Abstract
Purpose: Real-time tracking of respiratory target motion during radiation therapy is technically challenging, owing to rapid and possibly irregular breathing variations. The authors report on a method to predict and correct respiration-averaged drift in target position by means of couch adjustments on an accelerator equipped with such capability.
Methods: Dose delivery is broken up into a sequence of 10 s field segments, each followed by a couch adjustment based on analysis of breathing motion from an external monitor as a surrogate of internal target motion. Signal averaging over three respiratory cycles yields a baseline representing target drift. A Kalman filter predicts the baseline position 5 s in advance, for determination of the couch correction. The method's feasibility is tested with a motion phantom programmed according to previously recorded patient signals. Computed couch corrections are preprogrammed into a research mode of an accelerator capable of computer-controlled couch translations synchronized with the motion phantom. The method's performance is evaluated with five cases recorded during hypofractionated treatment and five from respiration-correlated CT simulation, using a root-mean-squared deviation (RMSD) of the baseline from the treatment planned position.
Results: RMSD is reduced in all 10 cases, from a mean of 4.9 mm (range 2.7–9.4 mm) before correction to 1.7 mm (range 0.7–2.3 mm) after correction. Treatment time is increased ∼5% relative to that for no corrections.
Conclusions: This work illustrates the potential for reduction in baseline respiratory drift with periodic adjustments in couch position during treatment. Future treatment machine capabilities will enable the use of “on-the-fly” couch adjustments during treatment.
Keywords: respiratory motion, tracking, mean-position estimation, adaptive radiotherapy
INTRODUCTION
Uncompensated respiratory tumor motions in the thorax or upper abdomen can be large, requiring margins of 1 cm or more.1, 2, 3 An active area of investigation for mitigating respiratory motion during treatment is motion tracking.4, 5 Correction of respiratory motion can be done either by repositioning the beam6, 7, 8, 9 or repositioning the patient. Patient repositioning methods focus on the use of dynamic couch motion to compensate for physiological motion during beam delivery. D’Souza et al.10 constructed a miniature adaptive couch motion system, consisting of two platforms to simulate tumor and couch motion, and evaluated its performance in a phantom driven by a sinusoidal oscillator. D’Souza and McAvoy11 analyzed the dynamics of a specialized couch (Hexapod, Medical Intelligence, Inc., Germany) and control system to determine the requirements for respiration-induced motion compensation; however, measurements of couch performance were limited to the response to a single step input. Qui et al.12 proposed a predictive feedback control method for real-time motion compensation using couch adjustments, and retrospectively evaluated it with recorded patient traces of abdominal displacement. Haas et al.13 used simulation and experiment to evaluate couch based motion compensation. Wilbert et al.14 described an approach for real-time tumor tracking and motion compensation tested with a HexaPOD couch and robotic 4D phantom. In another study, they examined the influence of breathing-correlated and uncorrelated couch motion on the breathing amplitude and patterns of volunteers.15 The steering control of the robotic couch attempted to compensate for optically detected abdominal breathing motion, but did not use any predictive model. Buzurovic et al.16 investigated a tumor motion prediction technique by means of computer simulations of two types of robotic couches. A variety of different motion prediction algorithms17, 18, 19 have been studied for target tracking. Evaluation of all these methods involved retrospective analysis of respiratory traces including those that, like the Cyberknife Synchrony system, use a marker of internal tumor motion so that the robotically controlled Linac can follow the target.8
In practice, implementing methods to track and correct for cyclical breathing is challenging due to sometimes rapid motion requirements, system latencies, and irregular breathing fluctuations.20 Therefore, some investigators have proposed mean-position tracking of the respiratory motion as an alternative to full motion tracking.21, 22, 23 In such scenarios, the cyclical motion is accounted for by margins or robust IMRT optimization while the slowly varying intrafraction drift in mean position is handled by electromechanical features of the treatment delivery.24 We report here on a feasibility study to track and correct for respiration-averaged drift in a respiratory signal by means of programmable couch adjustments on a new type of computer-controlled Linac (Varian TrueBeam).
We investigate an intratreatment correction strategy for drift consisting of short (10 s) intervals of dose delivery alternating with couch adjustment while dose delivery is briefly interrupted. Our study uses an external surrogate signal to demonstrate feasibility but the methodology can be applied just as well to an internal motion signal.
MATERIALS AND METHODS
Overview
Our strategy is to correct for slow changes (relative to cyclical breathing motion) in target position using couch adjustments at 10 s intervals. As a proof of principle, we investigate this strategy in motion phantom studies with synthetic and recorded patient traces, and correct for baseline drift of the external respiratory signal as a surrogate for target motion. The studies are carried out in the Developer mode of a computer-controlled Linac (TrueBeam, Varian Medical Systems, Palo Alto, CA), which provides capabilities for scripted couch motions during treatment delivery. A motion phantom (Quasar, Modus Medical Devices, London, ON) is programmed according to previously recorded patient external motion traces. The traces are acquired either during a respiratory-correlated simulation procedure using a commercial external tracking camera [Real-time Position Management (RPM), Varian Medical Systems] at 30 frames/s, or during hypofractionated treatment using an inhouse optical patient monitoring system (camera: Polaris, Northern Digital, Inc., Waterloo, ON) at 10 frames/s. Reflective markers on the phantom are tracked and recorded using the inhouse system. The recorded signal is processed to calculate a respiration-averaged baseline and input to a Kalman filter algorithm, which predicts the position of the baseline 5 s ahead (i.e., at a time midway between consecutive couch corrections). The predictions are used to calculate couch corrections to compensate for drift every 10 s.
Respiration-averaged baseline
We define the baseline as the average of the cyclical breathing trace over a user-set integral number of cycles, which requires computation of the breathing period. The breathing period is computed every second and the baseline is calculated as the respiration trace averaged retrospectively over three times the current period, which largely removes respiratory oscillations The period (τ) is calculated by finding the value of τ that minimizes a summation of normalized breathing trace amplitude differences: , where Pt is the signal at time t and A is the mean peak-to-trough signal amplitude during the initial 30 s of data. The initial 30 s is also used to set initial conditions for the Kalman filter and determine statistics from the raw breathing signal (described in Sec. 2C); no treatment beam or couch motion occurs during this “learning” period. Starting from the current time (t0) we sum over the last N data samples; N = 210 (for data recorded at 30 frames/s) is chosen to correspond to 7 s, which we have found sufficient to successfully detect the period in all patient traces tested (up to 9 s period). Since multiples of the respiration period will also minimize F(τ), we look for the smallest value to do so in a range from 2 to 14 s. The sequence of computed baseline positions at 1 s intervals are input to the Kalman filter algorithm (described in Sec. 2C).
Baseline prediction and correction
A Kalman filter is described by a set of equations that provides an estimate of the state of a discrete time process by combining its theoretical time evolution with periodic corrections based on noisy measurements. In this application, we estimate the one-dimensional (1D) position and velocity of the respiration-averaged (i.e., drift) signal.25
The state with position xk and velocity at a given discrete time k is represented as a vector (symbols in bold font indicate vector or matrix quantities). We assume that changes in drift velocity are associated with random fluctuations representing process noise (wk); thus, the current velocity is related to the previous velocity as The current position is expressed as the previous position plus the average velocity times the time increment (Δ): The state equation that describes the process is given by where α controls how sensitive the predictions are to the drift velocity, i.e., for smaller α values the contribution of the velocity to the prediction is reduced. The measured baseline position (zk) is related to the state vector by , where vk is the measurement noise. Details of the Kalman filter update equations and parameters are given in an appendix in the supplementary material.26 The process noise (wk) and the measurement noise (vk) are assumed to be independent Gaussian distributions with zero mean and are calculated for each patient using the position variance () and velocity variance () from our initial 30 s of training data. The parameters that are determined to tune the filter are the velocity scaling term α, measurement noise covariance R, and process reliability constant Qc. The constant Qc is a coefficient in the process noise covariance . The measurement noise covariance is estimated as , reflecting the positional accuracy of our tracking system and other measurement noise and is a scalar since our measurements are one-dimensional [superior-inferior (SI) or anterior-posterior (AP) position]. We perform an exhaustive search of combinations of the Kalman filter parameters R, Qc, and α so as to minimize the root-mean-square deviation (RMSD) of 5 s predictions from the true position. The parameters are tuned separately for 100 RPM traces from 4D-simulation sessions and for 22 traces acquired with the Polaris camera at hypofractionated treatments. The optimized parameters are found to be very similar for the two types of data sets, with R = 0.05 cm2 (corresponding to one standard deviation measurement error of about 2 mm), Qc = 0.01, and α = 0.4.
Evaluation with motion phantom
We investigate the feasibility of correcting for baseline drift by means of computer-controlled couch adjustments. The study uses a programmable motion phantom (Quasar, Modus Medical Devices, Inc.) whose motion follows that of a respiratory motion trace previously recorded from a patient's simulation or treatment. A cylindrical insert moving in the SI direction is linked to a platform moving AP, mimicking the respiratory motion of a patient's internal thoracic or abdominal target and his chest wall. For most examples in this study, couch shifts were applied in the SI direction as would be the case for a drifting tumor in 1D. Infrared reflective markers, placed on the cylindrical insert and couch, are tracked with the Polaris camera system.
The motion is first recorded with a stationary couch. The recorded trace is input to the baseline calculation and Kalman filter, and the couch shifts for baseline drift correction are calculated. This must be done because the current TrueBeam system does not enable “on-the-fly” couch adjustments from an external program during beam delivery. To test the method with the motion phantom, the TrueBeam system is preprogrammed for computer-controlled treatment setup and delivery in a nonclinical Developer mode, using XML scripts which direct the TrueBeam to deliver beam segments of 50 MU at a dose rate of 300 MU/min between couch shifts in the SI (longitudinal) direction. That is, the XML scripts deliver a mock treatment as a series of alternating, precalculated couch adjustments and field segments, where each field segment lasts approximately 10 s. The start of the programmed phantom motion and the XML script are manually synchronized and the resultant motion with couch corrections is recorded with the Polaris camera system. Each couch shift requires a finite amount of time, which causes systematic drift in time between the phantom motion and the XML script. We measure this as a function of the number of couch shifts and determine it to be approximately 0.2 s per couch shift for our machine. We account for this delay by reducing the field segment time to 9.8 s, such that couch adjustments occur at 10 s intervals, consistent with the intervals between the Kalman-predicted corrections. We selected 10 representative patient traces from the 122 traces used to tune the Kalman filter (described in Sec. 2C): five of them are 10 min segments recorded at hypofractionated treatment, and five are 2–5 min traces recorded at CT simulations. Efficacy of drift correction is evaluated by comparing the RMSD of the baseline from its intended position (i.e., the initial baseline position) before and after couch compensation.
We also investigated the effect of load on the couch shift response to understand its application to patient treatment. One of the mock treatment XML scripts, of 10 min duration from one of the hypofractionated treatments, was repeated with and without a 62 kg load on the couch. The couch motion in each case was recorded using the inhouse optical tracking system. In another experiment to investigate the effect of load on vertical couch motions, the same breathing trace was used to drive the AP motion of the phantom's “chest wall” platform, with reflective markers placed on the platform and the couch. This platform motion on a stationary couch was used to generate an XML file for compensating AP couch shifts which were then applied with the 62 kg load on the couch.
RESULTS
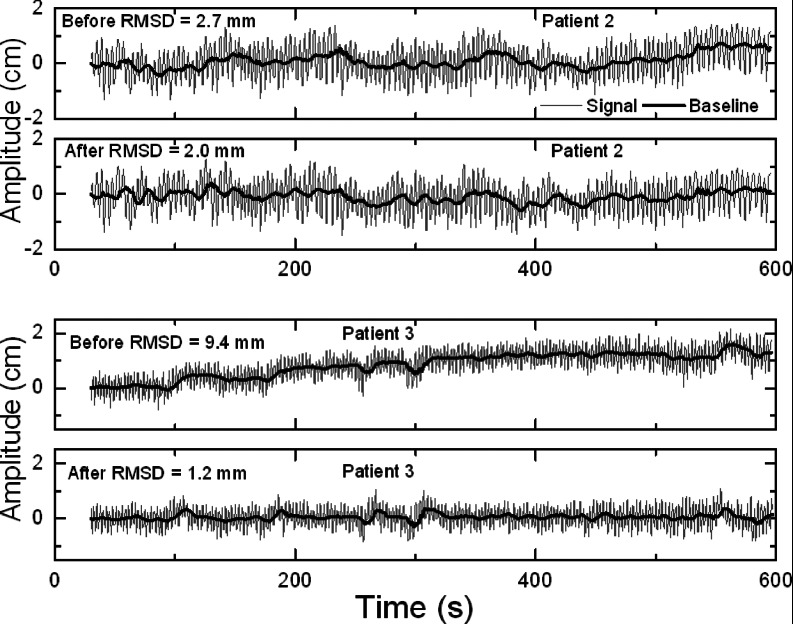
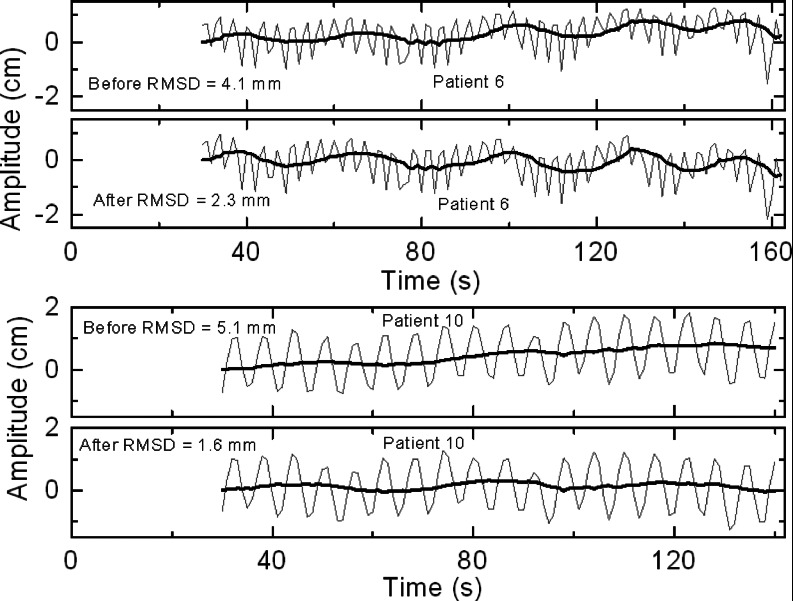
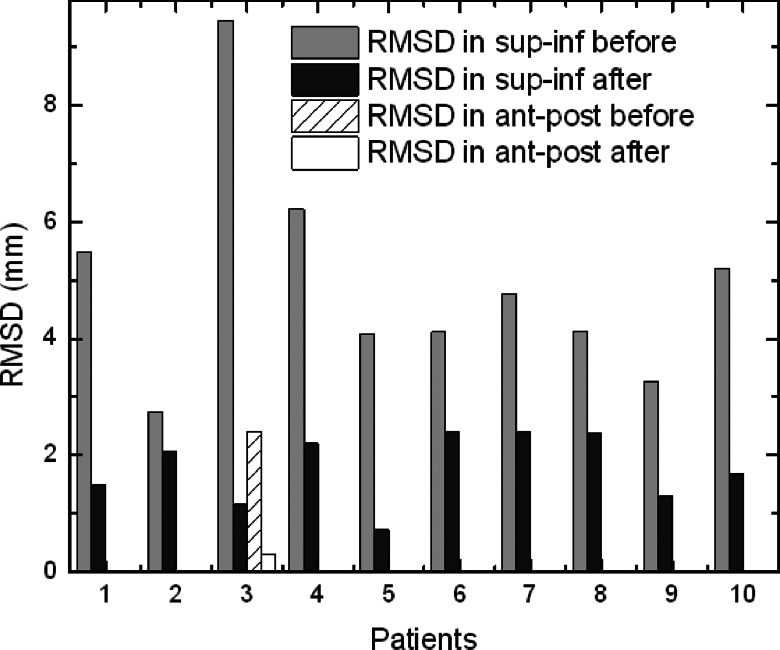
Figure 1 shows examples of the phantom's motion before and after preprogrammed couch corrections for two different traces from hypofractionated treatment. Figure 2 shows examples before and after corrections for two traces from CT simulation. In both figures, the two examples show the smallest and largest improvement with correction, respectively, from each patient group. Figure 3 summarizes the RMSD results before and after correction in SI direction, in which the phantom in cases 1–5 was programmed with traces from hypofractionated treatment and in cases 6–10 from CT simulation. Drift in the baseline (“before”) is evident and clearly reduced with scripted couch adjustments (“after”). Baseline drift is reduced by more than a factor of 2 in six of ten patients and slightly reduced in the remaining patients who had little drift to begin with. These patients had less systematic drift and more short-term fluctuations that are comparable to or smaller than the 10 s interval between couch corrections. For the ten patient cases representing our proof-of-principle study, RMSD is reduced from a mean of 4.9 mm (range 2.7–9.4 mm) before correction to 1.7 mm (range 0.7–2.3 mm) after correction. Treatment time with this scheme is increased ∼5% relative to that for no corrections due to the time required for the system to initiate the programmed couch shifts; corresponding to about 0.2 s per couch adjustment. This is factored into our synchronization of the motion phantom and the treatment machine as explained in Sec. 2D. In addition, comparison of SI couch motions from a 10-min script (patient 3), with and without a couch load of 62 kg, show differences of less than 0.3 mm (data not shown). This indicates that, at least for typical patient weights, the accuracy of SI couch adjustments is not affected. RMSD result before and after correction in the AP direction for the same patient is also shown in Fig. 3. Because of the phantom construction, the AP platform motion is smaller than the SI cylinder motion. Thus, the AP RMSD is smaller than that for SI. However, in both directions, correction reduces RMSD by an approximate factor of 8 implying that for a typical patient weight, the couch correction strategy is effective in both directions.
Figure 1.
Examples of motion phantom position vs time (gray), programmed with external motion traces from hypofractionated treatment (patient #2 and #3) and recorded with the Polaris camera system, before and after application of preprogrammed couch corrections at 10-s intervals on a TrueBeam Linac. Bold black curves show the deviation of the respiration-averaged baseline from the initial setup position.
Figure 2.
Examples of motion phantom position vs time (gray), programmed with external motion traces from CT simulation (patients #6 and #10) before and after application of preprogrammed couch corrections at 10-s intervals on a TrueBeam Linac. Bold black curves show the deviation of the respiration-averaged baseline from the initial setup position.
Figure 3.
Summary of motion phantom results. RMSD of the respiration-averaged baseline before and after preprogrammed couch corrections, based on 5 s Kalman filter predictions. Motion phantom is programmed with external motion traces from 10 patients (#1–5: 10 min traces from hypofractionated treatment, #6–10: 2–5 min traces from CT simulation).
DISCUSSION AND CONCLUSION
This study shows the potential for reduction in baseline respiratory drift with periodic adjustments in couch position during treatment. Its feasibility is demonstrated using programmed couch adjustments interleaved with short intervals of dose delivery on a computer-controlled Linac. The drift occurs over longer time scales than the breathing period, which allows more accurate prediction and makes couch-based correction clinically feasible. We calculate the baseline of the oscillatory breathing signal as an average over an integral number of prior breathing cycles (three in this study) and use a Kalman filter to predict its 5 s future position. An alternative definition of a baseline could be the end-expiration values of the breathing signal, particularly if used in conjunction with treatment gated about end-exhale.
The choice of correction frequency is a trade-off between the desire for prompt response to changes in drift velocity on the one hand, and increased treatment time introduced by couch adjustments on the other. Our choice of 10-s correction intervals yields a mean reduction in baseline RMSD of more than a factor of 2 while modestly increasing treatment time by approximately 5%. It should be noted that for very irregular breathers, the proposed correction strategy should be supplemented with temporary suspension of treatment delivery during intervals of irregular respiratory motion. In such cases, treatment time could be increased by more than 5%. We note that our strategy is to correct for positional drift that occurs over time scales longer than the 10-s correction interval. In this study, system latency (i.e., couch adjustment time of 0.2 s) was small relative to the correction interval and was compensated by a corresponding shortening of the field segment irradiation time between corrections. In actual clinical application of this strategy, the same circumstances would apply, i.e., alternating 0.2 s couch adjustment during beam hold and 9.8 s dose delivery, thus maintaining 10 s correction intervals but resulting in slightly longer treatment time. In applications that attempt to compensate real-time motion by means of continuously applied couch corrections, system latency is significant and must be taken into account by the predictive motion model.10, 11, 13
The choice of 30 s as the initial learning period of the respiration traces is based on the longest possible breathing period of about 14 s. At least two cycles are desirable to determine that the breathing signal is periodic, calculate the breathing period, establish the initial position of the respiration-averaged baseline, and train the Kalman filter. The 30-s learning period is a conservative number and could be reduced for shorter breathing periods although we did not investigate this possibility.
There are several limitations in our study. First, the current capabilities of the TrueBeam Linac do not permit on-the-fly couch adjustments by an external computer program during treatment. Therefore, our study used preprogrammed couch adjustments during treatment delivery implemented by means of an XML script in Developer mode. A future software upgrade of the machine capabilities will permit on-the-fly couch correction by an external predictive program via an interface to the machine control system. Second, our study examined couch corrections in one dimension only, but clinical implementation will require three-dimensional corrections.
Finally, the study assumes that the external respiratory trace represents target motion. There is a need to develop a correspondence model, in which the external signal is correlated with internal motion of the target or a nearby surrogate. One approach, used in the Cyberknife system (Accuray, Inc., Sunnyvale, CA), is to correlate the external motion signal with internal information from periodic x-ray imaging.8 The TrueBeam system has capabilities for acquiring kV images during treatment delivery that are triggered by the RPM gate. Such images could be used in combination with RPM to update a correspondence model. Alternatively, other real-time signals such as those from an electromagnetic tracking system (Calypso Medical Technologies, Inc., Seattle, WA) could be used.
ACKNOWLEDGMENTS
Research is supported by Award Nos. T32 CA61801 and R01 CA126993 from the National Cancer Institute, and by a research grant from Varian Medical Systems. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- Gierga D. P., Chen G. T. Y., Kung J. H., Betke M., Lombardi J., and Willett C. G., “Quantification of respiration-induced abdominal tumor motion and its impact on IMRT dose distributions,” Int. J. Radiat. Oncol., Biol., Phys. 58(5), 1584–1595 (2004). 10.1016/j.ijrobp.2003.09.077 [DOI] [PubMed] [Google Scholar]
- Jiang S. B., Pope C., Al Jarrah K. M., Kung J. H., Bortfeld T., and Chen G. T. Y., “An experimental investigation on intra-fractional organ motion effects in lung IMRT treatments,” Phys. Med. Biol. 48, 1773–1784 (2003). 10.1088/0031-9155/48/12/307 [DOI] [PubMed] [Google Scholar]
- Blomgren H., Lax I., Naslund I., and Svanström R., “Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: Clinical experience of the first thirty-one patients,” Acta Oncol. 34(6), 861–870 (1995). 10.3109/02841869509127197 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Kini V. R., Vedam S. S., and Mohan R., “Motion adaptive x-ray therapy: A feasibility study,” Phys. Med. Biol. 46(1), 1–10 (2001). 10.1088/0031-9155/46/1/301 [DOI] [PubMed] [Google Scholar]
- Dieterich S., Cleary K., D’Souza W., Murphy M., Wong K. H., and Keall P., “Locating and targeting moving tumors with radiation beams,” Med. Phys. 35(12), 5684–5694 (2008). 10.1118/1.3020593 [DOI] [PubMed] [Google Scholar]
- Sawant A., Venkat R., Srivastava V., Carlson D., Povzner S., Cattell H., and Keall P., “Management of three-dimensional intrafraction motion through real-time DMLC tracking,” Med. Phys. 35(5), 2050–2061 (2008). 10.1118/1.2905355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B. Y., Han-Oh S., Lerma F., Berman B. L., and Yu C., “Real-time tumor tracking with preprogrammed dynamic multileaf-collimator motion and adaptive dose-rate regulation,” Med. Phys. 35(9), 3955–3962 (2008). 10.1118/1.2965261 [DOI] [PubMed] [Google Scholar]
- Ozhasoglu C., Saw C. B., Chen H., Burton S., Komanduri K., Yue N. J., Huq S. M., and Heron D. E., “Synchrony-cyberknife respiratory compensation technology,” Med. Dosim. 33(2), 117–123 (2008). 10.1016/j.meddos.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Kamino Y., Takayama K., Kokubo M., Narita Y., Hirai E., Kawawda N., Mizowaki T., Nagata Y., Nishidai T., and Hiraoka M., “Development of a four-dimensional image-guided radiotherapy system with a gimbaled X-ray head,” Int. J. Radiat. Oncol., Biol., Phys. 66(1), 271–278 (2006). 10.1016/j.ijrobp.2006.04.044 [DOI] [PubMed] [Google Scholar]
- D’Souza W. D., Naqvi S. A., and Yu C. X., “Real-time intra-fraction-motion tracking using the treatment couch: A feasibility study,” Phys. Med. Biol. 50, 4021–4033 (2005). 10.1088/0031-9155/50/17/007 [DOI] [PubMed] [Google Scholar]
- D’Souza W. D. and McAvoy T. J., “An analysis of the treatment couch and control system dynamics for respiration-induced motion compensation,” Med. Phys. 33(12), 4701–4709 (2006). 10.1118/1.2372218 [DOI] [PubMed] [Google Scholar]
- Qui P., D’Souza W. D., McAvoy T. J., and Ray Liu K. J., “Inferential modeling and predictive feedback control in real-time motion compensation using the treatment couch during radiotherapy,” Phys. Med. Biol. 52, 5831–5854 (2007). 10.1088/0031-9155/52/19/007 [DOI] [PubMed] [Google Scholar]
- Haas O. C. L., Skworcow P., Paluszczyszyn D., Sahih A., Ruta M., and Mills J. A., “Couch-based motion compensation: Modeling, simulation and real-time experiments,” Phys. Med. Biol. 57, 5787–5807 (2012). 10.1088/0031-9155/57/18/5787 [DOI] [PubMed] [Google Scholar]
- Wilbert J., Meyer J., Baier K., Guckenberger M., Herrmann C., Heb R., Janka C., Ma L., Mersebach T., Richter A., Roth M., Schilling K., and Flentje M., “Tumor tracking and motion compensation with an adaptive tumor tracking system (ATTS): System description and prototype testing,” Med. Phys. 35, 3911–3921 (2008). 10.1118/1.2964090 [DOI] [PubMed] [Google Scholar]
- Wilbert J., Baier K., Richter A., Herrmann C., Ma L., Flentje M., and Guckenberger M., “Influence of continuous table motion on patient breathing patterns,” Int. J. Radiat. Oncol., Biol., Phys. 77(2), 622–629 (2010). 10.1016/j.ijrobp.2009.08.033 [DOI] [PubMed] [Google Scholar]
- Buzurovic I., Huang K., Yu Y., and Podder T. K., “A robotic approach to 4D real-time tumor tracking for radiotherapy,” Phys. Med. Biol. 56, 1299–1318 (2011). 10.1088/0031-9155/56/5/005 [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Jiang S. B., Shimizu S., and Shirato H., “Prediction of respiratory tumor motion for real-time image-guided radiotherapy,” Phys. Med. Biol. 49, 425–440 (2004). 10.1088/0031-9155/49/3/006 [DOI] [PubMed] [Google Scholar]
- Vedam S. S., Keall P. J., Docef A., Todar D. A., Kini V. R., and Mohan R., “Predicting respiratory motion for four-dimensional radiotherapy,” Med. Phys. 31(8), 2274–2283 (2004). 10.1118/1.1771931 [DOI] [PubMed] [Google Scholar]
- Ernst F., Schlaefer A., and Schweikard A., “Predicting the outcome of respiratory motion prediction,” Med. Phys. 38(10), 5569–5581 (2011). 10.1118/1.3633907 [DOI] [PubMed] [Google Scholar]
- Ruan D., Fessler J. A., Balter J. M., and Sonke J. J., “Exploring breathing pattern irregularity with projection-based method,” Med. Phys. 33(7), 2491–2499 (2006). 10.1118/1.2207253 [DOI] [PubMed] [Google Scholar]
- Ruan D., Fessler J. A., and Balter J. M., “Mean position tracking of respiratory motion,” Med. Phys. 35(2), 782–792 (2008). 10.1118/1.2825616 [DOI] [PubMed] [Google Scholar]
- Trofimov A., Vrancic C., Chan T. C., Sharp G. C., and Bortfeld T., “Tumor trailing strategy for intensity-modulated radiation therapy of moving targets,” Med. Phys. 35(5), 1718–1733 (2008). 10.1118/1.2900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R., Suh Y., Murphy M., Williamson J., Weiss E., and Keall P., “On the accuracy of a moving average algorithm for target tracking during radiation therapy treatment delivery,” Med. Phys. 35(6), 2356–2365 (2008). 10.1118/1.2921131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierga D. P., Brewer J., Sharp G. C., Betke M., Willett C. G., and Chen G. T., “The correlation between internal and external markers for abdominal tumors: Implications for respiratory gating,” Int. J. Radiat. Oncol., Biol., Phys. 61(5), 1551–1558 (2005). 10.1016/j.ijrobp.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Anderson B. O. and Moore J. B., Optimal Filtering (Dover, Mineola, New York, 2005), pp. 53–59. [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1118/1.4802736 for an appendix.