Abstract
Aldose reductase (AR) catalyzes the reduction of toxic lipid aldehydes to their alcohol products and mediates inflammatory signals triggered by lipopolysaccharide (LPS). Beta-glucogallin (BGG), a recently described AR inhibitor, was purified from extracts of the Indian gooseberry (Emblica officinalis). In this study, we found that BGG showed low cytotoxicity in Raw264.7 murine macrophages and effectively inhibited AR activity as measured by a decrease in sorbitol accumulation. In addition, BGG-mediated inhibition of AR prevented LPS-induced activation of JNK and p38 and lowered ROS levels, which could inhibit LPS-induced apoptosis. Uveitis is a disease of the eye associated with chronic inflammation. In this study, we also demonstrated that treatment with BGG decreased the number of inflammatory cells that infiltrate the ocular media of mice with experimental uveitis. Accordingly, these results suggest BGG is a potential therapy for inflammatory diseases.
Keywords: Aldose reductase, BGG, Macrophage, LPS, ROS, Uveitis
1. Introduction
The Indian gooseberry (Emblica officinalis), commonly known as Amla, is used in the practice of Indian traditional medicine (Ayurveda) to minimize the effects of diabetes and its complications. We previously showed that treatment of experimentally diabetic rats with crude extracts from Amla fruit delayed the onset and progression of cataracts and prevented the accumulation of sorbitol and diabetes-induced markers of lipid peroxidation and protein oxidation products in the lens [1,2]. Because these results were consistent with the effects of aldose reductase (AR) inhibition, we used a bioassay-guided scheme to search for putative AR inhibitors (ARI), using human AR (AKR1B1) activity as an assay read out. Fractionation of materials in an Amla extract resolved several compounds with ARI activity. Structure elucidation by 1H NMR identified the major inhibitor as 1-O-galloyl-beta-d-glucose, also known as Beta-glucogallin (BGG) [3]. Inhibition studies demonstrated an IC50 of approximately 17 µM against AKR1B1, and virtually no inhibition when assayed under similar conditions with the other major human AKR1 family members AKR1B10 (small intestine reductase, HSIR) and AKR1A1 (aldehyde reductase). In organ culture assays of lenses dissected from AKR1B1 transgenic mice, BGG decreased sorbitol accumulation by about 75% and protected against the loss of glutathione [3]. Ramana and others have shown that AR inhibition by sorbinil and a variety of other validated ARIs lowered the levels of inflammatory markers associated with exposure to lipopolysaccharide (LPS) endotoxins [4–6]. To determine whether BGG holds promise as an anti-inflammatory agent, we conducted studies to measure its toxicity toward mammalian cells in a tissue culture setting. Studies have also been carried out to explore the efficacy of BGG in prevention of lymphocyte infiltration into the anterior and posterior compartments of the mouse eye in the endotoxin-induced uveitis model. We further probed the ability of BGG to reduce the expression of some pro-inflammatory markers in macrophages following exposure to endotoxin. Our results demonstrate consistency with other validated ARIs and confirm that it has favorable bioavailability in the intact animal. The results of our studies substantiate the case for BGG as a potent and effective natural anti-inflammatory agent.
2. Materials and methods
2.1. Materials and cell culture
BGG was obtained and purified as previously reported [3]. LPS (Salmonella enterica serotype typhimurium) was purchased from Sigma–Aldrich (St. Louis, MO). Sorbinil ((4S)-6-Fluoro-2,3-dihydro-spiro[4H-1-benzopyran-4,4′-imidazolidine]-2′,5′-dione) was generously provided by Pfizer Central Research (Groton, CT). Raw264.7 murine macrophages were cultured in complete Dulbecco’s Modified Eagle Medium supplemented with 4 mM l-glutamine, 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin. Cells were maintained in a humidified incubator containing 5% carbon dioxide at 37 °C.
2.2. Cell viability assay
Cells (104 cells) were incubated in a 96-well plate and treated with Sorbinil, H2O2 and the indicated concentration of BGG. After 24 h treatment, the cell viability was determined by LIVE/DEAD® viability/cytotoxicity Kit (Invitrogen, Carlsbad, CA) as previously described [7]. Live cells were quantified by determining the Calcein fluorescence (F528) collected at 528 ± 20 nm from excitation at 485 ± 20 nm using a BioTek Synergy™ 4 Hybrid Microplate Reader (Bio Tek, Winooski, VT). Fluorescent data were analyzed as outlined in the manufacturer’s instructions.
2.3. Sorbitol colorimetric assay
Cells (107 cells) were incubated in T75 flasks. After treatment with ARIs and LPS, cells were collected and washed with cold PBS twice. The cell lysates were deproteinized with Deproteinizing Sample Preparation Kit (BioVision, Milpitas, CA). Sorbitol in neutralized samples was measured using a d-Sorbitol Colorimetric Assay Kit as described by the manufacturer (BioVision).
2.4. Western blotting
Lysates were prepared by suspending cells in Laemmli sample buffer (Sigma–Aldrich) and heating to 100 °C for 10 min. After a brief centrifugation, materials were resolved by SDS–PAGE (Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The following primary antibodies were used for immunodetection: rabbit antimouse p-JNK, p-p38, JNK and p38 (Cell Signaling Technology, Inc., Danvers, MA). Secondary antibodies conjugated to horseradish peroxidase (Millipore, Bedford, MA) and the Western Blot Substrate kit (Bio-Rad Laboratories) were used to detect chemiluminescence using a BioRad ChemiDoc™ XRS + imaging system.
2.5. Detection of ROS levels
Cells (104 cells) were incubated in a 96-well plate without or with Sorbinil or BGG and treated with the ROS-sensitive dye fluorophore 2′,7′-dichlorofluorescein diacetate (Sigma) for 30 min. Subsequently, the cells were exposed to LPS for 60 min and the fluorescence was measured with a BioTek Synergy™ 4 Hybrid Microplate Reader at excitation of 485 nm and emission of 528 nm.
2.6. Uveitis
Experimental uveitis was induced in male C57BL/6 mice with a single intraperitoneal injection of lipopolysaccharide, essentially as described by Tuo et al. [8]. Animals were randomly assigned to different treatment groups (in all cases, N = 3) including a control group receiving PBS, and sorbinil and BGG groups treated at 10 mg/kg. Treatments were carried out by intraperitoneal injections of 0.1 ml solution immediately after the LPS treatment. Mice were euthanized 24 h later and eyes fixed in formalin and embedded with paraffin. Tissue slices were mounted on coded glass slides, stained with hematoxylin–eosin and used to manually count inflammatory cells in the vitreous cavity and anterior chamber. Cells were counted with the observer masked to the treatment group.
2.7. Statistical analysis
Results are shown as the Means ± SEM of at least three experiments. Data were analyzed by Student’s t test with P value of <0.05 considered significant.
3. Results
3.1. BGG exhibits low cytotoxicity in Raw264.7 murine macrophages
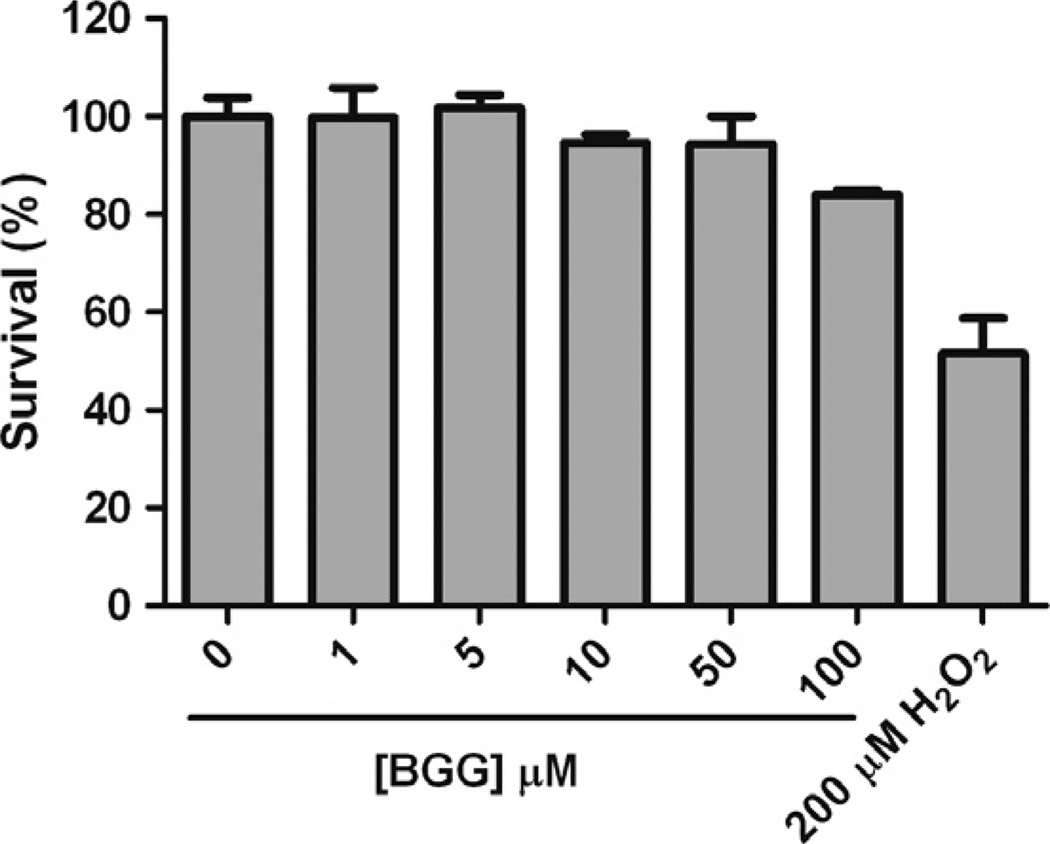
Although BGG has been implicated as a therapeutic agent against diabetic cataract [3], its cytotoxicity has not been assessed. In this study, we examined the cytotoxicity of BGG in Raw264.7 murine macrophages. After 24 h treatment, we found there is virtually no cytotoxicity with BGG up to a concentration of 50 µM, whereas some slight toxicity was apparent when cells were incubated in the presence of 100 µM BGG (84 ± 1.6% survival). In contrast, 200 µM H2O2 (positive control) shows 50% cytotoxicity (Fig. 1). The relatively low cytotoxicity of BGG suggests it may be tolerated over a broad range of doses.
Fig. 1.
The effect of BGG on the viability of Raw264.7 murine macrophages. Macrophages were treated with various concentrations of BGG for 24 h as described under Materials and Methods. As a positive control for toxicity, cells were incubated with 200 µM H2O2. Cell viability was determined by the Calcein Live assay. Data shown are means ± SEM (N = 3).
3.2. Aldose reductase inhibitors decrease sorbitol levels in macrophages
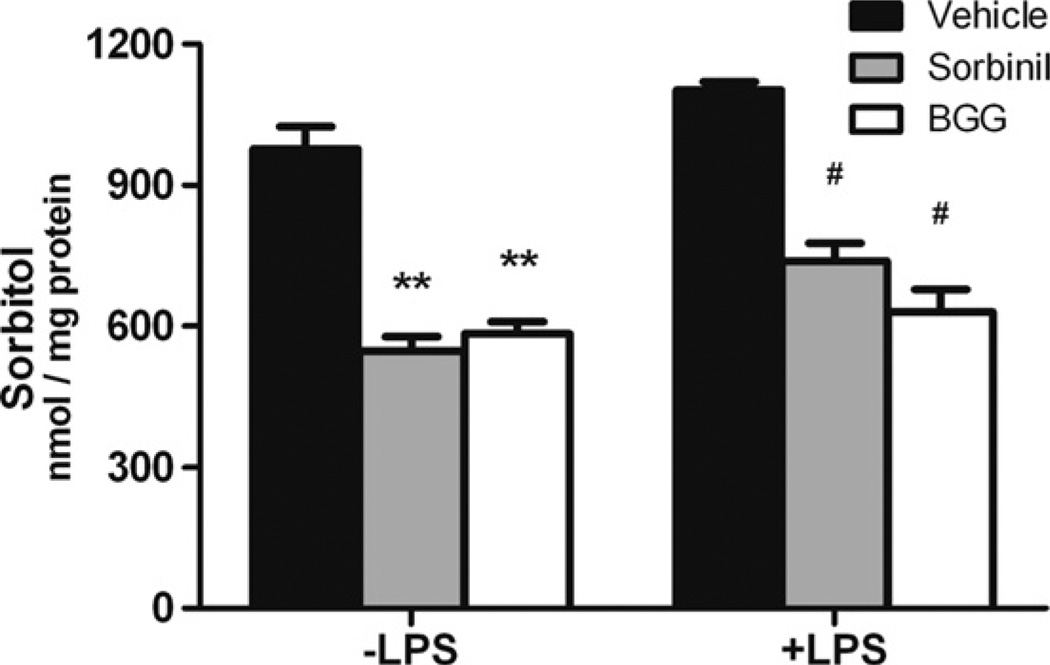
To determine the AR inhibition efficiency in macrophages, we measured the amount of sorbitol, which is produced by AR from glucose, after treatment of cells with BGG. For comparison, we also measured sorbitol levels in cells incubated with Sorbinil, a well-characterized ARI shown previously to diminish sorbitol accumulation in cell culture models and animal tissues [3,9]. We found that Sorbinil or BGG treatment decreased sorbitol levels by 44% or 40% compared to vehicle in macrophages without LPS exposure, respectively (Fig. 2). In addition, LPS induced a 13% increase in sorbitol levels as compared to cells not exposed to LPS (vehicle group). In the LPS group, Sorbinil or BGG treatment reduced sorbitol levels by 33% or 43%, respectively, as compared to vehicle (Fig. 2). These results are consistent with a role for BGG and Sorbinil in reduction of sorbitol accumulation by inhibition of AR in macrophages.
Fig. 2.
The effect of aldose reductase inhibitors on regulation of sorbitol level in macrophages. Macrophages were incubated with LPS (100 ng/ml) for 12 h following 24 h pretreatment with either Sorbinil (10 µM) or BGG (50 µM). ARI-supplemented culture medium was added fresh every 12 h. The sorbitol level in the macrophage cell lysates was measured using a sorbitol colorimetric assay. The amount of sorbitol in cell lysates was normalized to total protein. *Represents the statistical significance compared to vehicle of the no LPS group. #Represents the statistical significance compared to vehicle of the LPS group. Data shown are means ± SEM (N = 3). #P < 0.05; **P < 0.01.
3.3. BGG reduces LPS-induced activation of JNK and p38
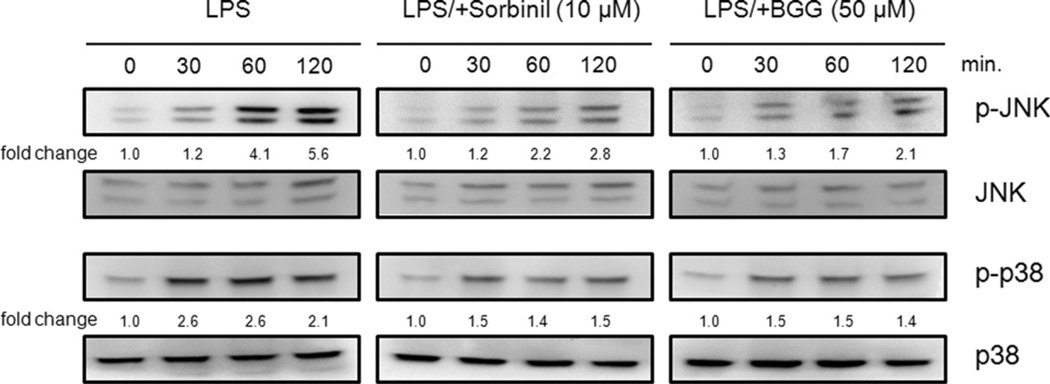
LPS has been shown to induce JNK and p38 activation [6]. Therefore, we investigated whether blockade of AR could have an impact on activation of these kinases in response to LPS exposure. As shown in Fig. 3, BGG treatment attenuated the LPS-induced activation of JNK and p38. Consistent with previous reports [6], a similar pattern was observed when LPS-exposed cells were pretreated with Sorbinil (Fig. 3). Therefore, inhibition of AR with BGG has similar effects to Sorbinil with regard to lowering JNK and p38 activation in response to LPS stimulation of macrophages.
Fig. 3.
The effect of aldose reductase inhibition on LPS-induced activation of p38 and JNK in Raw264.7 murine macrophages. Macrophages were pretreated with 10 µM Sorbinil or 50 µM BGG for 24 h followed by LPS (20 ng/ml) for the indicated times. The fold activation of p-JNK and p-p38 were normalized to total JNK and p38, respectively. For changes over time, the normalized values are expressed relative to samples taken at time zero.
3.4. BGG prevents LPS-induced oxidative stress
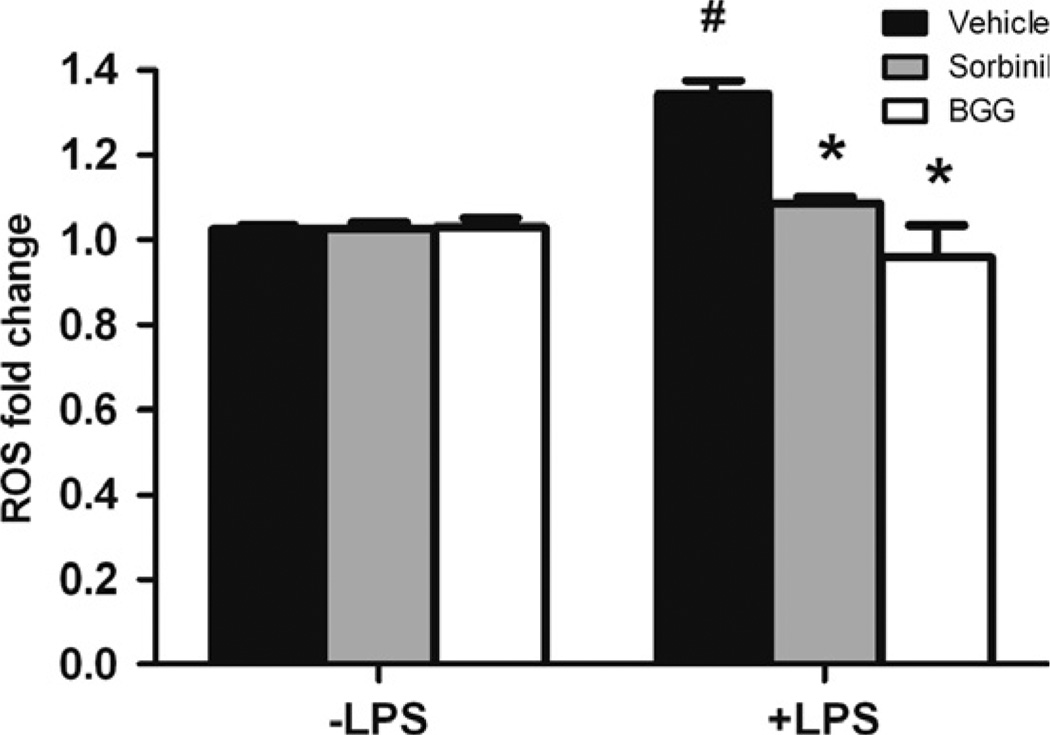
Increased production of ROS is implicated as a major cause of LPS-induced cytotoxicity [10,11]. As shown in Fig. 4, LPS exposure caused an increase in ROS levels as measured by the ROS-sensitive dye fluorophore 2′,7′-dichlorofluorescein diacetate. Pretreatment of cells with either BGG or Sorbinil resulted in a significant lowering of ROS production following LPS stimulation. Lowering of oxidative stress would be expected to curtail the activation of redox-sensitive transcription factors NF-κB required for increased expression of genes for pro-inflammatory cytokines such as TNF-α and IL-1β.
Fig. 4.
The effect of aldose reductase inhibition on LPS-induced oxidative stress in Raw264.7 murine macrophages. Macrophages were pretreated with 10 µM Sorbinil or 50 µM BGG for 24 h followed by stimulation with LPS (1 µg/ml) for 60 min. The level of ROS was measured fluorometrically and the fold change was compared to control. *Represents the statistical significance compared to vehicle of LPS group. #Represents the statistical significance compared to vehicle between no LPS and LPS groups. Data shown are means ± SEM (N = 3). *P < 0.05; #P < 0.05.
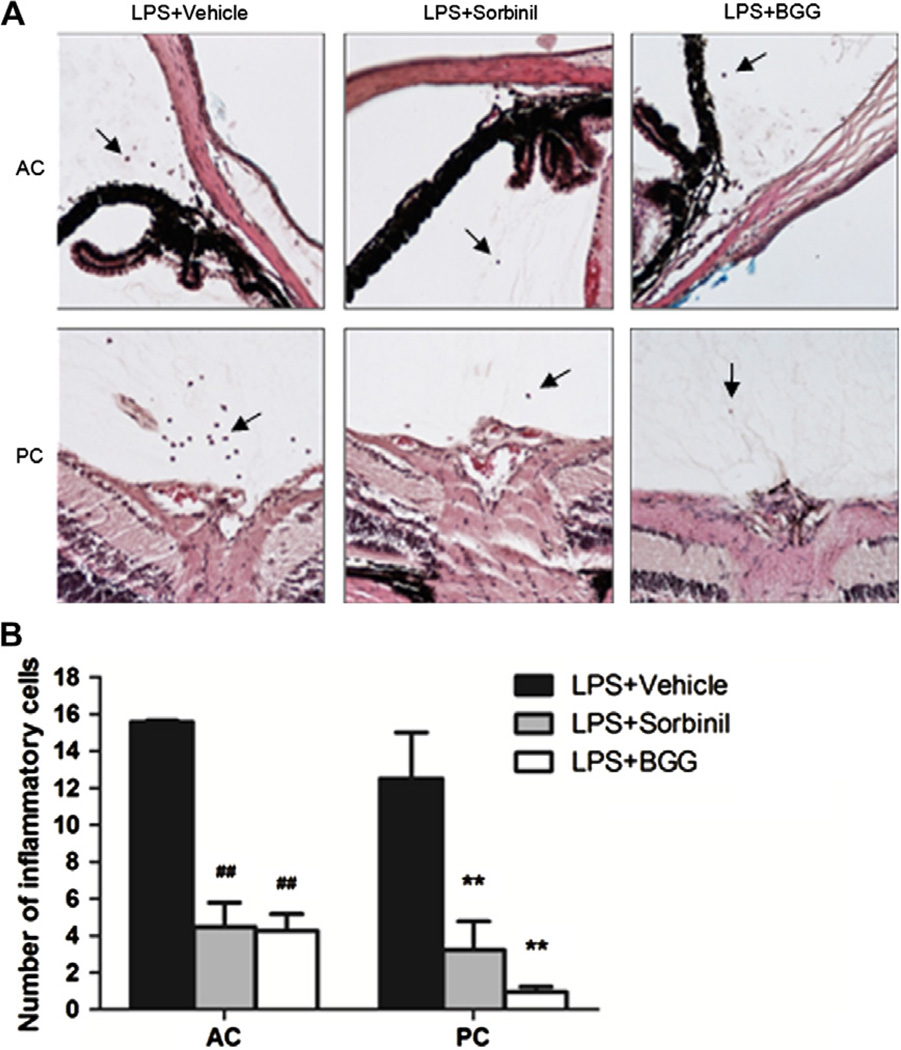
3.5. BGG attenuates LPS-induced uveitis in mice eyes
Treatment of mice with a single intraperitoneal injection of LPS induced a marked response in the anterior (aqueous) and posterior (vitreous) chambers of the eye, as measured by the infiltration of inflammatory cells (Fig. 5). We rarely observed inflammatory cells in either compartment of the eye in animals not treated with LPS (not shown). Administration of sorbinil or BGG to animals dramatically reduced the number of infiltrating cells infiltrates in this model.
Fig. 5.
The effect of BGG on LPS-induced uveitis in mice. (A) Inflammatory cells were stained in serial histological sections of the mouse eye as described in Materials and Methods. Cell counts were tabulated according to the anatomical location in the anterior (or aqueous) chamber (AC) or posterior (or vitreous) chamber (PC). The inflammatory cells are indicated by arrows. (B) The number of inflammatory cells is displayed as a bar chart. #Represents the statistical significance compared to vehicle of the AC group. *Represents the statistical significance compared to vehicle of the PC group. Data shown are means ± SEM (N = 3). ##P < 0.01; **P < 0.01.
4. Discussion
The plant E. officinalis, also known as Amla or in Western culture as the Indian gooseberry, is a traditional Indian medicine used for the treatment diabetes [12,13]. Extracts from fruits of E. officinalis have been shown to delay streptozotocin-induced diabetic cataract in rats [2]. It has also been shown that there is an increase of AR activity in streptozotocin-induced diabetes [14]. Multiple studies showed that AR inhibition is effective in the prevention of diabetic complications [15–17]. Recently, we isolated BGG from E. officinalis, characterized it as a potent inhibitor of AR, and showed this inhibition to be selective for AR when compared to other aldo–keto reductases [3]. In the present study, we sought to evaluate the efficacy of BGG in cell culture and animal models. This allowed us to determine if the inhibitory properties predicted from isolated enzyme measurements could be validated in living cells.
Because inflammation has been known to be a crucial feature in the pathogenesis of some diabetic complications [18], we sought to evaluate the efficacy of BGG in several different inflammation models. Ramana and Srivastava and their coworkers have shown that ARIs such as sorbinil and zopolrestat are capable of reducing LPS-induced inflammation and cytotoxicity in macrophages [4–6]. These ARIs also lowered the expression of inflammatory markers associated with LPS-induced uveitis in rats [19]. The purpose of our study was to extend these observations to include BGG as representative of a new class of ARI that has never been studied in this context of inflammation.
Exposure of macrophages to LPS leads to an increase in AR activity, and this can be largely prevented by ARIs [5]. In our study, we measured sorbitol accumulation as an index of AR activity in macrophages. Our results showed that BGG inhibited AR activity as evidenced by the reduction of sorbitol accumulation. Treatment of macrophage cultures with BGG also resulted in the attenuation of LPS-induced ROS activation, thus providing a possible mechanistic explanation for the anti-inflammatory effects of BGG.
In addition to effects on production of ROS, we explored the effect of BGG on mitogen-activated protein kinases such as ERK and p38, which become activated during inflammation [20]. In the present study, we showed that BGG inhibited the LPS-induced activation of p38, suggesting a possible mechanism by which AR inhibition reduces inflammation. Pandey et al. have hypothesized that blockade of AR helps to reduce the production of ROS and associated oxidative stress, which are important components that drive the pro-inflammatory response following exposure to cells to LPS [21]. Our results showing the BGG-mediated reduction in ROS levels are in complete agreement with this hypothesis. The low toxicity associated with BGG exposure to mammalian cells, together with the favorable cell penetration capabilities, suggest that it may find potential use as an anti-inflammatory agent.
Acknowledgements
This study is supported by NIH grants EY005856 (JMP) and EY021498 (JMP, DVL). We thank Dr. Cynthia Ju (Skaggs School of Pharmacy and Pharmaceutical Science, University of Colorado) for Raw264.7 murine macrophages. We also acknowledge Skaggs School of Pharmacy core facilities for NMR (Dr. Michael Wempe) and Mass Spectral (Joe Gomez) data acquisition, and the CCTSI for their support of these core facilities. AE was supported by a Collaborative Research Fellowship awarded by the ARVO Foundation for Eye Research (Bethesda, MD).
Abbreviations
- AR
aldose reductas
- ARI
aldose reductase inhibitor
- LPS
lipopolysaccharide
- BGG
beta-glucogallin
- ROS
reactive oxygen species
- DR
diabetic retinopathy
Footnotes
Conflict of interest statement
Portions of this work are disclosed in a patent application submitted by DVL and JMP. The other authors declare that there are no conflicts of interest.
References
- 1.Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB. Inhibition of aldose reductase by tannoid principles of Emblica officinalis: implications for the prevention of sugar cataract. Mol. Vis. 2004;10:148–154. [PubMed] [Google Scholar]
- 2.Suryanarayana P, Saraswat M, Petrash JM, Reddy GB. Emblica officinalis and its enriched tannoids delay streptozotocin-induced diabetic cataract in rats. Mol. Vis. 2007;13:1291–1297. [PubMed] [Google Scholar]
- 3.Puppala M, Ponder J, Suryanarayana P, Reddy GB, Petrash JM, Labarbera DV. The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS One. 2012;7:e31399. doi: 10.1371/journal.pone.0031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J. Biol. Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 5.Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radical Bio. Med. 2007;42:1290–1302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammar DA, Kahook MY. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol. Vis. 2011;17:1806–1813. [PMC free article] [PubMed] [Google Scholar]
- 8.Tuo J, Tuaillon N, Shen D, Chan CC. Endotoxin-induced uveitis in cyclooxygenase-2-deficient mice. Invest. Ophthalmol. Vis. Sci. 2004;45:2306–2313. doi: 10.1167/iovs.03-0756. [DOI] [PubMed] [Google Scholar]
- 9.Wu LY, et al. The anti-necrosis role of hypoxic preconditioning after acute anoxia is mediated by aldose reductase and sorbitol pathway in PC12 cells. Cell Stress Chaperones. 2010;15:387–394. doi: 10.1007/s12192-009-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BH, Cho SM, Reddy AM, Kim YS, Min KR, Kim Y. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem. Pharmacol. 2005;69:1577–1583. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 12.Akhtar MS, Ramzan A, Ali A, Ahmad M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food. Sci. Nutr. 2011;62:609–616. doi: 10.3109/09637486.2011.560565. [DOI] [PubMed] [Google Scholar]
- 13.Chen TS, Liou SY, Wu HC, Tsai FJ, Tsai CH, Huang CY, Chang YL. Efficacy of epigallocatechin-3-gallate and Amla (Emblica officinalis) extract for the treatment of diabetic-uremic patients. J. Med. Food. 2011;14:718–723. doi: 10.1089/jmf.2010.1195. [DOI] [PubMed] [Google Scholar]
- 14.Ghahary A, Luo JM, Gong YW, Chakrabarti S, Sima AA, Murphy LJ. Increased renal aldose reductase activity, immunoreactivity, and mRNA in streptozocin-induced diabetic rats. Diabetes. 1989;38:1067–1071. doi: 10.2337/diab.38.8.1067. [DOI] [PubMed] [Google Scholar]
- 15.Banditelli S, et al. A new approach against sugar cataract through aldose reductase inhibitors. Exp. Eye Res. 1999;69:533–538. doi: 10.1006/exer.1999.0729. [DOI] [PubMed] [Google Scholar]
- 16.Kador PF, Robison WG, Jr, Kinoshita JH. The pharmacology of aldose reductase inhibitors. Annu. Rev. Pharmacol. Toxicol. 1985;25:691–714. doi: 10.1146/annurev.pa.25.040185.003355. [DOI] [PubMed] [Google Scholar]
- 17.Sestanj K, Bellini F, Fung S, Abraham N, Treasurywala A, Humber L, Simard-Duquesne N, Dvornik D. N-[5-(Trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]-N-methylglycine (Tolrestat), a potent, orally active aldose reductase inhibitor. J. Med. Chem. 1984;27:255–256. doi: 10.1021/jm00369a003. [DOI] [PubMed] [Google Scholar]
- 18.Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Drager AM, Doni A, van Hinsbergh VW, Stehouwer CD. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia. 1999;42:351–357. doi: 10.1007/s001250051162. [DOI] [PubMed] [Google Scholar]
- 19.Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim AS, et al. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey S, Srivastava SK, Ramana KV. A potential therapeutic role for aldose reductase inhibitors in the treatment of endotoxin-related inflammatory diseases. Expert Opin. Invest. Drugs. 2012;21:329–339. doi: 10.1517/13543784.2012.656198. [DOI] [PMC free article] [PubMed] [Google Scholar]