Abstract
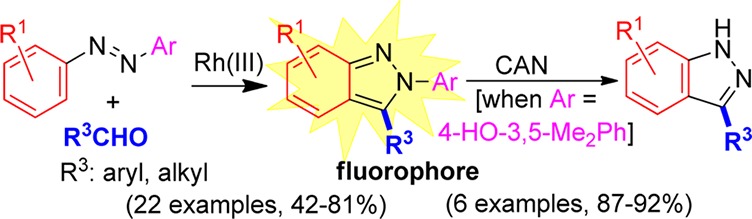
An efficient, one-step, and highly functional group-compatible synthesis of substituted N-aryl-2H-indazoles is reported via the rhodium(III)-catalyzed C–H bond addition of azobenzenes to aldehydes. The regioselective coupling of unsymmetrical azobenzenes was further demonstrated and led to the development of a new removable aryl group that allows for the preparation of indazoles without N-substitution. The 2-aryl-2H-indazole products also represent a new class of readily prepared fluorophores for which initial spectroscopic characterization has been performed.
Rhodium(III)-catalyzed addition of sp2-hybridized C–H bonds to polarized π-bonds, including those present in aldehydes,1 imines,2 isocyanates,3 aziridines,4 carbon monoxide,5 and isonitriles,6 has proven to be a versatile and highly functional group-compatible synthetic approach.7 Aldehydes are particularly attractive inputs because a vast number of derivatives are commercially available. However, the addition of C–H bonds to aldehydes is often reversible with the alcohol addition products typically favored when highly electron-deficient aldehydes are used.1c,1d,1h We have previously demonstrated that this inherent challenge can be overcome by cyclative capture of the hydroxyl group to obtain valuable heterocycle products (Figure 1).1a,1b,8 Herein, we introduce a new cyclative capture approach where the initially generated hydroxyl serves as a leaving group rather than a nucleophile to afford pharmaceutically important N-aryl-2H-indazoles by formal [4+1] annulation (Figure 1).9,10 Substituents on unsymmetrical azobenzenes that effect a high level of regiocontrol have been established to significantly enhance the scope of the method. Moreover, by capitalizing on substituent control of regioselectivity, a readily cleavable 2-aryl substituent has been developed that provides straightforward access to indazoles lacking N-substitution. Finally, we have determined that appropriately substituted 2-aryl-2H-indazoles represent a new class of fluorophores that may find utility as fluorescent probes for biological study or as specialized materials. Initial spectroscopic characterization of representative derivatives has therefore been performed.
Figure 1.
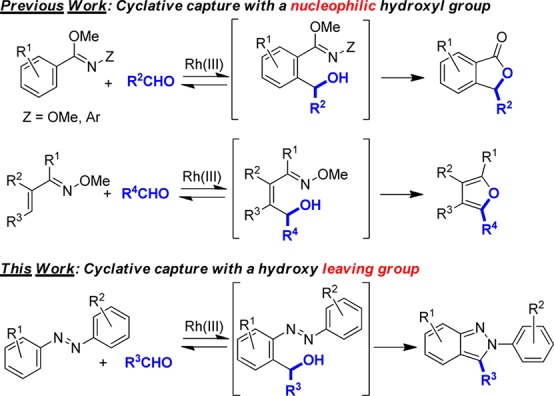
Two distinct approaches for cyclative capture.
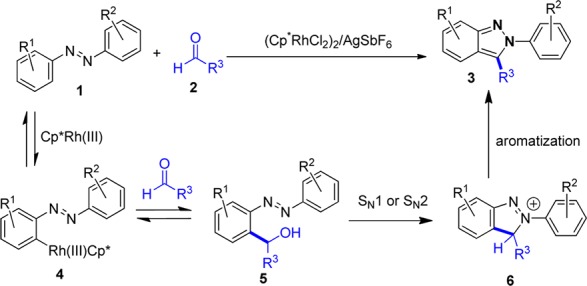
Indazole is recognized as a privileged structure in the pharmaceutical industry with multiple drugs and drug candidates in clinical trials incorporating this pharmacophore.11 The more difficult to prepare 2-substituted 2H-indazole class has in particular begun to attract the increased attention of the synthetic community due to the promise of drug candidates that contain this motif.12,13 We envisioned that convergent entry to 2-substituted 2H-indazoles 3 should be possible by Rh(III)-catalyzed addition of azobenzenes 1 to aldehydes 2 (Scheme 1). We rationalized that the azo functional group would direct ortho C–H bond activation, followed by the reversible addition to aldehyde 2 to provide alcohol 5. Cyclative capture by an intramolecular nucleophilic substitution to give 6 followed by rapid aromatization should provide the desired 2H-indazole 3.
Scheme 1. Proposed Reaction Pathway for 2H-Indazole Synthesis.
An enormous number of azobenzenes have been prepared due to their many applications in the chemical industry,14 and it is therefore not surprising that the cyclometalation of azobenzene was one of first examples of this type of transformation.15 Despite this, there have been few reports on the catalytic C–H functionalization of azobenzenes,16 and none have relied on Rh(III) catalysis. Moreover, except for one substrate example,16c the regioselective functionalization of unsymmetrical azobenzenes is unexplored.
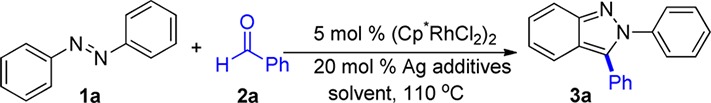
We first chose to evaluate azobenzene 1a and benzaldehyde 2a as model substrates and surveyed conditions that had previously been determined to be optimal for Rh(III) catalyzed additions to aldehydes (Table 1).1a The use of 5 mol% of [Cp*RhCl2]2 and 20 mol% of AgSbF6 in DCE afforded the desired product 3a in 46% yield (entry 1). The preformed cationic rhodium catalyst [Cp*Rh(CH3CN)3(SbF6)2] is often effective for aldehyde additions,1c,1g but for this substrate combination this catalyst completely shut down the reaction (entry 2). Because solvent can play a key role in the reaction outcome,1b a variety of solvents were screened. THF maintained the same yield as DCE (entry 3), HOAc improved the yield to 64% (entry 4), and dioxane proved to be the most effective, affording the product in 81% yield (entry 5). Replacing AgSbF6 with other silver salts reduced the yield of product (entries 6–8). Rationalizing the change in reaction yield upon variation of the counterion and solvent is complicated by the potential for differential effects upon C–H bond-functionalization and cyclization. No reaction was observed when either [Cp*RhCl2]2 or AgSbF6 alone was used (entries 9 and 10), which suggests that a cationic Rh(III) species is required for this C–H bond functionalization process.
Table 1. Optimization of Reaction Conditionsa.
| entry | solvent | Ag salt | yield (external)b |
|---|---|---|---|
| 1 | DCE | AgSbF6 | 46 |
| 2 | DCE | none | tracec |
| 3 | THF | AgSbF6 | 46 |
| 4 | HOAc | AgSbF6 | 64 |
| 5 | dioxane | AgSbF6 | 81 |
| 6 | dioxane | AgBF4 | 52 |
| 7 | dioxane | AgPF6 | 15 |
| 8 | dioxane | AgBC24F20 | 40 |
| 9 | dioxane | none | 0 |
| 10 | dioxane | AgSbF6 | 0d |
Conditions: 1 (0.10 mmol), 2 (0.20 mmol) in 0.5 mL of solvent for 24 h.
Determined by 1H NMR relative to 2,6-dimethoxytoluene as external standard.
In place of (Cp*RhCl2)2, Cp*Rh(CH3CN)3(SbF6)2 was used.
No (Cp*RhCl2)2 was added.
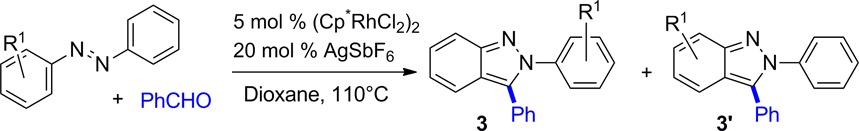
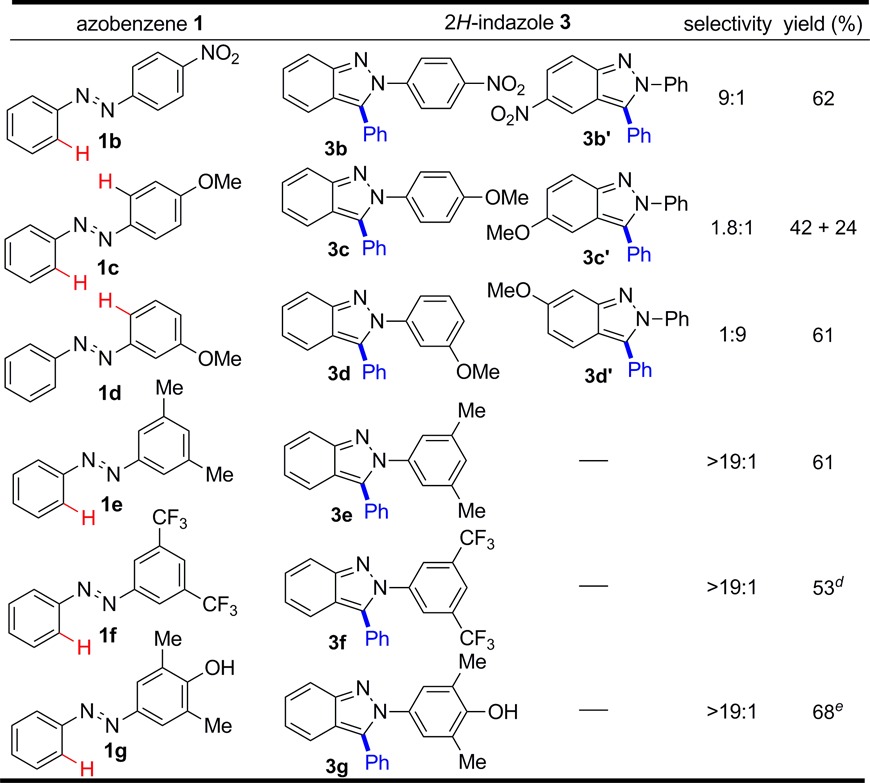
Having defined an effective catalyst and reaction conditions for the synthesis of 2H-indazole 3a, we next explored substrate scope for unsymmetrical azobenzenes. The 4-nitro-substituted azobenzene 1b led to the indazole products in 62% combined yield with 9:1 regioselectivity favoring 3b with C–H functionalization of the more electron-rich phenyl ring (Table 2). In contrast, the 4-methoxy-substituted azobenzene 1c generated the regioisomeric products 3c and 3c′ with low selectivity. The major product 3c was obtained by C–H functionalization on the less electron-rich phenyl ring of the azobenzene, which is opposite to the selectivity observed with the 4-nitro derivative 1b. For 3c′ the methoxy group is meta to the site of reaction and therefore contributes an inductive effect but does not provide resonance stabilization. The inductive effect could either slow the rate of C–H functionalization or affect the rate of cyclative capture of the reversibly formed alcohol addition product. To test this assumption, the 3-methoxy substitution pattern in 1d with the methoxy group capable of providing resonance stabilization was evaluated. As expected, the reaction favored 3d′ with a 9:1 regioselectivity for C–H functionalization occurring on the more electron-rich aromatic ring. The exclusive functionalization of azobenzene 1d para rather than ortho to the methoxy group suggests that steric effects are important. For this reason, azobenzenes 1e and 1f, which are 3,5-disubstituted with electron-rich methyl or electron-deficient CF3 groups, respectively, were evaluated, and each exclusively reacted at the less hindered ring to give indazoles 3e and 3f as single regioisomers.
Table 2. Electronic and Steric Effects on 2H-Indazole Regioselectivitya,b,c.
Conditions: azobenzene (0.20 mmol), benzaldehyde (0.40 mmol) in 1.0 mL of dioxane for 24 h.
Isolated yield.
Ratio was determined by crude 1H NMR.
Reaction was conducted in 0.4 mL of dioxane.
Reaction was conducted in 1.0 mL of THF with 100 mg of MgSO4.
The overriding importance of steric effects upon regioselectivity led to the design of azobenzene derivative 1g. This approach provides 2-aryl-2H-indazoles with an electron-rich aryl substituent that can readily be cleaved by straightforward oxidative methods to provide indazole products lacking substitution on nitrogen. Specifically, coupling azobenzene 1g with benzaldehyde smoothly afforded indazole 3g as a single regioisomer in good yield. For this hydroxy-substituted azobenzene, addition of MgSO4 as a drying agent was found to modestly improve the reaction yield. Moreover, THF provided a higher yield than dioxane, 68% vs 48%, respectively.
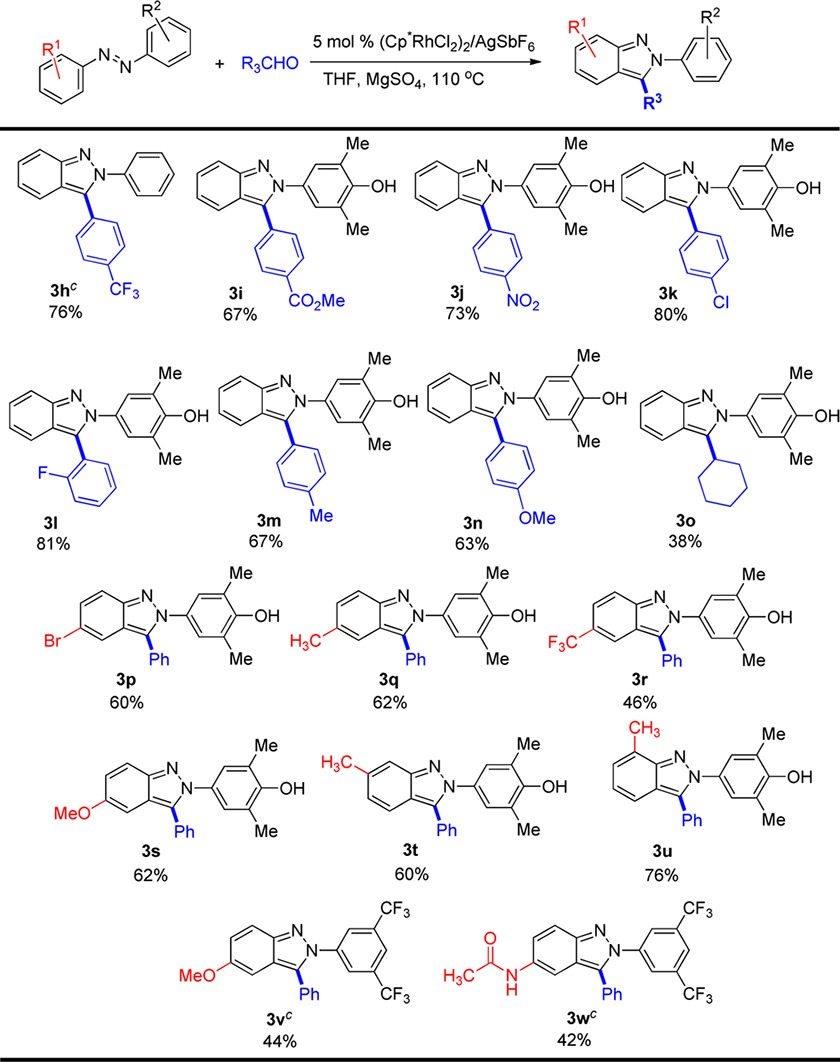
With an understanding of how electronic and steric effects control regioselectivity, we next explored substrate scope with a diverse set of azobenzenes 1 and aldehydes 2 (Table 3). We primarily focused on reactions of 4-hydroxy-3,5-dimethylphenyl derivatives of 1 because this group can be oxidatively removed from the 2H-indazole products 3 to provide the corresponding indazoles lacking N-substitution (vide infra). Both electron-poor and electron-rich aldehydes afforded the indazole products (3h–3n) in comparable yields, and ortho-substituents (3l) were also well tolerated. However, extension of the reaction to an aliphatic aldehyde (3o) resulted in a lower yield.
Table 3. Substrate Scopea,b.
Conditions: azobenzene (0.20 mmol), aldehyde (0.40 mmol), 100 mg of MgSO4 in 1.0 mL of THF for 24 h.
Isolated yield.
Reaction was conducted in 0.4 mL of dioxane.
The reaction is sensitive to electronic effects introduced by substitution on azobenzenes 1. Electron rich and neutral substituents led to indazole products in good yields (3p, 3q and 3s to 3u), while the electron-deficient CF3 group decreased the yield to 46% (3r). Consistent with the regiochemistry observed for m-methoxy substitution in 1d, m-methyl substitution exclusively afforded the 2H-indazole functionalized at the less hindered C–H site (3t). It is also significant that even for an ortho-substituted azobenzene, the indazole product was obtained in high yield (3u). Azobenzenes 3v and 3w were prepared to evaluate substituent effects on fluorescence (vide infra), and were obtained in somewhat lower yield due to the electron-deficient 3,5-bis-trifluoromethyl groups (see 3v vs 3s). Excellent functional group compatibility was observed with bromo (3p), chloro (3k), fluoro (3l), ester (3i), nitro (3j), methyl (3m), methoxy (3n), acetamido (3w), trifluoromethyl (3h), and phenol groups (3i–3u) all tolerated for this one step 2H-indazole synthesis.
We hypothesized that indazoles lacking N-substitution should be accessible from those 2H-indazoles substituted with the N-4-hydroxy-3,5-dimethylphenyl group. Although oxidative cleavage of N-hydroxyphenyl groups from 2H-indazoles had not previously been reported, oxidation with ceric ammonium nitrate (CAN) in methanol provided the indazoles 7 in excellent yield (Table 4). Good functional group compatibility for the oxidation step was observed, with chloro, methyl, nitro, and methoxy groups all being well tolerated.
Table 4. Oxidative Cleavage of Phenol by CANa,b.
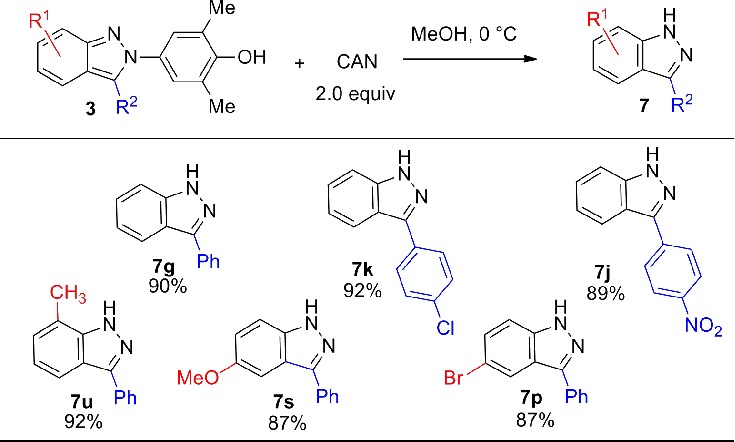
Conditions: azobenzene (0.10 mmol), CAN (0.20 mmol) in 4.0 mL of methanol at 0 °C for 10 min.
Isolated yield.
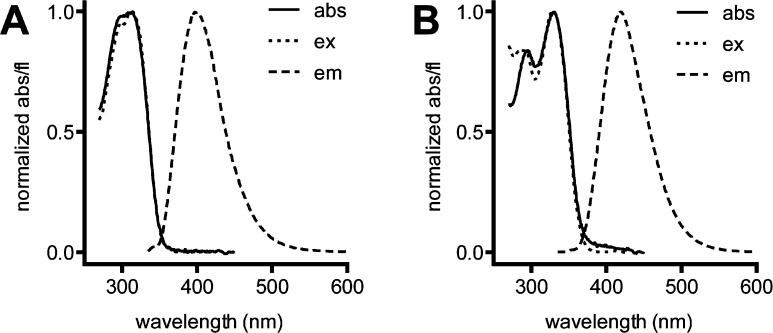
A number of the synthesized N-aryl-2H-indazoles were fluorescent. We therefore chose to characterize the fluorescence properties of representative derivatives (Table 5), in particular because our single-step synthesis strategy enables the preparation of a wide range of N-aryl 2H-indazoles with varied substitution patterns for facile optimization of fluorophore properties. The indazole fluorophores exhibited high extinction coefficients (∼104 M–1 cm–1) comparable to the widely used coumarin fluorophores.17 These novel dyes also showed large Stokes shifts (∼100 nm; Figure 2), which is an attractive property for biological probes. Electron-donating substituents on the 5-position of the indazole ring (3c′ and 3w, Figure 2B) and 3,5-bis-trifluoromethyl groups on the N-phenyl ring (3f) resulted in bathochromic shifts in emission wavelength and increases in quantum yield. However, with either the 4-hydroxy-3,5-dimethyl (3g) or 4-nitro (3b) substitution patterns on the N-phenyl group, minimal fluorescence was observed. Replacement of the 3-phenyl group with a 3-(4-trifluoromethylphenyl) group on the indazole had only a modest effect on the fluorescent properties (see 3a vs 3h). Substitution on both the indazole and the N-phenyl group (3v) did not prove cumulative but instead resulted in a compound exhibiting complex optical properties and modest quantum yield.
Table 5. Spectral Properties of Fluorophoresa.
| compd | λmax (nm) | λem (nm) | ε (M–1 cm–1) | Φ |
|---|---|---|---|---|
| 3a | 314 | 398 | 11700 | 0.08 |
| 3b | 344 | nd | 15600 | <0.01 |
| 3c | 308 | 419 | 14700 | 0.10 |
| 3c′ | 331 | 420 | 9800 | 0.26 |
| 3d′ | 303 | 412 | 16800 | 0.06 |
| 3f | 303 | 418 | 13400 | 0.17 |
| 3g | 309 | nd | 15300 | <0.01 |
| 3h | 317 | 408 | 12600 | 0.11 |
| 3w | 301 | 434 | 7700 | 0.14 |
| 3v | 309, 351 | 438 | 14300, 8900 | 0.08 |
Fluorescence measurements taken in 10 mM HEPES pH 7.3. Full spectra are given in the Supporting Information.
Figure 2.
Absorbance (abs), fluorescence excitation (ex), and fluorescence emission (em) spectra for (A) 3a and (B) 3c′.
In summary, a formal [4+1] annulation for the preparation of N-aryl-2H-indazoles from azobenzenes and aldehydes has been developed. The reaction is initiated by Rh(III)-catalyzed direct addition of an azobenzene C–H bond to an aldehyde with subsequent cyclization and aromatization. In addition to enabling the Rh(III)-catalyzed activation of ortho-C–H bonds, the azo moiety serves as a nucleophile to trap the initial aldehyde addition product. A broad range of aldehydes and azobenzenes participate in the reaction to provide access to a large variety of differently substituted indazoles. Regioselective functionalization of unsymmetrical azobenzenes can be controlled by either electronic or steric effects. The N-4-hydroxy-3,5-dimethylphenyl substituent can readily be oxidatively cleaved from 2H-indazoles to provide indazoles lacking N-substitution. Moreover, N-aryl-2H-indazoles represent a new class of fluorophores with high extinction coefficients and large Stokes shifts. The preparation and evaluation of 2H-indazoles with extended chromophores for near-infrared applications18 and with appropriate substitution for use as fluorogenic substrates19 are under active investigation.
Acknowledgments
This work was supported by NIH Grant GM069559 (to J.A.E.). R.G.B. acknowledges funding from The Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC02-05CH11231. L.D.L. is supported by the Howard Hughes Medical Institute.
Supporting Information Available
Experimental procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For Rh(III)-catalyzed direct C–H bonds addition to aldehydes, see:; a Lian Y.; Bergman R. B.; Ellman J. A. Chem. Sci. 2012, 3, 3088. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lian Y.; Huber T.; Hesp K. D.; Bergman R. G.; Ellman J. A. Angew. Chem., Int. Ed. 2013, 52, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yang L.; Correia C. A.; Li C.-J. Adv. Synth. Catal. 2011, 353, 1269. [Google Scholar]; d Li Y.; Zhang X.-S.; Chen K.; He K.-H.; Pan F.; Li B.-J.; Shi Z.-J. Org. Lett. 2012, 14, 636. [DOI] [PubMed] [Google Scholar]; e Park J.; Park E.; Kim A.; Lee Y.; Chi K. W.; Kwak J. H.; Jung Y. H.; Kim I. S. Org. Lett. 2011, 13, 4390. [DOI] [PubMed] [Google Scholar]; f Sharma S.; Park E.; Park J.; Kim I. S. Org. Lett. 2012, 14, 906. [DOI] [PubMed] [Google Scholar]; g Zhou B.; Yang Y.; Li Y. Chem. Commun. 2012, 48, 5163. [DOI] [PubMed] [Google Scholar]; h Shi X.-Y.; Li C.-J. Adv. Synth. Catal. 2012, 354, 2933. [Google Scholar]
- a Tsai A. S.; Tauchert M. E.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2011, 133, 1248. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li Y.; Li B.-J.; Wang W.-H.; Huang W.-P.; Zhang X.-S.; Chen K.; Shi Z.-J. Angew. Chem., Int. Ed. 2011, 50, 2115. [DOI] [PubMed] [Google Scholar]; c Tauchert M. E.; Incarvito C. D.; Rheingold A. L.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2012, 134, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Hesp K. D.; Bergman R. G.; Ellman J. A. Org. Lett. 2012, 14, 2304. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li Y.; Zhang X.-S.; Li H.; Wang W.-H.; Chen K.; Li B.-J.; Shi Z.-J. Chem. Sci. 2012, 3, 1634. [Google Scholar]; f Li Y.; Zhang X.-S.; Zhu Q.-L.; Shi Z.-J. Org. Lett. 2012, 14, 4498. [DOI] [PubMed] [Google Scholar]; g Zhou B.; Yang Y.-X.; Lin S.; Li Y.-C. Adv. Synth. Catal. 2013, 355, 360. [Google Scholar]
- a Hesp K. D.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2011, 133, 11430. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhou B.; Hou W.; Yang Y.-X.; Li Y.-C. Chem.—Eur. J. 2013, 19, 4701. [DOI] [PubMed] [Google Scholar]
- Li X.-W.; Yu S.-J.; Wan F.; Wan B.-S.; Yu X.-Z. Angew. Chem., Int. Ed. 2013, 52, 2577. [DOI] [PubMed] [Google Scholar]
- Du Y.; Hyster T. K.; Rovis T. Chem. Commun. 2011, 47, 12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.; Xie W.; Falck J, R. Chem.—Eur. J. 2011, 17, 12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews on Rh(III)-catalyzed C–H functionalization, see:; a Satoh T.; Miura M. Chem.—Eur. J. 2010, 16, 11212. [DOI] [PubMed] [Google Scholar]; b Wencel-Delord J.; Droge T.; Liu F.; Glorius F. Chem. Soc. Rev. 2011, 40, 4740. [DOI] [PubMed] [Google Scholar]; c Song G.; Wang F.; Li X. Chem. Soc. Rev. 2012, 41, 3651. [DOI] [PubMed] [Google Scholar]
- Other groups have also trapped the alcohol products generated from Rh(III)-catalyzed direct addition of C–H bonds to aldehydes by adding excess of an external oxidant to give ketones, see refs (1e, 1f, and 1g).
- For recent examples of Rh(III)-catalyzed nitrogen heterocycle synthesis via coupling to carbon–carbon π-bonds, see:; a Neely J. M.; Rovis T. J. Am. Chem. Soc. 2013, 135, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhen W.; Wang F.; Zhao M.; Du Z.; Li X. Angew. Chem., Int. Ed. 2013, 51, 11819. [DOI] [PubMed] [Google Scholar]; c Hyster T. K.; Knörr L.; Ward T. R.; Rovis T. Science 2012, 338, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ye B.; Cramer N. Science 2012, 338, 504. [DOI] [PubMed] [Google Scholar]; e Neely J. M.; Rovis T. J. Am. Chem. Soc. 2013, 135, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wang H.; Grohmann C.; Nimphius C.; Glorius F. J. Am. Chem. Soc. 2012, 134, 19595. [DOI] [PubMed] [Google Scholar]; g Wang H.; Glorius F. Angew. Chem., Int. Ed. 2012, 51, 7318. [DOI] [PubMed] [Google Scholar]
- For selected reviews on heterocycle synthesis through C–H functionalization, see:; a Yamaguchi J.; Yamaguchi A. D.; Itami K. Angew. Chem,. Int. Ed. 2012, 51, 8960. [DOI] [PubMed] [Google Scholar]; b Colby D. A.; Bergman R. G.; Ellman J. A. Chem. Rev. 2010, 110, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lyons T. W.; Sanford M. S. Chem. Rev. 2010, 110, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Seregin I. V.; Gevorgyan V. Chem. Soc. Rev. 2007, 36, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typing the names of the following drug and drug candidates into PubChem (http://pubchem.ncbi.nlm.nih.gov/) provides the compound structure, bioactivity, published studies, and information regarding ongoing clinical trials, applications, and usage: niraparib, granisetron, pazopanib, linifanib, axitinib, and GDC-0941.
- a Information on 2-substituted 2H-indazole clinical candidates niraparib and pazopanib can be accessed according to the instructions provided in reference (11).; b De Angelis M.; Stossi F.; Carlson K. A.; Katzenellenbogen B. S.; Katzenellenbogen K. A. J. Med. Chem. 2005, 48, 1132. [DOI] [PubMed] [Google Scholar]
- For reviews on 2H-indazole synthesis, see:; a Haddadin M. J.; Conrad W. E.; Kurth M. J. Mini-Rev. Med. Chem. 2012, 12, 1293. [DOI] [PubMed] [Google Scholar]; b Sapeta K.; Kerr M. A. Sci. Synth., Knowledge Updates 2011, 1, 15. [Google Scholar]; For some recent selected syntheses of 2H-indazoles, see:; c Hu J.-T.; Cheng Y.-F.; Yang Y.-Q.; Rao Y. Chem. Commun. 2011, 47, 10133. [DOI] [PubMed] [Google Scholar]; d Kumar M. R.; Park A.; Park N.; Lee S. Org. Lett. 2011, 13, 3542. [DOI] [PubMed] [Google Scholar]; e Halland N.; Nazare M.; R’kyek O.; Alonso J.; Urmann M.; Lindenschmidt A. Angew. Chem., Int. Ed. 2009, 48, 6879. [DOI] [PubMed] [Google Scholar]; f Stokes B. J.; Vogel C. V.; Urnezis L. K.; Pan M.; Driver T. G. Org. Lett. 2010, 12, 2884. [DOI] [PMC free article] [PubMed] [Google Scholar]; g See also ref (16d).
- For selected reviews, see:; a Beharry A. A.; Woolley G. A. Chem. Soc. Rev. 2011, 40, 4422. [DOI] [PubMed] [Google Scholar]; b Natansohn A.; Rochon P. Chem. Rev. 2002, 102, 4139. [DOI] [PubMed] [Google Scholar]; c Ichimura K. Chem. Rev. 2000, 100, 1847. [DOI] [PubMed] [Google Scholar]
- a Kleiman J. P.; Dubeck M. J. Am. Chem. Soc. 1963, 85, 1544. [Google Scholar]; b Murahashi S.; Horiie S. J. Am. Chem. Soc. 1956, 78, 4816. [Google Scholar]
- a Kakiuchi F.; Matsumoto M.; Tsuchiya K.; Igi K.; Hayamizu T.; Chatani N.; Murai S. J. Organomet. Chem. 2003, 686, 134. [Google Scholar]; b Dick A. R.; Hull K. L.; Sanford M. S. J. Am. Chem. Soc. 2004, 126, 2300. [DOI] [PubMed] [Google Scholar]; c Ma X.-T.; Tian S.-K. Adv. Synth. Catal. 2013, 355, 337. [Google Scholar]; d Li H.-J.; Li P.-H.; Wang L. Org. Lett. 2013, 15, 620. [DOI] [PubMed] [Google Scholar]; In this publication, a Pd-catalyzed coupling of only symmetrical azobenzenes with aldehydes was performed in the presence of excess TBHP as an oxidant to provide ketone products. In a second step, reductive cyclization with Zn, NH4Cl was performed to provide 2-aryl-2H-indazoles.
- Lavis L. D.; Raines R. T. ACS Chem. Biol. 2008, 3, 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyose K.; Kojima H.; Nagano T. Chem. Asian J. 2008, 3, 506–515. [Google Scholar]
- a Grimm J. B.; Heckman L. M.; Lavis L. D. Prog. Mol. Biol. Transl. Sci. 2013, 113, 1–34. [DOI] [PubMed] [Google Scholar]; b Harris J. L.; Backes B. J.; Leonetti F.; Mahrus S.; Ellman J. A.; Craik C. S. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.