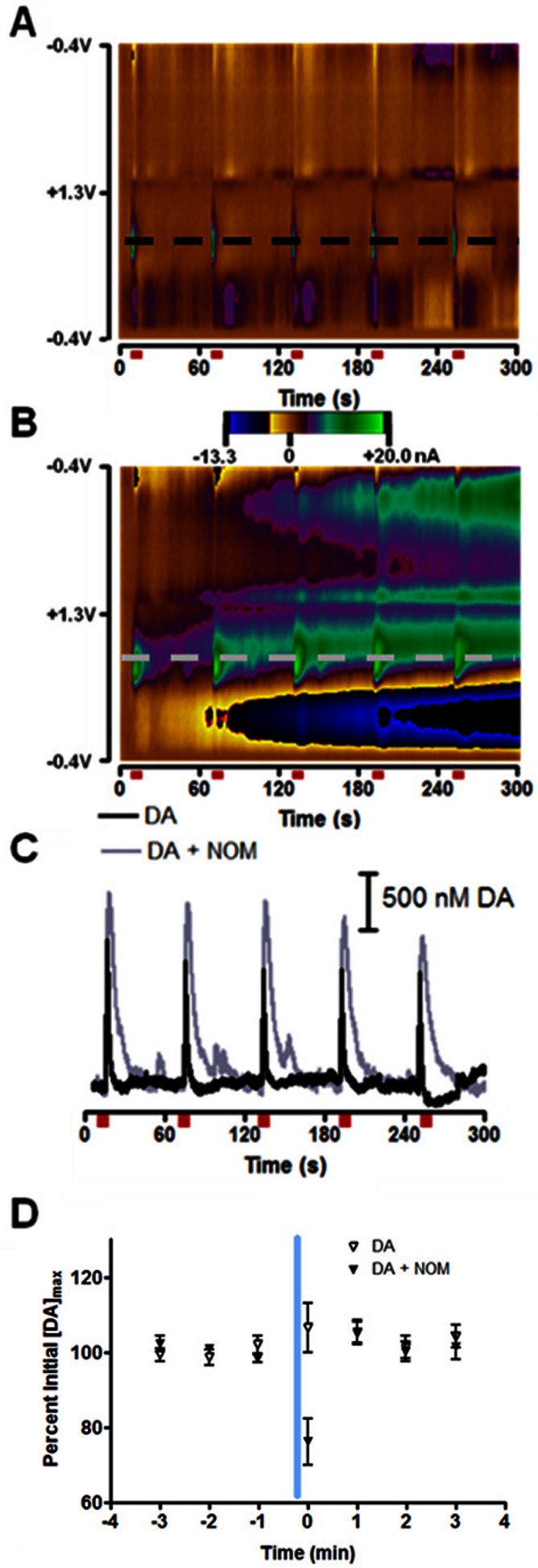
Effect of dopamine transporter on exogenous dopamine diffusion. (A) Two-dimensional color plot where current is shown in false color on the potential vs time axes for current changes in the nucleus accumbens (NAc). The positive (green) currents are indicative of dopamine release. Anesthetized rats received a 24 pulse, 125 μA, 60 Hz electrical stimulation every 60 s, and maximum dopamine concentration ([DA]max) evoked with each stimulation recorded. Stimulations are indicated by red bars below the color plot. (B) In the same animal, the same experiment was carried out as in (A) but 54 ± 2 μM nomifensine (NOM) was constantly iontophoresed during the stimulations. (C) Dopamine (DA) oxidation currents as a function of time. Traces were taken from the potentials marked with dashed lines in panels (A) and (B). (D) 15 s ejection of 16 ± 1 μM dopamine occurred at the vertical blue line and ended 10 s before electrical stimulation at t = 0 s. Open triangles indicate dopamine ejections, while black triangles indicate dopamine ejections made during constant application of nomifensine. [DA]max evoked was greater in the presence of nomifensine, but all data is presented as percent of pre-ejection [DA]max for each data set.
We also iontophoresed the D2 receptor antagonist raclopride in these same locations to ensure [DA]max responded as we previously showed to autoreceptor antagonism.18 The 15 s ejection consisted of 21 ± 11 μM raclopride and terminated 10 s before the stimulation. Ejections were repeated three times 7–10 min apart during which electrical stimulation occurred every 60 s. [DA]max was significantly increased by 151 ± 13% (N = 4 animals, p < 0.05, paired t test). Even 70 s after the ejection, [DA]max was still increased by 132 ± 8% but returned to preraclopride levels 5 min after the conclusion of the raclopride ejection.
We also looked at the effect of autoreceptor antagonism on the amplitude of stimulated dopamine release in the NAc of awake, behaving animals. Awake animals were trained to press a lever that delivered an electrical stimulation to the ventral tegmental area (VTA). Rats quickly learn this behavior termed intracranial self-stimulation (ICSS) that was discovered almost 60 years ago.34 To ensure dopamine release was not depleted by rapid lever pressing,35 a variable time out (VTO) paradigm, with lever availability 18–27 s after a previous lever press was used. This VTO allowed for a 15 s drug ejection between presses as well. Data logging of [DA]max was initiated when it had reached a stable value.36 After multiple lever-pressing trials, raclopride was ejected for 15 s and was terminated 3 s before the next lever extension. This caused an immediate increase in the maximum concentration of evoked dopamine that was observed after each lever press (Figure 4). This increase occurred at all locations tested, with raclopride increasing [DA]max at each location to 280 ± 130% the preraclopride [DA]max (N = 10 animals). This broad range of responses to raclopride is likely due to the variability in the distance between the probe tip (and the fixed distance between the carbon fiber and point where drug is introduced into the system) and affected receptors. This approach is an important improvement over systemic injections of dopamine receptor antagonists which can alter behavior and confound investigation of receptor mechanisms; localized iontophoretic application of raclopride allows modulation of receptors without affecting an animal’s behavior.37
Figure 4.
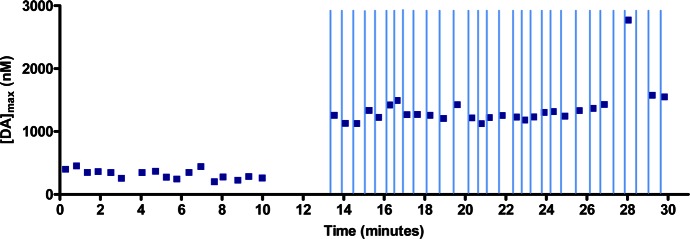
Consistent modulation of electrically evoked dopamine release with raclopride antagonism of the presynaptic D2 receptor during ICSS in a behaving rat. Stimulated release of dopamine occurred each time the animal pressed a lever, and the maximum amplitude was recorded as the maximum dopamine concentration ([DA]max). The lever was made available to the animal once every 18–27 s. Raclopride iontophoretic ejections (15 s, 100 nM) began at 13 min. The ejections terminated 3 s before the lever became available (indicated by vertical lines).
Modulation of Cell Firing with D1 and D2 Receptor Antagonists
We did not attempt measurements of MSN unit activity in anesthetized animals because firing rates are so low under these conditions that investigators typically need to use glutamate to increase activity.11,38,39 In the awake rat, we examined the effects of the D1 antagonist, SCH 23390, the D2 antagonist, raclopride, and dopamine on MSN firing rate (dopamine results are described later). Representative histograms for responses to iontophoretic drug applications are shown in Figure 5, all measured at the same cell. Figure 5A shows a sample MSN waveform, shape of the waveform remained constant for all test conditions. In these experiments the same concentration of drug was applied to the cell for 15 s once every 60 s. The histograms are the average firing rate centered around these 30 drug applications with cell firing placed in 1.0 s bins. Drug was introduced for 15 s starting at t = 0 s, and the average firing rate for 15 s before and after drug delivery also shown. The blue trace superimposed on each histogram is the average concentration of drug ejected over the time the histograms were collected. Figure 5B shows the average firing rate for the MSN over 30 trials during which no drug ejections occurred. This served to ensure that the cell was firing at a constant rate over a 30 min period.
Figure 5.
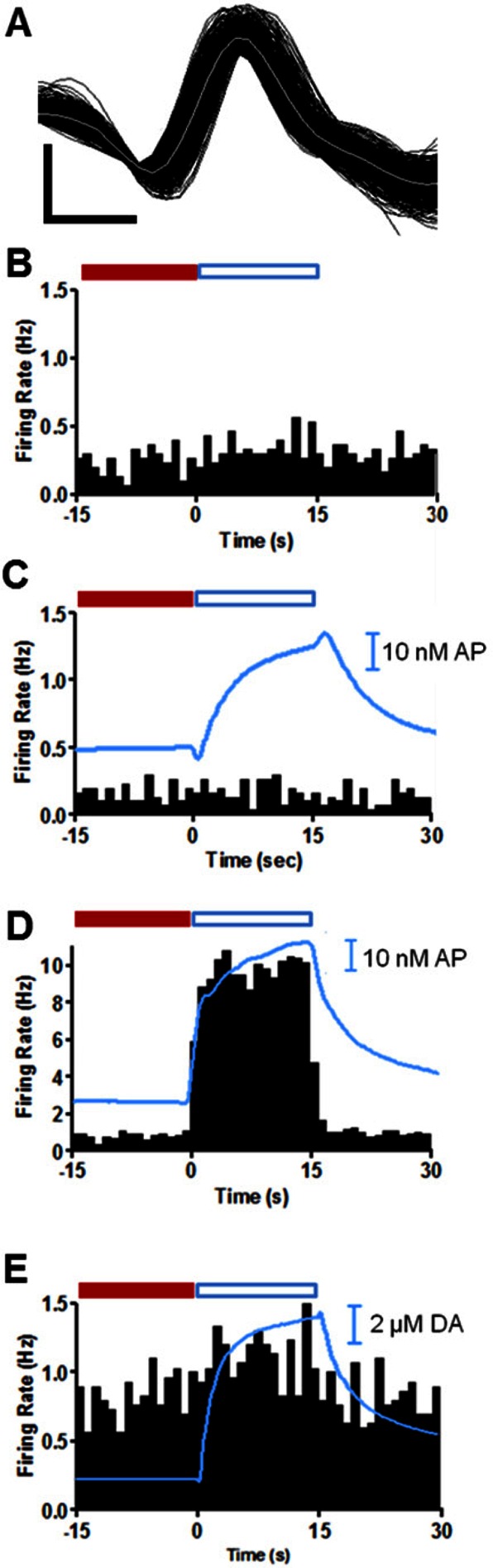
Immediate response of a single NAc MSN to iontophoretic application of dopamine receptor antagonists, SCH 23390 and raclopride, and dopamine in an awake animal. (A) Sample waveforms (150) shown in black with the average of the waveforms in gray for a NAc MSN collected in these experiments. The horizontal scale bar represents 0.3 ms, and vertical scale bar is 300 μV. (B) MSN firing with no iontophoretic ejections. (C) MSN firing during iontophoresis of 23 nM SCH 23390 for 15 s starting at t = 0 s. (D) MSN firing with 40 nM raclopride iontophoresed for 15 s starting at t = 0 s. (E) MSN firing with 12 μM of dopamine iontophoresed for 15 s starting at t = 0 s. Histograms are the average of 30 consecutive trials (60 s duration) and each bar is the average frequency recorded during the indicated 1 s bin. All histograms were collected in the same location in the NAc of the same animal. The blue traces superimposed over histograms are the average concentration of acetaminophen (AP) (C, D) or dopamine (DA) (E) ejected during the 45 s window shown for all 30 trials. In panels (C)–(E), iontophoretic drug delivery started at t = 0 s and ended at t = 15 s. Immediate changes in firing ratio were determined by comparison of cell firing during a baseline period (−15 to 0 s, solid red bar) to the average firing rate during the ejection (blue boxed region, 0 to 15 s). A quotient that differed from the control by ±0.5 was taken as an immediate change in cell firing rate.
To evaluate the immediate effect of iontophoretic drug application on MSN firing rates, we compared firing ratios. The firing ratio allows comparison of cell firing changes with respect to a defined baseline period. Here, baseline was defined as the average firing rate during the 15 s interval prior to each iontophoretic application (red bars in Figure 5). The firing ratio was determined by dividing the average firing rate during 30 consecutive drug ejections (the region shown by the blue open bars in Figure 5) by the baseline. The drug was deemed to have an immediate effect if it caused a change in firing ratio of 50% or more, a change never observed without drug application. At the location of the trials in Figure 5, the cell showed no immediate change in firing in response to application of 8.6 μM SCH 23390 (Figure 5C) whereas there was an abrupt increase in firing rate (and firing ratio) with the application of 14 μM raclopride (Figure 5D). This increase in cell firing is believed to be an excitation in response to blocking of the D2 mediated inhibition of MSN firing. The results from multiple units are summarized in Table 1 and show that only 12 out of the 30 cells that responded to one dopamine antagonist showed an immediate response.
Table 1. Distribution of Prolonged and Immediate Responses of MSNs to Dopamine Antagonistsa.
| no. of cells
(%) |
||
|---|---|---|
| type of response | raclopride | SCH 23390 |
| immediate | ||
| inhibitory | 0 (0) | 1 (3) |
| excitatory | 7 (23) | 4 (13) |
| prolonged | ||
| inhibitory | 5 (17) | 16 (53) |
| excitatory | 9 (30) | 0 (0) |
MSN = medium spiny neuron. MSN responses are tabulated from n = 35 cells (units) from 19 rats. One cell did not respond to either drug, and 4 cells responded to both. All 5 of these cells were excluded from the table.
The time required for a change in cell firing to occur is dependent on the time needed for ejected drug to diffuse to receptors on the MSN as well as the time delay between binding and the cascade of cell signaling that modulates MSN firing. Therefore, we also analyzed the data over a prolonged time scale. For prolonged firing ratios, baseline was taken as the average MSN firing rate during 30 trials with no drug ejections (red bar in Figure 6A). The prolonged firing ratio was then the average firing rate in the 15 s before and the 15 s after each of the 30 drug ejections (Figure 6B–E) divided the prolonged baseline. (The prolonged firing ratio purposely excludes cell firing during the 15 s period of drug ejection, which was used for determination of the immediate firing ratio.) Note that the −15 to 0 s period before an ejection was also the time period 45–60 s after an ejection after the first ejection.
Figure 6.
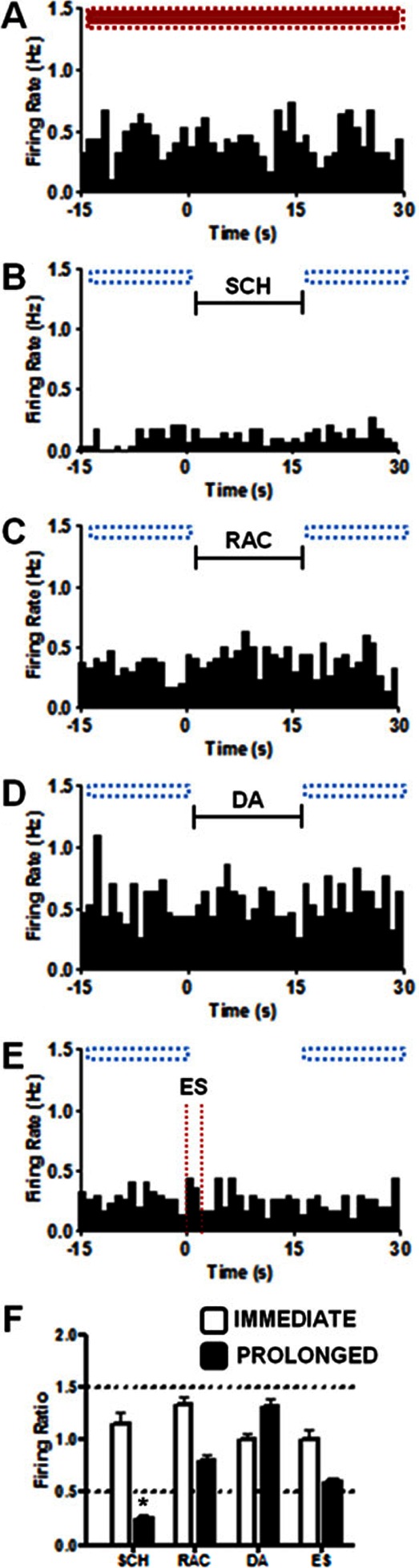
Firing rates measured at another MSN indicating the prolonged analysis method. Each histogram was constructed from the results of 30 trials for each condition, (A) Control where no event occurs (B) 15 s SCH 23390 (SCH) ejection, indicated by back bar (C) 15 s raclopride (RAC) ejection, indicated by back bar (D) 15s dopamine (DA) ejection, indicated by back bar (E) 60 Hz, 24 p electrical stimulation (ES), indicated with dashed vertical lines. The horizontal boxes above each histogram indicate where comparisons were made to determine prolonged changes in firing rate. Red indicates baseline (−15 to 30 s in panel A) and blue outlined boxes indicate regions compared to baseline (−15 to 0 s and 15 to 30 s). (F) Summary graph of immediate and prolonged changes in firing ratio from the single unit in panels (A)–(E). Prolonged firing ratios were determined by dividing the average firing rate during periods with blue boxes over them in a panel by the red boxed region in panel (A). Immediate firing ratios were determined as described in Figure 5 and the text. A quotient greater than 1.5 or less than 0.5 qualified as a change in frequency of cell firing. Horizontal dashed lines enclose the region where changes in firing ratio are less than 50% of the original signal. The only effect seen at this cell was a prolonged depression to SCH23390 as indicated by the asterisk (*) in panel (F).
Representative firing patterns of another single unit are shown in Figure 6 along with the time periods used to calculate the prolonged firing ratio. Figure 6A shows the baseline for the prolonged measure (no drug was ejected), Figure 6B shows trials where SCH 23390 was ejected, and Figure 6C shows trials where raclopride was ejected. The immediate and prolonged firing ratios for each condition at this location are seen in Figure 6F. At this cell, SCH 23390 had a prolonged inhibition on cell firing but no immediate effect (Figure 6B, F). Raclopride had no significant effect on the firing ratio on either time scale in this MSN (Figure 6C, F). These responses classify this cell as a D1 responsive MSN.
This analysis was repeated for 35 cells, and the results are also summarized as the prolonged changes in Table 1. Out of 35 cells, all but one showed a prolonged response to receptor antagonists. While 4 units showed a prolonged response to both antagonists, 16 were only responsive to the D1 antagonist and 14 were only responsive to the D2 antagonist. This breakdown of cells is remarkably similar to the ratio of D1 and D2 cells established in the NAc by Girault and co-workers.24 The 5 prolonged inhibitions evoked by raclopride all exhibited a robust, immediate excitation (like the example in Figure 5). The variability of the prolonged responses in MSNs that just responded to raclopride (5 out of 18 cells were inhibited while the remainder were excited) may have to do with the dual locations of D2 receptors.40−42 Activation of D2 receptors on MSNs tends to inhibit cell firing whereas activation of D2 autoreceptors on dopamine neurons decreases dopamine release, minimizing this inhibition.
Modulation of Cell Firing with Dopamine
At the same locations where the effects of raclopride and SCH 23390 were evaluated, we also evaluated the changes in firing rate induced by iontophoretically delivered dopamine and dopamine released by electrical stimulation (examples shown in Figure 6D and E). Iontophoresis of dopamine over a range of concentrations (10 nM to 20 μM) into the NAc caused no immediate change in MSN unit activity in 36 of the 40 cells tested (representative example in Figure 5E). However, only 18% of MSNs were unresponsive to either mode of dopamine delivery like the MSN in Figure 6. This means 82% of MSN showed a prolonged response to dopamine. In Table 2, the cells are grouped according to their prolonged responses to dopamine evoked by electrical stimulation or iontophoresis. Here, 75% of the MSNs monitored show the same type of prolonged response to both locally applied dopamine and electrical stimulation. Our result contrasts with that of Millar and co-workers who found electrically stimulated dopamine could excite or inhibit cell firing, but iontophoresed dopamine never excited cell firing.7 Our results also contrast with those of Gonon who found that transient electrical stimulations caused D1-mediated excitation of MSNs.43 However, both of the prior reports were in anesthetized preparations. The changes in firing rate induced by increased extracellular dopamine concentration show that MSN firing rates can be modulated even in the absence of a coordinated surge of glutamate or GABA. For the 25% of the cells in which dopamine and the electrical stimulation do not cause the same firing rate changes, it may be that the stimulation releases other neurotransmitters besides dopamine that contribute to the observed effect. For example, GABA44,45 and glutamate46 are released in conjunction with dopamine at some percentage of NAc synapses.
Table 2. Medium Spiny Neuron Responses to Electrically Stimulated Dopamine and Locally Applied Dopaminea.
| no. of cells (%) |
|||
|---|---|---|---|
| type of prolonged response | ES and DA | DA onlyb | ES onlyc |
| excitatory | 12 (30) | 5 (12) | 3 (8) |
| inhibitory | 14 (35) | 0 (0) | 0 (0) |
| no responsed | 4 (10) | – | – |
| mixed responsee | 2 (5) | – | – |
DA = locally applied dopamine; ES = electrically stimulated release. MSN responses are tabulated from n = 40 cells (units) from 21 rats.
Cells responded to DA only and had no response to ES.
Cells responded to ES only and had no response to DA.
Cells did not respond to ES or DA.
Cells with a mixed response responded differently to ES vs DA. One of the cells had an inhibitory response to ES and an excitatory response to DA, while the other cell had an excitatory response to ES and an inhibitory response to DA.
Conclusions
Here we characterized an improved procedure to combine iontophoresis with voltammetric/single-unit recording that eliminates much of the unpredictability seen in earlier applications of this technique. We have established that drug ejections 15 s in duration can alter both chemical release and cell firing on different time scales. Previous experiments with iontophoresis showed that long ejections of SCH 23390 inhibited cell firing.9 In agreement with this, here we found a population of MSNs that showed prolonged inhibition following exposure to SCH 23390. MSNs that showed a prolonged response to raclopride were more variable with only 40% of MSNs having a significant increase in firing rate. These changes in firing rate were induced by only 15 s of drug application (vs several minutes). In agreement with prior work in anesthetized animals, we find that dopamine may either increase or decrease firing rate at different MSNs.39
The results with raclopride on pre- and postsynaptic events differ in their onset time. Raclopride modulates dopamine release immediately and robustly after its application (Figure 3), but tends to be more effective in the prolonged modulation of MSN firing rate. These effects are mediated by two different isoforms of the D2 receptor that are found pre- and postsynaptically.47 Because of the high density of dopaminergic terminals, the presynaptic effects on dopamine release are readily observed by iontophoresis in the NAc. By contrast, spikes from MSN cell bodies are encountered less frequently. Because their dendrites extend several hundred micrometers away,48 this may explain why prolonged effects of dopamine antagonists were obtained more reliably than immediate effects. While both pre- and postsynaptic D2 receptors exhibit similar pharmacology,49 they differ in that the postsynaptic D2 receptor has a longer third intracellular loop that requires the presence of Gαi2 in order to inhibit adenylyl cyclase to the same extent that the presynaptic D2 receptor can with any Gαi protein.50,51 Functionally, this means more binding may be required at postsynaptic receptors to elicit comparable adenylyl cylase inhibitions.
Our results provide an interpretable view of dopamine–receptor interactions because of our ability to monitor the applied drug concentration. In previous studies of dopamine’s actions on MSNs, the dopamine concentration was not monitored directly but based on barrel concentrations and applied currents. The amount of dopamine applied in those experiments can be estimated to be considerably greater than the concentrations applied in this work.52 In studies where dopamine was exclusively inhibitory, the large concentrations of dopamine applied meant that uptake by the dopamine transporter could not make a significant change in the applied concentration.
Our initial iontophoresis experiments demonstrate at least three unique pharmacological responses that indicate the presence of different receptor populations on individual MSNs (Table 1) and these results are consistent with the current understanding of the distribution of D1 and D2 receptors in the NAc.25 This also supports the classic theory that the effect of dopamine on MSN intrinsic and synaptic channels will be excitatory at D1 MSNs and inhibitory at D2 MSNs.53−55 The prolonged effect of dopamine on MSN firing was independent of the receptor subtypes in these animals that were at rest.
We have presented a technique and methodology that is uniquely designed to determine not only what dopamine does in an awake animal under a number of conditions but that also has the potential to tease apart the role of other neurotransmitters in conjunction with dopamine. Characterization of this combined technique allows us to begin to differentiate between the function of MSNs based on their receptors. We can now investigate the argument that many different circuits within the NAc fire in different patterns specific to the aspect of goal-directed behavior for which they encode.56 These initial data are very promising and show the potential of this combined technique to contribute to a broader understanding of neuronal signaling.
Methods
Electrode Modifications
A capillary (Part # 624503, 0.60 mm o.d., 0.4 mm i.d., 4′′ long, A-M Systems, Sequim, WA) was loaded with a carbon fiber (T-650, Thornel, Amoco Corp., Greenville, SC) by aspiration and then loaded into one barrel of a prefused four-barrel glass (Part # 4FB100-75-100, 1.0 mm OD, 0.75 mm ID, 100 mm L, four-barrel omega dot tubing with filament, Friedrich and Dimmock, Millville, NJ). The four-barrel glass with attached heat shrink (to prevent microfractures in the glass) was pulled on a vertical micropipet puller (Narashige, Tokyo, Japan). When pulling, it is important that the multibarrel glass capillary is completely centered and straight within the coil of the puller. If the glass moved at all from the center of the coil when the chuck was rotated, the probe moved the same way when lowered into the brain. This greatly increased the likelihood of chipping or breaking the probe on the guide cannula or otherwise damaging the electrode seal.
In experiments described here, one iontophoresis barrel was filled with 5 mM dopamine dissolved in 5 mM NaCl. The additional two barrels were filled with either 5 mM of the D2-antagonist raclopride, 5 mM of the D1 antagonist SCH-23390, or 5 mM of the dopamine uptake inhibitor nomifensine. All drugs were dissolved in 5 mM NaCl containing 5 mM acetaminophen.18 Acetaminophen served as the electroactive marker that allowed ejections to be monitored and quantitated with FSCV the amount of electroinactive drug ejected was calculated indirectly from relative electrophoretic mobilities with respect to acetaminophen as done previously.17 Capillary electrophoresis and previously established methodology were used to determine that the relative electrophoretic mobilities of raclopride and SCH 23390 and nomifensine are 1.68, 1.90, and 2.24, respectively, compared to acetaminophen. Positive current was used to eject all drugs. The current was adjusted during “priming”18 to deliver the appropriate micromolar concentration (detected with FSCV at the carbon-fiber) of dopamine or acetaminophen during a 15 s ejection. Between probes and from barrel to barrel, the amount of current required to achieve a desired concentration varied from 10 to 450 nA. During experiments, drug application was randomized.
After barrels were loaded with drug solutions, the probe was inserted connection side first, into the modified Biela manipulator and fastened to the manipulator with a strip of parafilm. A gold pin was then soldered to the end of the silver wire connected to the carbon-fiber electrode; separate silver wires were inserted into each preloaded iontophoresis barrel, and all connections were secured to the probe with parafilm. A final check occurred to ensure that the electrode tip was centered and only moved straight up and down, not in circular motions, with turning of the manipulator.
For the range of acetaminophen concentrations used in these experiments, acetaminophen toxicity is considered unlikely. The first step in acetaminophen metabolism is its oxidation by cytochrome P450 2E1, which is predominately found in the liver.57 The enzyme is present in the brain but in smaller amounts (0.5–2% of liver concentration).58,59 This enzyme metabolizes acetaminophen into a toxic, reactive intermediate that under normal conditions is immediately broken down into a nontoxic compound by glutathione. The reactive intermediate can cause damage if there is an overexpression of the enzyme (normally due to chronic ethanol treatment) or if the glutathione concentration is low.60 Here, however, the plasma concentrations of acetaminophen were below 2 mM (the level for hepatoxicity).61
Cannula and Manipulator Modifications
Biela manipulators designed for four-barrel glass were obtained commercially (Crist Instrument Co, Part# 3-MMB-3D). Before surgical implantation of the guide cannula, the custom-made adapter was used to stretch the guide cannula (Bioanalytical Systems, West Lafayette, IN) slightly to ensure that the manipulator could easily slide into the cannula. Without this step, insertion of the manipulator into the cannula took more force which may stress the animal or electrode.
Combined Electrochemistry/Electrophysiology Instrumentation
A version of the custom build headstage27 with pins to connect the iontophoresis barrels to the current source was constructed by the UNC Electronics Facility. An iontophoretic constant current delivery system (NeuroPhore BH-2 System, Harvard Apparatus, Holliston, MA) was used for all iontophoretic ejections. All electrical signals were passed through an electrical swivel (Med-Associates, St. Albans, VT) that allowed the animal unrestrained movement within the behavioral chamber.8
Surgeries
For recovery surgeries, male Sprague–Dawley rats (225–350 g; Charles River, Wilmington, MA) were anesthetized with isofluorane (rats were induced at 4% and maintained at 1.5–2.0% during surgery) and placed in a stereotaxic frame (Kopf, Tujunga, CA). Surgeries were performed as previously described.36,62,63 The stretched guide cannula was implanted with the hub extending 2.5 mm into the brain directly above the NAc core (1.3 AP, 1.3 ML) or shell (1.7 AP, 0.8 ML, N = 4 animals, and n = 8 cells),64 and a bipolar stimulating electrode (Plastics One, Roanoke, VA) was lowered into the VTA (5.2 mm AP, 1.0 mm ML, and 7.8 mm DV). Coordinates for the bipolar stimulating electrode ensure activation of the neurons projecting to the NAc.65 A Ag/AgCl electrode was placed in the contralateral hemisphere to act as both reference for the carbon-fiber electrode and a ground for the iontophoresis barrels. All items were secured to the skull with stainless steel screws and dental cement. Rats inhaled 100% oxygen for a few minutes at the end of the surgery and were given tylenol (15 mg/kg) to aid in their recovery.
Experiments with anesthetized animals were similar except for anesthesia used. Rats were anesthetized with 1.5 g/kg urethane (50% urethane/50% saline, w/w). Identical coordinates were used for implantation of electrodes. All procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
Data Acquisition
After at least a 3 day recovery period, rats were anesthetized in an isofluorane chamber (4% for ∼2 min). Once lightly anesthetized, rats were removed from the chamber and the implanted cannula rinsed with 0.9% bacteriostatic saline (Animal Health International, Greely, CO). Isofluorane anesthetization lasts only a few minutes, and it has no measurable effect on an animal’s ability to perform ICSS or on electrically stimulated [DA]max.66,67 The loaded manipulator was then attached to the cannula, and the rat was placed into a behavioral chamber (Med Associates, St Albans, VT). Once in the chamber, the electrode and iontophoresis connections were made to the custom built headstage (Chemistry Department Electronics Facility, University of North Carolina, Chapel Hill).
The probe was slowly lowered to 6.0 mm in the brain (1 turn of the manipulator moved the electrode 0.4 mm). The electrode was then cycled for 15 min at 60 Hz from −0.4 to 1.3 V and back at 400 V/s68 using custom-built software (Tarheel CV, LABVIEW, National Instruments, Austin, TX), and then cycled for 10 min with the same waveform applied at 10 Hz. The iontophoresis barrels were primed as described previously,18 and pump currents to deliver the desired amount of each drug were determined at a depth of 6.0 mm into the brain. The electrode was then lowered into the NAc (7.0 mm DV), and the waveform was altered to allow FSCV and electrophysiological recordings.36 Figure 1 shows the circuitry that allowed this switching to occur along with the simultaneous outputs from each circuit. A solid-state relay in the headstage alternated between the current amplifier for voltammetric scans and a voltage follower for unit recording. This complementary metal–oxide–semiconductor (CMOS) switch (MAX 319CSA) was chosen due to its low leakage, low charge transfer, low and matched input capacitance, low resistance (10 Ω), and fast (<1 μs) switching time.27 The unit-recording interval had a 20 ms gap every 180 ms when voltammograms were collected. In the first 5.75 ms of this interval, a potential of −0.4 V was applied. The potential sweep for voltammetry remained the same as during the initial cycling.
Once in the NAc, data collection occurred at sites where a single unit was isolated. A single unit was classified as an MSN if baseline firing frequency was below 15 Hz and the waveform duration was less than 1.2 ms.70,71 After unit activity was monitored for 30 min to ensure a constant firing rate, drug was applied to the unit. The probe configuration allowed a maximum of three drugs applied per cell, so separate experiments were carried out prior to reported experiments where cell firing was monitored during ejections of 5 mM acetaminophen in 5 mM NaCl in rats during and prior to ICSS. Cell firing was not modulated by ejections of the neutral marker acetaminophen (N = 4 rats, n = 6 cells). Drugs were applied in a randomized order from cell to cell within an animal and from animal to animal to ensure affects were not due to the drug sequence. Positive currents were used for all ejections (10–450 nA). Multiple recording locations were used in a single animal provided they were at least 300 μm apart to ensure a new MSN was being recorded. Units recorded at carbon-fiber electrodes were amplified (×1000) and band-pass filtered (300–3000 Hz). All signals were digitized with commercially available software (DIGITIZER, Plexon, Dallas, TX), and isolation of a unit was accomplished with principal component analysis in commercially available software (OFFLINE SORTER, Plexon, Dallas, TX). Med-Associates software (St. Albans, VT) controlled iontophoresis ejections and provided digital outputs synchronized to behavioral cues that were recorded in both Tarheel CV (fast-scan voltammetry) and Plexon (electrophysiology) records.
Data Analysis
Dopamine was identified from the background subtracted cyclic voltammograms.72 Current traces showed changes in dopamine concentration over time at the oxidation potential of dopamine from the cyclic voltammograms collected every 200 ms. Neural activity was characterized with raster displays and peri-event histograms (PEHs) across distinct time domains that bracketed the iontophoretic ejections. Each PEH was partitioned into three parts [baseline (15 s before the ejection), response (15 s during ejection), and recovery (15 s after the ejection)] to allow measurement of changes relative to the drug application. We use N to indicate number of animals and n to equal the number units.
Acknowledgments
The authors thank Domenic Cerri for writing the code for the Med-Associates software. The authors also thank the UNC Electronics facility for design and manufacture of the specialized instrumentation necessary for these experiments.
The authors thank the National Institutes of Health for funding (NIH DA010900 and NIH DA017318).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Beyene M.; Carelli R. M.; Wightman R. M. (2010) Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience 169, 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. J.; Jones J. L.; Wightman R. M.; Carelli R. M. (2010) Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol. Psychiatry 68, 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White C. A.; Ariansen J.; Stuber G. D.; Cleaveland N. A.; Cheer J. F.; Wightman R. M.; Carelli R. M. (2009) Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur. J. Neurosci. 30, 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E.; Stuber G. D.; Heien M. L.; Wightman R. M.; Carelli R. M. (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618. [DOI] [PubMed] [Google Scholar]
- Robinson D. L., and Wightman R. M. (2007) Rapid dopamine release in freely moving rats. In Electrochemical Methods for Neuroscience (Michael A. C., and Borland L. M., Eds.), pp 17–36, CRC Press, Boca Raton, FL. [PubMed] [Google Scholar]
- Millar J.; Armstrong-James M.; Kruk Z. L. (1981) Polarographic assay of iontophoretically applied dopamine and low-noise unit recording using a multibarrel carbon fibre microelectrode. Brain Res. 205, 419–424. [DOI] [PubMed] [Google Scholar]
- Williams G. V.; Millar J. (1990) Differential actions of endogenous and iontophoretic dopamine in rat striatum. Eur.J.Neurosci. 2, 658–661. [DOI] [PubMed] [Google Scholar]
- Cheer J. F.; Heien M. L.; Garris P. A.; Carelli R. M.; Wightman R. M. (2005) Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: implications for intracranial self-stimulation. Proc. Natl. Acad. Sci. U.S.A. 102, 19150–19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer J. F.; Aragona B. J.; Heien M. L.; Seipel A. T.; Carelli R. M.; Wightman R. M. (2007) Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron 54, 237–244. [DOI] [PubMed] [Google Scholar]
- Grenhoff J., and Johnson S. W. (1997) Electrophysiological Effects of Dopamine Receptor Stimulation. In The Dopamine Receptors (Neve K. A., and Neve R. L., Eds.), 1st ed., Humana Press, Totowa, NJ. [Google Scholar]
- Chiodo L. A. (1988) Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci. Biobehav. Rev. 12, 49–91. [DOI] [PubMed] [Google Scholar]
- Rebec G. V. (1998) Real-time assessments of dopamine function during behavior: single-unit recording, iontophoresis, and fast-scan cyclic voltammetry in awake, unrestrained rats. Alcohol.: Clin. Exp. Res. 22, 32–40. [DOI] [PubMed] [Google Scholar]
- Kiyatkin E. A.; Rebec G. V. (1999) Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats. Brain Res. 822, 88–106. [DOI] [PubMed] [Google Scholar]
- Kiyatkin E. A.; Rebec G. V. (1996) Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J. Neurophysiol. 75, 142–153. [DOI] [PubMed] [Google Scholar]
- Pierce R. C.; Rebec G. V. (1995) Iontophoresis in the neostriatum of awake, unrestrained rats: differential effects of dopamine, glutamate and ascorbate on motor- and nonmotor-related neurons. Neuroscience 67, 313–324. [DOI] [PubMed] [Google Scholar]
- Stone. T. W.Microiontophoresis and Pressure Ejection; Smith, A. D., ed.; Wiley-Interscience: New York, NY, 1985. [Google Scholar]
- Herr N. R.; Kile B. M.; Carelli R. M.; Wightman R. M. (2008) Electroosmotic flow and its contribution to iontophoretic delivery. Anal. Chem. 80, 8635–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr N. R.; Daniel K. B.; Belle A. M.; Carelli R. M.; Wightman R. M. (2010) Probing presynaptic regulation of extracellular dopamine with iontophoresis. ACS Chem. Neurosci. 1, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. (2003) Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 17, 429–435. [DOI] [PubMed] [Google Scholar]
- Nicola S. M.; Hopf F. W.; Hjelmstad G. O. (2004) Contrast enhancement: a physiological effect of striatal dopamine?. Cell Tissue Res. 318, 93–106. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J.; Ding J.; Day M.; Wang Z.; Shen W. (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30, 228–235. [DOI] [PubMed] [Google Scholar]
- Yung K. K.; Bolam J. P.; Smith A. D.; Hersch S. M.; Ciliax B. J.; Levey A. I. (1995) Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65, 709–730. [DOI] [PubMed] [Google Scholar]
- Chang H. T.; Kitai S. T. (1985) Projection neurons of the nucleus accumbens: an intracellular labeling study. Brain Res. 347, 112–116. [DOI] [PubMed] [Google Scholar]
- Valjent E.; Bertran-Gonzalez J.; Herve D.; Fisone G.; Girault J. A. (2009) Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 32, 538–547. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R.; Surmeier D. J. (2011) Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. (2007) Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain. Res. Rev. 56, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takmakov P.; McKinney C. J.; Carelli R. M.; Wightman R. M. (2011) Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev. Sci. Instrum. 82, 074302.074301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. O.; Woodward D. J. (1984) A technique for microiontophoretic study of single neurons in the freely moving rat. J. Neurocsci. Meth. 11, 179–185. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M.; Borrelli E.; Gonon F. (2001) Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 21, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Reith M. E.; Walker Q. D.; Kuhn C. M.; Carroll F. I.; Garris P. A. (2002) Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J. Neurosci. 22, 6272–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. S.; Wightman R. M. (1987) Detection of dopamine overflow and diffusion with voltammetry in slices of rat brain. Brain Res. 423, 79–87. [DOI] [PubMed] [Google Scholar]
- Nicholson C. (1995) Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophys. J. 68, 1699–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. R.; Gainetdinov R. R.; Hu X. T.; Cooper D. C.; Wightman R. M.; White F. J.; Caron M. G. (1999) Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 2, 649–655. [DOI] [PubMed] [Google Scholar]
- Olds J.; Milner P. (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. . J. Comp. Physiol. Psychol. 47, 419–427. [DOI] [PubMed] [Google Scholar]
- Garris P. A.; Kilpatrick M.; Bunin M. A.; Michael D.; Walker Q. D.; Wightman R. M. (1999) Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature 398, 67–69. [DOI] [PubMed] [Google Scholar]
- Owesson-White C. A.; Cheer J. F.; Beyene M.; Carelli R. M.; Wightman R. M. (2008) Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc. Natl. Acad. Sci. U.S.A. 105, 11957–11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima I.; Carino M. A.; Horita A. (1995) Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacol., Biochem. Behav. 52, 737–741. [DOI] [PubMed] [Google Scholar]
- Hu X.-T.; Wang R. Y. (1988) Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: An electrophysiological study. J. Neurosci. 8, 4340–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. J.; Wang R. Y. (1986) Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J. Neurosci. 6, 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D.; Gubellini P.; Usiello A.; Rossi S.; Tscherter A.; Bracci E.; Erbs E.; Tognazzi N.; Bernardi G.; Pisani A.; Calabresi P.; Borrelli E. (2004) Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neuroscience 129, 157–166. [DOI] [PubMed] [Google Scholar]
- Centonze D.; Grande C.; Usiello A.; Gubellini P.; Erbs E.; Martín A. B.; Pisani A.; Tognazzi N.; Bernardi G.; Moratalla R.; Borrelli E.; Calabresi P. (2003) Receptor Subtypes Involved in the Presynaptic and Postsynaptic Actions of Dopamine on Striatal Interneurons. J. Neurosci. 23, 6245–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A.; Baik J.-H.; Rouge-Pont F.; Picetti R.; Dierich A.; LeMeur M.; Piazza P. V.; Borrelli E. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408, 199–203. [DOI] [PubMed] [Google Scholar]
- Gonon F. (1997) Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J. Neurosci. 17, 5972–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch N. X.; Ding J. B.; Sabatini B. L. (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen S. C.; Lee R.; Stobbs S. H.; Henriksen S. J. (2001) Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res. 906, 190–197. [DOI] [PubMed] [Google Scholar]
- Stuber G. D.; Hnasko T. S.; Britt J. P.; Edwards R. H.; Bonci A. (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 30, 8229–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B.; Sokoloff P.; Martres M.-P.; Riou J.-F.; Emorine L. J.; Schwartz J.-C. (1989) Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 342, 923–926. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. (1988) Synaptic organization of the striatum. J. Electron Microsc. Tech. 10, 265–281. [DOI] [PubMed] [Google Scholar]
- Elsworth J. D.; Roth R. H. (1997) Dopamine Synthesis, Uptake, Metabolism, and Receptors: Relevance to Gene Therapy of Parkinson’s Disease. Exp. Neurol. 144, 4–9. [DOI] [PubMed] [Google Scholar]
- Guiramand J.; Montmayeur J.-P.; Ceraline J.; Bhatia M.; Borrelli E. (1995) Alternative Splicing of the Dopamine D2 Receptor Directs Specificity of Coupling to G-proteins. J. Biol. Chem. 270, 7354–7358. [DOI] [PubMed] [Google Scholar]
- Montmayeur J. P.; Guiramand J.; Borrelli E. (1993) Preferential coupling between dopamine D2 receptors and G-proteins. Mol. Endocrinol. 7, 161–170. [DOI] [PubMed] [Google Scholar]
- Kiyatkin E. A.; Rebec G. V. (1999) Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and d2 dopamine receptors in freely moving rats. J. Neurosci. 19, 3594–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin R. L.; Young A. B.; Penney J. B. (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. [DOI] [PubMed] [Google Scholar]
- DeLong M. R. (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. [DOI] [PubMed] [Google Scholar]
- Kreitzer A. C.; Berke J. D. (2011) Investigating striatal function through cell-type-specific manipulations. Neuroscience 198, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli R. M.; Wightman R. M. (2004) Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr. Opin. Neurobiol. 14, 763–768. [DOI] [PubMed] [Google Scholar]
- Lee S. S. T.; Buters J. T. M.; Pineau T.; Fernandez-Salguero P.; Gonzalez F. J. (1996) Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 271, 12063–12067. [DOI] [PubMed] [Google Scholar]
- Upadhya S.; Tirumalai P.; Boyd M.; Mori T.; Ravindranath V. (2000) Cytochrome P4502E (CYP2E) in brain: constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Arch. Biochem. Biophys. 373, 23–34. [DOI] [PubMed] [Google Scholar]
- Hedlund E.; Gustafsson J. A.; Warner M. (2001) Cytochrome P450 in the brain; a review. Curr. Drug Metab. 2, 245–263. [DOI] [PubMed] [Google Scholar]
- Dong H.; Haining R. L.; Thummel K. E.; Rettie A. E.; Nelson S. D. (2000) Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab. Dispos. 28, 1397–1400. [PubMed] [Google Scholar]
- Rumack B. H.; Peterson R. G. (1978) Acetaminophen overdose: Incidence, diagnosis and management in 416 patients. Pediatrics 898–905. [PubMed] [Google Scholar]
- Cheer J. F.; Wassum K. M.; Heien M. L.; Phillips P. E.; Wightman R. M. (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J. Neurosci. 24, 4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman M. F.; Stuber G. D.; Phillips P. E.; Wightman R. M.; Carelli R. M. (2004) Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., and Watson C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th ed., Elsevier, Amsterdam. [Google Scholar]
- Ikemoto S.; Glazier B. S.; Murphy J. M.; McBride W. J. (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J. Neurosci. 17, 8580–8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. L.; Carelli R. M. (2008) Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur. J. Neurosci. 28, 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. L.; Howard E. C.; McConnell S.; Gonzales R. A.; Wightman R. M. (2009) Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol.: Clin. Exp. Res. 33, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien M. L.; Phillips P. E.; Stuber G. D.; Seipel A. T.; Wightman R. M. (2003) Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Carelli R. M.; Ijames S. G. (2001) Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 907, 156–161. [DOI] [PubMed] [Google Scholar]
- Kish L. J.; Palmer M. R.; Gerhardt G. A. (1999) Multiple single-unit recordings in the striatum of freely moving animals: effects of apomorphine and d-amphetamine in normal and unilateral 6-hydroxydopamine-lesioned rats. Brain Res. 833, 58–70. [DOI] [PubMed] [Google Scholar]
- Michael D. J.; Joseph J. D.; Kilpatrick M. R.; Travis E. R.; Wightman R. M. (1999) Improving data acquisition for fast-scan cyclic voltammetry. Anal. Chem. 71, 3941–3947. [DOI] [PubMed] [Google Scholar]