Abstract

L-DOPA is currently one of the best medications for Parkinson’s disease. It was assumed for several years that its benefits and side effects were related to the enhancement of dopamine release in the dopamine-depleted striatum. The use of intracerebral microdialysis combined with a pharmacological approach has led to the discovery that serotonergic neurons are responsible for dopamine release induced by L-DOPA. The subsequent use of multisite microdialysis has further revealed that L-DOPA-stimulated dopamine release is widespread and related to the serotonergic innervation. The present Review emphasizes the functional impact of extrastriatal release of dopamine induced by L-DOPA in both the therapeutic and side effects of L-DOPA.
Keywords: multisite intracerebral microdialysis, L-DOPA, DA and 5-HT release, 5-HT neurons, region-dependent effects, clearance mechanisms
L-DOPA is the best medication for Parkinson’s disease. The use of L-DOPA as a treatment for Parkinson’s disease began soon after the discovery that the striatal tissue concentration of dopamine (DA) was lower in Parkinsonian patients compared to age-matched individuals.1 The efficacy of L-DOPA was improved by combination with a peripheral inhibitor of l-aromatic amino acid decarboxylase (AADC) to prevent the conversion of L-DOPA into DA in the bloodstream, thus enhancing the brain penetration of L-DOPA. Although L-DOPA is highly effective in relieving motor symptoms, numerous motor and nonmotor side-effects including L-DOPA-induced dyskinesias and psychosis can gradually emerge over time.2 The functional impact of L-DOPA in the Parkinsonian brain may not be solely restricted to its ability to restore “physiological levels” of DA in the striatum. Further investigations of its mechanism of action are thoroughly needed in order to address the motor and nonmotor side-effects of L-DOPA.
Intracerebral microdialysis is a powerful sampling technique used to monitor steady-state extracellular levels of neurotransmitters in vivo. This technique has helped to decipher the mechanism of action of numerous drugs and medications with respect to their action toward neurotransmitter systems.3 Furthermore, the development of simultaneous implantation of multiple microdialysis probes in distinct brain regions (referred to here as multisite microdialysis) has proven its strength in revealing region-dependent dynamics of neurotransmitter release in response to pharmacological challenges and the neurochemical interactions between brain regions.
Here, we summarize data showing the numerous advantages of intracerebral microdialysis to decipher the diverse features of the mechanism of action of L-DOPA in the Parkinsonian brain by using both a classical neuropharmacological approach and a multisite microdialysis approach. The present Review emphasizes the effect of L-DOPA in extrastriatal brain regions and how the region-dependent pattern of DA release induced by L-DOPA through serotonergic (5-HT) neurons may be relevant to its therapeutic and/or side-effects.
I. The Use of Microdialysis to Study the Mechanism of Action of L-DOPA on DA Release in the Rat Hemiparkinsonian Brain
In an attempt to understand the efficacy of L-DOPA in restoring DA transmission in the Parkinsonian brain, initial studies measured DA levels in post-mortem brain tissue. However, these data led to numerous misunderstandings since tissue concentration of DA induced by L-DOPA does not reflect extracellular concentrations of DA. Moreover, these results were further confounded by the fact that high doses of L-DOPA were used that did not translate to the clinical situation. The more recent use of microdialysis to directly monitor extracellular levels of DA induced by L-DOPA has provided clear neurochemical data about its mechanism of action.4
A. Tissue Measurement of DA Gave Conflicting Results Regarding the Mechanism of Action of L-DOPA
The first evaluations of the mechanism of action of L-DOPA were performed using post-mortem tissue measurement of DA in the rodent brain (Figure 1A). The destruction of nigrostriatal DA neurons using the neurotoxin 6-hydroxydopamine (6-OHDA) has provided the opportunity to study in rodents (mainly rats) the biochemical and behavioral impact of L-DOPA in a model that recapitulates the main features of Parkinson’s disease.5 The use of L-DOPA in this model raised a major contradiction because L-DOPA, like agonists, and in contrast to amphetamine, induced contralateral rotations in unilaterally lesioned rats despite the loss of 90% or more DA neurons. Thus, how could L-DOPA be so efficient at restoring motor function through DA neurons when those neurons are lost?
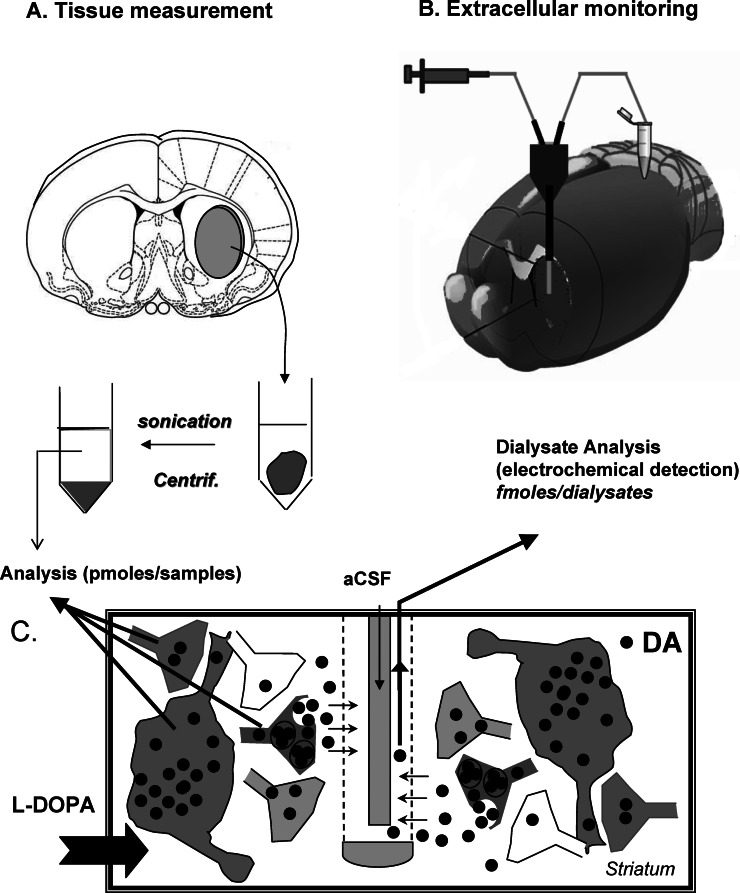
Figure 1.
Contribution of intracerebral microdialysis to the mechanism of action of L-DOPA toward dopamine responses. (A) Tissue measurement in response to L-DOPA administration in rodents was originally used. Immediately after the animal was sacrificed, the brain region of interest (here the striatum) was removed, placed in an acid medium, sonicated, and centrifuged. Monoamine tissue concentrations from the supernatant were quantified by high pressure liquid chromatography coupled to electrochemical detection (HPLC-ED). (B) Extracellular monitoring using intracerebral microdialysis is performed using microdialysis probes inserted in the brain region of interest (here the striatum of a living animal). The continuous flow rate of the artificial cerebrospinal fluid (aCSF) permits to collect samples at regular intervals. The dialysates are often analyzed with HPLC-ED due to the high sensitivity of this approach toward monoamines. (C) The panel illustrates the different origin of the DA signals analyzed with tissue measurement and intracerebral microdialysis after L-DOPA. L-DOPA will virtually enter all cells and/or terminals and, depending on the presence of L-DOPA decarboxylase, will be converted in DA in several loci. The magnitude of the DA signal is often large (picomoles) due to the contribution of multiple cellular systems. Using microdialysis probes, only DA reaching the extracellular space can be taken up by the probe. The contribution of cells to the DA signals, often low in magnitude (some femtomoles), is restricted to cells capable of releasing DA, e.g., the 5-HT neurons in DA-denervated rats.
The decarboxylation of L-DOPA into DA occurs in virtually all brain regions, but preferentially in the striatum.6 AADC is a ubiquitous enzyme expressed by many cellular subtypes including neurons, glial or endothelial cells in the brain where L-DOPA is converted to DA.7−9 The fact that newly synthesized DA is accumulating in a cell expressing AADC does not predict the ability of the cell to release DA.10 Indirect data suggested that 5-HT neurons may participate in the effects of L-DOPA,11 and supporting clinical data indicated that the efficacy of L-DOPA correlated with a decrease in tissue concentration of the main metabolite of 5-HT, 5-hydroxyindolacetic acid (5-HIAA).12,13 Nevertheless, the role of 5-HT neurons has been controversial with respect to the dose of L-DOPA used. In 6-OHDA rats, the selective destruction of 5-HT neurons reduced the ability of 30 mg/kg, but not 100 mg/kg L-DOPA to increase striatal DA tissue concentration and to induce contralateral rotations.8,14−16 These data indicate that cellular systems other than 5-HT terminals (Figure 1C) substantially contribute to the total amount of newly synthesized DA that accumulates in the tissue at high doses of L-DOPA, and this may impact upon motor output through an unidentified mechanism. Some studies have shown that L-DOPA contributes to the recovery of sensorimotor function17 and procedural motor learning in patients with stroke.18 Such recovery may involve DA receptors expressed by glial cells.19 Although these data suggest a role for glia in modulating L-DOPA-induced DA transmission, its functional contribution may occur at doses of L-DOPA far beyond the therapeutic range used in clinical settings, that is, 1–6 mg/kg.4
B. Contribution of Intracerebral Microdialysis to Understanding the Function of Neurotransmitter Systems
The use of intracerebral microdialysis coupled with a classical pharmacological approach has been unequivocal in determining the origin of DA release induced by L-DOPA. Figure 1B shows the implantation of a microdialysis probe into the striatum and Figure 1C illustrates that, among the many systems able to produce DA from exogenous L-DOPA, few have the cellular machinery to release DA into the extracellular space. Figure 2 displays a logical construction of the pharmacological evidence showing that the main cellular system responsible for the release of DA induced by L-DOPA in 6-OHDA rats is 5-HT neurons. First, the magnitude of the increase in L-DOPA-induced DA release is low in nonlesioned rats and comparatively large in DA neuron-lesioned rats (Figure 2A).20−23 Second, the increase in DA release induced by L-DOPA (50 mg/kg) in 6-OHDA rats is sensitive to tetrodotoxin (TTX), a blocker of fast voltage-dependent sodium channels (Figure 2B),21,23 to the vesicular monoaminergic transporter type 2 (VMAT2) blocker reserpine (Figure 2C),24 and to an almost complete lesion (95–98%) of 5-HT neurons (Figure 2D).16,25 The role of 5-HT neurons is further supported by numerous data showing that 5-HT1A agonists, selective serotonin reuptake inhibitors (SSRI), or deep brain stimulation of the subthalamic nucleus, which directly or indirectly reduce the firing rate of 5-HT neurons,26−28 inhibit L-DOPA-induced DA release.21,29−32
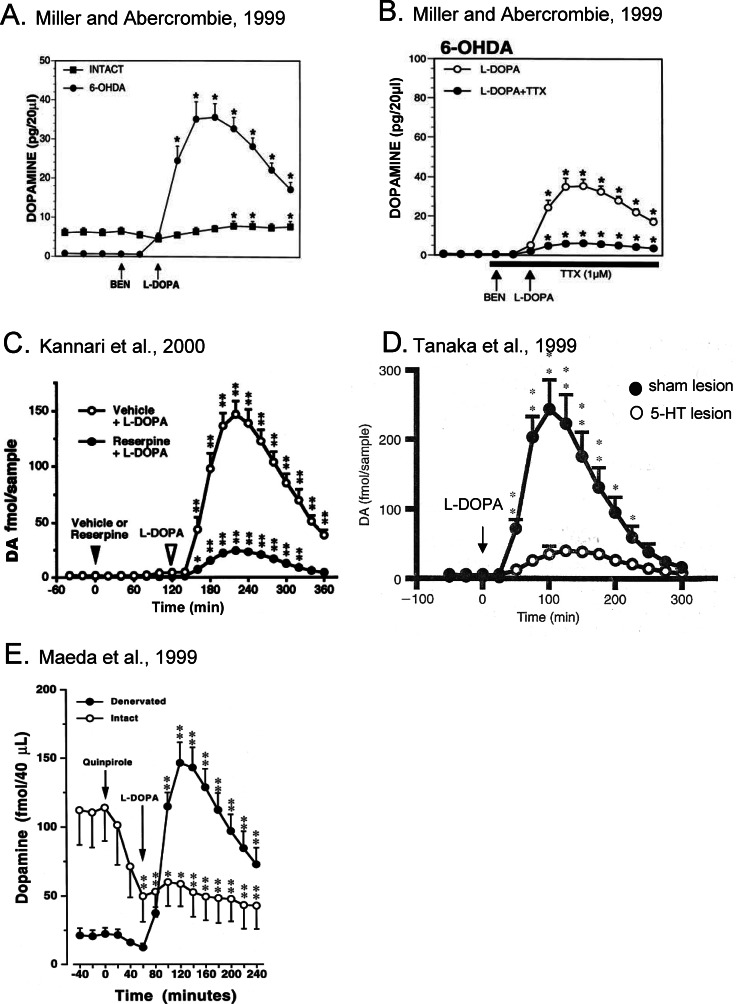
Figure 2.
Summary of the pioneering data establishing the main role of 5-HT neurons in L-DOPA-stimulated striatal DA release. All the panels report the results of different studies and the results correspond to the mean ± SEM of raw data (DA, quantity in picogram or femtomole per volume unit). In all cases, the injection of L-DOPA is preceded by the peripheral injection of the L-DOPA decarboxylase inhibitor benserazide (evidenced in panel A and B only). (A) The data of Miller and Abercrombie23 show that the effect of 50 mg/kg L-DOPA is minimal in intact rats and extremely high in 6-OHDA rats. (B) In the same study, they showed that the infusion of the fast sodium voltage-dependent channel blocker tetrodotoxin (TTX) dramatically reduced the effect of L-DOPA in 6-OHDA rats. The origin of DA released by L-DOPA is neuronal. (C) Using reserpine, a blocker of the monoamine vesicular transporter, administered 2 h before L-DOPA, Kannari et al.24 showed that the effect of L-DOPA required monoamine vesicles for exocytosis. (D) Tanaka et al.25 reported that the effect of 50 mg/kg L-DOPA was dramatically reduced in rats bearing a lesion of 5-HT neurons. (E) The release of DA induced by L-DOPA (30 mg/kg) is no longer sensitive (same magnitude of effect without quinpirole, not shown here) to the previous injection of the DA agonist quinpirole (1 mg/kg) in 6-OHDA rats.22 In intact rats, quinpirole limits the effect of L-DOPA on DA release. Figures have been taken and adapted from refs (23−)(25) with permission from Editors Wiley and Elsevier.
These data strongly suggest that DA terminals that remain after a drastic 6-OHDA lesion are not involved in the release of DA induced by L-DOPA.20 Indeed, L-DOPA-stimulated DA release in DA-depleted rats is no longer altered by the D2 agonist quinpirole (Figure 2E), the nonselective D2 antagonist haloperidol,22 an inhibitor of the DA transporter (DAT),23 or an inhibitor of monoamine oxidase A (MAOA).33 These data indicate that the impulse-dependent or -independent increase in DA release induced by L-DOPA is not sensitive to DA-dependent feedback mechanisms. Moreover, L-DOPA inhibits DA neuron firing rates in intact34,35 and partially DA-depleted rats,36 which would effectively counteract any impulse-dependent release from DA neurons.
Therefore, what could be the contribution of DA neurons to the increase in DA induced by L-DOPA in normal or partially DA-depleted rats? Interestingly, blockade of monoamine oxidase B (MAOB), an enzyme mainly present in 5-HT neurons which is involved in the metabolic degradation of DA, similarly enhances L-DOPA-induced DA release in intact and 6-OHDA rats. This suggests that 5-HT neurons also contribute to release DA from exogenous L-DOPA in intact rats. Finally, the main role of DA neurons in regulating DA extracellular levels that are raised by another system may rely on the presence of DAT.37 Indeed, blockade of DAT in intact animals has been shown to potentiate the increase in striatal DA extracellular levels induced by L-DOPA to a similar extent to that induced in DA-depleted rats. The authors suggested that DAT on spared DA terminals would maintain pseudophysiological DA tone by clearing excess extracellular levels of DA.20 Therefore, DA clearance by DAT may dampen the impact of L-DOPA-induced DA release from 5-HT neurons in the striatum as long as DA terminals are spared. However, this raises the question as to whether similar mechanisms are involved in extrastriatal regions that receive poor DA innervation and dense 5-HT innervation.
II. Multisite Implantation of Dialysis Probes to Study the Region-Dependent Neurochemical Pattern Induced by L-DOPA in the Rat Hemiparkinsonian Brain
Most microdialysis studies have focused on the striatum to assess the effect of L-DOPA on DA release. However, in light of the evidence that 5-HT neurons are responsible for the release of DA induced by L-DOPA, this brain region is probably not the unique target of L-DOPA’s effects. Indeed, 5-HT neurons originating from the dorsal (DR) and median (MR) raphe nuclei send widespread innervation throughout the entire brain.38,39 Moreover, increases in DA release induced by systemic or focal application of L-DOPA have also been observed in various brain regions using single microdialysis probe implantation including the hippocampus,40 the entopeduncular nucleus,41 the substantia nigra,42 and the hypothalamus.43,44 The implantation of several dialysis probes is interesting to simultaneously monitor DA extracellular levels induced by L-DOPA within multiple brain regions and to study the regional sensitivity to L-DOPA upon several neurotransmitter systems. In the next section, we review data showing how the use of a multisite microdialysis approach has shed light on the importance of extrastriatal DA release induced by L-DOPA.
A. L-DOPA Induces a Region-Dependent Neurochemical Pattern
1. DA Release
Using four microdialysis probes implanted ipsilaterally to the 6-OHDA-lesioned side (Figure 3), we showed that L-DOPA induced an ectopic release of DA in a pattern that follows the widespread innervation of 5-HT neurons. The increase in DA extracellular levels was not restricted to the striatum as it also occurred in the prefrontal cortex (PFC), hippocampus (HIPP) and substantia nigra pars reticulata (SNr).16,30,45 These brain regions receive various density levels of 5-HT innervation and express DA receptors (46) upon which the newly synthesized DA from L-DOPA can promote DA transmission. The increase in DA release in all brain regions is dependent upon the integrity of 5-HT neurons, as partial and total 5,7-dihydroxytryptamine (5,7-DHT) lesions respectively reduced and fully abolished the effect of L-DOPA.16 Furthermore, the systemic administration of citalopram, a blocker of 5-HT transporters (SERT), also blocked the increase in DA release induced by L-DOPA in all brain regions, confirming the role of 5-HT neurons in this widespread effect of L-DOPA.16,21
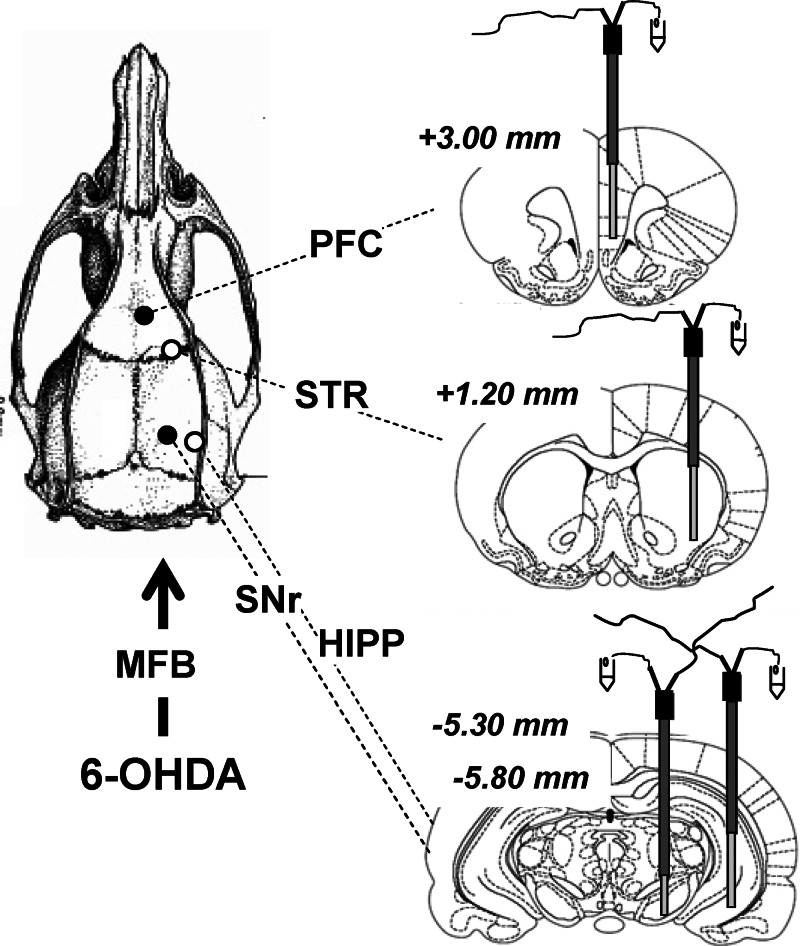
Figure 3.
Multisite intracerebral microdialysis. Schematic representation of the simultaneous implantation of four dialysis probes in the prefrontal cortex (PFC), striatum (STR), substantia nigra pars reticulata (SNr), and hippocampus (HIPP) on the ipsilateral DA-depleted side. Dialysis probes were implanted in isoflurane-anesthetized rats 3 weeks after the injection of 6-hydroxydopamine (6-OHDA) in the right medial forebrain bundle (MFB). One stereotaxic arm holds two dialysis probes for the STR and HIPP (4 mm long) and another holds two dialysis probes for the PFC (4 mm long) and SNr (2 mm long). The stereotaxic coordinates (in mm) were as follows: PFC, anteroposteriority from bregma (AP) = 3.00, laterality (L) = 0.4, ventrality (V) = 4; STR, AP = 1.20, L = 3, V = 2.4; SNr, AP = −5.30, L = 2.2, V = 1.2; HIPP, AP = −5.80, L = 5, V = 2.4. AP coordinates from bregma are indicated in italics.
The ectopic release of DA induced by L-DOPA via 5-HT neurons creates an ‘inverted’ striatal-extrastriatal balance in DA chemistry throughout the Parkinsonian brain. In physiological conditions, basal DA concentrations are 30 times higher in the striatum compared to other brain regions,47,48 in line with the preferential innervation of mesencephalic DA neurons in striatal territories. Using microdialysis techniques, monitored DA extracellular levels are between 4.6 and 7.8 fmol/μL in the striatum, while they are barely detectable in the PFC, SNr, and HIPP (below 0.2 fmol/μL), depending on dialysis and analytical conditions. In Parkinsonian conditions, the magnitude of the increase in extracellular DA concentrations induced by therapeutic doses of L-DOPA (3–12 mg/kg) is far higher in extrastriatal brain regions than in the striatum. At 3 mg/kg, L-DOPA enhanced DA levels to similar amounts (0.7–1.3 fmol) in the PFC, SNr, HIPP, and striatum. While the dose of 12 mg/kg L-DOPA may “restore” physiological levels of DA concentrations in the DA-denervated striatum, it induced a “hyperdopaminergy” in other brain regions by increasing DA concentrations to about 10–25 times higher than physiological levels. Therefore, it appears that L-DOPA dramatically favors extrastriatal DA transmission by releasing huge amounts of DA beyond the striatum49 that may impact on DA receptors throughout the Parkinsonian brain. Such an ‘inverted’ balance in DA transmission between the striatum and other brain regions may participate in the emergence of both short-term benefits and long-term side effects of L-DOPA treatment (see section III.B).
2. 5-HT Release
One of the advantages of the microdialysis technique is the ability to quantify, with an appropriate coupled analytical detection system, other neurotransmitters in the same dialysates. With respect to the crucial involvement of 5-HT neurons in the mechanism of action of L-DOPA, simultaneous monitoring of 5-HT and DA extracellular levels has provided important information about the region-dependent neurochemical pattern of L-DOPA. We showed that systemic administration of L-DOPA induced distinct effects on 5-HT release depending on the dose and the brain region dialysated (Figure 4). While an acute injection of 3 mg/kg L-DOPA barely altered 5-HT release in all brain regions, the dose of 6 mg/kg decreased 5-HT levels in the SNr and HIPP only. L-DOPA at 12 mg/kg decreased 5-HT extracellular levels in the PFC and SNr and induced a biphasic effect in the HIPP, while still not affecting 5-HT levels in the striatum.30,45 Different mechanisms have been proposed to account for the dose- and region-dependent effects of L-DOPA. The substitution of 5-HT by L-DOPA-derived DA could result in both a decrease in 5-HT exocytotic release and a nonexocytotic efflux of 5-HT from SERT reversal.42,50−52 The relative contribution of both mechanisms is currently unknown, but indirect evidence suggests that they may depend upon the dose of L-DOPA and the functional heterogeneity of 5-HT terminals in these brain regions.53
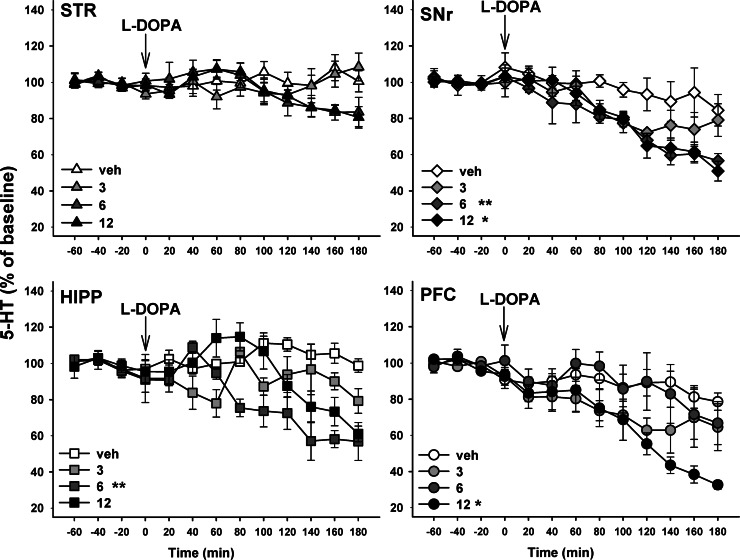
Figure 4.
Time course of the region- and dose-dependent effect of L-DOPA on extracellular levels of 5-HT in hemiparkinsonian rats. Three to four weeks after the unilateral injection of 6-hydroxydopamine into the medial forebrain bundle, rats were anesthetized with isoflurane and placed in a stereotaxic frame for the simultaneous and ipsilateral implantation on the lesioned side of four microdialysis probes in the striatum (STR), substantia nigra pars reticulata (SNr), hippocampus (HIPP) and prefrontal cortex (PFC) (see Figure 3). L-DOPA or its vehicle (veh) was administered intraperitoneally (i.p.) at 3, 6, and 12 mg/kg 20 min after the i.p. administration of benserazide (15 mg/kg), an inhibitor of peripheral decarboxylase. Data represent the mean ± SEM percentages of baseline in each sample (n = 4–5 rats/group) along the time course of the study. Statistical comparisons are shown for the overall effect over 3 h of monitoring, *p < 0.05, **p < 0.01 versus veh group (Fisher’s PLSD test).
3. Region-Dependent Mechanisms Revealed by Microdialysis
Both impulse-dependent and -independent components of DA release, and possibly of 5-HT, may participate in the region-dependent neurochemical pattern of L-DOPA.45 The impulse-dependent component may be differentially regulated in each brain region due to the distinct features of 5-HT innervation originating from the DR or MR nuclei.38,54 Indeed, the electrical activity of 5-HT neurons and the release of 5-HT from 5-HT neurons of the DR and MR are differentially controlled by 5-HT1A/1B autoreceptors.55−57 DR neurons display a greater sensitivity to 5-HT1A activation55,58−61 and preferentially innervate the striatum, PFC, and SNr.62 MR neurons may be under the control of another, as yet unidentified mechanism63 and mainly innervate the HIPP and PFC.64 Another mechanism that may impact upon the exocytotic component of DA and 5-HT release in a region-dependent manner is the expression of the vesicular glutamate transporter VGLUT3 in a subset of 5-HT terminals.65 The presence of VGLUT3 has been shown, at least in the raphe, PFC, lateral septum, and HIPP, to promote 5-HT transmission by increasing vesicular-filling synergy. Interestingly, VGLUT3 expression is up-regulated in the SNr but not in the striatum of 6-OHDA rats.66 This higher VGLUT3 expression may potentiate the capacity of 5-HT terminals in the SN to release DA from L-DOPA, and this may contribute to the region-dependent efficacy of L-DOPA to increase DA and/or 5-HT release, although this remains to be confirmed.
The impulse-independent release of DA induced by L-DOPA could occur via the reversal of the SERT at the level of 5-HT terminals. The relative contribution of such a mechanism may depend upon SERT density among different brain regions. A nonexocytotic (TTX-independent) efflux of DA has been observed at a moderate dose (6 mg/kg) of L-DOPA within the striatum and SN.21 This nonexocytotic component is 2-fold higher in the SNr compared with the striatum, in line with the higher density of 5-HT terminals and SERT in the SNr. Furthermore, a high dose of L-DOPA (100 mg/kg) has been shown to induce a nonexocytotic (TTX-independent) efflux of DA in the HIPP of naïve rats,40 a region particularly enriched with SERT.67 However, the blockade of SERT by citalopram fully blocked DA release induced by L-DOPA at 3 mg/kg and partially reduced that induced by L-DOPA at 100 mg/kg in the striatum, PFC, SNr, and HIPP, irrespective of the relative SERT density in these brain regions.16 Citalopram could affect both the impulse-dependent and -independent components of DA release by activating 5-HT negative feedback through the increase in 5-HT extracellular levels (decrease in exocytotic DA release) and by blocking SERT (decrease in nonexocytotic DA release by SERT reversal) respectively.
B. Involvement of Distinct Clearance Mechanisms in the Region-Dependent Neurochemical Pattern of L-DOPA
Distinct clearance mechanisms of L-DOPA-derived DA may contribute to the region-dependent neurochemical pattern of L-DOPA. As previously discussed (see section I.B), the contribution of DAT from spared DA neurons in the clearance of newly synthesized DA may operate mainly within the striatum at early stages of the disease. In extrastriatal brain regions like the PFC and HIPP, the impact of DA release from 5-HT neurons should be enhanced, in line with the lower expression of DAT in these regions.68 Due to the progressive loss of DA neurons throughout the course of the disease, the contribution of DAT in DA clearance mechanisms may in fact be minimal.69
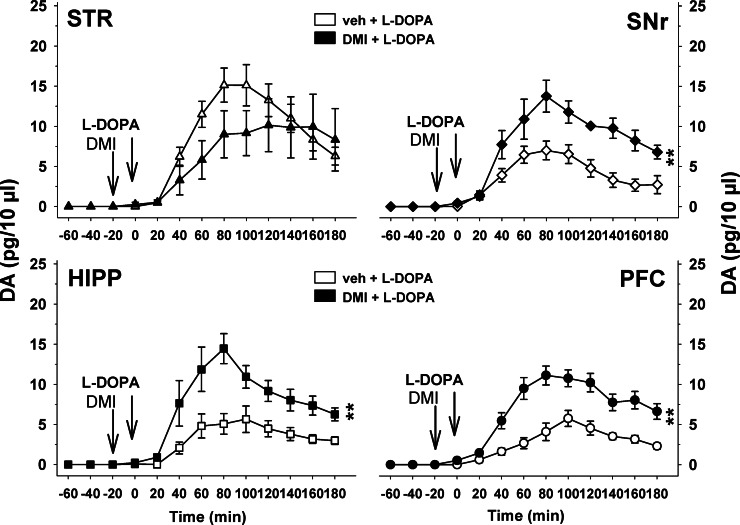
The absolute levels of extracellular DA induced by L-DOPA are greater in the striatum than in other areas for doses higher than 3 mg/kg of L-DOPA. In addition to the DAT, numerous data have shown that the SERT and the noradrenaline transporter (NET) are capable of robust DA reuptake70−72 with moderate to high affinity respectively (Km = 78 μM for SERT and 0.67 μM for NET compared to 2.54 μM for DAT). It has been proposed that SERT may contribute to the clearance of striatal DA release enhanced by L-DOPA.73 However, the fact that citalopram, a blocker of SERT, suppresses L-DOPA-induced DA release (16) supports a role for SERT in the release (see section II.A.3) rather than the clearance of extracellular DA. In contrast, NET may substantially participate in the clearance of DA within extrastriatal brain regions that have lower absolute levels of extracellular DA compared to the striatum. Indeed, NET is known to play a major role in the reuptake of extracellular DA in brain regions poorly innervated by DA fibers.74 NA fibers and NET are poorly represented in the striatum compared to other brain regions.75 Accordingly, we have found that administration of the NET blockers desipramine (Figure 5, unpublished observations) and reboxetine (data not shown, unpublished observations) enhanced L-DOPA-induced DA release in the SNr, PFC, and HIPP, but not in the striatum. Therefore, in the presence of NET blockers, the magnitude of the increase in DA release induced by L-DOPA becomes similar across all brain regions. This suggests that the greater effect of L-DOPA in the striatum is not only related to 5-HT terminals but also to the limited clearance of extracellular DA by NET in this brain region.
Figure 5.
Region-dependent influence of noradrenaline transporter blockade on DA release induced by L-DOPA in hemiparkinsonian rats. Three to four weeks after the unilateral injection of 6-hydroxydopamine into the medial forebrain bundle, rats were anesthetized with isoflurane and placed in a stereotaxic frame for the simultaneous and ipsilateral implantation on the lesioned side of four microdialysis probes in the striatum (STR), substantia nigra pars reticulata (SNr), hippocampus (HIPP), and prefrontal cortex (PFC) (see Figure 3). Desipramine (DMI) or its vehicle (veh) was administered intraperitoneally (i.p.) at 10 mg/kg 20 min before the i.p. administration of L-DOPA (12 mg/kg). Data represent the mean ± SEM percentages of absolute extracellular levels of DA expressed in pg/10 μL of dialysate (n = 4–5 rats/group). **p < 0.01 for the overall effect of the DMI + L-DOPA group versus the veh + L-DOPA group (Fisher’s PLSD test).
Other mechanisms may participate in this region-dependent clearance of L-DOPA-derived DA, including degradation by catechol-O-methyl transferase or MAOB enzymes33,76 or other transporters showing affinity for monoamines such as the carbocation transporter OCT-3.77 The relative contribution of these processes within each brain region remains to be elucidated.
C. Evolution of the Region-Dependent Neurochemical Pattern after Chronic L-DOPA Treatment
We have shown that the reactivity of 5-HT terminals to a subsequent challenge of L-DOPA (3–12 mg/kg) is modified in a region-dependent manner in L-DOPA-treated rats (12 mg/kg/day for 10 days).52 The inhibitory effect of acute L-DOPA at 3 and 12 mg/kg on 5-HT release was potentiated in the SNr and HIPP but not in the PFC of L-DOPA-treated rats. In the striatum, 5-HT release was unaltered by L-DOPA whatever the dose administered. Importantly, the region-dependent reactivity of 5-HT terminals has a direct impact on the ability of L-DOPA to increase DA release after chronic treatment. Indeed, the lack of sensitivity of striatal 5-HT terminals to L-DOPA on 5-HT release is associated with a preserved increase in L-DOPA-induced DA release. Conversely, nigral 5-HT terminals exhibit the highest sensitivity to L-DOPA and the most profound loss of efficacy of L-DOPA to increase DA release.52
Furthermore, chronic treatment with L-DOPA (12 mg/kg) decreases both basal extracellular levels and tissue concentrations of 5-HT and 5-HIAA.45,78,79 These data suggest a negative impact of L-DOPA on 5-HT neuron integrity.45,80 At 6 mg/kg, L-DOPA also decreased tissue concentrations of 5-HT, but not 5-HIAA, in the striatum of L-DOPA-treated rats.21 It appears that, even at moderate doses, a detrimental impact of chronic L-DOPA on 5-HT neuron integrity may participate in the heterogeneous region-dependent loss of efficacy of L-DOPA on DA release in the Parkinsonian brain. These data suggest that chronic L-DOPA treatment leads to a new disequilibrium of DA transmission between the striatum and other brain regions compared to the DA imbalance observed following acute treatment (see section II.A.1). As discussed previously, different mechanisms may occur in the striatum compared to other brain structures that might account for the relative preservation of striatal DA effect of L-DOPA (i.e., absence of NET clearance; see section II.B) and the region-dependent reactivity of 5-HT terminals to acute and chronic L-DOPA treatments.45 Of note, chronic L-DOPA treatment by itself has been shown to alter the morphology of 5-HT neurons in the striatum as well as synaptic plasticity in various brain regions.81−86 The sprouting of 5-HT fibers has been specifically observed in the DA-lesioned striatum of dyskinetic animals53 and is positively correlated with the severity of L-DOPA-induced dyskinesias.85 However, no correlation could be found between this higher 5-HT nerve density and the magnitude of KCl-evoked striatal DA release measured by in vivo chronoamperometry after chronic L-DOPA treatment.87
D. Methodological Considerations
Other sampling methods have been used to study the effects of L-DOPA on DA release such as high speed in vivo chronoamperometry.87,88 In contrast to microdialysis, this technique allows for the measurement of DA kinetics with better time resolution (subseconds compared to minutes for microdialysis) of changes in uptake rate and clearance time.89 However, this technique requires the simultaneous implantation of a carbon fiber electrode with one micropipet filled with KCl solution to evoke DA release after conversion (decarboxylation) from L-DOPA, which limits the possibility to assess DA kinetics simultaneously in multiple brain regions. Chronoamperometry may be preferred over microdialysis to avoid altered presynaptic inhibition due to dialysis probe implantation.90 However, this altered presynaptic inhibition results in underestimation of extracellular DA concentrations in a somewhat proportional manner for both reuptake and release processes.90−92 Lower monoamine concentrations are detectable using microdialysis compared to in vivo chronoamperometry as they do not require KCl or electrical stimulation. Most importantly, the direct and relative comparison of L-DOPA’s effects in several brain regions indicates that this presynaptic inhibition may be of minimal influence. Indeed, a distinct and specific pattern was observed on DA versus 5-HT levels induced by L-DOPA after various pharmacological challenges (chronic L-DOPA treatment or acute NET blockade).
Although the data presented above have undoubtedly revealed the powerful spatial resolution of multisite microdialysis to study the biochemical action of L-DOPA in the Parkinsonian brain, the functional impact of these diffuse effects on motor and nonmotor symptoms still remains to be elucidated. Indeed, the use of a multisite approach necessitates the use of anesthesia for the implantation of multiple probes. All anesthetics will alter the activity of diverse neuronal populations and may affect the biochemistry of neurotransmitter systems. Yet, the magnitude of the effects elicited by L-DOPA on striatal or nigral DA release in anesthetized rats16 is similar to that induced in awake rats.21 Few studies have used a dual-site microdialysis approach to directly correlate the neurochemical (GABA and glutamate release) and behavioral changes induced by L-DOPA in freely moving 6-OHDA-lesioned rats.93,94 These studies are remarkable and not easy to implement routinely due the difficulty of collecting dialysate samples in animals subjected to contraversive rotations and/or dyskinesias induced by acute and chronic L-DOPA treatment, respectively.
III. Prospective Outcomes from Multisite Microdialysis Studies to Control the Region-Dependent Neurochemical Pattern of L-DOPA
Many therapeutic strategies developed over the years have focused on the use of additional treatments to enhance the efficacy of L-DOPA to release DA in the striatum. However, the use of multisite microdialysis techniques has brought new biochemical evidence to reappraise the mechanism of action of L-DOPA beyond the striatum. Both the involvement of 5-HT neurons and the region-dependent neurochemical pattern of L-DOPA have created new directions for therapeutic strategies aimed at controlling the effects of L-DOPA throughout the Parkinsonian brain.
A. Therapeutic Strategies to Control 5-HT Neuronal Function
1. Inhibitors of Monoamine Oxidase B
Given its toxic properties,95−98 L-DOPA may be a factor contributing to 5-HT neuronal damage along with the progression of the disease. Quinones formed from L-DOPA and newly synthesized DA inactivate tryptophan hydroxylase (TPH),99 the initial enzyme involved in the biosynthesis of 5-HT, by converting TPH to a redox-cycling quinoprotein that may participate in 5-HT neuronal toxicity.100−102 These mechanisms could be involved in the overall decrease in 5-HT function observed in Parkinsonian patients and DA-depleted rats receiving L-DOPA treatment.45,53,78,100,103,104 Rasagiline, a nonselective MAOB inhibitor, enhances the efficacy of L-DOPA.76 It acts to maintain substantial concentrations of intracellular DA levels inside 5-HT cells and prevents the potential destruction of 5-HT neurons by enhancing oxidative metabolism.80 The decrease in oxidative stress may participate in the ability of rasagiline to prolong the efficacy of L-DOPA on striatal DA release.105,106 Since 5-HT neurons represent the main site of action of both L-DOPA and rasagiline, the prolonged efficacy on L-DOPA-induced DA release may extend beyond the striatum.
2. 5-HT1A/1B Agonists
One of the first therapeutic approaches arising from evidence that newly synthesized DA from L-DOPA is released by 5-HT neurons was to develop strategies aimed at controlling the activity of 5-HT neurons. It has been shown that the decrease in therapeutic efficacy of L-DOPA treatment over time is associated with the development of numerous side effects including L-DOPA-induced dyskinesias (LIDs). LIDs are thought to emerge as a consequence of the dysregulated release of DA.21,78,107−111 The use of 5-HT drugs to control 5-HT nerve activity and the output of DA from 5-HT neurons appears promising and clinical trials are currently underway to assess the ability of these drugs to alleviate LIDs.
The stimulation of 5-HT1A/1B autoreceptors,21,29 the blockade of SERT by SSRIs112−114 and/or 5,7-DHT lesion16,25 are known to inhibit L-DOPA-induced DA release, an effect associated with a marked reduction in LIDs.21,78 However, these mechanisms have been described mostly in the striatum while other brain regions could be involved in the development of LIDs.115−121 The limit of such an approach is that a general decrease in DA release from 5-HT neurons, although counteracting LIDs, may aggravate Parkinsonism and/or exacerbate depressive and cognitive symptoms.100,122−127 In light of the neurochemical brain pattern of L-DOPA, strategies aimed at controlling the output of DA from 5-HT neurons in a region-specific manner would be more appropriate.
B. Functional Perspectives from a Tailored Strategy Aimed at Controlling the Region-Dependent Effects of L-DOPA
Data obtained from acute to chronic L-DOPA treatment has shown that the region-dependent pattern of L-DOPA evolves toward prominent striatal DA transmission. Although the increase in striatal DA levels at early stages of the disease has been associated with the efficacy of L-DOPA on motor symptoms, the dysregulated DA release at later stages has been directly implicated in LIDs.78,107 Given the diffuse action of L-DOPA in the brain at early stages of the disease, it is likely that the large increase in DA levels observed in extrastriatal regions contributes to the efficacy of L-DOPA (see section II.A.1). Consistent with this, the SN is known to participate in the motor effects of L-DOPA in 6-OHDA-lesioned rats.119,128 Moreover, enhanced DA transmission in the PFC can counteract aberrant DA signaling in subcortical areas.129 However, the loss of efficacy of L-DOPA in increasing cortical and nigral DA levels after chronic treatment45 may favor aberrant DA signaling in cortico-subcortical loops and promote the occurrence of LIDs. It seems that we could gain from a targeted strategy aimed at restoring the efficacy of L-DOPA specifically in extrastriatal areas after chronic treatment.
To adopt a region-dependent strategy, one possibility could be to further develop another advantage of using multisite microdialysis, namely, the ability to examine the functional interconnections between brain regions.130,131 A number of studies have used a dual-probe microdialysis approach to directly assess the relationship between two brain regions by pharmacologically modulating one brain region while simultaneously examining the responses in efferent areas.93,94 These studies have mainly focused on amino acid efflux in different areas of the basal ganglia and their contribution to L-DOPA-induced dyskinesias. Currently, it is unknown whether the local rise in DA release induced by L-DOPA in the SN, PFC, or HIPP does influence DA released by L-DOPA in distal sites including the striatum. Similarly, it would be worth trying to determine whether local application of 5-HT agents in one brain region alters L-DOPA-induced DA release in efferent projection areas. In physiological conditions, DA is known to play a fundamental role in gating the functional interactions between many brain regions.132−134 These relationships are likely evolving in a pathophysiological condition such as Parkinson’s disease where DA is released by 5-HT neurons in a region-dependent manner.
Conclusion
Multisite microdialysis has proven to be a powerful method for the in vivo simultaneous monitoring of DA and 5-HT pharmacokinetics induced by L-DOPA in several brain regions of the hemiparkinsonian rat. This method has revealed a region-dependent pattern of DA release from exogenous L-DOPA throughout 5-HT neurons. This significant discovery has unravelled new insights into the mechanism of action of L-DOPA and its diffuse action in the Parkinsonian brain. Although the DA released in extrastriatal brain regions has been neglected for several decades, the data presented above supports the need to thoroughly consider this ectopic DA effect of L-DOPA in the development of therapeutic strategies aimed at controlling its motor and nonmotor complications.
Acknowledgments
The authors wish to thank Dr. Heather Madsen for editing English language.
This work was supported by the “Fondation de France”, the “Centre National de la Recherche Scientifique”, Bordeaux 2 University, and the “Société Française de Physiologie”. Additional support was given by the European cooperation in science and technology (COST action CM1103).
The authors declare no competing financial interest.
References
- Hornykiewicz O. (1966) Dopamine (3-hydroxytyramine) and brain function. Pharmacol. Rev. 18, 925–964. [PubMed] [Google Scholar]
- Cotzias G. C.; Papavasiliou P. S.; Gellene R. (1969) Modification of Parkinsonism--chronic treatment with L-dopa. New Engl. J. Med. 280, 337–345. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. (1990) In-vivo brain dialysis of neurotransmitters. Trends Pharmacol. Sci. 11, 116–121. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdere P.; Navailles S. (2012) What can we expect from the serotonergic side of l-DOPA?. Rev. Neurol. 168, 927–938. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U.; Arbuthnott G. W. (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 24, 485–493. [DOI] [PubMed] [Google Scholar]
- Lloyd K.; Hornykiewicz O. (1970) Parkinson’s disease: activity of L-dopa decarboxylase in discrete brain regions. Science 170, 1212–1213. [DOI] [PubMed] [Google Scholar]
- Hefti F.; Melamed E.; Wurtman R. J. (1981) The site of dopamine formation in rat striatum after L-dopa administration. J. Pharmacol. Exp. Ther. 217, 189–197. [PubMed] [Google Scholar]
- Melamed E.; Hefti F.; Liebman J.; Schlosberg A. J.; Wurtman R. J. (1980) Serotonergic neurones are not involved in action of L-dopa in Parkinson’s disease. Nature 283, 772–774. [DOI] [PubMed] [Google Scholar]
- Tison F.; Mons N.; Geffard M.; Henry P. (1991) The metabolism of exogenous L-dopa in the brain: an immunohistochemical study of its conversion to dopamine in non-catecholaminergic cells of the rat brain. J. Neural Transm.: Parkinson's Dis. Dementia Sect. 3, 27–39. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Ahmed M.; Barr E.; Leiden J. M.; Kang U. J. (2000) The localization and functional contribution of striatal aromatic L-amino acid decarboxylase to L-3,4-dihydroxyphenylalanine decarboxylation in rodent parkinsonian models. Cell Transplant. 9, 567–576. [DOI] [PubMed] [Google Scholar]
- Ng K. Y.; Chase T. N.; Colburn R. W.; Kopin I. J. (1970) L-Dopa-induced release of cerebral monoamines. Science 170, 76–77. [DOI] [PubMed] [Google Scholar]
- Gumpert J.; Sharpe D.; Curzon G. (1973) Amine metabolites in the cerebrospinal fluid in Parkinson’s disease and the response to levodopa. J. Neurol. Sci. 19, 1–12. [DOI] [PubMed] [Google Scholar]
- Korf J.; van Praag H. M.; Schut D.; Nienhuis R. J.; Lakke J. P. (1974) Parkinson’s disease and amine metabolites in cerebrospinal fluid: implications for L-Dopa therapy. Eur. Neurol. 12, 340–350. [DOI] [PubMed] [Google Scholar]
- Hollister A. S.; Breese G. R.; Mueller R. A. (1979) Role of monoamine neural systems in L-dihydroxyphenylalanine-stimulated activity. J. Pharmacol. Exp. Ther. 208, 37–43. [PubMed] [Google Scholar]
- Lopez A.; Munoz A.; Guerra M. J.; Labandeira-Garcia J. L. (2001) Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience 103, 639–651. [DOI] [PubMed] [Google Scholar]
- Navailles S.; Bioulac B.; Gross C.; De Deurwaerdere P. (2010) Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol. Dis. 38, 136–143. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K.; Fries W.; Muller F.; Koenig E. (2001) Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 358, 787–790. [DOI] [PubMed] [Google Scholar]
- Rosser N.; Heuschmann P.; Wersching H.; Breitenstein C.; Knecht S.; Floel A. (2008) Levodopa improves procedural motor learning in chronic stroke patients. Arch. Phys. Med. Rehabil. 89, 1633–1641. [DOI] [PubMed] [Google Scholar]
- Ruscher K.; Kuric E.; Wieloch T. (2012) Levodopa treatment improves functional recovery after experimental stroke. Stroke 43, 507–513. [DOI] [PubMed] [Google Scholar]
- Abercrombie E. D.; Bonatz A. E.; Zigmond M. J. (1990) Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 525, 36–44. [DOI] [PubMed] [Google Scholar]
- Lindgren H. S.; Andersson D. R.; Lagerkvist S.; Nissbrandt H.; Cenci M. A. (2010) L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J. Neurochem. 112, 1465–1476. [DOI] [PubMed] [Google Scholar]
- Maeda T.; Kannari K.; Suda T.; Matsunaga M. (1999) Loss of regulation by presynaptic dopamine D2 receptors of exogenous L-DOPA-derived dopamine release in the dopaminergic denervated striatum. Brain Res. 817, 185–191. [DOI] [PubMed] [Google Scholar]
- Miller D. W.; Abercrombie E. D. (1999) Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J. Neurochem. 72, 1516–1522. [DOI] [PubMed] [Google Scholar]
- Kannari K.; Tanaka H.; Maeda T.; Tomiyama M.; Suda T.; Matsunaga M. (2000) Reserpine pretreatment prevents increases in extracellular striatal dopamine following L-DOPA administration in rats with nigrostriatal denervation. J. Neurochem. 74, 263–269. [DOI] [PubMed] [Google Scholar]
- Tanaka H.; Kannari K.; Maeda T.; Tomiyama M.; Suda T.; Matsunaga M. (1999) Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. NeuroReport 10, 631–634. [DOI] [PubMed] [Google Scholar]
- Arborelius L.; Nomikos G. G.; Grillner P.; Hertel P.; Hook B. B.; Hacksell U.; Svensson T. H. (1995) 5-HT1A receptor antagonists increase the activity of serotonergic cells in the dorsal raphe nucleus in rats treated acutely or chronically with citalopram. Naunyn-Schmiedeberg's Arch. Pharmacol. 352, 157–165. [DOI] [PubMed] [Google Scholar]
- Sprouse J. S.; Aghajanian G. K. (1987) Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1, 3–9. [DOI] [PubMed] [Google Scholar]
- Temel Y.; Boothman L. J.; Blokland A.; Magill P. J.; Steinbusch H. W.; Visser-Vandewalle V.; Sharp T. (2007) Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc. Natl. Acad. Sci. U.S.A. 104, 17087–17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannari K.; Yamato H.; Shen H.; Tomiyama M.; Suda T.; Matsunaga M. (2001) Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J. Neurochem. 76, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Navailles S.; De Deurwaerdere P. (2010) Presynaptic control of serotonin on striatal dopamine function. Psychopharmacology (Berlin, Ger.) 213, 213–242. [DOI] [PubMed] [Google Scholar]
- Tan S. K.; Hartung H.; Visser-Vandewalle V.; Steinbusch H. W.; Temel Y.; Sharp T. (2012) A combined in vivo neurochemical and electrophysiological analysis of the effect of high-frequency stimulation of the subthalamic nucleus on 5-HT transmission. Exp. Neurol. 233, 145–153. [DOI] [PubMed] [Google Scholar]
- Yamato H.; Kannari K.; Shen H.; Suda T.; Matsunaga M. (2001) Fluoxetine reduces L-DOPA-derived extracellular DA in the 6-OHDA-lesioned rat striatum. NeuroReport 12, 1123–1126. [DOI] [PubMed] [Google Scholar]
- Wachtel S. R.; Abercrombie E. D. (1994) L-3,4-dihydroxyphenylalanine-induced dopamine release in the striatum of intact and 6-hydroxydopamine-treated rats: differential effects of monoamine oxidase A and B inhibitors. J. Neurochem. 63, 108–117. [DOI] [PubMed] [Google Scholar]
- Bunney B. S.; Aghajanian G. K.; Roth R. H. (1973) Comparison of effects of L-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nature (London), New Biol. 245, 123–125. [DOI] [PubMed] [Google Scholar]
- Mercuri N. B.; Calabresi P.; Bernardi G. (1990) Responses of rat substantia nigra compacta neurones to L-DOPA. Br. J. Pharmacol. 100, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden D. G.; Grace A. A. (1995) Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. J. Neurosci. 15, 6157–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond M. J.; Abercrombie E. D.; Berger T. W.; Grace A. A.; Stricker E. M. (1990) Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 13, 290–296. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C.; Segal M. (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 179, 641–667. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W. (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In Handbook of Chemical Neuroanatomy – Classical transmitters and transmitters receptors in the CNS Part II (Björklund A., Hökflet T., and Kuhar M. J., Eds.), pp 68–125, Elsevier: Amsterdam. [Google Scholar]
- Mizoguchi K.; Yokoo H.; Yoshida M.; Tanaka T.; Tanaka M. (1993) Dopamine formation from L-dopa administered exogenously is independent of dopaminergic neuronal activity: studies with in vivo microdialysis. Brain Res. 611, 152–154. [DOI] [PubMed] [Google Scholar]
- Biggs C. S.; Starr M. S. (1997) Dopamine and glutamate control each other’s release in the basal ganglia: a microdialysis study of the entopeduncular nucleus and substantia nigra. Neurosci. Biobehav. Rev. 21, 497–504. [DOI] [PubMed] [Google Scholar]
- Thorre K.; Sarre S.; Smolders I.; Ebinger G.; Michotte Y. (1998) Dopaminergic regulation of serotonin release in the substantia nigra of the freely moving rat using microdialysis. Brain Res. 796, 107–116. [DOI] [PubMed] [Google Scholar]
- Alam M. R.; Yoshizawa F.; Sugahara K. (2011) L-DOPA induced extracellular dopamine increases in the ventromedial hypothalamus affects food intake by chickens on a lysine-free diet. Neurosci. Lett. 495, 126–129. [DOI] [PubMed] [Google Scholar]
- Alam M. R.; Yoshizawa F.; Sugahara K. (2012) Local administration of L-DOPA in the chicken ventromedial hypothalamus increases dopamine release in a dose-dependent manner. Neurosci. Lett. 529, 150–154. [DOI] [PubMed] [Google Scholar]
- Navailles S.; Bioulac B.; Gross C.; De Deurwaerdere P. (2011) Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol. Dis. 41, 585–590. [DOI] [PubMed] [Google Scholar]
- Seeman P. (1980) Brain dopamine receptors. Pharmacol. Rev. 32, 229–313. [PubMed] [Google Scholar]
- Hornykiewicz O. (1973) Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA). Br. Med. Bull. 29, 172–178. [DOI] [PubMed] [Google Scholar]
- Obeso J. A.; Marin C.; Rodriguez-Oroz C.; Blesa J.; Benitez-Temino B.; Mena-Segovia J.; Rodriguez M.; Olanow C. W. (2008) The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann. Neurol. 64(Suppl 2), S30–46. [DOI] [PubMed] [Google Scholar]
- Brown W. D.; Taylor M. D.; Roberts A. D.; Oakes T. R.; Schueller M. J.; Holden J. E.; Malischke L. M.; DeJesus O. T.; Nickles R. J. (1999) FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology 53, 1212–1218. [DOI] [PubMed] [Google Scholar]
- Hery F.; Simonnet G.; Bourgoin S.; Soubrie P.; Artaud F.; Hamon M.; Glowinski J. (1979) Effect of nerve activity on the in vivo release of [3H]serotonin continuously formed from L-[3H]tryptophan in the caudate nucleus of the cat. Brain Res. 169, 317–334. [DOI] [PubMed] [Google Scholar]
- Melamed E.; Zoldan J.; Friedberg G.; Ziv I.; Weizmann A. (1996) Involvement of serotonin in clinical features of Parkinson’s disease and complications of L-DOPA therapy. Adv. Neurol. 69, 545–550. [PubMed] [Google Scholar]
- Navailles S.; Carta M.; Guthrie M.; De Deurwaerdere P. (2011) L-DOPA and serotonergic neurons: functional implication and therapeutic perspectives in Parkinson’s disease. Cent. Nerv. Syst. Agents Med. Chem. 11, 305–320. [DOI] [PubMed] [Google Scholar]
- Navailles S.; De Deurwaerdere P. (2012) Imbalanced Dopaminergic Transmission Mediated by Serotonergic Neurons in L-DOPA-Induced Dyskinesia. Parkinson’s Dis. 2012, 323686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade R.; Sharp T. (1997) Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J. Neurochem. 69, 791–796. [DOI] [PubMed] [Google Scholar]
- Blier P.; de Montigny C.; ChaVput Y. (1990) A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. J. Clin. Psychiatry 51(Suppl), 14–20discussion 21.. [PubMed] [Google Scholar]
- Hervas I.; Bel N.; Fernandez A. G.; Palacios J. M.; Artigas F. (1998) In vivo control of 5-hydroxytryptamine release by terminal autoreceptors in rat brain areas differentially innervated by the dorsal and median raphe nuclei. Naunyn-Schmiedeberg's Arch. Pharmacol. 358, 315–322. [DOI] [PubMed] [Google Scholar]
- Kreiss D. S.; Lucki I. (1994) Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J. Pharmacol. Exp. Ther. 269, 1268–1279. [PubMed] [Google Scholar]
- Casanovas J. M.; Artigas F. (1996) Differential effects of ipsapirone on 5-hydroxytryptamine release in the dorsal and median raphe neuronal pathways. J. Neurochem. 67, 1945–1952. [DOI] [PubMed] [Google Scholar]
- Casanovas J. M.; Lesourd M.; Artigas F. (1997) The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. B. J. Pharmacol. 122, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi R.; Carli M.; Di Clemente A.; Samanin R. (1991) Administration of 8-hydroxy-2-(Di-n-propylamino)tetralin in raphe nuclei dorsalis and medianus reduces serotonin synthesis in the rat brain: differences in potency and regional sensitivity. J. Neurochem. 56, 243–247. [DOI] [PubMed] [Google Scholar]
- Romero L.; Artigas F. (1997) Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J. Neurochem. 68, 2593–2603. [DOI] [PubMed] [Google Scholar]
- van der Kooy D.; Hattori T. (1980) Bilaterally situated dorsal raphe cell bodies have only unilateral forebrain projections in rat. Brain Res. 192, 550–554. [DOI] [PubMed] [Google Scholar]
- Hopwood S. E.; Stamford J. A. (2001) Multiple 5-HT(1) autoreceptor subtypes govern serotonin release in dorsal and median raphe nucle. Neuropharmacology 40, 508–519. [DOI] [PubMed] [Google Scholar]
- Moore R. Y.; Halaris A. E.; Jones B. E. (1978) Serotonin neurons of the midbrain raphe: ascending projections. J. Comp. Neurol. 180, 417–438. [DOI] [PubMed] [Google Scholar]
- Amilhon B.; Lepicard E.; Renoir T.; Mongeau R.; Popa D.; Poirel O.; Miot S.; Gras C.; Gardier A. M.; Gallego J.; Hamon M.; Lanfumey L.; Gasnier B.; Giros B.; El Mestikawy S. (2010) VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 30, 2198–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E. K.; Chen L. W.; Chan Y. S.; Yung K. K. (2006) Up-regulation in expression of vesicular glutamate transporter 3 in substantia nigra but not in striatum of 6-hydroxydopamine-lesioned rats. Neurosignals 15, 238–248. [DOI] [PubMed] [Google Scholar]
- Invernizzi R.; Velasco C.; Bramante M.; Longo A.; Samanin R. (1997) Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology 36, 467–473. [DOI] [PubMed] [Google Scholar]
- Mazei M. S.; Pluto C. P.; Kirkbride B.; Pehek E. A. (2002) Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 936, 58–67. [DOI] [PubMed] [Google Scholar]
- Chotibut T.; Apple D. M.; Jefferis R.; Salvatore M. F. (2012) Dopamine transporter loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine uptake. PloS One 7, e52322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E.; Silvagni A. (2004) Dopamine reuptake by norepinephrine neurons: exception or rule?. Crit. Rev. Neurobiol. 16, 121–128. [DOI] [PubMed] [Google Scholar]
- Giros B.; Wang Y. M.; Suter S.; McLeskey S. B.; Pifl C.; Caron M. G. (1994) Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J. Biol. Chem. 269, 15985–15988. [PubMed] [Google Scholar]
- Larsen M. B.; Sonders M. S.; Mortensen O. V.; Larson G. A.; Zahniser N. R.; Amara S. G. (2011) Dopamine transport by the serotonin transporter: a mechanistically distinct mode of substrate translocation. J. Neurosci. 31, 6605–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannari K.; Shen H.; Arai A.; Tomiyama M.; Baba M. (2006) Reuptake of L-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci. Lett. 402, 62–65. [DOI] [PubMed] [Google Scholar]
- Moron J. A.; Brockington A.; Wise R. A.; Rocha B. A.; Hope B. T. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 22, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G.; Zhu Y.; Card J. P. (2004) Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J. Neurosci. 24, 2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg J. P.; Wang J.; Bankiewicz K.; Harvey-White J.; Kopin I. J.; Goldstein D. S. (1998) Increased striatal dopamine production from L-DOPA following selective inhibition of monoamine oxidase B by R(+)-N-propargyl-1-aminoindan (rasagiline) in the monkey. J. Neural Transm., Suppl. 52, 279–285. [DOI] [PubMed] [Google Scholar]
- Sader-Mazbar O., Loboda Y., and Finberg J. (2012) Role of the Organic Cation Transporter (OCT-3) and Monoamine Oxidase Types A and B in the Metabolism of Dopamine Derived from L-DOPA in Rat Striatum Depleted of Dopaminergic and Serotonergic Afferent Inputs. In The Tenth International Catecholamine Symposium, Asilomar, Pacific Grove, CA. [Google Scholar]
- Carta M.; Carlsson T.; Kirik D.; Bjorklund A. (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130, 1819–1833. [DOI] [PubMed] [Google Scholar]
- Everett G. M.; Borcherding J. W. (1970) L-DOPA: effect on concentrations of dopamine, norepinephrine, and serotonin in brains of mice. Science 168, 847–850. [PubMed] [Google Scholar]
- Stansley B. J.; Yamamoto B. K. (2013) l-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 67, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A.; Bezard E. (2009) Dopamine receptors and L-dopa-induced dyskinesia. Parkinsonism Relat. Disord. 15(Suppl 4), S8–12. [DOI] [PubMed] [Google Scholar]
- Picconi B.; Ghiglieri V.; Calabresi P. (2010) L-3,4-dihydroxyphenylalanine-induced sprouting of serotonin axon terminals: A useful biomarker for dyskinesias?. Ann. Neurol. 68, 578–580. [DOI] [PubMed] [Google Scholar]
- Picconi B.; Pisani A.; Barone I.; Bonsi P.; Centonze D.; Bernardi G.; Calabresi P. (2005) Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology 26, 779–783. [DOI] [PubMed] [Google Scholar]
- Prescott I. A.; Dostrovsky J. O.; Moro E.; Hodaie M.; Lozano A. M.; Hutchison W. D. (2009) Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 132, 309–318. [DOI] [PubMed] [Google Scholar]
- Rylander D.; Parent M.; O’Sullivan S. S.; Dovero S.; Lees A. J.; Bezard E.; Descarries L.; Cenci M. A. (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 68, 619–628. [DOI] [PubMed] [Google Scholar]
- Zeng B. Y.; Iravani M. M.; Jackson M. J.; Rose S.; Parent A.; Jenner P. (2010) Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol. Dis. 40, 599–607. [DOI] [PubMed] [Google Scholar]
- Lundblad M.; af Bjerken S.; Cenci M. A.; Pomerleau F.; Gerhardt G. A.; Stromberg I. (2009) Chronic intermittent L-DOPA treatment induces changes in dopamine release. J. Neurochem. 108, 998–1008. [DOI] [PubMed] [Google Scholar]
- Nevalainen N.; Af Bjerken S.; Lundblad M.; Gerhardt G. A.; Stromberg I. (2011) Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J. Neurochem. 118, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser N. R.; Dickinson S. D.; Gerhardt G. A. (1998) High-speed chronoamperometric electrochemical measurements of dopamine clearance. Methods Enzymol. 296, 708–719. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Michael A. C. (2012) Microdialysis probes alter presynaptic regulation of dopamine terminals in rat striatum. J. Neurosci. methods 208, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay P. M.; Newton-Vinson P.; Isele W.; Garris P. A.; Justice J. B. (2003) Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J. Neurochem. 86, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C. (2005) Evidence on extracellular dopamine level in rat striatum: implications for the validity of quantitative microdialysis. J. Neurochem. 92, 46–58. [DOI] [PubMed] [Google Scholar]
- Bido S.; Marti M.; Morari M. (2011) Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J. Neurochem. 118, 1043–1055. [DOI] [PubMed] [Google Scholar]
- Mela F.; Marti M.; Bido S.; Cenci M. A.; Morari M. (2012) In vivo evidence for a differential contribution of striatal and nigral D1 and D2 receptors to L-DOPA induced dyskinesia and the accompanying surge of nigral amino acid levels. Neurobiol. Dis. 45, 573–582. [DOI] [PubMed] [Google Scholar]
- Alexander T.; Sortwell C. E.; Sladek C. D.; Roth R. H.; Steece-Collier K. (1997) Comparison of neurotoxicity following repeated administration of l-dopa, d-dopa and dopamine to embryonic mesencephalic dopamine neurons in cultures derived from Fisher 344 and Sprague-Dawley donors. Cell Transplant. 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Basma A. N.; Morris E. J.; Nicklas W. J.; Geller H. M. (1995) L-dopa cytotoxicity to PC12 cells in culture is via its autoxidation. J. Neurochem. 64, 825–832. [DOI] [PubMed] [Google Scholar]
- Cheng N.; Maeda T.; Kume T.; Kaneko S.; Kochiyama H.; Akaike A.; Goshima Y.; Misu Y. (1996) Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res. 743, 278–283. [DOI] [PubMed] [Google Scholar]
- Pardo B.; Mena M. A.; de Yebenes J. G. (1995) L-dopa inhibits complex IV of the electron transport chain in catecholamine-rich human neuroblastoma NB69 cells. J. Neurochem. 64, 576–582. [DOI] [PubMed] [Google Scholar]
- Hashiguti H.; Nakahara D.; Maruyama W.; Naoi M.; Ikeda T. (1993) Simultaneous determination of in vivo hydroxylation of tyrosine and tryptophan in rat striatum by microdialysis-HPLC: relationship between dopamine and serotonin biosynthesis. J. Neural Transm.: Gen. Sect. 93, 213–223. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs K. L.; Angoa-Perez M.; Kuhn D. M.; Bishop C. (2011) Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of l-DOPA treatment. Neurosci. Biobehav. Rev. 35, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. M.; Arthur R. Jr. (1998) Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J. Neurosci. 18, 7111–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. M.; Arthur R. E. Jr. (1999) L-DOPA-quinone inactivates tryptophan hydroxylase and converts the enzyme to a redox-cycling quinoprotein. Brain Res. Mol. Brain Res. 73, 78–84. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs K. L.; Dupre K. B.; Ostock C. Y.; Button T.; Deak T.; Bishop C. (2010) Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav. Pharmacol. 21, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama W.; Naoi M.; Takahashi A.; Watanabe H.; Konagaya Y.; Mokuno K.; Hasegawa S.; Nakahara D. (1992) The mechanism of perturbation in monoamine metabolism by L-dopa therapy: in vivo and in vitro studies. J. Neural Transm.: Gen. Sect. 90, 183–197. [DOI] [PubMed] [Google Scholar]
- Rascol O.; Brooks D. J.; Melamed E.; Oertel W.; Poewe W.; Stocchi F.; Tolosa E. (2005) Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 365, 947–954. [DOI] [PubMed] [Google Scholar]
- Weinreb O.; Amit T.; Bar-Am O.; Youdim M. B. (2010) Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog. Neurobiol. 92, 330–344. [DOI] [PubMed] [Google Scholar]
- Carta M.; Carlsson T.; Munoz A.; Kirik D.; Bjorklund A. (2008) Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog. Brain Res. 172, 465–478. [DOI] [PubMed] [Google Scholar]
- Chase T. N. (1998) Levodopa therapy: consequences of the nonphysiologic replacement of dopamine. Neurology 50, S17–25. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R.; Sossi V.; Huang Z.; Furtado S.; Lu J. Q.; Calne D. B.; Ruth T. J.; Stoessl A. J. (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127, 2747–2754. [DOI] [PubMed] [Google Scholar]
- Munoz A.; Li Q.; Gardoni F.; Marcello E.; Qin C.; Carlsson T.; Kirik D.; Di Luca M.; Bjorklund A.; Bezard E.; Carta M. (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain 131, 3380–3394. [DOI] [PubMed] [Google Scholar]
- Ulusoy A.; Sahin G.; Kirik D. (2010) Presynaptic dopaminergic compartment determines the susceptibility to L-DOPA-induced dyskinesia in rats. Proc. Natl. Acad. Sci. U.S.A. 107, 13159–13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C.; George J. A.; Buchta W.; Goldenberg A. A.; Mohamed M.; Dickinson S. O.; Eissa S.; Eskow Jaunarajs K. L. (2012) Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats. Eur. J. Neurosci. 36, 2839–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durif F.; Vidailhet M.; Bonnet A. M.; Blin J.; Agid Y. (1995) Levodopa-induced dyskinesias are improved by fluoxetine. Neurology 45, 1855–1858. [DOI] [PubMed] [Google Scholar]
- Kuan W. L.; Zhao J. W.; Barker R. A. (2008) The role of anxiety in the development of levodopa-induced dyskinesias in an animal model of Parkinson’s disease, and the effect of chronic treatment with the selective serotonin reuptake inhibitor citalopram. Psychopharmacology (Berlin, Ger.) 197, 279–293. [DOI] [PubMed] [Google Scholar]
- Cenci M. A.; Lundblad M. (2006) Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J. Neurochem. 99, 381–392. [DOI] [PubMed] [Google Scholar]
- Di Matteo V.; Di Giovanni G.; Pierucci M.; Esposito E. (2008) Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog. Brain Res. 172, 7–44. [DOI] [PubMed] [Google Scholar]
- Marin C.; Aguilar E.; Rodriguez-Oroz M. C.; Bartoszyk G. D.; Obeso J. A. (2009) Local administration of sarizotan into the subthalamic nucleus attenuates levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Psychopharmacology (Berlin, Ger.) 204, 241–250. [DOI] [PubMed] [Google Scholar]
- Munoz A.; Carlsson T.; Tronci E.; Kirik D.; Bjorklund A.; Carta M. (2009) Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp. Neurol. 219, 298–307. [DOI] [PubMed] [Google Scholar]
- Orosz D.; Bennett J. P. (1992) Simultaneous microdialysis in striatum and substantia nigra suggests that the nigra is a major site of action of L-dihydroxyphenylalanine in the ″hemiparkinsonian″ rat. Exp. Neurol. 115, 388–393. [DOI] [PubMed] [Google Scholar]
- Sarre S.; Herregodts P.; Deleu D.; Devrieze A.; De Klippel N.; Ebinger G.; Michotte Y. (1992) Biotransformation of L-dopa in striatum and substantia nigra of rats with a unilateral, nigrostriatal lesion: a microdialysis study. Naunyn-Schmiedeberg's Arch. Pharmacol. 346, 277–285. [DOI] [PubMed] [Google Scholar]
- Sarre S.; Smolders I.; Thorre K.; Ebinger G.; Michotte Y. (1997) Biotransformation of locally applied precursors of dopamine, serotonin and noradrenaline in striatum and hippocampus: a microdialysis study. J. Neural Transm. 104, 1215–1228. [DOI] [PubMed] [Google Scholar]
- Eskow K. L.; Gupta V.; Alam S.; Park J. Y.; Bishop C. (2007) The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol., Biochem. Behav. 87, 306–314. [DOI] [PubMed] [Google Scholar]
- Goetz C. G.; Damier P.; Hicking C.; Laska E.; Muller T.; Olanow C. W.; Rascol O.; Russ H. (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov. Disord. 22, 179–186. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B.; Bedard P. J. (1993) Effect of nondopaminergic drugs on L-dopa-induced dyskinesias in MPTP-treated monkeys. Clin. Neuropharmacol. 16, 418–427. [DOI] [PubMed] [Google Scholar]
- Iravani M. M.; Tayarani-Binazir K.; Chu W. B.; Jackson M. J.; Jenner P. (2006) In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with\ increased motor disability. J. Pharmacol. Exp. Ther. 319, 1225–1234. [DOI] [PubMed] [Google Scholar]
- Kannari K.; Kurahashi K.; Tomiyama M.; Maeda T.; Arai A.; Baba M.; Suda T.; Matsunaga M. (2002) [Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease]. No To Shinkei 54, 133–137. [PubMed] [Google Scholar]
- Tomiyama M.; Kimura T.; Maeda T.; Kannari K.; Matsunaga M.; Baba M. (2005) A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson’s disease. Neurosci. Res. 52, 185–194. [DOI] [PubMed] [Google Scholar]
- Robertson G. S.; Robertson H. A. (1989) Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J. Neurosci. 9, 3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci S.; Fernandez R.; Baptista T.; Murzi E.; Hernandez L. (1994) Dopamine increase in the prefrontal cortex correlates with reversal of haloperidol-induced catalepsy in rats. Brain Res. Bull. 35, 125–133. [DOI] [PubMed] [Google Scholar]
- Mark K. A.; Soghomonian J. J.; Yamamoto B. K. (2004) High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J. Neurosci. 24, 11449–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari M.; O’Connor W. T.; Darvelid M.; Ungerstedt U.; Bianchi C.; Fuxe K. (1996) Functional neuroanatomy of the nigrostriatal and striatonigral pathways as studied with dual probe microdialysis in the awake rat--I. Effects of perfusion with tetrodotoxin and low-calcium medium. Neuroscience 72, 79–87. [DOI] [PubMed] [Google Scholar]
- Del Arco A.; Mora F. (2009) Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J. Neural Transm. 116, 941–952. [DOI] [PubMed] [Google Scholar]
- Goto Y.; Grace A. A. (2008) Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb. Cortex 18, 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A. (2002) Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 67, 53–83. [DOI] [PubMed] [Google Scholar]