Abstract
A previously uncharacterized form of stereochemistry, constellational isomerism, is described. The isomerism arises from different arrangements of small-molecule guests in the space of a self-assembled, cylindrical host. The cylindrical host detains three molecules each of CHCl3, 1,2-dichloroethane, or isopropyl chloride. The exchange of guests in and out of the host is slow on the NMR time scale. The dimensions of the capsular host and the sizes of the guests hinder the mobility of molecules inside, and separate NMR signals are seen for guests at the ends of the capsule and those near its center. When two different guests are encapsulated, the spectra show up to four additional species: two sets of constellational isomers. In every pairwise combination of the three guests, all isomers could be identified. The equilibrium distributions of isomers depended on the concentrations of the guests in the bulk solution. The relative stability of the constellational isomers was a function of the polarity of the guest molecule and its ability to interact with the components of the capsule. The different arrangements represent information, and some possibilities for their use in data storage are proposed.
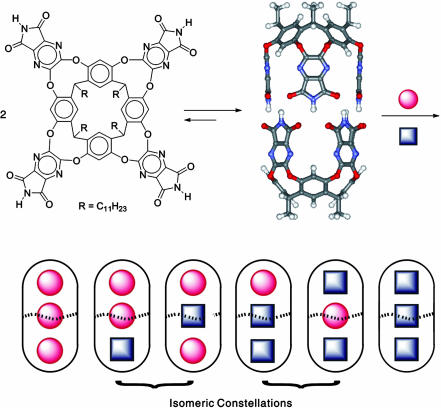
The stereochemistry of covalent bonds is older than a century, whereas that of mechanical bonds is younger than a decade. Carcerands (1) and catenanes (2, 3), for example, have been devised to show new forms of isomerism based on slowed molecular rotations and tumbling. Certain rotaxane (4, 5) isomers can interconvert through localized molecular translation. Weaker, intermolecular forces also can be recruited to restrain the motion, however temporarily, of molecules completely surrounded by other molecules. Reversibly formed capsules provide mechanical barriers to the tumbling of molecules detained within them; the energies separating isomers are modest but large enough to detect isomerism at ambient temperatures by NMR methods (for the preliminary communication see ref. 6). Here we develop a previously uncharacterized type of stereochemistry arising from the limited mobility of three molecules inside a cylindrical capsule (Fig. 1). Different arrangements exist for molecules in a well defined space, and we have proposed the term constellational isomerism to describe the phenomenon (7).
Fig. 1.
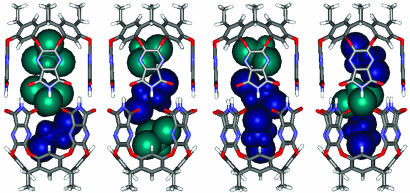
(Upper) Line drawing of the cavitand (Left) and a ball-and-stick representation of the self-assembled cylindrical capsule (Right). (Lower) Six distributions of two different guests in the capsule.
Experimental Methods
General. 1H NMR spectra were recorded on Bruker (Billerica, MA) DRX-600 (600 MHz) or DRX-800 (800 MHz) spectrometers using the solvent signals as internal references. The energy-minimized structures were calculated with hyperchem 7 (MM+ force field).
Titration. Isopropyl chloride (IPC) (300 μl) was added to a suspension of cylindrical capsule (3.0 mM) in mesitylene-d12. The appropriate volume of 1,2-dichloroethane (DCE)-d4 was titrated into the solution, and the 1H NMR spectrum was recorded at 273 K. The spectrum revealed IPCs in the capsule (arrays 13–17). The same titration using IPC-d7 and DCE showed DCE at the end of the capsule (arrays 14 and 16–18). The identity of each array was conformed by titrations and reverse titrations of the nondeuterated IPC and DCE in mesitylene-d12.
Results and Discussion
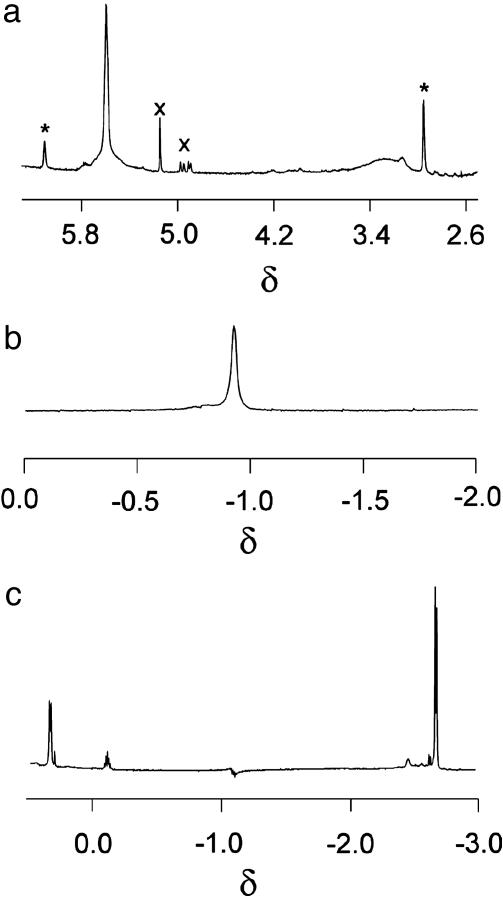
Individual Guests. We have described the NMR spectra of encapsulated CHCl3, DCE, and IPC elsewhere (8), and we revisit them here only as reference points for what follows. In brief, two signals are seen for the CHCl3 (Fig. 2a) in a 2:1 ratio. The signal at 3.52 (Δδ = -3.61 ppm, 2 protons) can be assigned to CHCl3 molecules at the ends of the capsule, whereas the resonance at 6.46 (Δδ = -0.77 ppm, 1 proton) can be assigned to the CHCl3 molecule located near the middle. The separate resonances indicate that the CHCl3 molecules do not exchange positions on this time scale: they are too large to slip past each other while within the capsule, and they enter and exit the capsule at a rate that is slow on the NMR time scale. The exchange rates could be slowed further at lower temperatures, and because sharper signals could be observed under these conditions, some subsequent spectra were taken at 253 K. The assignments were confirmed through 1D gradient-enhanced nuclear Overhauser effect spectroscopy (GOESY) techniques (9) but are obvious consequences of the shielding imparted by the aromatics of the host. The shielding is maximized near the ends of the capsule, at which the anisotropy of 8 aromatic rings converge on and affect the magnetic environment of any nuclei nearby. The resonance for CHCl3 in the center is also broadened and implies an increased rate of exchange of this guest with CHCl3 outside the capsule, in the bulk solvent. The three CHCl molecules (73 Å3 each) occupy ≈52% of the capsule's space (420 Å3), and the capsule is optimally filled (10).
Fig. 2.
NMR spectra of encapsulated solvents in CHCl3 (a) (the two locations of encapsulated solvent, marked with *, show different chemical shifts and slow exchange, and the signals at ≈5 ppm, marked with X, represent amylene stabilizers in the solvent), DCE (b) (the signal is of the guests at the ends of the capsule), and IPC (c) (with deuterated mesitylene as cosolvent, the upfield doublet and the heptet represent two IPC guests at the ends of the capsule, and the downfield doublet represents the IPC in the middle).
The longer DCE in the same capsule shows NMR characteristics similar to those of CHCl3 (Fig. 2b). The upfield signal at approximately -1.1 ppm is in a region free of other resonances, and it represents the two DCEs at the ends of the capsule and is considerably broadened. The signal for the DCE in the middle is obscured by aliphatic resonances of the capsule itself and somewhat distorted by the large signal for the DCE outside. A preparation of the capsule starting from perdeuterated dodecanal revealed the position of the centrally located DCE signal through a 1D GOESY experiment. Attempts to observe 2D NOESY signals between the guests failed, but exchange does take place (8). The shape of DCE is thinner than CHCl3, and its unbranched structure allows some slow slithering between the two positions. The volume of DCE (75 Å3) is marginally larger than CHCl3, and the occupancy is nearly 54%.
The NMR spectrum of IPC in the capsule using deuterated mesitylene as a cosolvent is shown in Fig. 2c. The signals also appear in an unobscured region of the spectrum and show the two types of encapsulated molecules: those at the ends of the capsule and the one at the middle. Again, energetic barriers prevent these guest molecules from exchanging positions; similar to CHCl3, which they resemble closely in size and shape, they are too large to snake past each other while within the capsule. The three IPC molecules (74 Å3 each) occupy ≈53% of the capsule's space. Energy-minimized structures for the three types of capsules are shown in Fig. 3.
Fig. 3.
Energy-minimized structures of encapsulation complexes: CHCl3 (Left), DCE (Center), and IPC (Right).
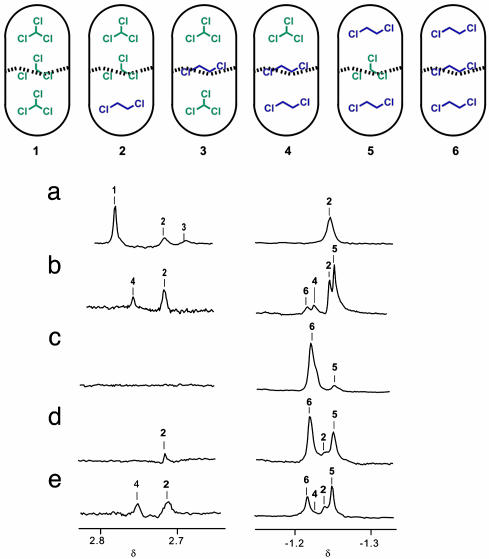
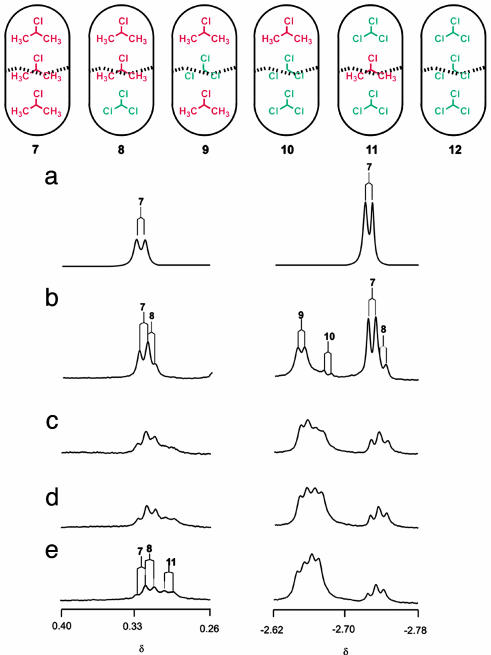
The CHCl3/DCE Pair. The titration of DCE into the CHCl3-filled capsule gave new signals that changed with the ratio of the each guest. The addition of 5 μl of DCE (60:1 ratio of CHCl3:DCE) gave a new signal in the -1-ppm region, caused by the DCE encapsulated at the end of the capsule, as expected for isomer 2. Another new signal at 2.7 ppm resulted from the CHCl3 at the end of the capsule. Further addition of DCE (15:1 ratio) provided a third signal at 2.7 ppm (Fig. 4a), representing isomer 3 or 4. If that was caused by 4, the presence of a new signal at -1 ppm was required. However, no new signal appeared in that region, so the species was assigned as isomer 3.
Fig. 4.
(Upper) Distributions of CHCl3 and DCE in the capsule. (Lower) NMR spectra (600 MHz, 273K) of encapsulated guests in deuterated mesitylene. (Right) DCE at the end of the capsule. (Left) CHCl3 at the end of the capsule. (a) DCE (10 μl) and CHCl3 (300 μl). (b) DCE (150 μl) and CHCl3 (300 μl). (c) DCE (300 μl) and CHCl3 (10 μl). (d) DCE (300 μl) and CHCl3 (75 μl). (e) DCE (300 μl) and CHCl3 (150 μl).
With an increase in the amount of DCE, isomer 3 disappeared. The stability of constellational isomers 2 and 3 is quite different: 2 is the better arrangement. It seems that the polar molecules tend to accumulate where they find their complements: at the middle of the capsule. A 2:1 ratio of CHCl3 to DCE caused four sets of signals to appear in the -1-ppm region, as expected for all distributions (Fig. 4b).
A key to the interpretation of the spectra lies in the identical stoichiometries of the constellational isomers (e.g., 2 and 3). The relative amounts of 2 and 3 need not be equal, but their ratio must remain constant at a given temperature regardless of the CHCl3 and DCE concentrations outside the capsule.
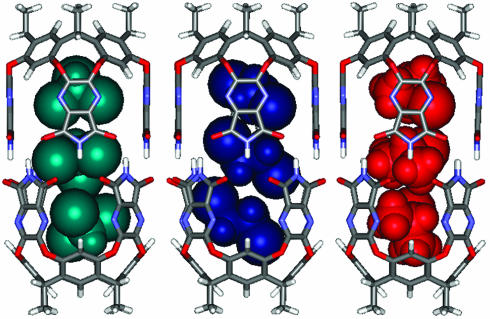
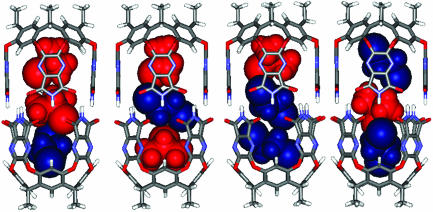
A titration in the reverse sense was carried out to assign each isomer completely. With a small amount of CHCl3 (≈30 DCE/1 CHCl3), a new signal in the -1-ppm region appears, representing isomer 2, 4, or 5. However, CHCl3 at the end of the capsule was not observed. This excludes isomers 2 and 4 and leaves isomer 5 (Fig. 4c). No new signal appeared until a 5:1 ratio of DCE and CHCl3 was reached, when 2 or 4 emerged (Fig. 4d). Further addition of CHCl3 caused a fourth signal in the -1-ppm region between the original (6) and the third (Fig. 4e). From the DCE titration, the second upfield signal at -1 ppm was assigned to 2, and the CHCl3 of the end of the capsule was more upfield-shifted than the isomer 4. Accordingly, the third signal belongs to isomer 2, and the fourth signal is isomer 4. The constellational isomers 4 and 5 also have much different stabilities. In this case, the more polar CHCl3 prefers the middle of the capsule. It is surprising that the isomer 2 appeared before 4 in this titration even though the concentration of DCE was high. Energy-minimized structures for these constellations are shown in Fig. 5.
Fig. 5.
Energy-minimized structures of two sets of constellational isomers (2 ≈ 5) due to CHCl3 and DCE coencapsulation.
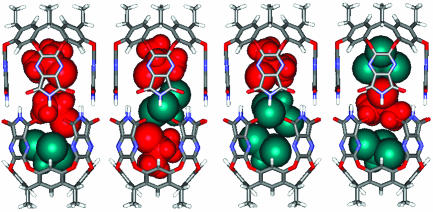
The IPC/CHCl3 Pair. The combination of IPC and CHCl3 in the capsule was followed by 800-MHz spectroscopy. Titration of CHCl3 into the IPC-filled capsule generated spectra shown in Fig. 6 b–e. The upfield regions of the spectra representing IPC at the ends and the middle of the capsule allowed identification of constellational isomers 8 and 9. The first to appear are those within which CHCl3 replaces a single IPC. For reasons noted previously, the relative amounts of 8 and 9 must remain constant throughout the titration. The assignments indicated meet this requirement with 9/8 = 2.5. The isomers with one IPC and two CHCl3 guests emerge next (10 and 11), but only 10 appears in the furthest upfield region of the spectrum. Centrally located IPC guests are in three arrays (7, 8, and 11), but the resonance for 7 was already known and that for 8 was deduced above (Fig. 6e). Accordingly, 11 can be identified. The titration was also performed in the reverse sense: IPC was added to the CHCl3-filled capsule. Signals for 10 and 11 appeared, followed by those for 8 and 9 in the NMR spectra.
Fig. 6.
(Upper) Distributions of IPC and CHCl3 in the capsule. (Lower) NMR spectra (800 MHz, 298K) of encapsulated guests in deuterated mesitylene. (Right) IPC at the end of the capsule. IPC in the middle of the capsule (left). (a) IPC (300 μl). (b) IPC (300 μl) and CHCl3 (50 μl). (c) IPC (300 μl) and CHCl3 (150 μl). (d) IPC (300 μl) and CHCl3 (300 μl). (e) IPC (300 μl) and CHCl3 (400 μl).
Again there is a marked preference for CHCl3 in the center; it is always present in greater amounts than 11. This is likely due to the weak hydrogen-bond-donating ability of CHCl3 as it skates along the acceptors near the center of the capsule. An equimolar mixture of CHCl3 and IPC showed the appropriate downfield signals for encapsulated CHCl3, including the CHCl3-filled capsule 12. These constellations are shown in Fig. 7.
Fig. 7.
Energy-minimized structures of two sets of constellational isomers (8 ≈ 11) due to IPC and CHCl3 coencapsulation.
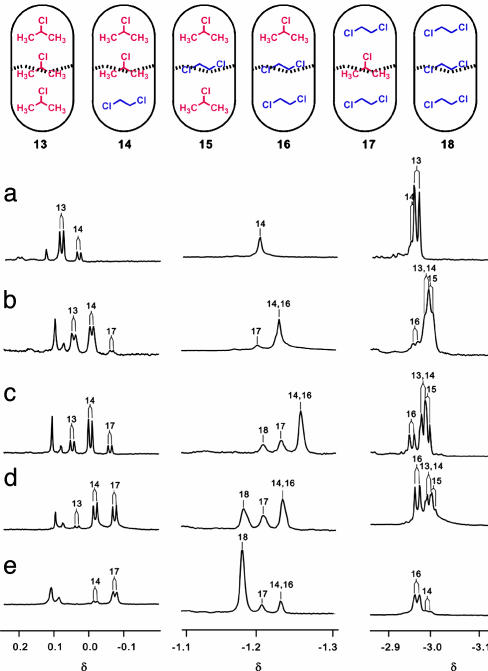
The IPC/DCE Pair. We also observed every combination of IPC and DCE in the capsule (Fig. 8). Here we paired a deuterated guest and a protio guest to obtain sharp NMR signals. Titration of DCE-d4 (DCE) into the IPC (IPC-d7)-filled capsule generated spectra shown in Fig. 8 a and b. Small amounts of DCE (DCE-d4) caused (Fig. 8a) a new signal that was assigned easily to isomer 14. The methyl protons at the end and middle of the capsule showed identical intensity, and the chemical shift of the signal of the DCE placed it at the end. Gradually, addition of more DCE provided a new doublet signal around -3.0 ppm, further upfield-shifted than the signals of isomers 13 and 14. That signal was presumed to be isomer 15, because no new signals appeared around -1 and 0 ppm. The 1D GOESY spectrum with irradiated free DCE showed the exchange of the -1-ppm signal (isomer 14) and also the 2.1-ppm signal resulting from the DCE in the middle of the capsule. This confirmed that the new doublet was caused by the isomer 15. Further addition of DCE gave two more doublet signals (Fig. 8b). The upper field signal was isomer 16, and the lower field signal was isomer 17.
Fig. 8.
(Upper) Distributions of IPC and DCE in the capsule. (Lower) NMR spectra (600Mhz, 273k) of encapsulated guests in deuterated mesitylene. (Right) IPC at the end of the capsule. (Center) DCE at the end of the capsule. (Left) IPC in the middle of the capsule. (a) IPC (300 μl) and DCE (20 μl). (b) IPC (300 μl) and DCE (150 μl). (c) IPC (150 μl) and DCE (150 μl). (d) IPC (100 μl) and DCE (300 μl). (e) IPC (20 μl) and DCE (300 μl).
The titration in the reverse sense (Fig. 8 d and e) initially gave a doublet signal at -3 ppm and singlet at -1 ppm. Those were assigned to the isomer 16. Further addition of a few drops of IPC provided isomer 17. Unfortunately, the encapsulated DCE's signal of the isomer 14 at the end of the capsule overlapped with others (Fig. 8e). By increasing the amount of IPC, the ratio of the isomer 14 increased, and it was identified by the growing doublet corresponding to the isomer 14 at ≈0 ppm (Fig. 8d).
The spectrum of 1:1 mixture of the IPC and DCE is shown in Fig. 8c. From the titration studies, it was clear that the spectrum reflects the presence of all six isomers, including the two sets of constellational isomers. These are shown in Fig. 9.
Fig. 9.
Energy minimized structures of two sets of constellational isomers (14 ≈ 17) due to IPC and DCE encapsulation.
Summary and Outlook
The existence of these isomers results from the restricted translations of the guests in the narrow cylinder of the host and a cheerful promiscuity of the host for these specific solvents: all combinations of two guests seem to be energetically acceptable and provide a good fit. The use of only two guests in the capsule to demonstrate isomeric constellations here is binary in nature, and the symmetry of the capsule limits the number of possibilities to six. Three different guests offer 18 possibilities including a constellational triplet with one of each guest inside. We have already encountered such a system (the  ion sandwiched between CHCl3 and IPC) and will report it in due course. Given the difficulties in analysis discussed above, it is unlikely that NMR will be able to unravel all 18 combinations of such a system. Rather, alternative spectroscopies must be recruited. There are additional possibilities for increasing the capacity with the use of polymeric capsules (11) and single-walled nanotubes. Inside the latter, the dimensions of substituted adamantanes should prevent their exchange of positions, and there are enough mono-, di-, and trisubstituted derivatives to map onto a typical computer keyboard. In short, writing at <1 nanometer per character would be possible. The problems of control, maintenance, and retrieval must be solved in step with the improvements in instrumentation to realize storage of data on the subnanometric scale. At present, we note that the three CHCl3 guests in the capsule remain inside after the solvent is evaporated and the complex is heated in vacuo overnight. This augurs well for the maintenance of a given constellational isomer in the solid state. Perhaps transmission electron microscopy will be able to scan through a single intact capsule or loaded nanotube to allow the retrieval of the information represented by the isomers and sequences within. The control of loading remains the most challenging feature for the use of these capsules for information storage.
ion sandwiched between CHCl3 and IPC) and will report it in due course. Given the difficulties in analysis discussed above, it is unlikely that NMR will be able to unravel all 18 combinations of such a system. Rather, alternative spectroscopies must be recruited. There are additional possibilities for increasing the capacity with the use of polymeric capsules (11) and single-walled nanotubes. Inside the latter, the dimensions of substituted adamantanes should prevent their exchange of positions, and there are enough mono-, di-, and trisubstituted derivatives to map onto a typical computer keyboard. In short, writing at <1 nanometer per character would be possible. The problems of control, maintenance, and retrieval must be solved in step with the improvements in instrumentation to realize storage of data on the subnanometric scale. At present, we note that the three CHCl3 guests in the capsule remain inside after the solvent is evaporated and the complex is heated in vacuo overnight. This augurs well for the maintenance of a given constellational isomer in the solid state. Perhaps transmission electron microscopy will be able to scan through a single intact capsule or loaded nanotube to allow the retrieval of the information represented by the isomers and sequences within. The control of loading remains the most challenging feature for the use of these capsules for information storage.
Acknowledgments
We thank the Skaggs Institute for Chemical Biology and the National Institutes of Health (Grant GM50174) for financial support. M.Y. thanks the Japan Society for the Promotion of Science Research Fellowships for Young Scientists. A.S. is a Skaggs Postdoctoral Fellow.
Abbreviations: DCE, 1,2-dichloroethane; IPC, isopropyl chloride; GOESY, gradient-enhanced nuclear Overhauser effect spectroscopy.
References
- 1.Timmerman, P., Verboom, W., van Veggel, F. C. J. M., van Duynhoven, J. P. M. & Reinhoudt, D. N. (1994) Angew. Chem. Int. Ed. Engl. 33, 2345-2348. [Google Scholar]
- 2.Chambron, J.-C., Sauvage, J.-P. & Mislow, K. (1997) J. Am. Chem. Soc. 119, 9558-9559 [Google Scholar]
- 3.Raymo, F. M., Houk, K. N. & Stoddart, J. F. (1998) J. Org. Chem. 63, 6523-6528. [Google Scholar]
- 4.Anelli, P. L., Spencer, N. & Stoddart, J. F. (1991) J. Am. Chem. Soc. 113, 5131-5133 [DOI] [PubMed] [Google Scholar]
- 5.Chiu, S.-H., Elizarov, A. M., Glink, P. T. & Stoddart, J. F. (2002) Org. Lett. 4, 3561-3564. [DOI] [PubMed] [Google Scholar]
- 6.Shivanyuk, A. & Rebek, J., Jr. (2002) J. Am. Chem. Soc. 124, 12074-12075. [DOI] [PubMed] [Google Scholar]
- 7.Shivanyuk, A. & Rebek, J., Jr. (2003) Angew. Chem. Int. Ed. Engl. 42, 684-686. [DOI] [PubMed] [Google Scholar]
- 8.Shivanyuk, A. & Rebek, J., Jr. (2002) Chem. Commun., 2326-2327. [DOI] [PubMed]
- 9.Stonehouse, J., Adell, P., Keeler, J. & Shaka, A. J. (1994) J. Am. Chem. Soc. 116, 6037-6038. [Google Scholar]
- 10.Mecozzi, S. & Rebek, J., Jr. (1998) Chem. Eur. J. 4, 1016-1022. [Google Scholar]
- 11.Castellano, R. K., Rudkevich, D. M. & Rebek, J., Jr. (1997) Proc. Natl. Acad. Sci. USA 94, 7132-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]