Abstract
Aims
It has been known for more than a century that pH changes can alter vascular tone. However, there is no consensus about the effects of pH changes on vascular response. In this study, we investigated the effects of extracellular pH (pHo) changes on intracellular pH (pHi) and intracellular nitric oxide concentration ([NO]i) in freshly isolated endothelial cells and cross sections from rat aorta.
Main Methods
The HCl was used to reduce the pHo from 7.4 to 7.0 and from 7.4 to 6.5; the NaOH was used to increase the pHo from 7.4 to 8.0 and from 7.4 to 8.5. The fluorescent dyes 5-(and-6)-carboxy SNARF-1, acetoxymethyl ester, acetate (SNARF-1) and diaminofluorescein-FM diacetate (DAF-FM DA) were employed to measure the pHi and [NO]i, respectively. The fluorescence intensity was measured in freshly isolated endothelial cells by flow cytometry and in freshly obtained aorta cross sections by confocal microscopy.
Key Findings
The endothelial and vascular smooth muscle pHi was increased at pHo 8.5. The extracellular acidification did not change the endothelial pHi, but the smooth muscle pHi was reduced at pHo 7.0. At pHo 8.5 and pHo 6.5, the endothelial [NO]i was increased. Both extracellular alkalinization and acidification increased the vascular smooth muscle [NO]i.
Significance
Not all changes in pHo did result in pHi changes, but disruption of acid-base balance in both directions induced NO synthesis in the endothelium and/or vascular smooth muscle.
Introduction
It has been known for more than a century that pH changes can alter vascular tone and thereby influence the circulation and blood pressure. Gaskell was probably the first to show the importance of pH in modulating the vascular tone. Studying mylohyoid muscle arteries and mesenteric arteries from frog, he demonstrated that acidification increased the vascular diameter while alkalinization decreased this diameter [1]. It has also been known that both extracellular pH (pHo) and intracellular pH (pHi) can alter vascular tone and that they influence each other [2], [3]. However, the following areas remain unclear: the expected vascular response to pH reduction or augmentation; the mechanisms responsible for pH-induced vasodilation or constriction; whether pHo changes pHi; and which of the compartment pH is the major modulator of the vascular tone.
Considering there is no consensus about the effects of pH changes on vascular response we have developed a research strategy to address this issue and have demonstrated that extracellular alkalinization causes endothelium-dependent relaxation through the nitric oxide (NO) pathway in rat aorta [4]. On the other hand, rat aorta response to extracellular acidification is more complex and involves endothelium-independent relaxation through NO and hyperpolarization pathways [5]. In an attempt to better understand these previous findings showing the role of NO in the relaxation induced by changes in pHo, the present study was carried out to investigate the effects of pHo changes on pHi and intracellular NO concentration ([NO]i) in freshly isolated endothelial cells and freshly obtained cross sections from rat aorta.
Materials and Methods
1. Materials
HEPES, NaOH, nigericin and poly-L-lysine were purchased from Sigma (St. Louis, MO, USA). The probes 5-(and-6)-carboxy SNARF-1, acetoxymethyl ester, acetate (SNARF-1) and diaminofluorescein-FM diacetate (DAF-FM DA) were acquired from Invitrogen (Carlsbad, CA, USA). HCl was purchased from Zilquímica (Ribeirão Preto, SP, Brazil). Thiopental sodium was purchased from Cristália (São Paulo, SP, Brazil). All the other salts were obtained from Vetec Química Fina (Duque de Caxias, RJ, Brazil). All drugs were prepared with distilled water.
2. Experimental design
Freshly isolated endothelial cells and freshly obtained cross sections from rat aorta were exposed to pHo changes for analyzing pHi and [NO]i by flow cytometry and confocal microscopy. The experimental protocol was designed to mimic metabolic alkalosis or acidosis, as we have previously done [4], [5]. Then, the extracellular alkalinization was induced by NaOH, whilst the extracellular acidification was induced by HCl. The most popular method for measuring pHi has involved the use of pH-sensitive fluoroprobes [6], and we choose SNARF-1. DAF-FM DA, a selective NO fluorescent probe, was chosen because it exhibits a stable fluorescence intensity in a large range of pH (above pH 5.8) [7].
3. Animals
The experimental procedures and animal handling were reviewed and approved by the Institutional Committee for Animal Care and Use of the School of Medicine of Ribeirão Preto, University of São Paulo, and were in accordance with the Directive 2010/63/EU (European Commission). Rats were housed under standard laboratory conditions (12 h light/dark cycle at 21°C), with free access to food and water.
Male Wistar rats (230–280 g) were anesthetized with thiopental sodium (40 mg/kg, intraperitoneal injection), and underwent laparotomy for exsanguination via the abdominal aorta and thoracotomy for thoracic aorta harvesting. The thoracic aorta was carefully dissected free of connective tissue and immediately immersed in Hanks solution (composition [mM]: NaCl 145.0, KCl 5.0, CaCl2 1.6, NaH2PO4 0.5, MgCl2 0.5, dextrose 10.0, HEPES 10.0; pH 7.4) to perform cytofluorographic and confocal microscopy analyses.
4. Endothelial pHi and [NO]i measurement by flow cytometry
The thoracic aorta, immersed in Hanks solution, was longitudinally opened, and the endothelial cells were isolated by gentle rake friction. The Hanks solution containing the isolated cells was centrifuged at 200 g for 2 min, and the cells were resuspended in 1 mL of Hanks buffer. The cells were then loaded with SNARF-1 (10 µM) or DAF-FM DA (5 µM) and maintained in a humidified 37°C incubator gassed with 5% CO2 for 30 or 20 min, respectively. The cytofluorographic analysis was performed using a FACScan (Becton-Dickinson, San Jose, CA, USA): the fluorescence was excited with the 488 nm line of an argon ion laser for both dyes, and the emitted fluorescence was measured at 580 nm for SNARF-1 in the acidification experiments, 640 nm for SNARF-1 in the alkalinization experiments, and 515 nm for DAF-FM DA. The fluorescence intensity was evaluated using CellQuest 1.2 software (Becton-Dickinson, Franklin Lakes, NJ, USA). The fluorescence intensity was measured before the unique stimulus and at different time points (t = 1, 3, 9 and 15 min) after the unique stimulus with HCl or NaOH. HCl was used to decrease the pH of Hanks solution containing endothelial cells from 7.4 to 7.0 (final concentration of HCl≈6 mM) and from 7.4 to 6.5 (final concentration of HCl≈10 mM). NaOH was used to increase the pH of Hanks solution containing endothelial cells from 7.4 to 8.0 (final concentration of NaOH≈15 mM) and from 7.4 to 8.5 (final concentration of NaOH≈27 mM); Hanks solution, at pH 7.4, served as control. The fluorescence intensity before the stimulus was designated F0, and the fluorescence intensity after the stimulus for each time point was designated Ft. In this way, the difference in fluorescence intensity (ΔF = Ft - F0) was obtained for each time point.
Before starting the pHi measurement with SNARF-1, we evaluated the specificity of this dye for acid and alkali stimuli using nigericin and NH4Cl at different emitted fluorescence wavelengths (580 and 640 nm), respectively.
5. Aorta pHi and [NO]i measurement by confocal microscopy
The fresh aorta cross sections (100 µm thick) were placed on a poly-L-lysine-coated slide. The tissue was loaded with 10 µM SNARF-1 or 5 µM DAF-FM DA and maintained in a humidified 37°C incubator gassed with 5% CO2 for 30 or 20 min, respectively. The pHi and [NO]i were assessed using a confocal scanning laser microscope (Leica TCS SP2, Leica Microsystems CMS GmbH, Mannheim, Baden-Württemberg, Germany). The fluorescence was excited with the 488 nm line of an argon ion laser for both dyes, and the emitted fluorescence was measured at 580 nm for SNARF-1 in the acidification experiments, 640 nm for SNARF-1 in the alkalization experiments, and 515 nm for DAF-FM DA. Time-course software was used to capture cross sections images at intervals of 2 s (xyt) in Live Data Mode acquisition at 512×512 pixels at 700 Hz. Aorta cross sections were stimulated at 3rd and 9th min with Hanks acidified or alkalinized solution and cross sections images were captured during 15 min. From 0 to 3rd min the cross sections were immersed in Hanks solution (pH 7.4), from 3rd to 9th min the cross sections were immersed in Hanks acidified (pH 7.0) or alkalinized (pH 8.0) solution, and from 9th to 15th min the cross sections were immersed in Hanks acidified (pH 6.5) or alkalinized (pH 8.5) solution. We used HCl or NaOH to change the pH of the Hanks solution; Hanks solution, at pH 7.4, served as the control. Using the Leica Microsystem LAS AF software (Leica Microsystems CMS GmbH, Mannheim, Baden-Württemberg, Germany), the fluorescence intensity was measured in the endothelial and the smooth muscle layers, separately. The fluorescence intensity before the stimulus (immediately before the 3rd min) was designated F, and the fluorescence intensity at 6th min after the stimulus for each pH value was designated FpH. In this way, the difference in fluorescence intensity (ΔF = FpH - F) was obtained for each pH value.
6. Statistical analysis
The data are expressed as means ± SEM. The statistical analysis was performed using two-way repeated-measures ANOVA or one-way ANOVA and Bonferroni post-test (Prism 4.0, GraphPad Software, San Diego, CA, USA). p values lower than 0.05 were considered statistically significant.
Results
1. Effect of extracellular alkalinization on pHi in isolated endothelial cells
NaOH-induced extracellular alkalinization increased the SNARF-1 ΔF (pH 8.5 compared with pH 7.4), showing that extreme extracellular alkalinization increased the pHi while less severe pHo augmentation did not change the pHi in freshly isolated endothelial cells (Figure 1).
Figure 1. Effect of extracellular alkalinization on pHi in isolated endothelial cells from rat aorta.
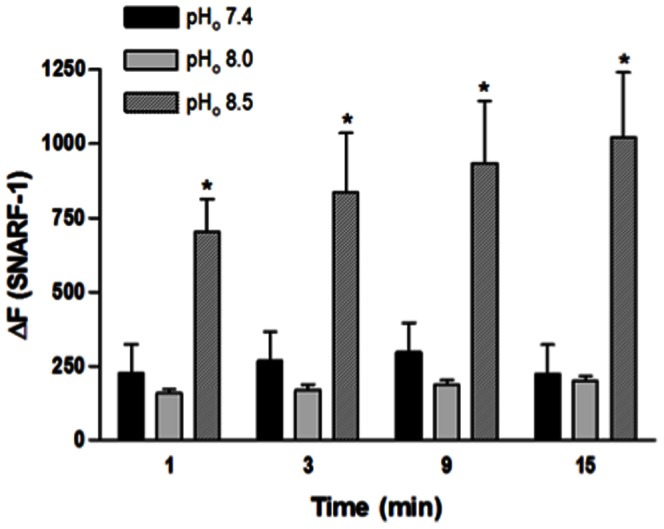
Cells were loaded with SNARF-1 (10 µM) and analyzed by flow cytometry. NaOH was used to increase pHo from 7.4 to 8.0 and from 7.4 to 8.5; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the NaOH stimulus (F0) and at different time points (t = 1, 3, 9 and 15 min) after this stimulus (Ft). Results are reported as ΔF = Ft - F0. All values are means ± SEM (n = 7). Two-way ANOVA, Bonferroni's post-test, * p<0.05 versus control.
2. Effect of extracellular alkalinization on pHi and [NO]i in aorta cross sections
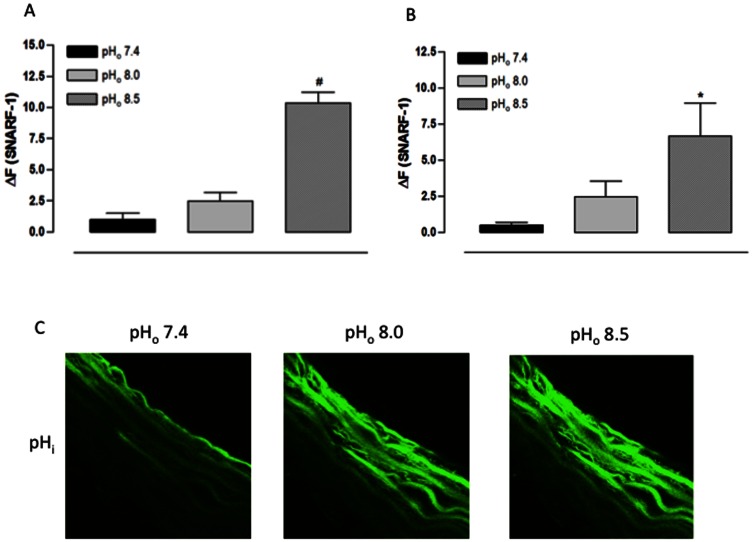
Confirming the result above, the pHo 8.5 increased the SNARF-1 ΔF in the endothelial layer of fresh aorta cross sections while the pHo 8.0 did not result in a change (Figures 2 A and 2 C). Furthermore, the result was the same in the muscular layer: the pHo 8.5 increased the SNARF-1 ΔF while the pHo 8.0 did not change it (Figures 2 B and 2 C).
Figure 2. Effect of extracellular alkalinization on pHi of rat aorta cross sections assessed using a confocal scanning laser microscope.
A) Fluorescence intensity for endothelial layer. B) Fluorescence intensity for muscular layer. C) Representative confocal photomicrograph of one aorta cross section. Aorta cross sections were loaded with SNARF-1 (10 µM) and analyzed by confocal microscopy. Aorta cross sections were stimulated at 3rd and 9th min with Hanks alkalinized solution pH 8.0 and 8.5, respectively. NaOH was used to change the pH of Hanks solution; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the stimulus (F) and at 6th min after the stimulus for each pHo value (FpH). Results are reported as ΔF = FpH – F. All values are means ± SEM (n = 7). One-way ANOVA, Bonferroni's post-test, # p<0.001 versus control, * p<0.05 versus control.
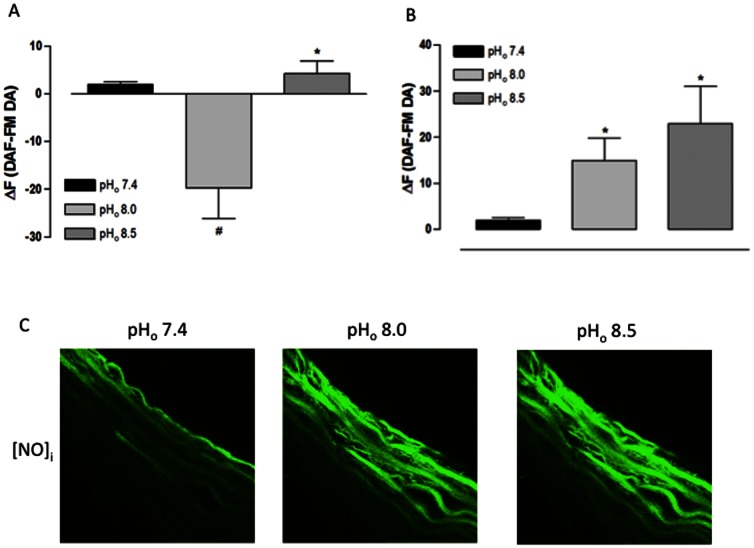
In the endothelial layer of freshly obtained aorta cross sections, the pHo 8.0 reduced while the pHo 8.5 increased the DAF-FM DA ΔF (Figures 3 A and 3 C). However, in the muscular layer, both pHo values increased the DAF-FM DA ΔF (Figures 3 B and 3 C). These data indicate that, at pHo 8.0, a reduction in the endothelial [NO]i was observed simultaneously with an increase in muscular [NO]i, and that, at pHo 8.5, the [NO]i increased in both vessel layers.
Figure 3. Effect of extracellular alkalinization on [NO]i of rat aorta cross sections assessed using a confocal scanning laser microscope.
A) Fluorescence intensity for endothelial layer. B) Fluorescence intensity for muscular layer. C) Representative confocal photomicrograph of one aorta cross section.. Aorta cross sections were loaded with DAF-FM DA (5 µM) and analyzed by confocal microscopy. Aorta cross sections were stimulated at 3rd and 9th min with Hanks alkalinized solution pH 8.0 and 8.5, respectively. NaOH was used to change the pH of Hanks solution; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the stimulus (F) and at 6th min after the stimulus for each pHo value (FpH). Results are reported as ΔF = FpH – F. All values are means ± SEM (n = 7). One-way ANOVA, Bonferroni's post-test, # p<0.001 versus control, * p<0.05 versus control.
3. Effect of extracellular acidification on pHi in isolated endothelial cells
HCl-induced extracellular acidification had no effect on the SNARF-1 fluorescence, showing that extracellular acidification did not change the pHi in freshly isolated endothelial cells (Figure 4).
Figure 4. Effect of extracellular acidification on pHi in isolated endothelial cells from rat aorta.
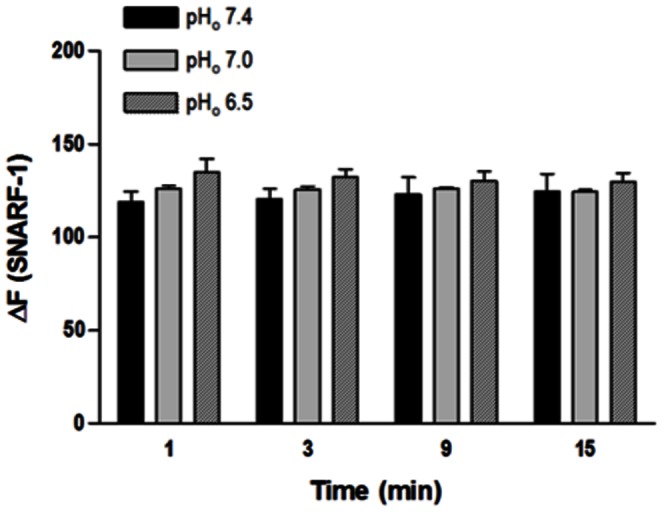
Cells were loaded with SNARF-1 (10 µM) and analyzed by flow cytometry. HCl was used to reduce pHo from 7.4 to 7.0 and from 7.4 to 6.5; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the HCl stimulus (F0) and at different time points (t = 1, 3, 9 and 15 min) after this stimulus (Ft). Results are reported as ΔF = Ft - F0. All values are means ± SEM (n = 7). Two-way ANOVA, Bonferroni's post-test.
4. Effect of extracellular acidification on pHi and [NO]i in aorta cross sections
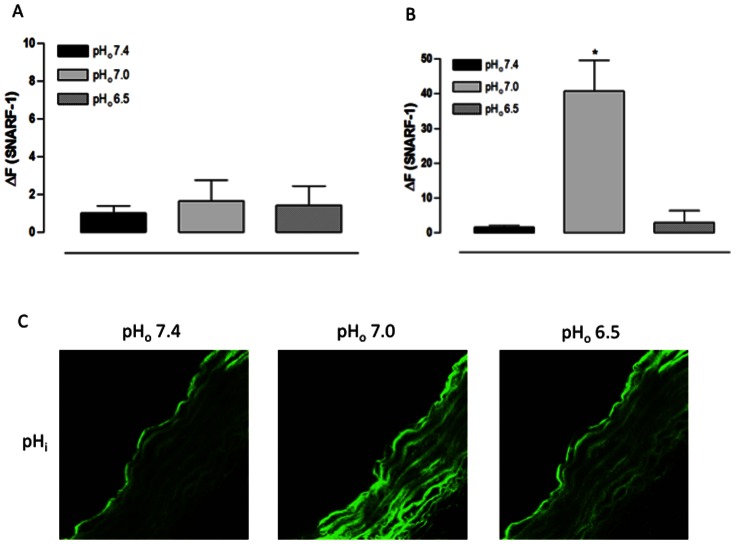
Confirming the result above, the SNARF-1 fluorescence in the endothelial layer of freshly obtained aorta cross sections did not change when the pHo was reduced to 7.0 or 6.5 (Figures 5 A and 5 C). However, in the muscular layer, the pHo 7.0 increased the SNARF-1 ΔF while the pHo 6.5 resulted in no change in the SNARF-1 ΔF, showing that the pHo 7.0 acidified the smooth muscle cells pHi which returned to the basal levels with extreme extracellular acidification (Figures 5 B and 5 C).
Figure 5. Effect of extracellular acidification on pHi of rat aorta cross sections assessed using a confocal scanning laser microscope.
A) Fluorescence intensity for endothelial layer. B) Fluorescence intensity for muscular layer. C) Representative confocal photomicrograph of one aorta cross section. Aorta cross sections were loaded with SNARF-1 (10 µM) and analyzed by confocal microscopy. Aorta cross sections were stimulated at 3rd and 9th min with Hanks acidified solution pH 7.0 and 6.5, respectively. HCl was used to change the pH of Hanks solution; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the stimulus (F) and at 6th min after the stimulus for each pHo value (FpH). Results are reported as ΔF = FpH – F. All values are means ± SEM (n = 7). One-way ANOVA, Bonferroni's post-test, * p<0.01 versus control.
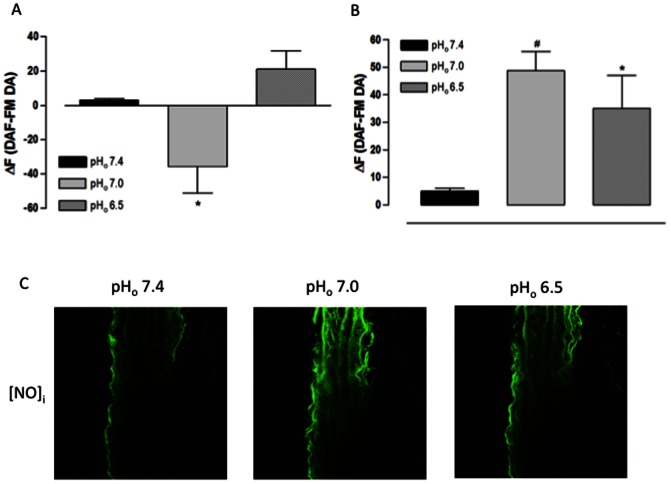
In the endothelial layer of fresh aorta cross sections, the pHo 7.0 reduced the DAF-FM DA ΔF while the pHo 6.5 increased it without statistical significance (Figures 6 A and 6 C). However, in the muscular layer, the DAF-FM DA ΔF was increased with both pHo values (Figures 6 B and 6 C). These data demonstrate that, at pHo 7.0, the [NO]i is reduced in the endothelial layer at the same time in which it is increased in the muscular layer. Moreover, at pHo 6.5, the [NO]i is increased in both vessel layers.
Figure 6. Effect of extracellular acidification on [NO]i of rat aorta cross sections assessed using a confocal scanning laser microscope.
A) Fluorescence intensity for endothelial layer. B) Fluorescence intensity for muscular layer. C) Representative confocal photomicrograph of one aorta cross section. Aorta cross sections were loaded with DAF-FM DA (5 µM) and analyzed by confocal microscopy. Aorta cross sections were stimulated at 3rd and 9th min with Hanks acidified solution pH 7.0 and 6.5, respectively. HCl was used to change the pH of Hanks solution; Hanks solution pH 7.4 served as control. Fluorescence intensity was measured before the stimulus (F) and at 6th min after the stimulus for each pHo value (FpH). Results are reported as ΔF = FpH – F. All values are means ± SEM (n = 7). One-way ANOVA, Bonferroni's post-test, * p<0.05 versus control, # p<0.001 versus control.
Discussion
The main findings of the present study are: a) extracellular alkalinization increased the pHi in the endothelium and vascular smooth muscle; b) extracellular acidification did not change the endothelial pHi but reduced the smooth muscle pHi; c) severe extracellular alkalinization (pHo 8.5) and acidification (pHo 6.5) increased the endothelial [NO]i; and d) both extracellular alkalinization and acidification increased the vascular smooth muscle [NO]i.
In the present investigation, we selected aorta because it is the most important conductance artery. In addition, we wanted to clarify previous results obtained with this artery. Another methodological concern that deserves consideration is the use of freshly isolated endothelial cells and fresh aorta cross sections. Studies using freshly isolated cells should be distinguished from those using cultured cells or cell lines, as these cells may no longer express an in vivo phenotype [2], [8], [9]. Another important methodological issue is the pH range. We decided to study the pHo from 6.5 to 8.5 because the literature shows that, in this pHo range, the cells are viable [8], [9], [10] and we confirmed this literature data in our previous studies [4], [5].
It is known that both pHo and pHi can alter vascular tone and that they can influence each other. The main factor responsible for this mutual interaction between pHo and pHi is the high H+ permeability of arterial vascular smooth muscle. An alternative route would be the flux of weak acids across the membrane. This acid flux can occur either by passive diffusion of the protonated form or via a transporter mechanism. Thus, it is believed that changes in the pHo can induce changes in the pHi in the same direction [2]. However, here we have demonstrated that not all changes in the pHo induce changes in the pHi, especially in the endothelium. The extracellular acidification did not produce any change in the endothelial pHi of isolated cells or cells covering the aorta internal surface. Concerning extracellular alkalinization, a great change in the pHo was necessary to induce an increase in the pHi in both isolated endothelial cells and endothelium-intact rings. This suggests that the endothelium, the largest functional organ responsible for regulating vascular tone, coagulation, inflammation and permeability [11], probably has efficient mechanisms of controlling the pHi to avoid changes in enzymatic function and signal transduction, which could impair the important endothelial control of body homeostasis or result in cell death. Moreover, these control mechanisms seem to be more efficient for acidification than for alkalinization. In contrast to the endothelial findings, the extracellular acidification reduced the vascular smooth muscle pHi. Actually, at pHo 7.0, the pHi was decreased, corroborating previous data in rat mesenteric arteries [12], [13], cerebral arteries [14] and portal vein [15], but the pHi returned to basal levels when the pHo was reduced to 6.5, probably due to slow recovery to the initial pHi as acid equivalents are removed from the fiber by the pHi regulating mechanism [16]. The vascular smooth muscle exhibited the same behavior as the endothelium when the pHo was increased. Oly extreme extracellular alkalinization increased the pHi, which is different from the findings of earlier studies showing that the pHo 7.9 was sufficient to increase the smooth muscle pHi [12], [13], [15]. These variations between endothelial and vascular smooth muscle cells and between distinct vessels can be due to different proton permeabilities or different mechanisms of controlling the pHi [2].
Although the mechanisms by which the pH influences vascular tone remain inconclusive, some evidences suggest the involvement of NO [17], [18], [19], [20], prostacyclin [20], [21], potassium channels [17], [22], [23] and calcium flux [14], [22], [23]. We have previously demonstrated that both extracellular alkalinization and acidification induce vasodilation, and although the mechanisms responsible for this relaxation are not the same in each acid-base disorder, the NO is a common pathway [4], [5]. Here, we have confirmed that changes in acid-base balance did increase the [NO]i in freshly obtained aorta cross sections. However, this [NO]i increase was not necessarily dependent on pHi changes, it could have been due to pHo changes. This observation came from our results showing that, in some cases, even without changing the endothelial or muscular pHi, the [NO]i was altered. Moreover, the pHo changes can alter cellular permeability and then activate different signaling pathways [24], [25].
In the present study, we showed that mild extracellular alkalinization (pHo 8.0) and acidification (pHo 7.0) reduced the [NO]i in the endothelial layer and increased the [NO]i in the smooth muscle layer, while severe extracellular alkalinization (pHo 8.5) and acidification (pHo 6.5) increased the [NO]i in the entire vascular wall. The reduction in endothelial [NO]i can be explained by the fast diffusion of this gas to the muscular layer. This event was clearly observed during the confocal experiments, when it was possible to see that the increase in fluorescence intensity in the endothelial layer coincided with the addition of the base or acid, and that, immediately after this addition, the fluorescence intensity in the endothelial layer decreased simultaneously with its increase in the muscular layer. This is confirmed by our previous results showing that the [NO]i in isolated endothelial cells was raised not only at pHo 8.5 and 6.5, but also at pHo 8.0 and 7.0 [4], [5]. Moreover, it was demonstrated that NaOH-induced extracellular alkalinization (pHo 8.0) stimulated the endothelial nitric oxide synthase (eNOS) activity in cultured human pulmonary arterial endothelial cells [8]. Conversely, in cultured human umbilical vein endothelial cells and freshly isolated porcine aortic endothelial cells, the extracellular alkalinization-induced intracellular alkalinization diminished the eNOS activity [26]. Regarding acidosis, it has been shown that the pH reduction predisposes to an increase in the [NO]i. In pig cerebellum, the acidification favored the NO synthesis, probably due to an increase in the enzymatic activity [27]. It has also been suggested that, in an acidic milieu, the nitrite can be non-enzymatically reduced to NO [28] and that the NO is more stable in this milieu as it is protected from degradation [19], [29]. However, there are some reports describing that acidification decreases the eNOS activity [8], [26]. Discrepancies between our results (obtained from both extracellular alkalinization and acidification) and this literature data may be attributed to the use of different animal species, different alkali or acid stimulus [2], [24] or different phenotypes (cultured versus fresh cells) [2], [8], [9] and the presence or absence of pHi change when the pHo is altered.
An important consideration about [NO]i is that although both extracellular alkalinization and acidification raised the [NO]i in the muscular layer, the NO synthesis was restricted to the endothelium during the augmentation of the pHo. On the other hand, during the acidification, the NO was synthesized by the endothelium and smooth muscle. These inferences are based on the fact that the extracellular alkalinization promoted an endothelium-dependent relaxation, which was exclusively mediated by NO [4], while the extracellular acidification-induced relaxation was partially reduced after inhibition of the NO synthesis in endothelium-denuded rings [5]. Another intriguing detail is the endothelial pHi change induced by extracellular alkalinization compared with the lack of change in the endothelial pHi when the pHo was reduced. Based on this observation, would be valid to speculate that this difference is responsible for the exclusive role of NO in the alkalinization-induced relaxation while the vasodilation elicited by extracellular acidification needs factors other than NO?
Conclusions
In summary, we have demonstrated that not all changes in pHo are reflected in pHi alterations and that both acid-base disorders induce NO synthesis in the endothelium and/or vascular smooth muscle.
Acknowledgments
We thank Agnes Afrodite Albuquerque Sumarelli and Fabiana Rossetto Morais for technical support.
Funding Statement
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA/HCFMRP/USP) for financial support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gaskell WH (1880) On the Tonicity of the Heart and Blood Vessels. J Physiology 3: 48–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith GL, Austin C, Crichton C, Wray S (1998) A review of the actions and control of intracellular pH in vascular smooth muscle. Cardiovasc Res 38: 316–331. [DOI] [PubMed] [Google Scholar]
- 3. Wray S (1988) Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol 254: C213–225. [DOI] [PubMed] [Google Scholar]
- 4. Celotto AC, Capellini VK, Restini CB, Baldo CF, Bendhack LM, et al. (2010) Extracellular alkalinization induces endothelium-derived nitric oxide dependent relaxation in rat thoracic aorta. Nitric Oxide 23: 269–274. [DOI] [PubMed] [Google Scholar]
- 5. Celotto AC, Restini CB, Capellini VK, Bendhack LM, Evora PR (2011) Acidosis induces relaxation mediated by nitric oxide and potassium channels in rat thoracic aorta. Eur J Pharmacol 656: 88–93. [DOI] [PubMed] [Google Scholar]
- 6. Bond J, Varley J (2005) Use of flow cytometry and SNARF to calibrate and measure intracellular pH in NS0 cells. Cytometry Part A : the journal of the International Society for Analytical Cytology 64: 43–50. [DOI] [PubMed] [Google Scholar]
- 7. Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, et al. (1999) Fluorescent Indicators for Imaging Nitric Oxide Production. Angewandte Chemie 38: 3209–3212. [DOI] [PubMed] [Google Scholar]
- 8. Mizuno S, Demura Y, Ameshima S, Okamura S, Miyamori I, et al. (2002) Alkalosis stimulates endothelial nitric oxide synthase in cultured human pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 283: L113–119. [DOI] [PubMed] [Google Scholar]
- 9. Nagy S, Harris MB, Ju H, Bhatia J, Venema RC (2006) pH and nitric oxide synthase activity and expression in bovine aortic endothelial cells. Acta Paediatr 95: 814–817. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JA, de Nucci G, Warner TD, Vane JR (1991) Alkaline buffers release EDRF from bovine cultured aortic endothelial cells. Br J Pharmacol 103: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crimi E, Taccone FS, Infante T, Scolletta S, Crudele V, et al. (2011) Effects of intracellular acidosis on endothelial function: An overview. Journal of critical care [DOI] [PubMed] [Google Scholar]
- 12. Austin C, Dilly K, Eisner D, Wray S (1996) Simultaneous measurement of intracellular pH, calcium, and tension in rat mesenteric vessels: effects of extracellular pH. Biochem Biophys Res Commun 222: 537–540. [DOI] [PubMed] [Google Scholar]
- 13. Austin C, Wray S (1993) Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol 466: 1–8. [PMC free article] [PubMed] [Google Scholar]
- 14. Peng HL, Jensen PE, Nilsson H, Aalkjaer C (1998) Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: is there a role for membrane potential? Am J Physiol 274: H655–662. [DOI] [PubMed] [Google Scholar]
- 15. Taggart M, Austin C, Wray S (1994) A comparison of the effects of intracellular and extracellular pH on contraction in isolated rat portal vein. J Physiol 475: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aickin CC (1984) Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol 349: 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U (2003) Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J Cereb Blood Flow Metab 23: 1227–1238. [DOI] [PubMed] [Google Scholar]
- 18. Gurevicius J, Salem MR, Metwally AA, Silver JM, Crystal GJ (1995) Contribution of nitric oxide to coronary vasodilation during hypercapnic acidosis. Am J Physiol 268: H39–47. [DOI] [PubMed] [Google Scholar]
- 19. Hattori K, Tsuchida S, Tsukahara H, Mayumi M, Tanaka T, et al. (2002) Augmentation of NO-mediated vasodilation in metabolic acidosis. Life Sci 71: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Leffler CW (2002) Compensatory role of NO in cerebral circulation of piglets chronically treated with indomethacin. American journal of physiology Regulatory, integrative and comparative physiology 282: R400–410. [DOI] [PubMed] [Google Scholar]
- 21. Niwa K, Haensel C, Ross ME, Iadecola C (2001) Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res 88: 600–608. [DOI] [PubMed] [Google Scholar]
- 22. Dabertrand F, Nelson MT, Brayden JE (2011) Acidosis Dilates Brain Parenchymal Arterioles by Conversion of Calcium Waves to Sparks to Activate BK Channels. Circ Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohra DK, Sharif HM, Zubairi HS, Sarfraz K, Ghayur MN, et al. (2005) Acidosis-induced relaxation of human internal mammary artery is due to activation of ATP-sensitive potassium channels. Eur J Pharmacol 514: 175–181. [DOI] [PubMed] [Google Scholar]
- 24. Celotto AC, Capellini VK, Baldo CF, Dalio MB, Rodrigues AJ, et al. (2008) Effects of acid-base imbalance on vascular reactivity. Braz J Med Biol Res 41: 439–445. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura K, Kamouchi M, Arimura K, Nishimura A, Kuroda J, et al. (2012) Extracellular acidification activates cAMP responsive element binding protein via Na+/H+ exchanger isoform 1-mediated Ca(2)(+) oscillation in central nervous system pericytes. Arteriosclerosis, thrombosis, and vascular biology 32: 2670–2677. [DOI] [PubMed] [Google Scholar]
- 26. Fleming I, Hecker M, Busse R (1994) Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ Res 74: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 27. Heinzel B, John M, Klatt P, Bohme E, Mayer B (1992) Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J 281 Pt 3: 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, et al. (2001) Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand 171: 9–16. [DOI] [PubMed] [Google Scholar]
- 29. Ignarro LJ (1989) Endothelium-derived nitric oxide: actions and properties. Faseb J 3: 31–36. [DOI] [PubMed] [Google Scholar]