Abstract
Approaches to imaging the breast with nuclear medicine and/or molecular imaging methods have been under investigation since the late 1980s when a technique called scintimammography was first introduced. This review charts the progress of nuclear imaging of the breast over the last 20 years, covering the development of newer techniques such as breast specific gamma imaging, molecular breast imaging, and positron emission mammography. Key issues critical to the adoption of these technologies in the clinical environment are discussed, including the current status of clinical studies, the efforts at reducing the radiation dose from procedures associated with these technologies, and the relevant radiopharmaceuticals that are available or under development. The necessary steps required to move these technologies from bench to bedside are also discussed.
Keywords: molecular breast imaging, positron emission mammography, dedicated breast PET
INTRODUCTION
Approaches to imaging the breast with nuclear medicine and/or molecular imaging methods have been under investigation since the late 1980s when a technique called scintimammography was first introduced. Nuclear medicine procedures, which detect the preferential uptake of a radiotracer in breast lesions, have the potential to offer valuable functional information that complements conventional anatomical imaging techniques such as mammography and ultrasound. By imaging the biochemical behavior of breast tissue, it was hoped that these techniques could offer improved detection and characterization of breast lesions.
Despite initial enthusiasm for scintimammography and the development of subsequent technologies over the last 20 years, nuclear medicine techniques are only recently finding their niche within the breast imaging community. This slow acceptance is likely attributable to multiple factors, but fundamentally hinges on the relatively poor spatial resolution of conventional Anger gamma cameras and positron emission tomography (PET) scanners that impedes their ability to reliably detect subcentimeter lesions in the breast. Without the ability to detect small lesions indicative of early stage disease, a breast imaging technique has limited clinical utility. The higher radiation burden associated with nuclear medicine procedures, which deliver a systemic dose to the body, relative to mammography is also an important concern, particularly if nuclear medicine tests are considered for use in the screening setting.1, 2 Additionally, previously proposed clinical indications for nuclear breast imaging technologies, such as problem solving of an equivocal mammogram or as an alternative to biopsy, have often been unwise, unclear, or lacking in sufficient evidence to support them.
Key changes in the field of nuclear imaging in recent years warrant a re-evaluation of the role of nuclear medicine in imaging breast cancer. There has been an emergence of several types of dedicated nuclear breast imaging systems (now often referred to as molecular breast imaging systems) that are optimized for breast imaging and offer substantially improved spatial resolution over conventional systems used for whole body SPECT or PET. The collection of dedicated systems currently in clinical use and under development includes a variety of detector designs for both single-photon and coincidence detection systems that are capable of detecting small breast tumors with high sensitivity. These improvements in instrumentation have in turn enabled lower administered doses of radiation to be used, such that the radiation risk from nuclear medicine breast procedures is approaching a level comparable to the extremely low risks associated with radiation exposure from mammography.
Recent clinical studies have demonstrated potential roles of nuclear methods in detecting breast disease that is occult on mammography,3 in providing a useful evaluation of both the ipsilateral and contralateral breast in the preoperative setting4, 5, 6 and in monitoring the response to neoadjuvant (primary) chemotherapy.7 These encouraging findings have led to a shift in thinking about nuclear medicine in breast imaging, and dedicated techniques are gradually becoming recognized as valid and beneficial tests for certain subsets of patients that are not served well by currently available modalities.
The purpose of this paper is to review recent developments in dedicated nuclear medicine instrumentation and discuss their potential clinical roles in the diagnosis and management of breast cancer. We also address some of the obstacles that have been encountered during the introduction of a new breast imaging modality into clinical practice and suggest strategies for success.
EARLY DEVELOPMENT OF NUCLEAR BREAST IMAGING
Like many scientific developments, the notion of imaging breast cancer with nuclear medicine began as an unanticipated finding. During the 1970s and the following two decades, a number of radiopharmaceuticals developed for purposes other than tumor imaging were observed to localize in breast disease.8
As early as 1973, the bone imaging agent technetium-99m diphosphonate (99mTc-MDP) was reported to localize in breast tumors of patients having bone scans performed for metastatic evaluation.9 The perfusion imaging agent thallium-201, developed for nuclear cardiology studies, was also found to concentrate in breast tumors as first reported in 1978.10201Tl was subsequently evaluated in investigations of breast scintigraphy over the next several years until the introduction of a new perfusion agent 99mTc-methoxyisobutylisonitrile, also known as 99mTc-sestamibi. The benefits of 99mTc-sestamibi over 201Tl included shorter half-life (6 h vs 73 h), better radiation dosimetry characteristics, and easier preparation (generator-produced vs cyclotron-produced).11 Shortly after 99mTc-sestamibi's effective replacement of 201Tl in nuclear cardiology, it was noted that sestamibi also localized in several types of tumors, primarily parathyroid adenomas, lung cancer, and breast cancer. The first report of avid 99mTc-sestamibi uptake in breast cancer is by Aktolun et al. in 1992.1299mTc-tetrofosmin, with properties very similar to 99mTc-sestamibi, was another technetium-labeled radiopharmaceutical developed for perfusion imaging reported to have avid uptake in breast tumors.13
These serendipitous discoveries of radiopharmaceutical uptake in breast cancer led to the development of scintimammography, the name given to nuclear medicine breast imaging performed with scintillating gamma cameras. The gamma cameras utilized for scintimammography were the same large field-of-view conventional gamma cameras used for nuclear cardiology and general nuclear medicine imaging. Both planar and single photon emission computed tomography (SPECT) scintimammographic methods with several options for radiopharmaceuticals were heavily investigated throughout the 1990s.8, 14
A meta-analysis of scintimammography literature performed prior to 1999 reported an aggregate sensitivity of 85% and specificity of 86% for the technique.14 However, when analysis was limited to only nonpalpable masses, aggregate sensitivity dropped to 67%.14 Several large multicenter trials were conducted to evaluate the diagnostic accuracy of 99mTc-sestamibi planar scintimammography by enrolling women with known breast masses who were scheduled to undergo excisional biopsy or surgery. These trials reported overall sensitivities between 71%–93% and specificities between 69%–87%.15, 16, 17 The sensitivity of scintimammography was substantially lower when only nonpalpable masses were considered: sensitivity for nonpalpable masses was 61% in the trial by Khalkhali et al.15 and 30% in the trial by Palmedo et al.16
The overall conclusion from examination of the numerous studies performed was that scintimammography was not able to provide reliable detection of nonpalpable, small breast tumors.8, 18 This decreased sensitivity for small breast tumors was attributed to the limited resolving power of conventional gamma cameras.14 At the time, investigators also suspected that 99mTc-sestamibi had poor uptake in some breast cancers.19, 20
The primary limitation of conventional gamma cameras for breast imaging is their inability to be easily positioned close to the breast. Conventional gamma camera detectors comprise a large single crystal of sodium iodide (NaI) coupled to an array of photomultiplier tubes (PMTs). Edge effects caused by reflecting scintillation photons at the border of the crystal result in an area of about 7–9 cm at the crystal edge that is unusable for imaging or “dead space.” Due to this large dead space at the edge of the detector, the breast cannot be imaged if positioned directly on the detector as in mammography. Anterior views of the breasts could be performed but are limited by activity in the chest and abdomen that is 2–3 orders of magnitude greater than breast activity. Hence, scintimammography was typically performed with the patient prone and with the camera positioned laterally, a technique pioneered by Khalkhali et al. to improve separation of the breast from the chest and abdomen.21, 22 Even with this positioning, the distance between the gamma camera and the pendant, uncompressed breast during planar scintimammography was ∼5 cm. As the average thickness of the pendant breast is ∼16 cm,23 a tumor in the middle of the breast could be 10–15 cm from the collimator face. Conventional gamma cameras equipped with high resolution collimators typically achieve spatial resolutions of 10–15 mm at this distance. Partial volume effects will diminish the apparent intensity of lesions below this spatial resolution, making their detection difficult.
It was hoped that the use of SPECT rather than planar imaging could improve the sensitivity of scintimammography for small breast tumors. While some studies demonstrated SPECT to offer improved sensitivity and specificity over planar methods,24, 25, 26, 27, 28 others demonstrated similar or better sensitivity with the planar technique.29, 30 Some studies recommended that planar and SPECT imaging in combination would yield the highest sensitivity.27, 29 Like the planar studies examined previously, the studies evaluating SPECT tended to include few patients with small (≤10 mm) lesions and in that subgroup, both methods showed limited sensitivity. The exception to these findings was work by Spanu et al. that demonstrated SPECT with 99mTc-tetrofosmin to be significantly better than planar scintimammography at detecting sub-cm lesions; in one study SPECT sensitivity for cancers ≤10 mm was 91% versus 45% for planar imaging (p < 0.0005), and in another study SPECT sensitivity for T1b cancers (>5 mm but ≤10 mm) was 95% versus 49% for planar (p < 0.0005).24, 25
In addition to the single photon emitting radiopharmaceuticals used for scintimammography, the positron emitter 18F-fluorodeoxyglucose (FDG) as imaged by PET accumulates in both primary breast cancer and metastatic disease. While the spatial resolution of PET is superior to that of conventional gamma cameras, current clinical PET scanners only achieve ∼4–6 mm resolution. Thus, unless uptake in the lesion is very high compared to surrounding tissues, many small, clinically relevant breast lesions are at the limit of detection of current whole body PET/CT systems. In a review of breast cancer detection with FDG PET, small tumor size (<10 mm) and low tumor grade were significant predictors of a false-negative FDG PET result.31 Currently, there is widespread agreement that whole-body FDG PET does not have a clinical role in detecting primary breast cancer, nor is it an alternative to histologic sampling to establish or exclude malignancy.32, 33
The inability of the nuclear medicine techniques discussed above to detect small breast tumors considerably limited their clinical value, especially in a field that was moving toward adoption of highly sophisticated, high resolution techniques such as breast magnetic resonance imaging. As a result, breast imaging with nuclear medicine was largely abandoned by the late 1990s. At the same time, however, researchers were just beginning to explore the potential of new dedicated nuclear systems to offer improved detection of small breast lesions.34
DEDICATED NUCLEAR MEDICINE IMAGING SYSTEMS
Single-photon gamma cameras
Over the last 15 years, several types of compact gamma camera systems have been developed for a variety of dedicated detection tasks, with breast imaging being perhaps the most widely used clinical application. Breast-dedicated gamma detectors have the ability to provide substantially improved spatial resolution compared to conventional gamma cameras due to several factors: use of pixelated arrays of small detector elements; coupling of the detector elements to electronics with better spatial discrimination; isolation of the breast from nearby organs with avid radiopharmaceutical uptake; and reduction in the lesion-to-detector distance through direct contact with the breast and application of mild breast compression.
Early dedicated detectors replaced the single NaI scintillating crystal of conventional gamma cameras with pixelated arrays of cesium iodide (CsI) or NaI coupled to position-sensitive photomultiplier tubes (PSPMTs).35, 36, 37, 38, 39, 40 Other designs have comprised multi-crystal arrays of CsI coupled to solid-state silicon photodiodes.41, 42, 43 Small multicrystal gamma cameras have typically been constructed with arrays of individual crystals ∼2 or 3 mm in size, in order to optimize detector efficiency and light collection. Loss of light at the sides of the small scintillating crystals can result in average energy resolutions in the range of 12%–17% (of 140 keV photopeak), which is poorer than conventional Anger gamma cameras,44, 45, 46 and results in decreased contrast due to the increased scatter content of events recorded in the energy window.
An alternative to the multicrystal design is a completely solid-state detector that utilizes semiconductor materials such as cadmium zinc telluride (CZT), which can provide better energy resolution and smaller pixel sizes.47, 48 Energy resolution of dedicated CZT detectors is currently in the 4%–6% range.48, 49 In theory, better energy discrimination should provide better ability to separate photopeak (unscattered) and Compton scattered counts and therefore provide better tumor contrast. However, phantom studies and simulations of CZT-based gamma cameras have suggested that the proportion of scattered counts in dedicated breast images, in which the breast is largely isolated from the chest, is relatively low.49, 50 Therefore, improvements in energy resolution below about 10% may be a less important factor in determining lesion detection than other factors such as spatial resolution and count sensitivity.
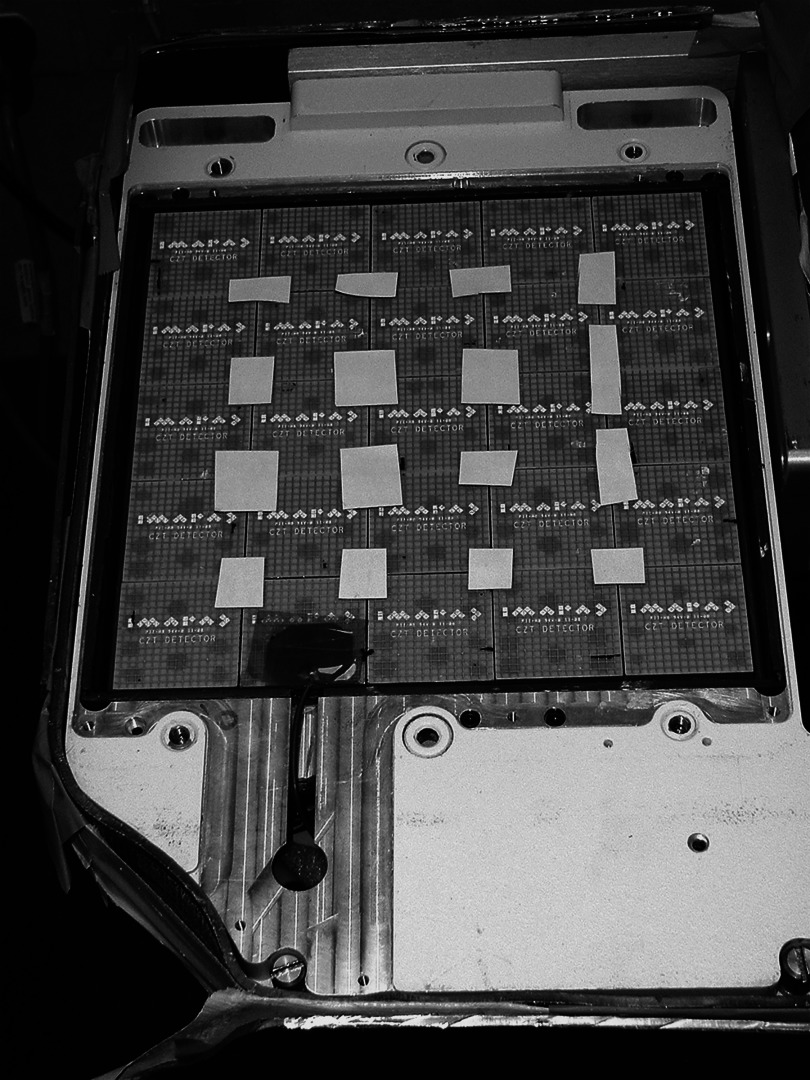
Pixel size in CZT detectors is determined by the placement of electrodes on the material and the design of the application specific integrated circuit card coupled to them. Current CZT detectors are composed of arrays of detector modules (Fig. 1), with each module containing a 16 × 16 array of individual pixels. Current systems have pixel sizes of 1.6 and 2.5 mm, with the possibility of even smaller pixel sizes in the future.51 The major disadvantage of CZT is that it is still relatively new and substantially more expensive to manufacture compared to NaI and CsI. The development of CZT-based gamma cameras is primarily being driven by the needs of the nuclear cardiology market where the use of unique CZT-based detector designs permits a factor of 3–4 reduction in either imaging time or administered dose of radiopharmaceutical.52 This new need for CZT in the clinical environment is likely to accelerate its large scale production, which will eventually result in lower costs of the material.
Figure 1.
A cadmium zinc telluride (CZT)-based detector. Collimator and cover plate have been removed to show the 5 × 5 array of CZT modules giving a 20 × 20 cm imaging detector. Each CZT module comprises 16 × 16 array of 2.5 × 2.5 mm pixels.
With appropriate collimation, both multicrystal and semiconductor-based gamma cameras are capable of providing a factor of ∼2 gain in spatial resolution compared to conventional gamma cameras at distances considered the “near-field,” that is within ∼6 cm from the collimator face.48, 53 While this improvement is of little benefit in conventional nuclear medicine imaging of the body, improved spatial resolution in the near-field has a major impact on image quality in breast imaging, where the breast is in close proximity or in contact with the detector.
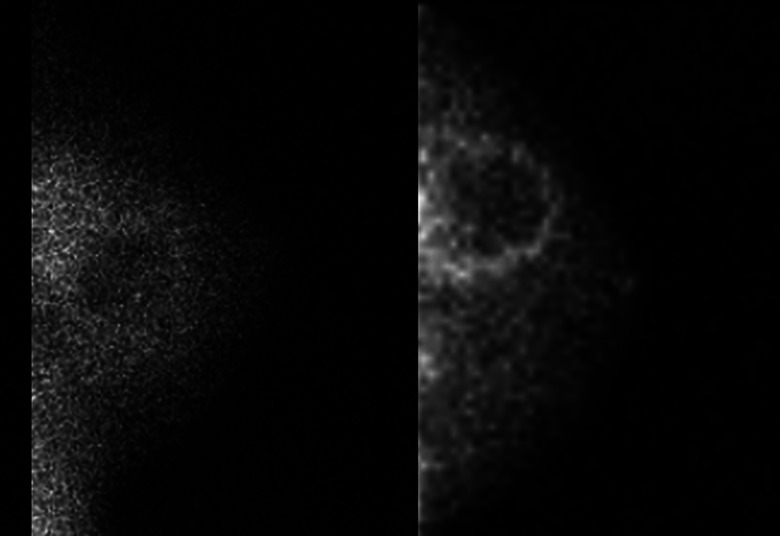
Further gains in spatial resolution are realized with dedicated detectors simply because of optimized breast positioning. Dead space at the detector edge of a dedicated detector is only about 5–8 mm, reduced from the 70–90 mm dead space of conventional systems. The compact size and minimal dead space allows the breast to be positioned directly on the camera in geometries similar to those used for mammography (e.g., craniocaudal [CC] and mediolateral oblique [MLO] views). Acquisition of these standard projections has been suggested to increase familiarity and make the study easier to read.54 Mild breast compression is also possible and is recommended to prevent motion and to further reduce distance between breast lesions and the detector. One study demonstrated that applying mild compression can reduce breast thickness from ∼16 cm for a pendant breast to ∼6 cm for a lightly compressed breast.23 Because spatial resolution of nuclear medicine detectors degrades with distance from the collimator face, bringing the lesion as close as possible to the camera is essential in improving lesion detection.34 Figure 2 shows a direct comparison between images acquired using a dedicated CZT detector and a conventional gamma camera (scintimammography) in the same patient.
Figure 2.
Lateral view of a benign breast cyst with inflammation. Images were acquired using a conventional gamma camera (scintimammography) (left) and a dedicated breast imaging gamma camera (right).
A number of names have been used to refer to the method of breast imaging with dedicated gamma camera systems, including high resolution scintimammography, single photon emission mammography (SPEM), breast specific gamma imaging (BSGI), molecular breast imaging (MBI), and others. The assignment of a unique name by each research team evaluating a particular technology has created some confusion within and outside of this field of study. The term “scintimammography” was firmly associated with the conventional technique of the 1990s that had unfortunately obtained a poor reputation in breast imaging. Thus, proponents of dedicated systems sought to distinguish the new compact cameras from scintimammography performed with conventional systems. Furthermore, the new semiconductor-based detectors no longer relied on a scintillating crystal detector so the term “scinti” mammography was actually inaccurate.
In recent years, developers of breast-dedicated nuclear medicine systems have begun to embrace “molecular breast imaging” or MBI as a collective term to describe compact detectors utilizing either single-photon- or positron-emitting (described next) radiopharmaceuticals. Although several dedicated gamma camera systems are actively undergoing development in the research setting, today three different types of single-photon MBI systems exist in the commercial market.
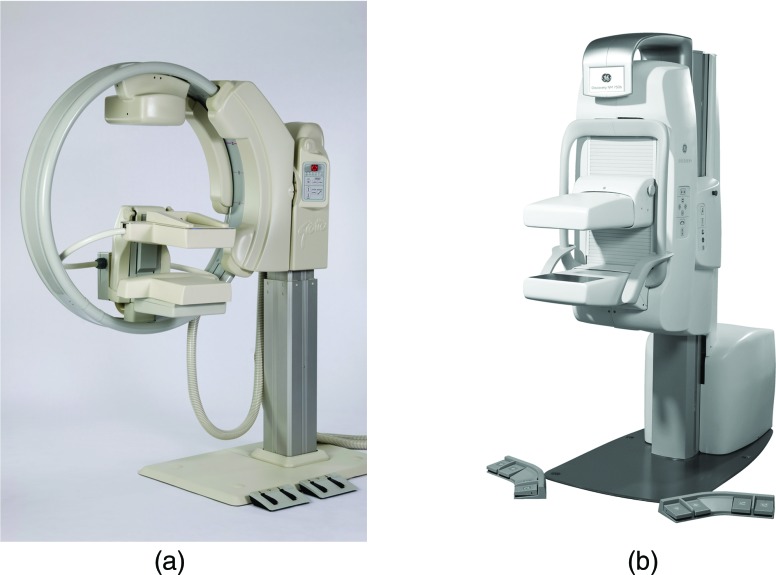
The dedicated system with the longest history of commercial use is the Dilon 6800 (Dilon Diagnostics, Newport News, VA), most commonly referred to as a breast specific gamma imaging (BSGI) system. The original BSGI system employs a single gamma camera with a 20 × 15 cm field of view (FOV) containing an array of NaI crystals (3 × 3 mm pixel size) coupled to an array of position sensitive photomultiplier tubes (PSPMTs). An initial report from a patient study conducted on this system showed the BSGI system to offer improved detection of smaller and nonpalpable lesions compared to scintimammography performed on a conventional gamma camera.19 The latest generation BSGI system, the Dilon 6800 Acella (Fig. 3), employs CsI crystals (3.2 × 3.2 mm pixel size) coupled to solid-state photodiodes and has a larger FOV of 25 ×20 cm.
Figure 3.
Dilon 6800 gamma camera for breast specific gamma imaging (BSGI). (courtesy of Dilon Diagnostics)
Two dedicated CZT-based detectors for MBI are commercially available: Discovery NM750b (GE Healthcare, Milwaukee, WI) [Fig. 4a] and LumaGem 3200s (Gamma Medica, Inc., Northridge, CA) [Fig. 4b]. Both of these systems employ two opposing CZT detectors in a dual-head configuration, where the breast is positioned between the two detectors. The current Discovery NM 750b has 2.5 mm-pixels and a FOV of 24 × 16 cm (recently changed from 20 × 20 cm). The LumaGem has 1.6 mm-pixels and 20 × 16 cm FOV. Manufacturers of these dedicated systems have recognized the need to increase detector FOV to accommodate larger breasts and in particular to increase detector width such that both the axilla and intramammary fold can be visualized in the MLO position.
Figure 4.
(a) Gamma Medica Lumagem 3200s system (courtesy of Gamma Medica, Inc.). (b) GE Discovery NM750b system (courtesy of GE Healthcare).
While a dual-head configuration is more expensive than a single head system, this arrangement has the advantage of ensuring that a breast lesion can never be more than ½ the breast thickness from either detector. Use of a dual-head CZT-based design was shown to improve sensitivity for the detection of small breast tumors (≤10 mm), particularly those located in the upper inner quadrant of the breast, compared to a single CZT detector: sensitivity was 82% for dual-head versus 68% for single head gamma imaging, p = 0.004).55 Work conducted with phantoms on multicrystal NaI-PSPMT cameras has also advocated for the use of a dual-head configuration to improve lesion detection.38, 56, 57, 58, 59
Besides improving lesion detection, the dual-detector configuration also allows a specialized method for quantitative analysis of tumor uptake to be performed.60 Both adipose and fibroglandular breast tissue have similar attenuation coefficients at energies of 140 keV. As breast thickness can be readily measured, an absolute estimate can be made of activity in a breast lesion and adjacent normal tissue. As these technologies become better integrated into breast imaging practices, the ability to quantitate tumor uptake may be of particular benefit for certain applications such as monitoring tumor response to neoadjuvant chemotherapy.
Coincidence detection systems
Just as compact gamma cameras were developed to overcome limitations of conventional scintimammography, several compact breast-specific PET systems were concurrently designed to overcome limitations of conventional PET scanners. The introduction of a positron emission mammography (PEM) system was initially met with some skepticism. At the time, only a small number of facilities were offering PET imaging as radiopharmaceutical production of FDG and required personnel were considered too expensive for routine use.61 Historically, enthusiasm for organ-specific PET devices, such as those specifically created for brain PET, has been low.62 However, PET has become widely incorporated into clinical practice at major medical centers during the last decade, FDG is now readily available, and dedicated equipment optimized for the breast is expected of breast imaging technologies.
The primary advantage of a breast-dedicated design over conventional PET scanners is substantially improved spatial resolution. Coincidence detectors operate by detecting two 511 keV gamma rays emitted during positron annihilation. To perform reconstruction, it must be assumed that the gamma rays travel in opposite directions 180° apart, which is not always the case if the positron is not entirely at rest when annihilation with an electron occurs. Compared to conventional PET scanners, made up of a ring of detectors about 1 m in diameter, a PEM system with two detectors in direct contact with the breast will have much smaller detector separation (the ∼5–6 cm thickness of a lightly compressed breast) and will be less prone to blurring due to angular noncolinearity of the emitted gamma rays. The use of smaller crystals in dedicated devices compared to conventional PET also aids in improved spatial resolution.
In 1994, Thompson et al. provided the earliest proposal of a PEM system by simulating two flat detector arrays of bismuth germinate (BGO) operating in coincidence that could be incorporated within a conventional mammography unit.61 Experiments of their proposed design suggested that a PEM device could theoretically achieve 2 mm spatial resolution (compared to 4–6 mm in-plane resolution of a conventional PET scanner).63 Because the compact detector design would subtend a larger solid angle of the emitted gamma rays, this PEM design was expected to achieve approximately 100 times the count sensitivity of conventional PET.64 The first evaluation of PEM in patients, using this BGO detector as an accessory to a mammography unit, was reported in 1996.65
By imaging only the breast rather than the entire chest (as with whole-body PET), the proportion of scattered events is low and tissue attenuation is so minimal that attenuation correction is simple or may not even be required.61, 66 An additional advantage of PEM over dedicated gamma cameras described above was the ability to reconstruct either limited-angle or tomographic 3D slices of the breast rather than only single-projection planar images.
Since that initial feasibility study, numerous other breast-specific PET designs were proposed with arrays of high density scintillating detectors coupled to PSPMTs or more recently, semiconductor photodiodes. The scintillators most commonly used include BGO, lutetium oxyorthosilicate (LSO), lutetium gadolinium oxyorthosilicate (LGSO), and lutetium yttrium oxyorthosilicate (LYSO), but CsI, NaI, and CZT have also been proposed.66, 67, 68, 69, 70, 71, 72, 73, 74 These reports have described primarily two types of designs: either two flat detectors placed on opposite sides of the breast that mimic mammographic positioning (typically referred to as PEM systems), or a small ring of detectors within which the breast hangs pendant (often referred to as a dedicated breast PET [DbPET] design). Results from simulations comparing PEM and DbPET designs demonstrated that both offered substantially better lesion visualization than whole-body PET, but showed pros and cons of each type of design.67 The two detector PEM design offered lower noise, but the DbPET ring design offered better contrast and signal-to-noise ratios. A recognized practical advantage of the PEM design is its flexibility in positioning that can accommodate various breast sizes and provide better visualization of the axilla and lesions near the chest wall.
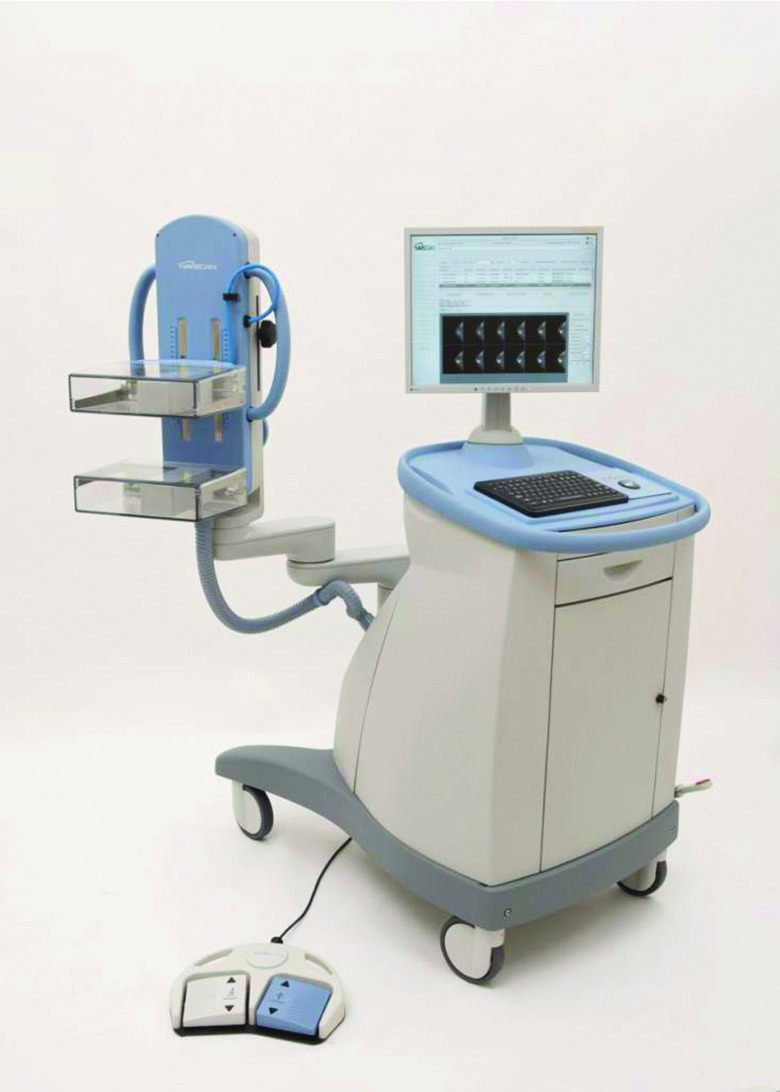
The first PEM system to become commercially available was the PEM-FLEX (Naviscan Corporation, San Diego, CA). The PEM-FLEX comprises two opposing detectors that are contained within transparent compression plates (Fig. 5). Analogous to mammographic positioning, the patient's breast is placed between the two plates, although only mild compression is applied. The two detectors are both long, narrow configurations of LYSO crystals (dimensions of 2 × 2 × 13 mm) that move in unison across the paddles to scan the entire 24 × 16.4 cm FOV. Limited-angle tomosynthetic reconstruction is performed to generate 3D slices. Performance evaluations of the PEM-FLEX demonstrated an in-plane spatial resolution of 1.8–2.4 mm, depending on the reconstruction mode used.75, 76 Resolution between planes is degraded due to the limited angle tomography and ranges from 6 to 9 mm.76 No attenuation correction is applied with this system.
Figure 5.
Naviscan PEM-FLEX Solo II system (courtesy of Naviscan).
The Mammi-PEM (Oncovision, Valencia, Spain), which is a true tomographic DbPET system, achieved CE certification in Europe in early 2011 and is commercially available. The Mammi-PEM (Fig. 6) comprises a ring of 12 detectors, each with 40 × 40 mm monolithic crystals of LYSO coupled to PSPMTs. In place of small individual crystals, this design opted for larger crystals to improve count sensitivity and relies on the PSPMT and Anger like electronics to calculate the location of the light pulse within each crystal. Reported intrinsic spatial resolution of the Mammi-PEM is 1.6 mm.77 For imaging, the patient lies prone on an imaging table and a breast hangs pendant through a table opening into the unit positioned directly beneath. Using an injected dose of ∼65 MBq (1.8 mCi) 18F-FDG, a full 3D tomographic dataset can be acquired of each breast in ∼5 min, with a resolution of ∼2 mm that is relatively isotropic.78 The system incorporates a calculated (rather than measured) attenuation correction method.79
Figure 6.
Oncovision Mammi-PEM system. Cover has been removed to show the 12 detector modules in a ring geometry (courtesy of Oncovision).
In addition to these commercial systems, there is a substantial amount of research activity focused on improving PEM and DbPET imaging technology. Several types of systems are currently under development by research groups around the world, including University of California-Davis,80, 81 Lawrence Berkeley National Laboratory,82 Brookhaven National Laboratory,83 Stanford University,84 Duke University,85 Thomas Jefferson National Accelerator Facility and West Virginia University,86, 87 MD Anderson,88 University of Pisa,89 the Crystal Clear Collaboration at CERN,90, 91, 92 and Shimadzu Corporation.93 The developers of these systems are beginning to collect initial patient data and rapidly moving toward clinical trials.
The Clear-PEM design under development by the Crystal Clear Collaboration, is a hybrid type of PEM design in that it comprises two parallel detectors on opposite sides of the breast, but, with the patient lying prone, the detectors can rotate around the breast during acquisition to allow tomographic reconstruction.91 The Clear-PEM detector uses LYSO crystals that are coupled to multipixel avalanche photodiodes. By using 2 photodiodes per crystal in the Clear-PEM, a double readout of emitted light is performed, which in turn enables a depth of interaction (DOI) calculation to be done. Using DOI, the Clear-PEM has reported spatial resolution of 1.4 mm at the center of its field of view.92
One system that is unique in design compared to other DbPET systems is the C-Shaped detector by Shimadzu that is a partial ring of 4096 LGSO crystals over a 300° arc. The patient is positioned semiprone into the C-shaped ring and a tomographic data set is acquired. The system is expected to achieve a spatial resolution of <1.5 mm.94 Recent work has shown the clinical feasibility of this system.95
Multimodality systems
One of the enduring messages learned from the development of PET over the last decade is that fused anatomical and functional information obtained using a combined PET/CT device is more accurate in evaluating patients with known or suspected malignancy than either PET or CT alone or PET and CT acquired separately but interpreted together.96, 97 Translation of that message into breast imaging has spurred the development of multimodality systems capable of providing both anatomical and functional information in a single examination.
As previously mentioned, early developers of breast-dedicated gamma cameras and coincidence detection systems intended for nuclear imaging to be incorporated as an accessory to mammography, rather than an independent device, to allow easy coregistration of anatomical and functional data. This was the case for the initial PEM designs61, 98, 99, 100 and for the original Gamma Medica LumaGem MBI detector.101
In the breast, coregistration of anatomy and function may be more important than in other organs because breast tissue is so deformable, with few anatomical landmarks. The three most-widely used breast imaging modalities use completely different positioning techniques: the patient is upright with breast tissue compressed during mammography, the patient is supine with breast tissue flat against chest wall during ultrasound, and the patient is prone with breasts hanging pendant during MRI. Even breast radiologists who are accustomed to correlating modalities often find that positioning differences make it difficult to correctly identify the same lesion(s) across modalities.
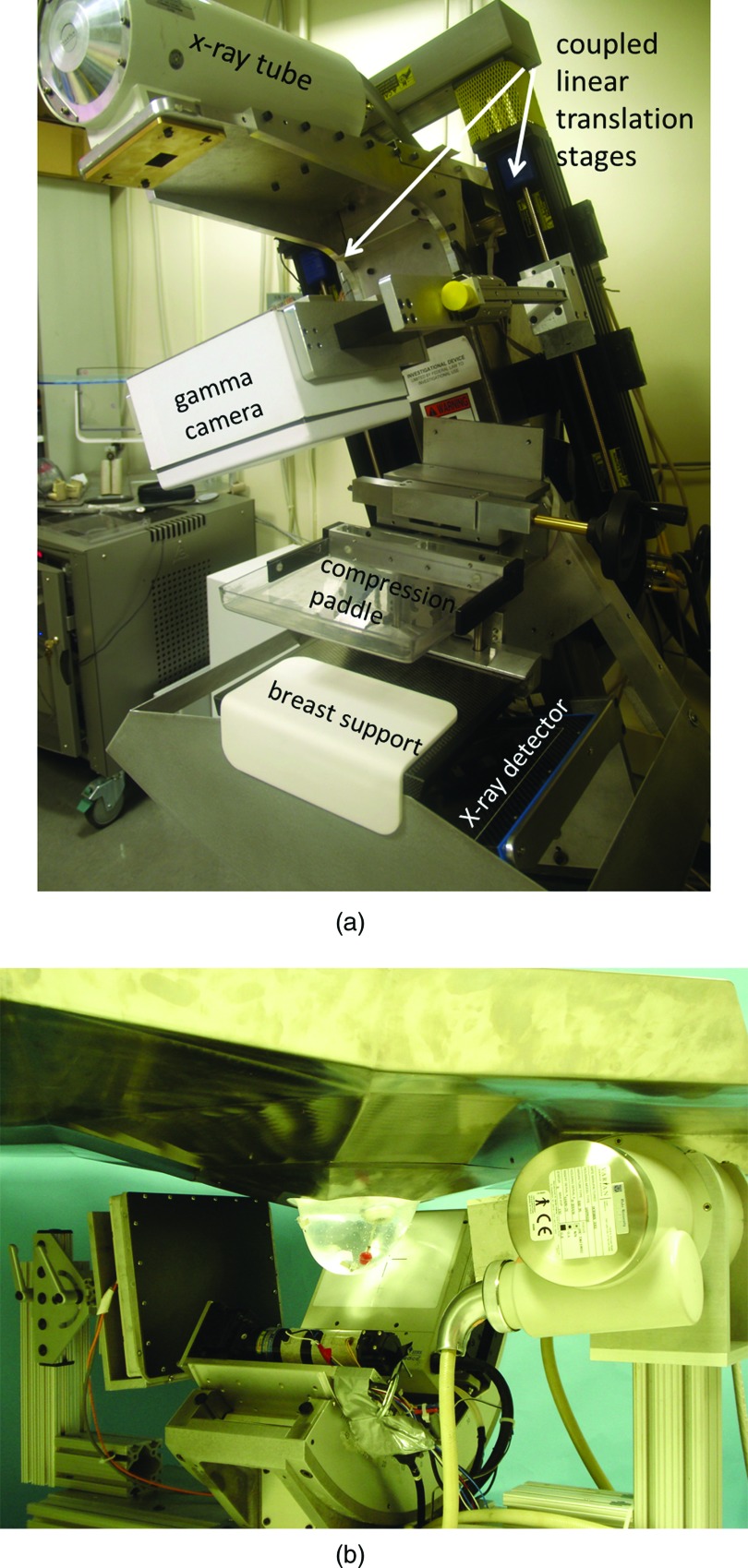
Researchers at the University of Virginia have worked on the development and evaluation of a hybrid system called dual-modality breast tomosynthesis (DMT) that combines digital x-ray tomosynthesis and limited-angle SPECT.102, 103 The system comprises a small 15 × 20 cm dedicated NaI gamma camera and a mammography system mounted on a tomosynthesis gantry [Fig. 7a]. The x-ray tube, x-ray detector, and gamma camera rotate ± 20° around a common axis, producing coregistered x-ray and gamma ray images in three dimensions. A prospective pilot study in 17 patients demonstrated the feasibility of this approach and suggested that the addition of unambiguous correlations between the anatomical and functional information could improve specificity.103
Figure 7.
Multimodality single-photon systems. (a) Dual-modality breast tomosynthesis (DMT) system combining digital x-ray tomosynthesis and limited-angle SPECT. A small high-resolution gamma camera is mounted on a translation stage attached to the gantry arm. The configuration during gamma imaging is shown here. The camera is positioned anteriorly out of the x-ray beam during x-ray imaging. Image courtesy of Dr. Mark Williams, University of Virginia. (b) Combined SPECT/CT system developed at Duke University. Patient is positioned prone and breast is imaged with a small CZT based detector and CT system. Image courtesy of Dr. Martin Tornai, Duke University Medical Center.
A breast-dedicated SPECT/CT system has been developed by researchers at Duke University.104 This system incorporates a small CZT detector mounted on a gantry that is capable of unique, complex acquisition trajectories [Fig. 7b]. Phantom work has demonstrated that these trajectories allow a larger volume of breast tissue to be imaged and permit better imaging of breast tissue near the chest wall.105 Pilot patient data has been reported in patients with this device.106
Several of the coincidence detection systems mentioned above are under investigation as multimodality devices. The system developed at University of California-Davis is a combined DbPET/CT system that acquires fully 3D images of the pendant breast using rotating LSO detectors and digital breast CT technology.80, 81, 107 Figure 8 shows an example of the combined DbPET/CT image of a patient with invasive breast cancer. Researchers at Brookhaven National Laboratory are developing a combined PET/MRI system that uses a DbPET design incorporated into a commercial breast-dedicated MRI system (Aurora Imaging Technology, North Andover, MA).83 The consortium at CERN that developed the Clear-PEM unit is working on the development of the Clear-PEM-Sonic unit that integrates PEM with an ultrasound system.108
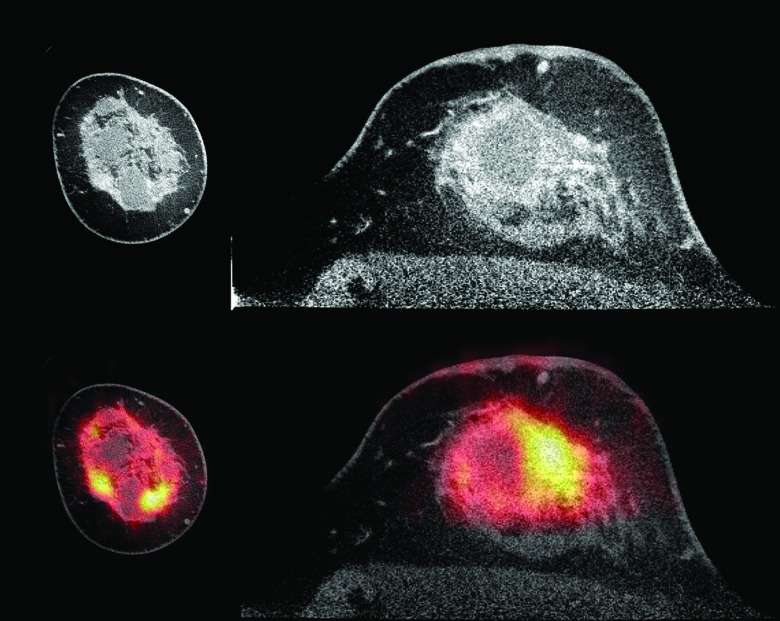
Figure 8.
Images of a patient with an invasive ductal carcinoma with lobular features obtained using a dedicated LYSO PET/CT system. The contrast enhanced CT, shown at top (coronal view at left and axial view at right), has been window to enhance appearance of benign cysts. The fused PET/CT image, at bottom, demonstrates F-18 FDG uptake in the extensive tumor. Image courtesy of Dr. Ramsey Badawi, University of California Davis.
CLINICAL EVALUATIONS
Establishing diagnostic accuracy
Of the many types of breast-specific detectors described above, few designs have progressed to evaluation with clinical studies. As initially done with conventional scintimammography, the first patient studies to determine clinical performance of dedicated gamma camera systems and PEM systems were conducted in patients with known breast lesions. The primary enrollment criterion was a lesion identified by mammography or clinical exam that was deemed suspicious and scheduled for biopsy. In most studies, the nuclear medicine imaging was performed prior to biopsy and the final surgical pathology findings were used as reference standard to determine the performance of imaging. This type of study design has been heavily utilized to establish baseline values for diagnostic accuracy (sensitivity and specificity) of the techniques and to investigate the relative advantages and limitations of dedicated nuclear imaging compared to other breast imaging modalities.
The diagnostic accuracy studies performed with independent participants are given in Table 1. Although notable differences exist between studies, in general their results demonstrate good sensitivity and specificity for breast cancer with dedicated nuclear medicine systems. In particular, the sensitivity for small breast cancers is respectable and improved for small lesions compared to values previously reported with conventional scintimammography. In the ten studies performed with single photon gamma camera systems in a total of 778 patients, the overall sensitivity was 556 of 605 (92%), the sensitivity for cancers ≤10 mm was 199 of 242 (82%), and the specificity was 218 of 294 (74%). In the four studies performed with PEM systems in a total of 130 patients, overall sensitivity was 75 of 86 (87%), sensitivity for cancers ≤10 mm was 11 of 15 (73%), and specificity was 53 of 62 (85%).
Table 1.
Findings from independent “diagnostic accuracy” studies of dedicated gamma cameras and PEM in which the primary enrollment criteria for subjects was a suspicious lesion identified by physical exam, mammography, or other imaging.
| Author | Year | Camera type | Radio-pharmaceutical | Administered activity (MBq) | Nuclear study interpretation | N | Sensitivity for all cancers3 | Sensitivity for cancers ≤10 mm3 | Sensitivity for DCIS3 | Specificity for benign lesions3 |
|---|---|---|---|---|---|---|---|---|---|---|
| De Vincentis (Ref. 35) | 1997 | Single-head CsI-PSPMT gamma camera | Tc-99m sestamibi | 740 | Not reported | 7 | 4/4 (100) | 3/3 (100) | – | 3/3 (100) |
| Pani (Ref. 44) | 1998 | Single-head CsI-PSPMT gamma camera | Tc-99m sestamibi | 740 | Not reported | 14 | 9/11 (82) | 5/7 (71) | – | 2/3 (67) |
| Maini (Ref. 211) | 1999 | Single-head CsI-PSPMT gamma camera | Tc-99m sestamibi | 740 | Not reported | 29 | 16/19 (84) | 6/9 (67) | – | 8/10 (80) |
| Scopinaro (Ref. 23) | 1999 | Single-head CsI-PSPMT gamma camera | Tc-99m sestamibi | 740 | Blinded | 53 | 27/31 (87) | 13/16 (81) | – | 19/22 (86) |
| Brem (Ref. 19) | 2002 | Single-head NaI-PSPMT gamma camera | Tc-99m sestamibi | 925 | Blinded | 50 | 22/28 (79) | 10/15 (67) | – | 28/30 (93) |
| O'Connor (Ref. 212) | 2007 | Single-head CZT gamma camera | Tc-99m sestamibi | 740 | Access to MG and US findings | 99 | 57/67 (85) | 26/35 (74) | 8/8 (100) | 36/47 (77) |
| Spanu (Ref. 213) | 2007 | single-head CZT gamma camera | Tc-99m tetrofosmin | 740 | Blinded | 85 | 87/90 (97) | 28/31 (90) | – | 11/12 (92) |
| Brem (Ref. 214) | 2008 | Single-head NaI-PSPMT gamma camera | Tc-99m sestamibi | 925–1110 | In clinical practice, access to available imaging | 146 | 80/83 (96) | 17/20 (85) | 15/16 (94) | 50/84 (60) |
| Hruska (Ref. 55) | 2008 | Dual-head CZT gamma camera | Tc-99m sestamibi | 740 | Blinded | 150 | 115/128 (90) | 50/61 (82) | 16/17 (94) | 42/61 (69) |
| Spanu (Ref. 215) | 2008 | Single-head CZT gamma camera | Tc-99m tetrofosmin | 740 | Blinded | 145 | 139/143 (97) | 41/45 (91) | – | 19/22 (86) |
| Total | 778 | 556/604 (92) | 199/242 (82) | 39/41 (95) | 218/294 (74) | |||||
| Murthy (Ref. 216) | 2000 | Dual-head BGO-PSPMT PEM system | F-18 FDG | 75 | Access to MG findings | 14 | 8/10 (80) | – | – | 4/4 (100) |
| Levine (Ref. 217) | 2003 | Dual-head GSO-PSPMT PEM system | F-18 FDG | 370 | Access to MG findings | 16 | 6/7 (86) | 4/4 (100) | 2/2 (100) | 10/11 (91) |
| Rosen (Ref. 85) | 2005 | Dual-head LGSO-PSPMT PEM system | F-18 FDG | 74–93 | Access to MG findings | 23 | 18/21 (86) | 2/3 (67) | 2/3 (100) | 1/3 (33) |
| Berg (Ref. 218) | 2006 | Dual-head LYSO-PSPMT PEM system | F-18 FDG | 300–795 | Access to MG findings | 77 | 39/42 (93) | 5/8 (63) | 10/11 (91) | 29/35 (83) |
| Total | 130 | 75/86 (87) | 11/15 (73) | 14/16 (88) | 53/62 (85) |
Numbers in parentheses are percentages and are rounded.
N = number of analyzable participants. MG = mammography. US = ultrasound. DCIS = ductal carcinoma in situ.
Sensitivity and specificity are reported on the per lesion level and some patients had more than one lesion.
Preoperative evaluation
Accurate evaluation of disease extent is critical for guiding appropriate surgical management of patients diagnosed with breast cancer. The addition of MRI to standard assessment with clinical exam, mammography, and ultrasound is increasingly being incorporated into preoperative planning, but this approach is controversial.109 Preoperative MRI has been associated with increasing rates of mastectomy over breast conservation therapy,110, 111 however, to date there is a lack of evidence to demonstrate that use of preoperative MRI can reduce rates of positive margins or local recurrence, or lead to improved survival. Despite this, data from prospective trials have shown that after conventional evaluation, use of preoperative MRI detects additional sites of mammographically-occult cancer in the ipsilateral breast of 7%–12% of women4, 112 and the contralateral breast of 3%–4% of women.5, 113 Preoperative measurements of disease extent on MRI have been shown to modestly correlate with pathologic tumor size; reported correlation (r) between tumor size and pathologic size is 0.53–0.81 but a number of studies have noted that MRI measurements tend to over- or underestimate true tumor size.4, 114, 115, 116
While preoperative MRI has been shown to have very high sensitivity that approaches 100% when combined with mammography and clinical evaluation,117 its specificity is highly variable, with reported values ranging from 26% to 90%.4, 5, 118, 119, 120 Nuclear medicine techniques have been proposed as useful tests for detection of multifocal, multicentric, or contralateral disease on the basis that they may offer better specificity than MRI, yet still offer better sensitivity than mammography, especially in radiographically dense breasts.
Several studies have been performed to investigate the efficacy of dedicated gamma cameras as an adjunct to conventional diagnostic workup (physical exam, mammography and/or ultrasound) in preoperative evaluation. In patients with newly diagnosed breast cancer, gamma imaging with BSGI identified additional sites of malignancy in 9%–11% of patients, while giving false positive results for additional sites in 7%–20% of patients.6, 121, 122 In a comparison of BSGI with MRI used in preoperative evaluation, the sensitivities of the two techniques were similar (89% and 92%) but BSGI demonstrated significantly better specificity of 90% compared to 39% for MRI (p < 0.0001).123
A study performed using a dedicated CZT-based MBI system and 99mTc-tetrofosmin in 264 patients showed gamma imaging to significantly improve detection of multifocal or multicentric disease compared to that detected with mammography (88% vs 48% of cases, p < 0.0005) and to correctly alter surgical management in 16% of patients.124 Preliminary results from a similar study using a dual-head version of the CZT-based MBI system and reduced doses of ∼300 MBq 99mTc-sestamibi were reported: gamma imaging detected additional disease not appreciated by mammography and ultrasound in 12 of 98 patients (12%), which altered surgical management.125 A comparison of measured tumor size on MBI and pathological size showed good correlation of r = 0.681.7
Preoperative evaluation has been one of the primary proposed indications for PEM technology and a number of studies have been performed to investigate the efficacy of PEM in this setting.4, 5, 126, 127, 128 Findings from a large multicenter trial comparing PEM to MRI in preoperative planning were recently reported by Berg et al.4, 5 In preoperative evaluation of the ipsilateral breast in 388 patients,4 the addition of either PEM or MRI to conventional imaging (mammography and/or ultrasound) improved sensitivity for additional malignancies compared to conventional imaging alone. PEM and MRI were found to be complementary: adding MRI to conventional imaging detected additional disease in 13% of patients, adding PEM to conventional imaging detected additional disease in 11% of patients, and the combination of conventional imaging with both MRI and PEM detected additional disease in 18% of patients. MRI demonstrated greater lesion-level sensitivity and more accurately depicted the need for mastectomy compared to PEM, but PEM had greater specificity at the breast and lesion levels and therefore prompted fewer biopsies that proved benign. Tumor size measured on imaging was better correlated with pathological size using MRI measurements (r = 0.81) compared to PEM measurements (r = 0.55, p < 0.001).
In a separate report of preoperative evaluation of the contralateral breast in 367 women with breast cancer,5 the sensitivity of MRI for contralateral malignancies was significantly higher than PEM (14 of 15 [93%] vs 3 of 15 [20%], p < 0.001). However, a subsequent retrospective blinded interpretation of PEM imaging by experienced readers detected 11 of 15 (73%) contralateral malignancies, citing interpretive errors or assignment of a probably benign assessment as factors for false negative results.
Monitoring neoadjuvant therapy
Closely tied with the need for accurate imaging in preoperative evaluation is the need for accurate imaging in monitoring response to neoadjuvant therapy. Neoadjuvant therapy, which is chemotherapy or hormonal therapy administered prior to surgical treatment, is increasingly being used as evidence suggests that it can improve patient outcomes by reducing cancer burden and potentially allowing less extensive surgery. Early assessment of the tumor's response to therapy can indicate if treatment should be altered or prolonged in order to achieve optimal response prior to surgery. Measurement of the residual disease at the completion of neoadjuvant therapy can also be used to guide surgical options. Thus, accurate assessment of response to neoadjuvant therapy through diagnostic imaging is critical to fully utilize the benefits of neoadjuvant therapy.
Similar to the setting of preoperative evaluation, there is no standard protocol that dictates which imaging tools should be used in monitoring neoadjuvant therapy. Physical exam, mammography, ultrasound have been shown to only moderately correlate with pathologic size and often over- or underestimate disease extent.129 All diagnostic imaging tests are inherently unable to identify with certainty the absence of all malignant cells and therefore have been demonstrated to unreliably predict pathologic complete response (pCR).130, 131 However, it has been proposed that functional imaging with nuclear medicine techniques may be able to better characterize residual tumor viability and reflect response at an earlier stage through its ability to depict a decrease in tumor function that precedes a decrease in anatomical size.
Initial studies performed with conventional gamma cameras in patients with locally advanced breast cancer provided evidence that 99mTc-sestamibi uptake could accurately differentiate responders from nonresponders to neoadjuvant chemotherapy.132, 133, 13499mTc-sestamibi uptake was also demonstrated to have prognostic significance: in a series of 62 patients, residual uptake observed on scintimammography studies performed after completion of neoadjuvant therapy was significantly associated with poorer disease free survival and poorer overall survival.135 It must be noted, however, that sestamibi has reduced uptake in tumors that express the P-glycoprotein-mediate multidrug resistance gene136 and therefore lack of uptake may in some patients reflect drug resistance rather than reduced tumor function. Conventional PET with 18F-FDG has also demonstrated potential for predicting response to neoadjuvant therapy.137, 138 Ultimately, because conventional scintimammography and PET lack the necessary spatial resolution to visualize very small residual tumor foci, they are limited in their ability to accurately determine whether residual disease is present.
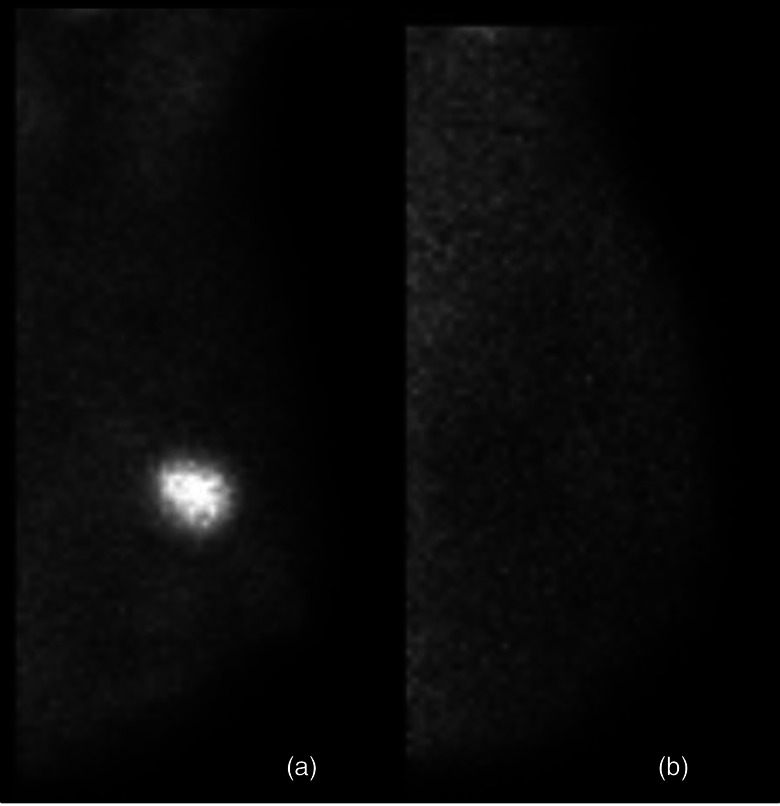
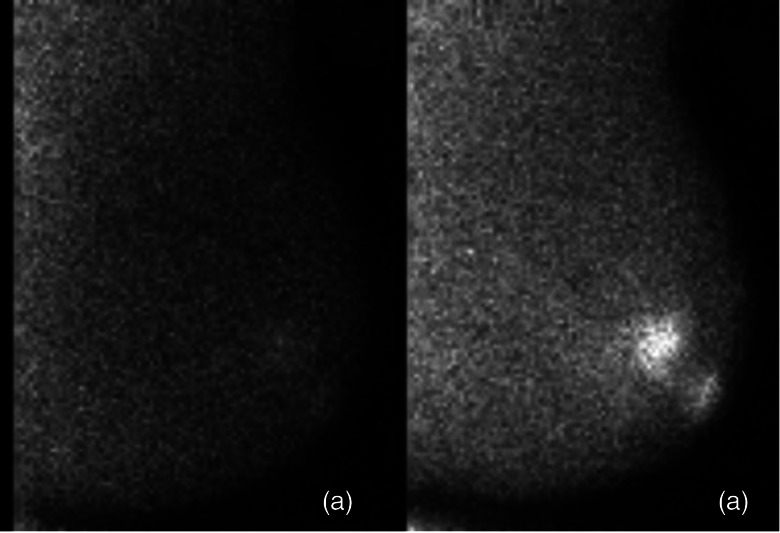
Dedicated systems with improved spatial resolution have thus allowed for a renewed consideration of using functional radiotracer uptake in assessing response. Spanu et al. demonstrated in a small series of patients that a dedicated MBI gamma camera could visualize uptake of 99mTc-tetrofosmin in multifocal, sub-cm scattered cancers remaining after neoadjuvant therapy when findings on a conventional gamma camera incorrectly demonstrated no residual disease.139 Pilot data from the Mayo Clinic has shown that MBI performed with 99mTc-sestamibi is capable of indicating response as early as 3 weeks following initiation of chemotherapy (Fig. 9).140 Additionally, measurements of tumor size have correlated well with pathology size, indicating that MBI could be useful in guiding surgical choices.7, 140
Figure 9.
A patient with a 2.2 cm invasive breast cancer was imaged with MBI prior to initiation of neoadjuvant chemotherapy (panel a) and again at 3 weeks after initiation (panel b). MBI performed at 3 weeks showed nearly complete resolution of the tumor, indicating a functional response to neoadjuvant chemotherapy. Surgery performed after four months of therapy showed complete pathological response (pCR), i.e., no residual in situ or invasive disease.
Encouraging findings showing the ability of PEM systems to monitor neoadjuvant chemotherapy have been reported. In trials underway at MD Anderson using the Naviscan PEM system, quantitative measurements of uptake on PEM were performed at baseline and 14 days into chemotherapy. Both higher baseline uptake and a decrease in uptake from baseline to 14 days into chemotherapy were significantly associated with pCR.141 The group at UC Davis is also performing a trial in neoadjuvant chemotherapy patients using their dedicated PET/CT system.142
Comparisons with MRI
The design and implementation of a research study that compares diagnostic accuracy of nuclear techniques and MRI in detection of breast cancer is challenging. Because MRI is capable of detecting very small lesions that may never be found at surgery or never reported by the pathologist, determining a reference standard for every diagnosed lesion is sometimes not possible, especially when analysis is performed retrospectively.
Most reports comparing performance of nuclear techniques to that of MRI have been done retrospectively and have noteworthy limitations such as small numbers of patients and unblinded interpretations of the imaging studies. In reviews comparing performance of dedicated gamma camera systems and MRI, findings have indicated that gamma imaging and MRI correlate well and have similar sensitivities for cancer, and gamma imaging demonstrated better specificity in a limited number of patients.123, 143, 144, 145, 146, 147 The only prospective trials yet performed to compare performance of a dedicated nuclear technology with MRI are the studies investigating preoperative evaluation with PEM compared to that with MRI that were previously discussed.4, 5, 127 Based on complementary findings between PEM and MRI, the authors concluded that PEM could be a useful option for patients who could not tolerate MRI.
Screening
Screening mammography has a long history as the best available screening test for the general population of women due to several important factors: wide accessibility; relatively low cost to patients and reimbursement by insurance carriers; low radiation risks; fast patient throughput; highly regulated systems for quality control of equipment and images (such as the American College of Radiology (ACR) Mammography Accreditation Program and the Mammography Quality and Standards Act [MQSA]); and an established and validated system for radiologist interpretation [ACR Breast Imaging Reporting and Data System (BI-RADS)148]. Mammography is also the only screening modality yet to demonstrate a breast cancer mortality benefit.149, 150 Thus, any new technology trying to gain the same widespread acceptance as mammography in the screening setting is at a distinct disadvantage, as many years and many patients studied are required to build up the necessary evidence and infrastructure to support its clinical implementation.
Despite widespread implementation of mammographic screening, it is well recognized that mammographic sensitivity is considerably limited in certain subsets of patients, such as women with radiographically dense breasts,151, 152, 153, 154, 155 those with a personal history of breast cancer,156 and those who are carriers of the breast cancer gene BRCA-1 or -2.157 The subset of women with dense breasts is particularly interesting due to current increases in public awareness and discussion about the topic of breast density.
It is estimated that over 30% of women age 40 and older have breast tissue considered dense on mammography—that is, their mammograms are classified as one of the BI-RADS density categories of heterogeneously dense or extremely dense.158 Density is not only associated with reduced sensitivity (reported sensitivity in extremely dense breasts is between 29% and 62% (Refs. 151, 152, 154), but epidemiological evidence also demonstrates that density is a strong independent risk factor for development of breast cancer. In a study by Boyd et al., women with the densest breasts on mammography (>75% fibroglandular tissue) were up to five times more likely to develop breast cancer than those with almost no density (<10% fibroglandular tissue).159 Due to efforts by patient advocate groups, legislation in several U.S. states now requires medical centers to notify patients if their screening mammogram demonstrates dense tissue and to recommend considering additional screening with another imaging modality. Digital tomosynthesis, whole-breast ultrasound (automated or hand-held), MRI, and nuclear medicine techniques have all been under study as supplemental screening tests to mammography for women with dense breasts. A major criticism of this legislation, however, is that there is not yet enough evidence to support recommendation of any of these particular modalities for adjunct screening of dense breasts.
Recent reader studies conducted in cancer-enriched populations indicate that adding tomosynthesis to mammography can significantly reduce the recall rate of noncancer cases by an average of 39% in one reader study and 17% in another.160 To date, there is little published on the performance of tomosynthesis in the screening setting and no evidence yet that it can lead to improved sensitivity specifically in the dense breast population. Interim results from the 12 600 examinations studied in the Oslo Tomosynthesis Screening trial demonstrated a significantly improved diagnostic yield with addition of digital tomosynthesis to mammography (6.1 for mammography alone vs 8.0 for mammography plus digital tomosynthesis, p = 0.001) and a 15% decrease in recall rate for the combined technologies (p < 0.001).161 The improvement in cancer detection with tomosynthesis was noted to be in invasive cancers but not ductal carcinoma in situ, and was across all breast densities, even fatty breasts.161
Results from the ACRIN 6666 clinical trial showed that adding physician-performed whole-breast screening ultrasound to screening mammography significantly increased diagnostic yield (number of cancers detected per 1000 women screened) from 8.1 for mammography alone to 11.8 for mammography and ultrasound combined in women with dense breasts and other risk factors. However, the addition of whole breast ultrasound to mammography also significantly increased the number of false positive findings. At the first year of screening with the combined modalities over one-quarter (27%) of women were recalled for additional workup and 10% were biopsied, and after three years of annual screening 17% of women were recalled and 7% were biopsied.162 The malignancy rate per biopsy (positive predictive value [PPV]3) for combined mammography and ultrasound (16%) was significantly worse than that of screening mammography alone (38%) after three years of screening.162 Other multicenter trial results from Italy demonstrated that physician-performed screening ultrasound in women with dense breasts who had negative screening mammograms increased diagnostic yield from 2.8 with mammography alone to 7.2 with combined ultrasound and mammography.163
A multicenter study of automated breast ultrasound (ABUS) in over 4000 women also showed a significant increase in cancers detected in dense breast tissue with only a small decrease in specificity.164 However, a number of obstacles need to be addressed with regard to the use of ABUS in clinical practice, including the time to read a study (3–12 min), increased recall rate and increased biopsy rate.
MRI is currently recommended by the American Cancer Society (ACS) as an annual screening method for women in the highest risk categories, such as those at greater than 20% lifetime risk by risk prediction models or those who carry a pathologic mutation in BRCA-1 or -2 genes. However, the ACS has stated that there is insufficient evidence to recommend for or against MRI screening for women in intermediate risk categories, such as those with dense breasts or personal history of breast cancer, when the high risk criteria are not met.165 The ACS also recognized the variable specificity of this modality, which can range from 50% to 90%. The addition of MRI to screening mammography in women with dense breasts in a cohort of 612 participants of the ACRIN 6666 trial resulted in significantly improved diagnostic yield (26.1 for combined MRI and mammography vs 8.2 for mammography alone, normalized per 1000 women screened) but significantly worse specificity (71% for combined modalities vs 92% for mammography alone).162 In the current economic climate, the high cost of breast MRI is likely to be a limiting factor in the widespread adoption of this imaging technique.
Nuclear medicine techniques have been considered as an option for those women with dense breasts who do not qualify for, do not have access to, or cannot afford MRI screening. The primary barrier to implementation of nuclear techniques in the screening setting has been concern that their radiation dose is too high for annual or biennial use in an asymptomatic population.1 However, more recent data showing that lowered activities of radiopharmaceuticals can be administered and still achieve acceptable diagnostic accuracy support further investigation into whether nuclear techniques could have a role in the screening setting.
Several patient studies have demonstrated the ability of dedicated gamma cameras to detect mammographically occult breast cancer in the screening setting.3, 101, 166, 167 The largest trial of screening with dedicated gamma imaging yet performed was a single-center trial that compared the efficacy of MBI performed with a dual-head CZT-based gamma camera and screening mammography in 936 asymptomatic women with dense breasts.3 Results showed that the addition of a one-time or prevalence screen MBI, performed using 740 MBq (20 mCi) 99mTc-sestamibi, to incident screening mammography in women with dense breasts significantly increased diagnostic yield from 3.2 with mammography alone to 10.7 with the combination of tests (p = 0.016). Although the recall rates of incident mammography and prevalent MBI used independently were similar (9% vs 8%, respectively), recall rate of the combination of techniques was increased to 15% (p < 0.001 when compared with mammography alone). The addition of MBI to mammography also increased the biopsy rate from 1.8% to 3.9%; absolute PPV3 was consequently raised from 18% with mammography alone to 24% with the combination of MBI and mammography, but this increase was not statistically significant (p = 0.516). It is expected that with repeated rounds of MBI screening (incidence screening), recall and biopsy rates of MBI would improve, as has been observed with all other breast imaging screening modalities, including mammography,162, 168 ultrasound,162 and MRI.169
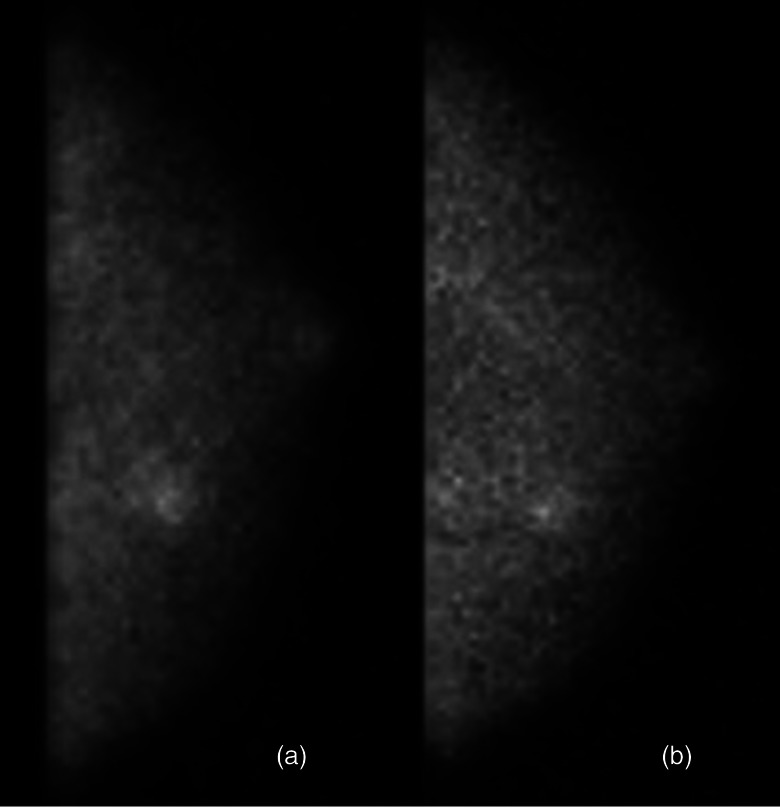
Recent improvements in the CZT-based MBI technology used in this screening MBI trial have resulted in a ∼3 fold reduction in the administered dose of 99mTc-sestamibi required for MBI.170, 171 Preliminary results from 600 patients screened with low-dose MBI performed with 300 MBq (8 mCi) 99mTc-sestamibi and mammography confirmed the findings of improved sensitivity of MBI relative to mammography in dense breasts at reduced administered dose.172 Early findings from trials underway at Mayo Clinic indicate feasibility of performing screening MBI using administered doses as low as 150 MBq (4 mCi) 99mTc-sestamibi. Figure 10 shows an example of a breast cancer detected on separate MBI studies performed following injections of 150 MBq 99mTc-sestamibi and 300 MBq 99mTc-sestamibi in the same patient.
Figure 10.
Two separate MBI studies performed in the same patient with a 10-mm invasive ductal carcinoma. MBI was performed using 150 MBq with a resolution recovery algorithm applied (panel a) and 300 MBq (panel b) Tc-99m sestamibi on a dual-head CZT-based gamma camera.
PEM has not been evaluated as a screening tool. The patient preparation requirements for PEM dictated by the use of 18F-FDG, including fasting for 4–6 h, blood glucose monitoring, and a 40–60 min wait time postinjection, reduce the likelihood of its adoption in the screening setting in the near future.
NECESSARY FACTORS FOR ADOPTION OF NUCLEAR MEDICINE BREAST IMAGING
Clear indications
After over 20 years of investigation and the development of a number of high-resolution dedicated detectors for nuclear medicine breast imaging, a vital question stills plagues the modality—where do these technologies fit within a clinical breast imaging practice?
The primary goal of any new modality is to perform better than existing techniques. Nuclear medicine breast imaging has been shown to offer improved detection of certain cancers within mammographically dense breast tissue compared to mammography. It promises to be better tolerated by patients, easier to interpret, less costly, and possibly more specific than breast MRI. The possible indications for nuclear medicine breast imaging are thus very broad.173 Some have suggested that a general indication for nuclear techniques for the breast is any patient in whom MRI is indicated, but cannot be performed due to lack of availability, inability to pay, or contraindications such as an implanted device, allergy to gadolinium, large body habitus, or inability to tolerate the exam.174
Based on current evidence presented above, three key patient groups have emerged as the most likely to benefit from imaging with new dedicated nuclear medicine techniques. The first is women with a diagnosis of breast cancer requiring preoperative evaluation, especially for women in whom MRI is contraindicated. PEM has been demonstrated to be particularly useful in this group.4 The second is women undergoing neoadjuvant therapy, although the true potential of functional nuclear medicine imaging to accurately show response and aid in tailoring treatment may not be realized until targeted radiopharmaceuticals are implemented in this setting.7 The third group, and by far the largest, is women with mammographically dense breasts who either do not meet the risk eligibility criteria for screening MRI or who decline MRI screening for a number of reasons.175 Women with additional risk factors (personal history of breast cancer, strong family history) in this subgroup may be the most likely to benefit from additional screening with nuclear medicine. Low-dose gamma imaging with dedicated CZT-based MBI systems has already shown promise in this setting.3, 171, 172
As discussed above, numerous studies investigating nuclear breast imaging systems have had the inclusion criteria bias of enrolling women with suspicious lesions that were scheduled to be biopsied. Although the intention of such a study design was to establish diagnostic accuracy of the specific technique using an enriched population, some have erroneously used such studies to infer that a negative nuclear study could prevent “unnecessary biopsies.”176, 177, 178
Indeterminate imaging findings (BIRADS 3) on mammography and/or ultrasound where biopsy is not indicated or a persistent clinical concern have also been proposed indications for nuclear medicine breast imaging. Little evidence supports this use other than knowledge that the nuclear techniques have a high probability of finding cancer (greater than 5 mm) if it exists and a negative study can offer additional reassurance to patients.
Biopsy capability
Precisely because nuclear techniques can visualize lesions that are occult on conventional imaging with mammography and directed ultrasound, a direct localization method is essential for widespread clinical acceptance. Several designs for direct biopsy have been introduced and evaluated with simulations and phantoms, however only two commercial systems offer an accessory biopsy unit.
Early approaches to localization for biopsy using needle localization and surgical removal were proposed for scintimammography and dedicated gamma cameras.101, 179, 180 Approaches of integrating a dedicated gamma camera detector or PEM detector into a mammographic stereotactic biopsy unit have been explored.86, 181, 182
The only commercial, Food and Drug Administration (FDA)-approved biopsy scheme for dedicated gamma cameras is the system developed by Dilon Technologies for BSGI.183 This system uses a sliding slant-hole collimator to acquire two separate gamma images and allow stereotactic localization the lesion. Biopsy is performed with the patient positioned upright at the BSGI imaging system and the needle is inserted through a grid-support and needle positioning block that also acts as a compression paddle. Preliminary findings demonstrated successful BSGI-guided biopsy performed in 17 patients, with a reported procedure time of 86 min.184
Direct biopsy capability has also been developed and FDA-approved for the Naviscan PEM system. Like the BSGI biopsy scheme, PEM-guided biopsy is performed with the patient upright while positioned at the imaging system. Because both detectors of the PEM system are required for coincidence imaging, the needle is inserted from the lateral side of the compressed breast. A prospective multicenter clinical trial showed successful biopsy of lesions in 22 patients, with an average procedure time of ∼30 min.185 Currently, there are no biopsy schemes available for the dedicated ring-based breast PET designs, and there is some concern that access to the breast for biopsy may be limited with such designs that utilize a ring of detectors.
In the Mayo Clinic practice and in other institutions that are exploring the use of MBI performed with dual-head CZT-based gamma cameras, the standard protocol for evaluation of newly detected lesions on nuclear studies is evaluation with diagnostic mammography and/or targeted ultrasound, and biopsy is typically performed under stereotactic or ultrasound guidance if warranted. If diagnostic workup by conventional imaging is negative, further evaluation is determined by the level of suspicion of the MBI-detected lesion: indeterminate lesions (MBI assessment = 3) are followed by MBI performed at six month intervals, suspicious lesions (MBI assessment = 4 or 5) are evaluated with MRI. A review of the diagnostic workup generated by adding screening MBI to mammography in women with dense breasts was recently presented.186 In 1640 women, 109 (6.6%) had newly detected lesions on MBI, of which 13 (0.8%) necessitated MRI evaluation, and 8 (0.5%) underwent MRI-guided biopsy. These findings suggest a limited role for direct MBI-guided biopsy capability, but such a device may have been useful for obviating the need to perform MRI in a small number of patients.
Although there is a FDA-approved method of imaging the obturator with PEM to verify its position after placement, a limitation of direct biopsy techniques for nuclear systems developed thus far is the lack of real-time feedback (as in ultrasound-guided biopsy) on the location of the biopsy needle or obturator. A recently proposed approach of using a high sensitivity focused ring-shaped collimator for dedicated gamma imaging may allow monitoring of the obturator that approaches real time (frames updated every 1 min), a substantial improvement over the current acquisition of 5–10 min frames during imaging.187 Use of a multimodality system could also overcome the real-time monitoring problem by providing anatomical localization of the functional abnormality along with real-time visualization of needle location. Systems such as the combined Clear-PEM Sonic system may allow the option of performing biopsy under ultrasound guidance.
Radiation dose considerations
Concern about radiation risk associated with nuclear medicine breast imaging techniques is one of the primary factors that have limited their clinical adoption. The breast is considered one of the most radiosensitive organs in the body.188 Hence there will always be considerable scrutiny of any breast imaging procedure that involves ionizing radiation. Numerous reports have documented the very low risk of any harmful effects associated with radiation received from mammography, and that risk is even lower with today's digital mammography detectors.189 Hence, researchers are striving to implement dose reduction strategies that allow the nuclear techniques to be performed at radiation doses comparable to that used in mammography.
Initial studies with dedicated gamma cameras employed doses of 99mTc-sestamibi that were comparable to those originally employed by conventional scintimammography.19 Doses usually ranged from 740 MBq up to 1110 MBq 99mTc-sestamibi resulting in an estimated absorbed dose to breast tissue of up to 2 mGy. Initial PEM studies typically used administered doses up to 370 MBq 18F-FDG, resulting in dose to the breast of 3.4 mGy. By comparison, the mean glandular dose (to the breast) from digital mammography is ∼4 mGy.1
However, with nuclear medicine techniques, the administered radiotracer delivers a systemic dose and most of the radiation burden is to organs other than the breast, mainly the upper and lower intestines for 99mTc-sestamibi, and the spleen, heart and bladder for 18F-FDG. The effective (whole-body) dose from administration of 740–1100 MBq 99mTc-sestamibi is 5.9–9.4 mSv and the effective dose from administration of 370 MBq 18F-FDG is 6.2–7.1 mSv.1 These effective doses are a factor of 10–20 times greater than the average effective dose of 0.5 mSv for digital mammography.1
The higher effective radiation doses from these nuclear medicine breast imaging techniques compared to mammography have raised concerns about the cumulative radiation burden to patients, particularly if these procedures become widely adopted and their use is expanded into screening. An analysis by O'Connor et al.,2 which calculated the lifetime attributable risk (LAR) of cancer incidence and mortality from annual mammography and annual nuclear medicine procedures, concluded that in order for the radiation-induced cancer risks from nuclear medicine techniques to be comparable to that of mammography, the injected radiotracer activities need to be reduced to 150 MBq or less of 99mTc-sestamibi and 70 MBq or less of 18F-FDG. These administered activities both correspond to an effective radiation dose of ∼1.2 mSv, which is comparable to the effective dose from combined mammography and breast tomosynthesis.
The low doses received from annual screening mammography programs have been determined to be of low risk compared with expected mortality reductions achievable through mammographic screening. Hence, it is reasonable to assume that a more sensitive screening technique would likewise be considered to be low risk relative to the potential mortality reduction. Recent efforts at optimization of current CZT-based MBI gamma camera technology have yielded a reduction in the administered dose of 99mTc-sestamibi to 300 MBq as currently routinely used at our institution and 150 MBq in the research setting.170, 171 Further work with some of the newer radiopharmaceuticals may yield an additional factor of 2–3 reduction, which would bring the effective dose down below that of mammography. At these dose levels, it is now reasonable to consider the incorporation of MBI into a screening regime: this could be accomplished by alternating annually between mammography and MBI or the addition of MBI as an adjunct screening tool on a biennial basis.
Clinical studies with the Naviscan PEM unit have typically employed a 370 MBq dose of 18F-FDG but some recent data suggest that adequate diagnostic image quality can be obtained using 185 MBq 18F-FDG.190 The next generation of PEM systems that incorporate either fully populated detector plates such as the ClearPEM unit, or dedicated breast PET systems full ring detectors such as the MammiPEM system, are likely to be considerably more sensitive than the current PEM-Flex system and should permit a corresponding reduction in the administered dose of 18F-FDG required for clinical studies.191, 192 An evaluation of several types of dedicated PET configurations recently demonstrated the sensitivity to true coincidence events at the center of a box-shaped or polygon-shaped ring detector to be 33%–35% compared to 8% for a dual-detector PEM system.192
Targeted radiopharmaceuticals
In nuclear medicine, the successful creation of a useful imaging test requires two components; the right instrumentation, which is now available, and the appropriate radiopharmaceuticals that can target and characterize breast cancer while delivering a low radiation burden to the patient. The lack of suitable instrumentation over the last 10–15 years has effectively undercut any major attempts to develop better radiopharmaceuticals for breast imaging. Consider that 99mTc-sestamibi became the first radiopharmaceutical specifically approved for breast imaging as a second-line diagnostic test by the FDA as far back as 1997. 99mTc-tetrofosmin was FDA approved in 1999, but never received specific approval for breast imaging in the U.S. 99mTc-tetrofosmin did, however, receive European approval as a breast imaging agent in 2002. Since then no other radiopharmaceuticals have been approved for breast imaging in either the U.S. or Europe.
As discussed earlier, from the studies performed in the 1990s in both scintimammography and PET, it was assumed that the failure of both techniques to detect small breast tumors was partly due to variable uptake of 99mTc-sestamibi and 18F-FDG by some tumors. As is now apparent from more recent clinical results with dedicated systems, both radiopharmaceuticals appear to demonstrate good to excellent uptake in small breast lesions, and the problem was not with the radiopharmaceutical, but with the limitations of the imaging technology.
Quantitative measurements from our laboratory in breast tumors with 99mTc-sestamibi have indicated that tumor to background concentration ratio are in the range of 10:1–40:1,60, 193 considerably higher than reported in prior scintimammography studies,194 and more in-line with in vitro studies of the uptake of 99mTc-sestamibi in carcinoma cell lines.195 This is good news for both single photon and positron emitting technologies as the development and regulatory approval of new radiopharmaceuticals is a long and expensive process and one that few manufacturers would be willing to undertake in the absence of a proven clinical market.
99mTc-sestamibi and 18F-FDG serve the role as generic markers of increased metabolic function, and hence surrogates of breast cancer, but are relatively nonspecific in their uptake and are often unable to distinguish between benign and malignant processes. Several alternative radiopharmaceuticals may provide more specific information on the functional aspects of breast cancers. While a full review of other potential radiotracers for breast imaging is outside the scope of this paper, Table 2 presents a partial list of the radiolabeled compounds that have been used to detect breast cancer in humans (adapted from Kong et al.).196
Table 2.
Radiotracers that have been used in human studies to detect breast cancer. Listed by tumor-specific target humans [adapted from Kong et al. (Ref. 196)].
| Target | Single-photon (MBI/BSGI) | Positron-emitter (PET or PEM) |
|---|---|---|
| Perfusion | Tl-201 Thallous Chloride | O-15 Water |
| Tc-99m Sestamibi | ||
| Tc-99m tetrofosmin | ||
| Glucose metabolism | Tc-99m EC-glucosamine | F-18 FDG |
| Hormone receptor | I-123 estradiol | F-18 FES |
| F-18 fluoro-tamoxifen | ||
| F-18 fluoro-norprogesterone | ||
| F-18 fluoromoxestol | ||
| HER2 | In-111 trastuzumab | Zr-89 trastuzumab |
| Cell proliferation/angiogenesis | Tc-99m maraciclatide | F-18 flucuclatide |
| In-111 bevacizumab | F-18 galacto-RGD | |
| F-18 fluorothymidine | ||
| Zr-80 bevacizumab | ||
| Amino acid transporters & protein synthesis | Tc-99m methionine | C-11 methionine |
| I-123 methyltyrosine | C-11 tyrosine | |
| F-18 fluoro-ethyl-tyrosine | ||
| Epidermal growth factor receptor | C-11 Iressa | |
| Cell surface receptor (VPAC1) | Tc-99m VPAC1 | Cu-64 VPAC1 |
| Hypoxia | F-18 fluoromisonidazole | |
| F-18 fluoroetanidazole | ||
| F-18 fluoroazomycin-arabinoside | ||
| Cu-64 diacetyl-bis-N4-methylthiosemicarbazone | ||
| Apoptosis | Tc-99m EC-annexin V | F-18 annexin V |
| Somatostatin receptors | In-111 octreotide | |
| Membrane synthesis | C-11 choline |
The development of new blood vessels (angiogenesis) around tumors is essential for tumor growth. Protein-based receptors known as integrins play a key role in the process of angiogenesis. The expression of the integrin αvβ3 on vascular endothelium has a key role in this process. This integrin has been successfully labeled with both 99mTc and 18F,197, 198 and initial studies have shown uptake of radiolabeled integrin in primary breast cancer and metastases.199, 200 Figure 11 compares images of a breast cancer obtained in the same patient with 99mTc-sestamibi and 99mTc-radiolabeled integrin (99mTc-NC100692) on a CZT-based MBI system. Compared to the 99mTc-sestamibi images, the 99mTc-radiolabeled integrin images demonstrated higher uptake of the radiopharmaceutical in both the normal breast tissue and in the tumor. 99mTc-NC100692 imparts an effective dose of 7.8 μSv/MBq,201 which is slightly lower than the effective dose of sestamibi (8.1–8.5 μSv/MBq).1 The higher relative uptake in breast tissue obtained with 99mTc-radiolabeled integrin may allow for a reduction in the administered dose required for acceptable image quality.
Figure 11.
Images from a patient with two areas of confirmed ductal carcinoma in situ who had MBI performed with 300 MBq Tc-99m sestamibi (panel a) and 300 MBq Tc-99m alphaV beta 3 integrin (panel b).
Estrogen exerts an important effect in breast tissue, causing growth and enhancing many metabolic pathways cells while inhibiting others. One of the earliest changes of breast cancer is the inability of a breast cell to maintain the appropriate estrogen receptor levels. Excess levels of estrogen receptor cause sustained, prolonged, and unopposed estrogen stimulation of the breast cell. A large percentage of breast cancers express the estrogen receptor (ER). Knowledge of a patient's ER status has important consequences for treatment decision making, because patients with ER-positive tumors are likely to benefit from antiestrogen therapy. Several groups have developed radiolabeled derivatives of estradiol using either 123I or 18F.202, 203 Early work by Preston et al.204 and Bennink et al.205 indicated that 123I estradiol may be able to detect current disease and be a sensitive tool for the detection of estrogen receptors in patients with breast cancer. Also, several studies showed that 18F fluoroestradiol (FES) PET could assess the in vivo pharmacodynamics of ER-targeted agents and may give insight into the activity of established therapeutic agents.206
Radiopharmaceuticals targeting angiogenesis and estrogen receptor status are just two of the myriad of new radiolabeled compounds that offer the promise of providing better functional and metabolic information on breast cancer. Other potential compounds include F-18 fluorodeoxythymidine and 99mTc-(V) dimercaptosuccinic acid (DMSA), both with uptake in the breast that appears to mirror cell proliferation,207, 208 and 99mTc-Annexin V which mimics tumor cell death.209 In a recent review of novel geonomic biomarkers for molecular imaging of cancer, Thakur210 discussed the numerous biomarkers that are under development and that have the potential promise to better identify and characterize breast cancer.
The promise of all of these radiolabeled compounds can only be realized with high resolution SPECT and PET instrumentation. This is a symbiotic relationship that can only succeed if developments in instrumentation are matched by developments in new radiopharmaceuticals. With the developments in coincidence and single-photon detector instrumentation over the last 5–10 years, the time is now right for a renaissance in radiopharmaceuticals optimized for both the detection of breast cancer and the evaluation of breast function.
CONCLUSION
A number of single-photon and coincidence-detection nuclear breast imaging technologies and their potential clinical roles were discussed. A summary of these technologies is given in Table 3.
Table 3.
Comparison of single-photon and coincidence-detection nuclear medicine technologies as applied to breast imaging.
| MBI | MBI | PEM | DbPET | |
|---|---|---|---|---|
| (Dual-head CZT-based) | (BSGI) | |||
| Primary proposed clinical indications | Preoperative evaluation | Preoperative evaluation | ||
| Monitoring response to neoadjuvant therapy | Monitoring response to neoadjuvant therapy | |||
| Patients with contraindications to MRI | Patients with contraindications to MRI | |||
| Screening in dense breasts (at low radiation dose) | ||||
| Radiopharmaceutical | Tc-99m sestamibi or Tc-99m tetrofosmin | F-18 FDG | ||
| Administered activityt1n1 | 300 MBq | 740–1110 MBq | 370 MBq | 370 MBq |
| Fasting period prior to imaging | None | None | 4–6 h | 4–6 h |
| Wait period between injection and imaging | 5 min | 5 min | 40–60 min | 40–60 min |
| Acquisition duration | 10 min/view × 4 views | 10 min/view × 4 views | 10 min/view × 4 views | Variable |
| Biopsy guidance | In development | FDA-approved | FDA-approved | Not available |
| FOV | 20 × 16 cm or | 20 cm by 15 cm or | 24 × 16 cm | Variable |
| 24 × 16 cm | 25 cm × 20 cm | |||
| Standardized interpretive criteria | Yes (Refs. 219 and 220) | Yes (Ref. 173) | Yes (Ref. 221) | No |
Although a range of values are used in clinical and research settings, typical administered doses used in clinical exams are given.
The future of nuclear breast imaging technologies is dependent upon several key factors. The primary factor is clinical relevance: these technologies need to establish a place as cost-effective tools in either the screening environment or the diagnostic work-up of patients with breast concerns. Failure to establish a role for these technologies in the routine care of breast cancer and in the minds of the breast radiologists and ordering physicians will be a critical obstacle to their future development.
Over the last ten years our research group at Mayo Clinic has worked on the development of MBI with dedicated CZT-based gamma cameras. An enduring lesson from those ten years has been that development of the technical aspects of a new procedure is only the tip of the iceberg. Technical journals are rife with excellent papers on new techniques and technologies that demonstrate notable improvements in image quality. Few of these advancements ever progress from a physics project to a clinical tool. Unfortunately having a great technology is no guarantee that it will ever go from the research work bench into routine clinical use.
Many of us working in the field of medical physics naively assume that our job is finished once we complete the technical aspects, but as the people who best understand the technology, we also need to stay deeply involved in all the additional steps that are required to translate it into clinical use. These steps include active participation in the initial patient studies, development of clinical trials, and education of the end users to the strengths and limitations of the technology. As physicists and engineers we also need to understand where a new technology fits with competing technologies, and to recognize that a new technology, particularly if it is disruptive, may not be welcomed, even if it is demonstrably superior to current technologies. Much of the above discussion requires us to move out of our comfort zone into a world where decisions are influenced by economic pressures and bias toward the status quo. However, participation in translating the new technology from bench to bedside is equally as important as the physics work that went into the original design.
Nuclear techniques have already been evaluated in breast imaging and found wanting. The emergence of dedicated nuclear breast imaging systems in recent years presents a second chance to demonstrate that these technologies have an important role to play in the detection and understanding of breast cancer. The continued involvement of physicists and engineers beyond the workbench and into the clinical studies is important to ensure good science and that the capabilities of the technologies are appropriately represented. Failure to understand how our clinical colleagues employ these technologies and failure to adequately realize the technologies' strengths and weaknesses may lead to a repeat of what occurred with scintimammography in the 1990s. The strength of molecular imaging is its ability to detect pathophysiological changes at the onset of disease. While molecular imaging technology is commonly used for imaging many disease processes today, it has lagged behind in clinical use for imaging of breast cancer. We believe that with the latest generation of PET and SPECT imaging technologies dedicated to breast imaging, we can now begin to explore how molecular imaging can improve our understanding and treatment of this disease.
ACKNOWLEDGMENTS
This work was funded in part by grants from the Susan G. Komen Foundation, National Institutes of Health (CA 128407), and the Mayo Foundation. C. Hruska and M. O'Connor received royalties per licensing agreement between Mayo Clinic and Gamma Medica, Inc. M. O'Connor acknowledges research grant from GE Healthcare.
References
- Hendrick R. E., “Radiation doses and cancer risks from breast imaging studies,” Radiology 257, 246–253 (2010). 10.1148/radiol.10100570 [DOI] [PubMed] [Google Scholar]
- O'Connor M. K., Li H., Rhodes D. J., Hruska C. B., Clancy C. B., and Vetter R. J., “Comparison of radiation exposure and associated radiation-induced cancer risks from mammography and molecular imaging of the breast,” Med. Phys. 37, 6187–6198 (2010). 10.1118/1.3512759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. J., Hruska C. B., Phillips S. W., Whaley D. H., and O'Connor M. K., “Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts,” Radiology 258, 106–118 (2011). 10.1148/radiol.10100625 [DOI] [PubMed] [Google Scholar]
- Berg W. A., Madsen K. S., Schilling K., Tartar M., Pisano E. D., Larsen L. H., Narayanan D., Ozonoff A., Miller J. P., and Kalinyak J. E., “Breast cancer: Comparative effectiveness of positron emission mammo- graphy and MR imaging in presurgical planning for the ipsilateral breast,” Radiology 258, 59–72 (2011). 10.1148/radiol.10100454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg W. A., Madsen K. S., Schilling K., Tartar M., Pisano E. D., Larsen L. H., Narayanan D., and Kalinyak J. E., “Comparative effectiveness of positron emission mammography and MRI in the contralateral breast of women with newly diagnosed breast cancer,” AJR, Am. J. Roentgenol. 198, 219–232 (2012). 10.2214/AJR.10.6342 [DOI] [PubMed] [Google Scholar]
- Brem R. F., Shahan C., Rapleyea J. A., Donnelly C. A., Rechtman L. R., Kidwell A. B., Teal C. B., McSwain A., and Torrente J., “Detection of occult foci of breast cancer using breast-specific gamma imaging in women with one mammographic or clinically suspicious breast lesion,” Acad. Radiol. 17, 735–743 (2010). 10.1016/j.acra.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Wahner-Roedler D. L., Boughey J. C., Hruska C. B., Chen B., Rhodes D. J., Tortorelli C. L., Maxwell R. W., Cha S. S., and O'Connor M. K., “The use of molecular breast imaging to assess response in women undergoing neoadjuvant therapy for breast cancer: a pilot study,” Clin. Nucl. Med. 37, 344–350 (2012). 10.1097/RLU.0b013e31824437b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillefer R., “The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis,” Semin Nucl. Med. 29, 16–40 (1999). 10.1016/S0001-2998(99)80027-0 [DOI] [PubMed] [Google Scholar]
- Berg G. R., Kalisher L., Osmond J. D., Pendergrass H. P., and Potsaid M. S., “99mTc-diphosphonate concentration in primary breast carcinoma,” Radiology 109, 393–394 (1973). 10.1148/109.2.393 [DOI] [PubMed] [Google Scholar]
- Hisada K., Tonami N., Miyamae T., Hiraki Y., Yamazaki T., Maeda T., and Nakajo M., “Clinical evaluation of tumor imaging with 201 TI chloride,” Radiology 129, 497–500 (1978). 10.1148/129.2.497 [DOI] [PubMed] [Google Scholar]
- Ghosh P. R., “FDA approves two new technetium-labeled cardiac agents and a pharmacologic alternative to exercise in stress-thallium studies,” J. Nucl. Med. 32, 11N–19N (1991). [PubMed] [Google Scholar]
- Aktolun C., Bayhan H., and Kir M., “Clinical experience with Tc-99m MIBI imaging in patients with malignant tumors. Preliminary results and comparison with Tl-201,” Clin. Nucl. Med. 17, 171–176 (1992). 10.1097/00003072-199203000-00003 [DOI] [PubMed] [Google Scholar]
- Rambaldi P. F., Mansi L., Procaccini E., Di Gregorio F., and Del Vecchio E., “Breast cancer detection with Tc-99m tetrofosmin,” Clin. Nucl. Med. 20, 703–705 (1995). 10.1097/00003072-199508000-00009 [DOI] [PubMed] [Google Scholar]
- Liberman M., Sampalis F., Mulder D. S., and Sampalis J. S., “Breast cancer diagnosis by scintimammography: A meta-analysis and review of the literature,” Breast Cancer Res. Treat. 80, 115–126 (2003). 10.1023/A:1024417331304 [DOI] [PubMed] [Google Scholar]
- Khalkhali I., Villanueva-Meyer J., Edell S. L., Connolly J. L., Schnitt S. J., Baum J. K., Houlihan M. J., Jenkins R. M., and Haber S. B., “Diagnostic accuracy of 99mTc-sestamibi breast imaging: multicenter trial results,” J. Nucl. Med. 41, 1973–1979 (2000). [PubMed] [Google Scholar]
- Palmedo H., Biersack H. J., Lastoria S., Maublant J., Prats E., Stegner H. E., Bourgeois P., Hustinx R., Hilson A. J., and Bischof-Delaloye A., “Scintimammography with technetium-99m methoxyisobutylisonitrile: Results of a prospective European multicentre trial,” Eur. J. Nucl. Med. 25, 375–385 (1998). 10.1007/s002590050235 [DOI] [PubMed] [Google Scholar]
- Sampalis F. S., Denis R., Picard D., Fleiszer D., Martin G., Nassif E., and Sampalis J. S., “International prospective evaluation of scintimammography with (99m)technetium sestamibi,” Am. J. Surg. 185, 544–549 (2003). 10.1016/S0002-9610(03)00077-1 [DOI] [PubMed] [Google Scholar]
- Waxman A. D., “The role of (99m)Tc methoxyisobutylisonitrile in imaging breast cancer,” Semin Nucl. Med. 27, 40–54 (1997). 10.1016/S0001-2998(97)80035-9 [DOI] [PubMed] [Google Scholar]
- Brem R. F., Schoonjans J. M., Kieper D. A., Majewski S., Goodman S., and Civelek C., “High-resolution scintimammography: A pilot study,” J. Nucl. Med. 43, 909–915 (2002). [PubMed] [Google Scholar]
- Papantoniou V., Christodoulidou J., Papadaki E., Valotassiou V., Stipsanelli A., Louvrou A., Lazaris D., Sotiropoulou M., Pampouras G., Keramopoulos A., Michalas S., and Zerva C., “99mTc-(V)DMSA scintimammography in the assessment of breast lesions: Comparative study with 99mTc-MIBI,” Eur. J. Nucl. Med. 28, 923–928 (2001). 10.1007/s002590100545 [DOI] [PubMed] [Google Scholar]
- Khalkhali I., Mena I., and Diggles L., “Review of imaging techniques for the diagnosis of breast cancer: A new role of prone scintimammography using technetium-99m sestamibi,” Eur. J. Nucl. Med. 21, 357–362 (1994). 10.1007/BF00947973 [DOI] [PubMed] [Google Scholar]
- Aktolun C., “Exaggerated role of prone technique in breast cancer imaging with technetium-99m methoxyisobutylisonitrile,” Eur. J. Nucl. Med. 21, 1257–1260 (1994). 10.1007/BF00182366 [DOI] [PubMed] [Google Scholar]
- Scopinaro F., Pani R., De Vincentis G., Soluri A., Pellegrini R., and Porfiri L. M., “High-resolution scintimammography improves the accuracy of technetium-99m methoxyisobutylisonitrile scintimammography: Use of a new dedicated gamma camera,” Eur. J. Nucl. Med. 26, 1279–1288 (1999). 10.1007/s002590050584 [DOI] [PubMed] [Google Scholar]
- Spanu A., Dettori G., Nuvoli S., Porcu A., Falchi A., Cottu P., Solinas M. E., Scanu A. M., Chessa F., and Madeddu G., “(99)mTc-tetrofosmin SPET in the detection of both primary breast cancer and axillary lymph node metastasis,” Eur. J. Nucl. Med. 28, 1781–1794 (2001). 10.1007/s00259-001-0657-5 [DOI] [PubMed] [Google Scholar]
- Spanu A., Schillaci O., Meloni G. B., Porcu A., Cottu P., Nuvoli S., Falchi A., Chessa F., Solinas M. E., and Madeddu G., “The usefulness of 99mTc-tetrofosmin SPECT scintimammography in the detection of small size primary breast carcinomas,” Int. J. Oncol. 21, 831–840 (2002). [PubMed] [Google Scholar]
- Aziz A., Hashmi R., Ogawa Y., and Hayashi K., “Tc-99m-MIBI scintimammography; SPECT versus planar imaging,” Cancer Biother. Radiopharm. 14, 495–500 (1999). 10.1089/cbr.1999.14.495 [DOI] [PubMed] [Google Scholar]
- Myslivecek M., Koranda P., Kaminek M., Husak V., Hartlova M., Duskova M., and Cwiertka K., “Technetium-99m-MIBI scintimammography by planar and SPECT imaging in the diagnosis of breast carcinoma and axillary lymph node involvement,” Nucl. Med. Rev. Cent. East. Eur. 7, 151–155 (2004). [PubMed] [Google Scholar]
- Schillaci O., Danieli R., Filippi L., Romano P., Cossu E., Manni C., and Simonetti G., “Scintimammography with a hybrid SPECT/CT imaging system,” Anticancer Res. 27, 557–562 (2007). [PubMed] [Google Scholar]
- Tiling R., Tatsch K., Sommer H., Meyer G., Pechmann M., Gebauer K., Munzing W., Linke R., Khalkhali I., and Hahn K., “Technetium-99m-sestamibi scintimammography for the detection of breast carcinoma: comparison between planar and SPECT imaging,” J. Nucl. Med. 39, 849–856 (1998). [PubMed] [Google Scholar]
- Danielsson R., Bone B., Agren B., Svensson L., and Aspelin P., “Comparison of planar and SPECT scintimammography with 99mTc-sestamibi in the diagnosis of breast carcinoma,” Acta Radiol. 40, 176–180 (1999). 10.3109/02841859909177734 [DOI] [PubMed] [Google Scholar]
- Rosen E. L., Eubank W. B., and Mankoff D. A., “FDG PET, PET/CT, and breast cancer imaging,” Radiographics 27(Suppl 1), S215–S229 (2007). 10.1148/rg.27si075517 [DOI] [PubMed] [Google Scholar]
- Buscombe J. R., Holloway B., Roche N., and Bombardieri E., “Position of nuclear medicine modalities in the diagnostic work-up of breast cancer,” Q. J. Nucl. Med. Mol. Imaging 48, 109–118 (2004). [PubMed] [Google Scholar]
- Samson D. J., Flamm C. R., Pisano E. D., and Aronson N., “Should FDG PET be used to decide whether a patient with an abnormal mammogram or breast finding at physical examination should undergo biopsy?,” Acad. Radiol. 9, 773–783 (2002). 10.1016/S1076-6332(03)80347-1 [DOI] [PubMed] [Google Scholar]
- Weinberg I. N., Pani R., Pellegrini R., Scopinaro F., De Vincentis G., Pergola A., and Soluri A., “Small lesion visualization in scintimammo- graphy,” IEEE Trans. Nucl. Sci. 44, 1398–1402 (1997). 10.1109/23.597019 [DOI] [Google Scholar]
- De Vincentis G., Scopinaro F., Pani R., Pellegrini R., Soluri A., Ierardi M., Ballesio L., Weinberg I. N., and Pergola A., “99mTc MIBI scintimammography with a high resolution single tube gamma camera: Preliminary study,” Anticancer Res. 17, 1627–1630 (1997). [PubMed] [Google Scholar]
- Williams M. B., Goode A. R., Galbis-Reig V., Majewski S., Weisenberger A. G., and Wojcik R., “Performance of a PSPMT based detector for scintimammography,” Phys. Med. Biol. 45, 781–800 (2000). 10.1088/0031-9155/45/3/315 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Choi Y., Joo K. S., Sihn B. S., Chong J. W., Kim S. E., Lee K. H., Choe Y. S., and Kim B. T., “Development of a miniature scintillation camera using an NaI(Tl) scintillator and PSPMT for scintimammography,” Phys. Med. Biol. 45, 3481–3488 (2000). 10.1088/0031-9155/45/11/326 [DOI] [PubMed] [Google Scholar]
- Majewski S., Kieper D., Curran E., Keppel C., Kross B., Palumbo A., Popov V., Wisenberger A. G., Welch B., Wojcik R., Williams M. B., Goode A. R., More M., and Zhang G., “Optimization of dedicated scintimammography procedure using detector prototypes and compressible phantoms,” IEEE Trans. Nucl. Sci. 48, 822–829 (2001). 10.1109/23.940170 [DOI] [Google Scholar]
- Wojcik R., Majewski S., Kross B., Steinbach D., and Weisenberger A. G., “High spatial resolution gamma imaging detector based on a 5″ diameter R3292 Hamamatsu PSPMT,” IEEE Trans. Nucl. Sci. 45, 487–491 (1998). 10.1109/23.682432 [DOI] [Google Scholar]
- Pani R., Soluri A., Scafe R., Pergola A., Pellegrini R., De Vincentis G., Trotta G., and Scopinaro F., “Multi-PSPMT scintillation camera,” IEEE Trans. Nucl. Sci. 46, 702–708 (1999). 10.1109/23.775602 [DOI] [Google Scholar]
- Patt B. E., Iwanczyk J. S., Rossington Tull C., Wang N. W., Tornai M. P., and Hoffman E. J., “High resolution CsI(Tl)/Si-PIN detector development for breast imaging,” IEEE Trans. Nucl. Sci. 45, 2126–2131 (1998). 10.1109/23.708319 [DOI] [Google Scholar]
- Gruber G. J., Moses W. W., Derenzo S. E., Wang N. W., Beuville E., and Ho H., “A discrete scintillation camera module using silicon photodiode readout of CsI(TI) crystals for breast cancer imaging,” IEEE Trans. Nucl. Sci. 45, 1063–1068 (1998). 10.1109/23.681979 [DOI] [Google Scholar]
- Trinci G., Massari R., Scandellari M., Scopinaro F., and Soluri A., “Super spatial resolution (SSR) method for scintigraphic imaging,” Nucl. Instrum. Methods Phys. Res. A 626–627, 120–127 (2011). 10.1016/j.nima.2010.10.077 [DOI] [Google Scholar]
- Pani R., De Vincentis G., Scopinaro F., Pellegrini R., Soluri A., Weinberg I. N., Pergola A., Scafe R., and Trotta G., “Dedicated gamma camera for single photon emission mammography (SPEM),” IEEE Trans. Nucl. Sci. 45, 3127–3133 (1998). 10.1109/23.737675 [DOI] [Google Scholar]
- Hruska C. B., O'Connor M. K., and Collins D. A., “Comparison of small field of view gamma camera systems for scintimammography,” Nucl. Med. Commun. 26, 441–445 (2005). 10.1097/00006231-200505000-00008 [DOI] [PubMed] [Google Scholar]
- More M. J., Goodale P. J., Majewski S., and Williams M. B., “Evaluation of gamma cameras for use in dedicated breast imaging,” IEEE Trans. Nucl. Sci. 53, 2675–2679 (2006). 10.1109/TNS.2006.876003 [DOI] [Google Scholar]
- Butler J. F., Lingren C. L., Friesenhahn S. J., Doty F. P., Ashburn W. L., Conwell R. L., Augustine F. L., Apotovsky B., Pi B., Collins T., Zhao S., and Isaacson C., “CdZnTe solid-state gamma camera,” IEEE Trans. Nucl. Sci. 45, 359–363 (1998). 10.1109/23.682408 [DOI] [Google Scholar]
- Mueller B., O'Connor M. K., Blevis I., Rhodes D. J., Smith R., Collins D. A., and Phillips S. W., “Evaluation of a small cadmium zinc telluride detector for scintimammography,” J. Nucl. Med. 44, 602–609 (2003). [PubMed] [Google Scholar]
- Hruska C. B. and O'Connor M. K., “A Monte Carlo model for energy spectra analysis in dedicated nuclear breast imaging,” IEEE Trans. Nucl. Sci. 55, 491–500 (2008). 10.1109/TNS.2007.910882 [DOI] [Google Scholar]
- Hruska C. B. and O'Connor M. K., “CZT detectors: How important is energy resolution for nuclear breast imaging?,” Phys. Med. 21(Suppl 1), 72–75 (2006). 10.1016/S1120-1797(06)80029-3 [DOI] [PubMed] [Google Scholar]
- Robert C., Montemont G., Rebuffel V., Buvat I., Guerin L., and Verger L., “Simulation-based evaluation and optimization of a new CdZnTe gamma-camera architecture (HiSens),” Phys. Med. Biol. 55, 2709–2726 (2010). 10.1088/0031-9155/55/9/019 [DOI] [PubMed] [Google Scholar]
- Slomka P. J., Dey D., Duvall W. L., Henzlova M. J., Berman D. S., and Germano G., “Advances in nuclear cardiac instrumentation with a view towards reduced radiation exposure,” Curr. Cardiol. Rep. 14, 208–216 (2012). 10.1007/s11886-012-0248-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann A. L., Hruska C. B., and O'Connor M. K., “Design of optimal collimation for dedicated molecular breast imaging systems,” Med. Phys. 36, 845–856 (2009). 10.1118/1.3077119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastoria S., Piccolo S., and Muto P., “Invited commentary: One step forward,” J. Nucl. Med. 43, 916–917 (2002). [PubMed] [Google Scholar]
- Hruska C. B., Phillips S. W., Whaley D. H., Rhodes D. J., and O'Connor M. K., “Molecular breast imaging: use of a dual-head dedicated gamma camera to detect small breast tumors,” AJR, Am. J. Roentgenol. 191, 1805–1815 (2008). 10.2214/AJR.07.3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S., Black R., Kross B., Popov V., Welch B., Wojecik R., Williams M. B., More M. J., and Goodale P., “Phantom evaluations of a dedicated dual-head scintimammography system,” Phys. Med. 21(Suppl 1), 35–38 (2006). 10.1016/S1120-1797(06)80021-9 [DOI] [PubMed] [Google Scholar]
- Judy P. G., Welch B., Saviour T. St., Kieper D., Majewski S., McKisson J., Kross B., Proffitt J., Stolin A., More M. J., Dinion N. L., and Williams M. B., “Molecular breast imaging with directly opposing compact gamma cameras,” in Nuclear Science Symposium Conference Record, 2007. NSS ‘07. IEEE, Vol. 6 (IEEE, 2007), pp. 4040–4043.
- Garibaldi F., Cisbani E., Colilli S., Cusanno F., Fratoni R., Giuliani F., Gricia M., Lucentini M., Magliozzi M. L., Santavenere F., Torrioli S., Musico P., Argentieri A., Cossu E., Padovano F., Simonetti G., Schillaci O., and Majewski S., “A novel high resolution and high efficiency dual head detector for molecular breast imaging: New results from clinical trials,” Nucl. Instrum. Methods Phys. Res. A 617, 227–229 (2010). 10.1016/j.nima.2009.10.015 [DOI] [Google Scholar]
- Judy P. G., Zongyi G., Dinion N. L., Welch B. L., Saviour T. S., Kieper D., Majewski S., McKisson J., Kross B., Proffitt J., Stolin A. V., More M. J., and Williams M. B., “Analysis of image combination methods for conjugate breast scintigraphy,” IEEE Trans. Nucl. Sci. 57, 1146–1154 (2010). 10.1109/TNS.2009.2038472 [DOI] [Google Scholar]
- Hruska C. B. and O'Connor M. K., “Quantification of lesion size, depth, and uptake using a dual-head molecular breast imaging system,” Med. Phys. 35, 1365–1376 (2008). 10.1118/1.2885371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Murthy K., Weinberg I. N., and Mako F., “Feasibility study for positron emission mammography,” Med. Phys. 21, 529–538 (1994). 10.1118/1.597169 [DOI] [PubMed] [Google Scholar]
- Weinberg I. N., “Applications for positron emission mammography,” Phys. Med. 21(Suppl 1), 132–137 (2006). 10.1016/S1120-1797(06)80045-1 [DOI] [PubMed] [Google Scholar]
- Joshi A., Koeppe R. A., and Fessler J. A., “Reducing between scanner differences in multi-center PET studies,” Neuroimage 46, 154–159 (2009). 10.1016/j.neuroimage.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg I., “The promise of PEM,” in Advance for Imaging & Radiation Oncology (Merion Matters, 2004), Vol. 14, p. 91. [Google Scholar]
- Weinberg I., Majewski S., Weisenberger A., Markowitz A., Aloj L., Majewski L., Danforth D., Mulshine J., Cowan K., Zujewski J., Chow C., Jones E., Chang V., Berg W., and Frank J., “Preliminary results for positron emission mammography: Real-time functional breast imaging in a conventional mammography gantry,” Eur. J. Nucl. Med. 23, 804–806 (1996). 10.1007/BF00843710 [DOI] [PubMed] [Google Scholar]
- Doshi N. K., Shao Y., Silverman R. W., and Cherry S. R., “Design and evaluation of an LSO PET detector for breast cancer imaging,” Med. Phys. 27, 1535–1543 (2000). 10.1118/1.599019 [DOI] [PubMed] [Google Scholar]
- Freifelder R. and Karp J. S., “Dedicated PET scanners for breast imaging,” Phys. Med. Biol. 42, 2463–2480 (1997). 10.1088/0031-9155/42/12/012 [DOI] [PubMed] [Google Scholar]
- Huber J. S., Moses W. W., Derenzo S. E., Ho M. H., Andreaco M. S., Paulus M. J., and Nutt R., “Characterization of a 64 channel PET detector using photodiodes for crystal identification,” IEEE Trans. Nucl. Sci. 44, 1197–1201 (1997). 10.1109/23.596987 [DOI] [Google Scholar]
- Williams M. B., Sealock R. M., Majewski S., and Weisenberger A. G., “PET detector using waveshifting optical fibers and microchannel plate PMT with delay line readout,” IEEE Trans. Nucl. Sci. 45, 195–205 (1998). 10.1109/23.664171 [DOI] [Google Scholar]
- Worstell W., Johnson O., Kudrolli H., and Zavarzin V., “First results with high-resolution PET detector modules using wavelength-shifting fibers,” IEEE Trans. Nucl. Sci. 45, 2993–2999 (1998). 10.1109/23.737655 [DOI] [Google Scholar]
- Raylman R. R., Majewski S., Wojcik R., Weisenberger A. G., Kross B., Popov V., and Bishop H. A., “The potential role of positron emission mammography for detection of breast cancer. A phantom study,” Med. Phys. 27, 1943–1954 (2000). 10.1118/1.1287439 [DOI] [PubMed] [Google Scholar]
- Smith M. F., Raylman R. R., Majewski S., and Weisenberger A. G., “Positron emission mammography with tomographic acquisition using dual planar detectors: Initial evaluations,” Phys. Med. Biol. 49, 2437–2452 (2004). 10.1088/0031-9155/49/11/022 [DOI] [PubMed] [Google Scholar]
- Zhang J., Foudray A. M. K., Olcott P. D., Farrell R., Shah K., and Levin C. S., “Performance Characterization of a novel thin position-sensitive avalanche photodiode for 1 mm resolution positron emission tomography,” IEEE Trans. Nucl. Sci. 54, 415–421 (2007). 10.1109/TNS.2007.894128 [DOI] [Google Scholar]
- Peng H. and Levin C. S., “Design study of a high-resolution breast-dedicated PET system built from cadmium zinc telluride detectors,” Phys. Med. Biol. 55, 2761–2788 (2010). 10.1088/0031-9155/55/9/022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald L., Edwards J., Lewellen T., Haseley D., Rogers J., and Kinahan P., “Clinical imaging characteristics of the positron emission mammography camera: PEM Flex Solo II,” J. Nucl. Med. 50, 1666–1675 (2009). 10.2967/jnumed.109.064345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Anashkin E., and Matthews C. G., “Performance evaluation of a pem scanner using the NEMA NU 4-2008 small animal PET standards,” IEEE Trans. Nucl. Sci. 57, 94–103 (2010). 10.1109/TNS.2009.2036847 [DOI] [Google Scholar]
- Moliner L. et al. , “Design and evaluation of the MAMMI dedicated breast PET,” Med. Phys. 39, 5393–5404 (2012). 10.1118/1.4742850 [DOI] [PubMed] [Google Scholar]
- Moliner L., Benlloch J. M., Carles M., Correcher C., Gonzalez A. J., Orero A., Sanchez F., and Soriano A., “Performance characteristics of the MAMMI PEMT scanner based on NEMA NU 2–2007,” Nuclear Science Symposium Conference Record (NSS/MIC) (IEEE, 2010), pp. 2591–2594. 10.1109/NSSMIC.2010.5874256 [DOI]
- Soriano A., González A., Orero A., Moliner L., Carles M., Sánchez F., Benlloch J. M., Correcher C., Carrilero V., and Seimetz M., “Attenuation correction without transmission scan for the MAMMI breast PET,” Nucl. Instrum. Methods Phys. Res. A 648(Suppl 1), S75–S78 (2011). 10.1016/j.nima.2010.12.138 [DOI] [Google Scholar]
- Wu Y., Bowen S. L., Yang K., Packard N., Fu L., Burkett G., Qi J., Boone J. M., Cherry S. R., and Badawi R. D., “PET characteristics of a dedicated breast PET/CT scanner prototype,” Phys. Med. Biol. 54, 4273–4287 (2009). 10.1088/0031-9155/54/13/020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S. L., Wu Y., Chaudhari A. J., Fu L., Packard N. J., Burkett G. W., Yang K., Lindfors K. K., Shelton D. K., Hagge R., Borowsky A. D., Martinez S. R., Qi J., Boone J. M., Cherry S. R., and Badawi R. D., “Initial characterization of a dedicated breast PET/CT scanner during human imaging,” J. Nucl. Med. 50, 1401–1408 (2009). 10.2967/jnumed.109.064428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. C., Huber J. S., Moses W. W., Qi J., and Choong W. S., “Characterization of the LBNL PEM camera,” IEEE Trans. Nucl. Sci. 53, 1129–1135 (2006). 10.1109/TNS.2006.874956 [DOI] [Google Scholar]
- Ravindranath B., Maramraju S. H., Junnarkar S. S., Southekal S. S., Stoll S. P., Pratte J. F., Purschke M. L., Hong X., Bennett D., Cheng K., Tomasi D., Smith D. S., Krishnamoorthy S., Vaska P., Woody C. L., and Schlyer D. J., “A simultaneous PET/MRI breast scanner based on the RatCAP,” Nuclear Science Symposium Conference Record, 2008. NSS '08 (IEEE, 2008), pp. 4650–4655. 10.1109/NSSMIC.2008.4774460 [DOI]
- Zhang J., Chinn G., Foudray A. M., Habte F., Olcott P., and Levin C. S., “Evaluation of a dual-panel PET camera design to breast cancer imaging,” Phys. Med. 21(Suppl 1), 94–98 (2006). 10.1016/S1120-1797(06)80035-9 [DOI] [PubMed] [Google Scholar]
- Rosen E. L., Turkington T. G., Soo M. S., Baker J. A., and Coleman R. E., “Detection of primary breast carcinoma with a dedicated, large-field-of-view FDG PET mammography device: Initial experience1,” Radiology 234, 527–534 (2005). 10.1148/radiol.2342040654 [DOI] [PubMed] [Google Scholar]
- Raylman R. R., Majewski S., Smith M. F., Proffitt J., Hammond W., Srinivasan A., McKisson J., Popov V., Weisenberger A., Judy C. O., Kross B., Ramasubramanian S., Banta L. E., Kinahan P. E., and Champley K., “The positron emission mammography/tomography breast imaging and biopsy system (PEM/PET): Design, construction and phantom-based measurements,” Phys. Med. Biol. 53, 637–653 (2008). 10.1088/0031-9155/53/3/009 [DOI] [PubMed] [Google Scholar]
- Raylman R. R., Abraham J., Hazard H., Koren C., Filburn S., Schreiman J. S., Kurian S., Majewski S., and Marano G. D., “Initial clinical test of a breast-PET scanner,” J. Med. Imaging Radiat. Oncol. 55, 58–64 (2011). 10.1111/j.1754-9485.2010.02230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ramirez R. A., Hongdi L., Shitao L., Shaohui A., Chao W., Baghaei H., and Wai-Hoi W., “The system design, engineering architecture, and preliminary results of a lower-cost high-sensitivity high-resolution positron emission mammography camera,” IEEE Trans. Nucl. Sci. 57, 104–110 (2010). 10.1109/TNS.2009.2031644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda M., Belcari N., Guerra A. D., Galeotti S., Morsani F., Herbert D. J., and Vaiano A., “Development of the YAP-PEM scanner for breast cancer imaging,” Phys. Med. 21(Suppl 1), 114–116 (2006). 10.1016/S1120-1797(06)80040-2 [DOI] [PubMed] [Google Scholar]
- Lecoq P. and Varela J., “Clear-PEM, a dedicated PET camera for mammography,” Nucl. Instrum. Methods Phys. Res. A 486, 1–6 (2002). 10.1016/S0168-9002(02)00666-6 [DOI] [Google Scholar]
- Abreu M. C., Aguiar D., Albuquerque E., Almeida F. G., Almeida P., Amaral P., Auffray E., Bento P., Bruyndonckx P., Bugalho R., Carriço B., Cordeiro H., Ferreira M., Ferreira N. C., Gonçalves F., Lecoq P., Leong C., Lopes F., Lousã P., Luyten J., Martins M. V., Matela N., Mendes P. R., Moura R., Nobre J., Oliveira N., Ortigão C., Peralta L., Rego J., Ribeiro R., Rodrigues P., Santos A. I., Silva J. C., Silva M. M., Tavernier S., Teixeira I. C., Teixeira J. P., Trindade A., Trummer J., and Varela J., “Clear-PEM: A PET imaging system dedicated to breast cancer diagnostics,” Nucl. Instrum. Methods Phys. Res. A 571, 81–84 (2007). 10.1016/j.nima.2006.10.034 [DOI] [Google Scholar]
- Varela J., “A PET imaging system dedicated to mammography,” Radiat. Phys. Chem. 76, 347–350 (2007). 10.1016/j.radphyschem.2006.03.065 [DOI] [Google Scholar]
- Kitamura K., Junichi O., Hiromichi T., Yoshihiro Y., Tetsuo F., Masafumi F., Masanobu S., Tomoaki T., Masayuki N., Nobuya H., Yoshiyuki Y., Ayako K., and Yoshihiko K., “Development of a C-shaped breast PET scanner equipped with four-layer DOI detectors,” Nuclear Science Symposium Conference Record, 2008, NSS '08 (IEEE, 2008), pp. 5662–5665. 10.1109/NSSMIC.2008.4774517 [DOI]
- Furuta M., Kitamura K., Ohi J., Tonami H., Yamada Y., Furumiya T., Satoh M., Tsuda T., Nakazawa M., Hashizume N., Yamakawa Y., Kawashima A., and Kumazawa Y., “Basic evaluation of a C-shaped breast PET scanner,” Nuclear Science Symposium Conference Record (NSS/MIC) (IEEE, 2009), pp. 2548–2552. 10.1109/NSSMIC.2009.5402027 [DOI]
- Iima M., Nakamoto Y., Kanao S., Sugie T., Ueno T., Kawada M., Mikami Y., Toi M., and Togashi K., “Clinical performance of 2 dedicated PET scanners for breast imaging: Initial evaluation,” J. Nucl. Med. 53, 1534–1542 (2012). 10.2967/jnumed.111.100958 [DOI] [PubMed] [Google Scholar]
- Cohade C., Osman M., Leal J., and Wahl R. L., “Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma,” J. Nucl. Med. 44, 1797–1803 (2003). [PubMed] [Google Scholar]
- Lardinois D., Weder W., Hany T. F., Kamel E. M., Korom S., Seifert B., von Schulthess G. K., and Steinert H. C., “Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography,” N Engl. J. Med. 348, 2500–2507 (2003). 10.1056/NEJMoa022136 [DOI] [PubMed] [Google Scholar]
- Bergman A. M., Thompson C. J., Murthy K., Robar J. L., Clancy R. L., English M. J., Loutfi A., Lisbona R., and Gagnon J., “Technique to obtain positron emission mammography images in registration with x-ray mammograms,” Med. Phys. 25, 2119–2129 (1998). 10.1118/1.598408 [DOI] [PubMed] [Google Scholar]
- Adler L. P., Weinberg I. N., Bradbury M. S., Levine E. A., Lesko N. M., Geisinger K. R., Berg W. A., and Freimanis R. I., “Method for combined FDG-PET and radiographic imaging of primary breast cancers,” Breast J. 9, 163–166 (2003). 10.1046/j.1524-4741.2003.09306.x [DOI] [PubMed] [Google Scholar]
- Murthy K., Aznar M., Bergman A. M., Thompson C. J., Robar J. L., Lisbona R., Loutfi A., and Gagnon J. H., “Positron emission mammographic instrument: Initial results.,” Radiology 215, 280–285 (2000). [DOI] [PubMed] [Google Scholar]
- Coover L. R., Caravaglia G., and Kuhn P., “Scintimammography with dedicated breast camera detects and localizes occult carcinoma,” J. Nucl. Med. 45, 553–558 (2004). [PubMed] [Google Scholar]
- Williams M. B., More M. J., Narayanan D., Majewski S., Weisenberger A. G., Wojcik R., Stanton M., Phillips W., and Stewart A., “Combined structural and functional imaging of the breast,” Technol. Cancer Res. Treat. 1, 39–42 (2002). [DOI] [PubMed] [Google Scholar]
- Williams M. B., Judy P. G., Gunn S., and Majewski S., “Dual-modality breast tomosynthesis,” Radiology 255, 191–198 (2010). 10.1148/radiol.09091160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzymialkiewicz C. N., Tornai M. P., McKinley R. L., Cutler S. J., and Bowsher J. E., “Performance of dedicated emission mammotomography for various breast shapes and sizes,” Phys. Med. Biol. 51, 5051–5064 (2006). 10.1088/0031-9155/51/19/021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty D. J., Cutler S. J., McKinley R. L., Madhav P., Perez K. L., and Tornai M. P., “Improved chest wall imaging through combined complex trajectories in dedicated dual modality SPECT-CT breast molecular imaging,” Nuclear Science Symposium Conference Record, 2008, NSS '08 (IEEE, 2008), pp. 2650–5656. 10.1109/NSSMIC.2008.4774525 [DOI]
- Madhav P., Cutler S. J., Perez K. L., Crotty D. J., McKinley R. L., Wong T. Z., and Tornai M. P., “Comparison of reduced angle and fully 3D acquisition sequencing and trajectories for dual-modality mammotomography,” Nuclear Science Symposium Conference Record, 2007, NSS '07 (IEEE, 2007), Vol. 6, pp. 4044–4050. 10.1109/NSSMIC.2007.4437016 [DOI]
- Lindfors K. K., Boone J. M., Nelson T. R., Yang K., Kwan A. L., and Miller D. F., “Dedicated breast CT: initial clinical experience,” Radiology 246, 725–733 (2008). 10.1148/radiol.2463070410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E., Almeida F. G., Almeida P., Auffray E., Barbosa J., Bastos A. L., Bexiga V., Bugalho R., Carmona S., Carrico B., Ferreira C. S., Ferreira N. C., Ferreira M., Frade M., Godinho J., Goncalves F., Guerreiro C., Lecoq P., Leong C., Lousa P., Machado P., Martins M. V., Matela N., Moura R., Neves P., Oliveira N., Ortigao C., Piedade F., Pinheiro J. F., Relvas P., Rivetti A., Rodrigues P., Rolo I., Sampaio J., Santos A. I., Santos J., Silva M. M., Tavernier S., Teixeira I. C., Teixeira J. P., Silva R., Silva J. C., Trindade A., and Varela J., “An overview of the Clear-PEM breast imaging scanner,” Nuclear Science Symposium Conference Record, 2008. NSS '08 (IEEE, 2008), pp. 5616–5618. 10.1109/NSSMIC.2008.4774518 [DOI]
- Hede K., “Preoperative MRI in breast cancer grows contentious,” J. Natl. Cancer Inst. 101, 1667–1669 (2009). 10.1093/jnci/djp461 [DOI] [PubMed] [Google Scholar]
- Katipamula R., Degnim A. C., Hoskin T., Boughey J. C., Loprinzi C., Grant C. S., Brandt K. R., Pruthi S., Chute C. G., Olson J. E., Couch F. J., Ingle J. N., and Goetz M. P., “Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging,” J. Clin. Oncol. 27, 4082–4088 (2009). 10.1200/JCO.2008.19.4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssami N., Turner R., and Morrow M., “Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes,” Ann. Surg. 257, 249–255 (2013). 10.1097/SLA.0b013e31827a8d17 [DOI] [PubMed] [Google Scholar]
- Schnall M. D., Blume J., Bluemke D. A., Deangelis G. A., Debruhl N., Harms S., Heywang-Kobrunner S. H., Hylton N., Kuhl C. K., Pisano E. D., Causer P., Schnitt S. J., Smazal S. F., Stelling C. B., Lehman C., Weatherall P. T., and Gatsonis C. A., “MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883,” J. Surg. Oncol. 92, 32–38 (2005). 10.1002/jso.20381 [DOI] [PubMed] [Google Scholar]
- Lehman C. D., Gatsonis C., Kuhl C. K., Hendrick R. E., Pisano E. D., Hanna L., Peacock S., Smazal S. F., Maki D. D., Julian T. B., DePeri E. R., Bluemke D. A., and Schnall M. D., “MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer,” N. Engl. J. Med. 356, 1295–1303 (2007). 10.1056/NEJMoa065447 [DOI] [PubMed] [Google Scholar]
- Khan A. N., Hoosein M., Khan H., Grosvenor L., and Al-Attar M., “Does breast magnetic resonance imaging measurement correlate with pathology in assessment of primary breast cancer? [abstract],” Breast Cancer Res. 11, P3 (2009). 10.1186/bcr2373 [DOI] [Google Scholar]
- Caprio K. A., Chagpar A. B., Hooley R., Tavassoli F., Honarpishe H., Lannin D. R., Killelea B. K., and Horowitz N. R., “Accuracy of breast MRI in predicting pathologic tumor size [abstract],” J. Clin. Oncol. 30, 1109 (2012). [Google Scholar]
- Onesti J. K., Mangus B. E., Helmer S. D., and Osland J. S., “Breast cancer tumor size: Correlation between magnetic resonance imaging and pathology measurements,” Am. J. Surg. 196, 844–850 (2008). 10.1016/j.amjsurg.2008.07.028 [DOI] [PubMed] [Google Scholar]
- Berg W. A., Gutierrez L., NessAiver M. S., Carter W. B., Bhargavan M., Lewis R. S., and Ioffe O. B., “Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer,” Radiology 233, 830–849 (2004). 10.1148/radiol.2333031484 [DOI] [PubMed] [Google Scholar]
- Malur S., Wurdinger S., Moritz A., Michels W., and Schneider A., “Comparison of written reports of mammography, sonography and magnetic resonance mammography for preoperative evaluation of breast lesions, with special emphasis on magnetic resonance mammography,” Breast Cancer Res. 3, 55–60 (2001). 10.1186/bcr271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone B., Wiberg M. K., Szabo B. K., Szakos A., and Danielsson R., “Comparison of 99mTc-sestamibi scintimammography and dynamic MR imaging as adjuncts to mammography in the diagnosis of breast cancer,” Acta Radiol. 44, 28–34 (2003). 10.1034/j.1600-0455.2003.00012.x [DOI] [PubMed] [Google Scholar]
- Liberman L., Morris E. A., Dershaw D. D., Abramson A. F., and Tan L. K., “MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer,” AJR, Am. J. Roentgenol. 180, 901–910 (2003). 10.2214/ajr.180.4.1800901 [DOI] [PubMed] [Google Scholar]
- Killelea B. K., Gillego A., Kirstein L. J., Asad J., Shpilko M., Shah A., Feldman S., and Boolbol S. K., “George Peters Award: How does breast-specific gamma imaging affect the management of patients with newly diagnosed breast cancer?,” Am. J. Surg. 198, 470–474 (2009). 10.1016/j.amjsurg.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Zhou M., Johnson N., Gruner S., Ecklund G. W., Meunier P., Bryn S., Glissmeyer M., and Steinbock K., “Clinical utility of breast-specific gamma imaging for evaluating disease extent in the newly diagnosed breast cancer patient,” Am. J. Surg. 197, 159–163 (2009). 10.1016/j.amjsurg.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Kim B., “Usefulness of breast-specific gamma imaging as an adjunct modality in breast cancer patients with dense breast: A comparative study with MRI,” Ann. Nucl. Med. 26, 131–137 (2012). 10.1007/s12149-011-0544-5 [DOI] [PubMed] [Google Scholar]
- Spanu A., Chessa F., Battista Meloni G., Sanna D., Cottu P., Manca A., Nuvoli S., and Madeddu G., “Scintimammography with high resolution dedicated breast camera and mammography in multifocal, multicentric and bilateral breast cancer detection: A comparative study,” Q J. Nucl. Med. Mol. Imaging 53, 133–143 (2009). [PubMed] [Google Scholar]
- O'Connor M. K., Hruska C. B., Rhodes D. J., Conners A. L., Tortorelli C. L., Maxwell R., and Boughey J. C., “Molecular breast imaging (MBI) in the preoperative evaluation of women with biopsy-proven breast cancer [abstract],” J. Nucl. Med. 52, 660–675 (2011). [Google Scholar]
- Tafra L., Cheng Z., Uddo J., Lobrano M. B., Stein W., Berg W. A., Levine E., Weinberg I. N., Narayanan D., Ross E., Beylin D., Yarnall S., Keen R., Sawyer K., Van Geffen J., Freimanis R. L., Staab E., Adler L. P., Lovelace J., Shen P., Stewart J., and Dolinsky S., “Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer,” Am. J. Surg. 190, 628–632 (2005). 10.1016/j.amjsurg.2005.06.029 [DOI] [PubMed] [Google Scholar]
- Schilling K., Narayanan D., Kalinyak J. E., The J., Velasquez M. V., Kahn S., Saady M., Mahal R., and Chrystal L., “Positron emission mammography in breast cancer presurgical planning: Comparisons with magnetic resonance imaging,” Eur. J. Nucl. Med. Mol Imaging 38, 23–36 (2011). 10.1007/s00259-010-1588-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo J. S., Chun I. K., Paeng J. C., Kang K. W., Lee S. M., Han W., Noh D.-Y., Chung J.-K., and Lee D. S., “Imaging sensitivity of dedicated positron emission mammography in relation to tumor size,” Breast 21, 66–71 (2012). 10.1016/j.breast.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Chagpar A. B., Middleton L. P., Sahin A. A., Dempsey P., Buzdar A. U., Mirza A. N., Ames F. C., Babiera G. V., Feig B. W., Hunt K. K., Kuerer H. M., Meric-Bernstam F., Ross M. I., and Singletary S. E., “Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy,” Ann. Surg. 243, 257–264 (2006). 10.1097/01.sla.0000197714.14318.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helvie M. A., Joynt L. K., Cody R. L., Pierce L. J., Adler D. D., and Merajver S. D., “Locally advanced breast carcinoma: Accuracy of mammography versus clinical examination in the prediction of residual disease after chemotherapy,” Radiology 198, 327–332 (1996). [DOI] [PubMed] [Google Scholar]
- Croshaw R., Shapiro-Wright H., Svensson E., Erb K., and Julian T., “Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients,” Ann. Surg. Oncol. 18, 3160–3163 (2011). 10.1245/s10434-011-1919-5 [DOI] [PubMed] [Google Scholar]
- Maini C. L., Tofani A., Sciuto R., Semprebene A., Cavaliere R., Mottolese M., Benevolo M., Ferranti F., Grandinetti M. L., Vici P., Lopez M., and Botti C., “Technetium-99m-MIBI scintigraphy in the assessment of neoadjuvant chemotherapy in breast carcinoma,” J. Nucl. Med. 38, 1546–1551 (1997). [PubMed] [Google Scholar]
- Mankoff D. A., Dunnwald L. K., Gralow J. R., Ellis G. K., Drucker M. J., and Livingston R. B., “Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99m]-sestamibi scintimammography,” Cancer 85, 2410–2423 (1999). [DOI] [PubMed] [Google Scholar]
- Tiling R., Kessler M., Untch M., Sommer H., Linke R., Brinkbaumer K., and Hahn K., “Breast cancer: monitoring response to neoadjuvant chemotherapy using Tc-99m sestamibi scintimammography,” Onkologie 26, 27–31 (2003). 10.1159/000069860 [DOI] [PubMed] [Google Scholar]
- Dunnwald L. K., Gralow J. R., Ellis G. K., Livingston R. B., Linden H. M., Lawton T. J., Barlow W. E., Schubert E. K., and Mankoff D. A., “Residual tumor uptake of [99mTc]-sestamibi after neoadjuvant chemotherapy for locally advanced breast carcinoma predicts survival,” Cancer 103, 680–688 (2005). 10.1002/cncr.20831 [DOI] [PubMed] [Google Scholar]
- Fuster D., Munoz M., Pavia J., Palacin A., Bellet N., Mateos J. J., Martin F., Ortega M., Setoain F. J., and Pons F., “Quantified 99mTc-MIBI scintigraphy for predicting chemotherapy response in breast cancer patients: Factors that influence the level of 99m Tc-MIBI uptake,” Nucl. Med. Commun. 23, 31–38 (2002). 10.1097/00006231-200201000-00006 [DOI] [PubMed] [Google Scholar]
- Smith I. C., Welch A. E., Hutcheon A. W., Miller I. D., Payne S., Chilcott F., Waikar S., Whitaker T., Ah-See A. K., Eremin O., Heys S. D., Gilbert F. J., and Sharp P. F., “Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy,” J. Clin. Oncol. 18, 1676–1688 (2000). [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kim S. K., Lee E. S., Ro J., and Kang S., “Predictive value of [18F]FDG PET for pathological response of breast cancer to neo-adjuvant chemotherapy,” Ann. Oncol. 15, 1352–1357 (2004). 10.1093/annonc/mdh345 [DOI] [PubMed] [Google Scholar]
- Spanu A., Farris A., Chessa F., Sanna D., Pittalis M., Manca A., and Madeddu G., “Planar scintimammography and SPECT in neoadjuvant chemo or hormonotherapy response evaluation in locally advanced primary breast cancer,” Int. J. Oncol. 32, 1275–1283 (2008). [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Wahner-Roedler D. L., Boughey J. C., Conners A. L., Tortorelli C. L., Maxwell R., O'Connor M. K., and Rhodes D. J., “Molecular breast imaging (MBI) in monitoring treatment response to neoadjuvant chemotherapy [abstract],” J. Nucl. Med. 52, 660–675 (2011). [Google Scholar]
- Yang W. T., Chang J., Cristofanilli M., Wei W., Krishnamurthy S., Rohren E., Ueno N., Valero V., and Buchholz T., “Evaluation of response to targeted therapy using positron emission mammography (PEM) [abstract],” presented at Radiological Society of North America Annual Meeting (unpublished).
- Ferrero A., Chaudhari A. J., Bowen S. L., Yang K., Lindfors K. K., Boone J. M., Martinez S. R., and Badawi R. D., “Quantification of early response to neoadjuvant chemotherapy in breast cancer: initial studies in humans using a high resolution PET/CT scanner [abstract],” J. Nucl. Med. 52, 660–675 (2011). [Google Scholar]
- Brem R. F., Petrovitch I., Rapelyea J. A., Young H., Teal C., and Kelly T., “Breast-specific gamma imaging with 99mTc-Sestamibi and magnetic resonance imaging in the diagnosis of breast cancer–A comparative study,” Breast J. 13, 465–469 (2007). 10.1111/j.1524-4741.2007.00466.x [DOI] [PubMed] [Google Scholar]
- Brem R. F., Fishman M., and Rapelyea J. A., “Detection of ductal carcinoma in situ with mammography, breast specific gamma imaging, and magnetic resonance imaging: A comparative study,” Acad. Radiol. 14, 945–950 (2007). 10.1016/j.acra.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Brem R. F., Ioffe M., Rapelyea J. A., Yost K. G., Weigert J. M., Bertrand M. L., and Stern L. H., “Invasive lobular carcinoma: Detection with mammography, sonography, MRI, and breast-specific gamma imaging,” AJR, Am. J. Roentgenol. 192, 379–383 (2009). 10.2214/AJR.07.3827 [DOI] [PubMed] [Google Scholar]
- Keto J., Kirstein L., Sanchez D., Fulop T., McPartland L., Cohen I., and Boolbol S., “MRI versus breast-specific gamma imaging (BSGI) in newly diagnosed ductal cell carcinoma insitu: A prospective head-to-head trial,” Ann. Surg. Oncol. 19, 249–252 (2012). 10.1245/s10434-011-1848-3 [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Phillips S. W., Rhodes D. J., and O'Connor M. K., “Concordance of molecular breast imaging and breast MRI findings: A retrospective review [abstract],” Eur. J. Nucl. Med. Mol. Imaging 35, 199–210 (2008). 10.1007/s00259-008-0906-y [DOI] [Google Scholar]
- D'Orsi C. I., Bassett L. W., and Berg W. A., Breast Imaging Reporting and Data System, BI-RADS: Mammography (American College of Radiology, Reston, VA, 2003). [Google Scholar]
- de Koning H. J., “Mammographic screening: Evidence from randomised controlled trials,” Ann. Oncol. 14, 1185–1189 (2003). 10.1093/annonc/mdg319 [DOI] [PubMed] [Google Scholar]
- Nelson H. D., Tyne K., Naik A., Bougatsos C., Chan B. K., and Humphrey L., “Screening for breast cancer: An update for the U.S. Preventive Services Task Force,” Ann. Intern Med. 151, 727–737 (2009). 10.7326/0003-4819-151-10-200911170-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelson M. T., Oestreicher N., Porter P. L., White D., Finder C. A., Taplin S. H., and White E., “Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers,” J. Natl. Cancer Inst. 92, 1081–1087 (2000). 10.1093/jnci/92.13.1081 [DOI] [PubMed] [Google Scholar]
- Kolb T. M., Lichy J., and Newhouse J. H., “Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations,” Radiology 225, 165–175 (2002). 10.1148/radiol.2251011667 [DOI] [PubMed] [Google Scholar]
- Carney P. A., Miglioretti D. L., Yankaskas B. C., Kerlikowske K., Rosenberg R., Rutter C. M., Geller B. M., Abraham L. A., Taplin S. H., Dignan M., Cutter G., and Ballard-Barbash R., “Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography,” Ann. Intern Med. 138, 168–175 (2003). 10.7326/0003-4819-138-3-200302040-00008 [DOI] [PubMed] [Google Scholar]
- Buist D. S., Porter P. L., Lehman C., Taplin S. H., and White E., “Factors contributing to mammography failure in women aged 40-49 years,” J. Natl. Cancer Inst. 96, 1432–1440 (2004). 10.1093/jnci/djh269 [DOI] [PubMed] [Google Scholar]
- Pisano E. D., Hendrick R. E., Yaffe M. J., Baum J. K., Acharyya S., Cormack J. B., Hanna L. A., Conant E. F., Fajardo L. L., Bassett L. W., D'Orsi C. J., Jong R. A., Rebner M., Tosteson A. N., and Gatsonis C. A., “Diagnostic accuracy of digital versus film mammography: Exploratory analysis of selected population subgroups in DMIST,” Radiology 246, 376–383 (2008). 10.1148/radiol.2461070200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssami N. et al. , “Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer,” JAMA, J. Am. Med. Assoc. 305, 790–799 (2011). 10.1001/jama.2011.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigenwald R. Z., Warner E., Gunasekara A., Hill K. A., Causer P. A., Messner S. J., Eisen A., Plewes D. B., Narod S. A., Zhang L., and Yaffe M. J., “Is Mammography Adequate for Screening Women with Inherited BRCA Mutations and Low Breast Density?,” Cancer Epidem. Biomarkers Prev. 17, 706–711 (2008). 10.1158/1055-9965.EPI-07-0509 [DOI] [PubMed] [Google Scholar]
- Vachon C. M., van Gils C. H., Sellers T. A., Ghosh K., Pruthi S., Brandt K. R., and Pankratz V. S., “Mammographic density, breast cancer risk and risk prediction,” Breast Cancer Res. 9, 217–225 (2007). 10.1186/bcr1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N. F., Guo H., Martin L. J., Sun L., Stone J., Fishell E., Jong R. A., Hislop G., Chiarelli A., Minkin S., and Yaffe M. J., “Mammographic density and the risk and detection of breast cancer,” N Engl. J. Med. 356, 227–236 (2007). 10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- Rafferty E. A., Park J. M., Philpotts L. E., Poplack S. P., Sumkin J. H., Halpern E. F., and Niklason L. T., “Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: Results of a multicenter, multireader trial,” Radiology 266, 104–113 (2013). 10.1148/radiol.12120674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaane P., Bandos A. I., Gullien R., Eben E. B., Ekseth U., Haakenaasen U., Izadi M., Jebsen I. N., Jahr G., Krager M., Niklason L. T., Hofvind S., and Gur D., “Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program,” Radiology 267, 47–56 (2013). 10.1148/radiol.12121373 [DOI] [PubMed] [Google Scholar]
- Berg W. A., Zhang Z., Lehrer D., Jong R. A., Pisano E. D., Barr R. G., Bohm-Velez M., Mahoney M. C., W. P.Evans3rd, Larsen L. H., Morton M. J., Mendelson E. B., Farria D. M., Cormack J. B., Marques H. S., Adams A., Yeh N. M., and Gabrielli G., “Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk,” JAMA, J. Am. Med. Assoc. 307, 1394–1404 (2012). 10.1001/jama.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti V., Houssami N., Ghirardi M., Ferrari A., Speziani M., Bellarosa S., Remida G., Gasparotti C., Galligioni E., and Ciatto S., “Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1 year follow-up,” Eur. J. Cancer 47, 1021–1026 (2011). 10.1016/j.ejca.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Kelly K., Dean J., Comulada W., and Lee S.-J., “Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts,” Eur. Radiol. 20, 734–742 (2010). 10.1007/s00330-009-1588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow D., Boetes C., Burke W., Harms S., Leach M. O., Lehman C. D., Morris E., Pisano E., Schnall M., Sener S., Smith R. A., Warner E., Yaffe M., Andrews K. S., and Russell C. A., “American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography,” Ca-Cancer J. Clin. 57, 75–89 (2007). 10.3322/canjclin.57.2.75 [DOI] [PubMed] [Google Scholar]
- Brem R. F., Rapelyea J. A., Zisman G., Mohtashemi K., Raub J., Teal C. B., Majewski S., and Welch B. L., “Occult breast cancer: Scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer,” Radiology 237, 274–280 (2005). 10.1148/radiol.2371040758 [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Rhodes D. J., Collins D. A., Tortorelli C. L., Askew J. W., and O'Connor M. K., “Evaluation of molecular breast imaging in women undergoing myocardial perfusion imaging with tc-99m sestamibi,” J. Womens Health (Larchmt) 21, 730–738 (2012). 10.1089/jwh.2011.3267 [DOI] [PubMed] [Google Scholar]
- Schell M. J., Yankaskas B. C., Ballard-Barbash R., Qaqish B. F., Barlow W. E., Rosenberg R. D., and Smith-Bindman R., “Evidence-based target recall rates for screening mammography,” Radiology 243, 681–689 (2007). 10.1148/radiol.2433060372 [DOI] [PubMed] [Google Scholar]
- Kriege M., Brekelmans C. T., Boetes C., Muller S. H., Zonderland H. M., Obdeijn I. M., Manoliu R. A., Kok T., Rutgers E. J., de Koning H. J., and Klijn J. G., “Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition,” Cancer 106, 2318–2326 (2006). 10.1002/cncr.21863 [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Weinmann A. L., and O'Connor M. K., “Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part I. Evaluation in phantoms,” Med. Phys. 39, 3466–3475 (2012). 10.1118/1.4718665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska C. B., Weinmann A. L., Tello Skjerseth C. M., Wagenaar E. M., Conners A. L., Tortorelli C. L., Maxwell R. W., Rhodes D. J., and O'Connor M. K., “Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part II. Evaluation in patients,” Med. Phys. 39, 3476–3483 (2012). 10.1118/1.4719959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Hruska C., Conners A., Tortorelli C., Maxwell R., and O'Connor M., “Low-dose molecular breast imaging with Tc-99m sestamibi for screening in women with mammographically dense breasts [abstract],” presented at Radiological Society of North America Annual Meeting (unpublished).
- Goldsmith S. J., Parsons W., Guiberteau M. J., Stern L. H., Lanzkowsky L., Weigert J., Heston T. F., Jones E., Buscombe J., and Stabin M. G., “SNM practice guideline for breast scintigraphy with breast-specific gamma-cameras 1.0,” J. Nucl. Med. Technol. 38, 219–224 (2010). 10.2967/jnmt.110.082271 [DOI] [PubMed] [Google Scholar]
- Silverstein M. J., Recht A., Lagios M. D., Bleiweiss I. J., Blumencranz P. W., Gizienski T., Harms S. E., Harness J., Jackman R. J., Klimberg V. S., Kuske R., Levine G. M., Linver M. N., Rafferty E. A., Rugo H., Schilling K., Tripathy D., Vicini F. A., Whitworth P. W., and Willey S. C., “Special report: Consensus conference. III. Image-detected breast cancer: state-of-the-art diagnosis and treatment,” J. Am. Coll. Surg. 209, 504–520 (2009). 10.1016/j.jamcollsurg.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Berg W. A., Blume J. D., Adams A. M., Jong R. A., Barr R. G., Lehrer D. E., Pisano E. D., Evans W. P., 3rd, Mahoney M. C., Hovanessian Larsen L., Gabrielli G. J., and Mendelson E. B., “Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666,” Radiology 254, 79–87 (2010). 10.1148/radiol.2541090953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillaci O. and Buscombe J. R., “Breast scintigraphy today: Indications and limitations,” Eur. J. Nucl. Med. Mol Imaging 31(Suppl 1), S35–S45 (2004). 10.1007/s00259-004-1525-x [DOI] [PubMed] [Google Scholar]
- Zhou M., Johnson N., Blanchard D., Bryn S., and Nelson J., “Real-world application of breast-specific gamma imaging, initial experience at a community breast center and its potential impact on clinical care,” Am. J. Surg. 195, 631–635 (2008). 10.1016/j.amjsurg.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Kessler R., Sutcliffe J. B., Bell L., Bradley Y. C., Anderson S., and Banks K. P., “Negative predictive value of breast-specific gamma imaging in low suspicion breast lesions: A potential means for reducing benign biopsies,” Breast J. 17, 319–321 (2011). 10.1111/j.1524-4741.2011.01077.x [DOI] [PubMed] [Google Scholar]
- Khalkhali I., Mishkin F. S., Diggles L. E., and Klein S. R., “Radionuclide-guided stereotactic prebiopsy localization of nonpalpable breast lesions with normal mammograms,” J. Nucl. Med. 38, 1019–1022 (1997). [PubMed] [Google Scholar]
- Coover L. R. and Malaspina P. J., “Breast tumor localization using sestamibi,” Clin. Nucl. Med. 27, 161–164 (2002). 10.1097/00003072-200203000-00001 [DOI] [PubMed] [Google Scholar]
- Weisenberger A. G., Barbosa F., Green T. D., Hoefer R., Keppel C., Kross B., Majewski S., Popov V., Wojcik R., and Wymer D. C., “Small field of view scintimammography gamma camera integrated to a stereotactic core biopsy digital x-ray system,” IEEE Trans. Nucl. Sci. 49, 2256–2261 (2002). 10.1109/TNS.2002.803778 [DOI] [Google Scholar]
- Welch B., Brem R., Kross B., Popov V., Wojcik R., and Majewski S., “Gamma-guided stereotactic breast biopsy system,” IEEE Trans. Nucl. Sci. 53, 2690–2697 (2006). 10.1109/TNS.2006.876002 [DOI] [Google Scholar]
- Moadel R. M., “Breast Cancer Imaging Devices,” Semin Nucl. Med. 41, 229–241 (2011). 10.1053/j.semnuclmed.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Lorino C., Chiarella D. A., Welch B., Hodge T., and Kieper D., “Clinical performance of a stereotactic breast-specific gamma imaging apparatus in the localization of breast lesions for vacuum-assisted needle biopsy procedure [abstract],” presented at Radiological Society of North America Annual Meeting (unpublished).
- Kalinyak J. E., Schilling K., Berg W. A., Narayanan D., Mayberry J. P., Rai R., Dupree E. B., Shusterman D. K., Gittleman M. A., Luo W., and Matthews C. G., “PET-guided breast biopsy,” Breast J. 17, 143–151 (2011). 10.1111/j.1524-4741.2010.01044.x [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Conners A. L., Jones K. N., Tortorelli C. L., Maxwell R. M., Rhodes D. J., and O'Connor M. K., “Diagnostic workup of new findings on screening molecular breast imaging [abstract],” presented at Radiological Society of North America Annual Meeting (unpublished).
- Weinmann A. L., Hruska C. B., Conners A. L., and O'Connor M. K., “Collimator design for a dedicated molecular breast imaging-guided biopsy system: Proof-of-concept,” Med. Phys. 40, 012503 (12pp.) (2013). 10.1118/1.4770274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2 (The National Academies Press, 2006). [PubMed] [Google Scholar]
- Yaffe M. J. and Mainprize J. G., “Risk of radiation-induced breast cancer from mammographic screening,” Radiology 258, 98–105 (2011). 10.1148/radiol.10100655 [DOI] [PubMed] [Google Scholar]
- Mercier G., Vassilakis N., Brennan J., DeVita F., and Kachnic L., “Clinical performance of low dose positron emission mammography [abstract],” J. Nucl. Med. 53, 1231 (2012). [Google Scholar]
- Koolen B. B., Vogel W. V., Vrancken Peeters M. J., Loo C. E., Rutgers E. J., and Valdes Olmos R. A., “Molecular imaging in breast cancer: From whole body PET/CT to dedicated breast PET,” J. Oncol. (2012) (available online). 10.1155/2012/438647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar P. and Lois C., “Analytical study of the effect of the system geometry on photon sensitivity and depth of interaction of positron emission mammography,” J. Oncol. 2012, 605076. 10.1155/2012/605076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska C. B. and O'Connor M. K., “Effect of collimator selection on tumor detection for dedicated nuclear breast imaging systems,” IEEE Trans. Nucl. Sci. 53, 2680–2689 (2006). 10.1109/TNS.2006.879824 [DOI] [Google Scholar]
- Maublant J., de Latour M., Mestas D., Clemenson A., Charrier S., Feillel V., Le Bouedec G., Kaufmann P., Dauplat J., and Veyre A., “Technetium-99m-sestamibi uptake in breast tumor and associated lymph nodes,” J. Nucl. Med. 37, 922–925 (1996). [PubMed] [Google Scholar]
- Maublant J. C., Zhang Z., Rapp M., Ollier M., Michelot J., and Veyre A., “In vitro uptake of technetium-99m-teboroxime in carcinoma cell lines and normal cells: comparison with technetium-99m-sestamibi and thallium-201,” J. Nucl. Med. 34, 1949–1952 (1993). [PubMed] [Google Scholar]
- Kong F. L., Kim E. E., and Yang D. J., “Targeted nuclear imaging of breast cancer: status of radiotracer development and clinical applications,” Cancer Biother. Radiopharm. 27, 105–112 (2012). 10.1089/cbr.2011.1025 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Miao W., Li Q., Dai H., Ma Q., Wang F., Yang A., Jia B., Jing X., Liu S., Shi J., Liu Z., Zhao Z., and Li F., “99mTc-3PRGD2 for integrin receptor imaging of lung cancer: a multicenter study,” J. Nucl. Med. 53, 716–722 (2012). 10.2967/jnumed.111.098988 [DOI] [PubMed] [Google Scholar]
- Liu Z., Liu S., Wang F., and Chen X., “Noninvasive imaging of tumor integrin expression using (18)F-labeled RGD dimer peptide with PEG (4) linkers,” Eur. J. Nucl. Med. Mol Imaging 36, 1296–1307 (2009). 10.1007/s00259-009-1112-2 [DOI] [PubMed] [Google Scholar]
- Bach-Gansmo T., Bogsrud T. V., and Skretting A., “Integrin scintimammography using a dedicated breast imaging, solid-state gamma-camera and (99m)Tc-labelled NC100692,” Clin. Physiol. Funct. Imaging 28, 235–239 (2008). 10.1111/j.1475-097X.2008.00801.x [DOI] [PubMed] [Google Scholar]
- Axelsson R., Bach-Gansmo T., Castell-Conesa J., and McParland B. J., “An open-label, multicenter, phase 2a study to assess the feasibility of imaging metastases in late-stage cancer patients with the alpha v beta 3-selective angiogenesis imaging agent 99mTc-NC100692,” Acta Radiol. 51, 40–46 (2010). 10.3109/02841850903273974 [DOI] [PubMed] [Google Scholar]
- “NC100692 Kit (for the preparation of Technetium (99mTc) NC100692 Injection) [package insert],” (GE Healthcare Limited, Little Chalfont, Buckinghamshire, UK).
- Mortimer J. E., Dehdashti F., Siegel B. A., Katzenellenbogen J. A., Fracasso P., and Welch M. J., “Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy,” Clin. Cancer Res. 2, 933–939 (1996). [PubMed] [Google Scholar]
- Kenady D. E., Pavlik E. J., Nelson K., van Nagell J. R., Gallion H., DePriest P. D., Ryo U. Y., and Baranczuk R. J., “Images of estrogen-receptor-positive breast tumors produced by estradiol labeled with iodine I 123 at 16 alpha,” Arch. Surg. 128, 1373–1381 (1993). 10.1001/archsurg.1993.01420240081016 [DOI] [PubMed] [Google Scholar]
- Preston D. F., Spicer J. A., Baranczuk C., Fabian C., Baxter N. L., Martin W. R., and Jewell R. G., “Clinical results of breast cancer detedtion by imageable estradiol (I-123 E2) [abstract],” Eur. J. Nucl. Med. Mol. Imaging 16, S123 (1990). [Google Scholar]
- Bennink R. J., Rijks L. J., van Tienhoven G., Noorduyn L. A., Janssen A. G., and Sloof G. W., “Estrogen receptor status in primary breast cancer: Iodine 123–labeled cis-11β-Methoxy-17α-iodovinyl Estradiol Scintigraphy1,” Radiology 220, 774–779 (2001). 10.1148/radiol.2203001639 [DOI] [PubMed] [Google Scholar]
- Sundararajan L., Linden H. M., Link J. M., Krohn K. A., and Mankoff D. A., “18F-Fluoroestradiol,” Semin Nucl. Med. 37, 470–476 (2007). 10.1053/j.semnuclmed.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Pio B. S., Park C. K., Pietras R., Hsueh W. A., Satyamurthy N., Pegram M. D., Czernin J., Phelps M. E., and Silverman D. H., “Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy,” Mol. Imaging Biol. 8, 36–42 (2006). 10.1007/s11307-005-0029-9 [DOI] [PubMed] [Google Scholar]
- Papantoniou V. J., Souvatzoglou M. A., Valotassiou V. J., Louvrou A. N., Ambela C., Koutsikos J., Lazaris D., Christodoulidou J. K., Sotiropoulou M. G., Melissinou M. J., Perperoglou A., Tsiouris S., and Zerva C. J., “Relationship of cell proliferation (Ki-67) to 99mTc-(V)DMSA uptake in breast cancer,” Breast Cancer Res. 6, R56–R62 (2004). 10.1186/bcr751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H., Yang D. J., Cristofanilli M., Erwin W. D., Yu D. F., Kohanim S., Mendez R., and Kim E. E., “Imaging and dosimetry of 99mTc EC annexin V: Preliminary clinical study targeting apoptosis in breast tumors,” Appl. Radiat. Isot. 66, 1175–1182 (2008). 10.1016/j.apradiso.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M. L., “Genomic biomarkers for molecular imaging: Predicting the future,” Semin. Nucl. Med. 39, 236–246 (2009). 10.1053/j.semnuclmed.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini C. L., de Notaristefani F., Tofani A., Iacopi F., Sciuto R., Semprebene A., Malatesta T., Vittori F., Frezza F., Botti C., Giunta S., and Natali P. G., “99mTc-MIBI scintimammography using a dedicated nuclear mammograph,” J. Nucl. Med. 40, 46–51 (1999). [PubMed] [Google Scholar]
- O'Connor M. K., Phillips S. W., Hruska C. B., Rhodes D. J., and Collins D. A., “Molecular breast imaging: Advantages and limitations of a scintimammographic technique in patients with small breast tumors,” Breast J. 13, 3–11 (2007). 10.1111/j.1524-4741.2006.00356.x [DOI] [PubMed] [Google Scholar]
- Spanu A., Cottu P., Manca A., Chessa F., Sanna D., and Madeddu G., “Scintimammography with dedicated breast camera in unifocal and multifocal/multicentric primary breast cancer detection: A comparative study with SPECT,” Int. J. Oncol. 31, 369–377 (2007). [PubMed] [Google Scholar]
- Brem R. F., Floerke A. C., Rapelyea J. A., Teal C., Kelly T., and Mathur V., “Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer,” Radiology 247, 651–657 (2008). 10.1148/radiol.2473061678 [DOI] [PubMed] [Google Scholar]
- Spanu A., Chessa F., Meloni G. B., Sanna D., Cottu P., Manca A., Nuvoli S., and Madeddu G., “The role of planar scintimammography with high-resolution dedicated breast camera in the diagnosis of primary breast cancer,” Clin. Nucl. Med. 33, 739–742 (2008). 10.1097/RLU.0b013e318187ee75 [DOI] [PubMed] [Google Scholar]
- Murthy K., Aznar M., Thompson C. J., Loutfi A., Lisbona R., and Gagnon J. H., “Results of preliminary clinical trials of the positron emission mammography system PEM-I: A dedicated breast imaging system producing glucose metabolic images using FDG,” J. Nucl. Med. 41, 1851–1858 (2000). [PubMed] [Google Scholar]
- Levine E. A., Freimanis R. I., Perrier N. D., Morton K., Lesko N. M., Bergman S., Geisinger K. R., Williams R. C., Sharpe C., Zavarzin V., Weinberg I. N., Stepanov P. Y., Beylin D., Lauckner K., Doss M., Lovelace J., and Adler L. P., “Positron emission mammography: initial clinical results,” Ann. Surg. Oncol. 10, 86–91 (2003). 10.1245/ASO.2003.03.047 [DOI] [PubMed] [Google Scholar]
- Berg W. A., Weinberg I. N., Narayanan D., Lobrano M. E., Ross E., Amodei L., Tafra L., Adler L. P., Uddo J., W.Stein3rd, and Levine E. A., “High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer,” Breast J. 12, 309–323 (2006). 10.1111/j.1075-122X.2006.00269.x [DOI] [PubMed] [Google Scholar]
- Conners A. L., Hruska C. B., Tortorelli C. L., Maxwell R. W., Rhodes D. J., Boughey J. C., and Berg W. A., “Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy,” Eur. J. Nucl. Med. Mol. Imaging 39, 971–982 (2012). 10.1007/s00259-011-2054-z [DOI] [PubMed] [Google Scholar]
- Conners A. L., Maxwell R. W., Tortorelli C. L., Hruska C. B., Rhodes D. J., Boughey J. C., and Berg W. A., “Gamma camera molecular breast imaging lexicon: A pictoral essay,” AJR, Am. J. Roentgenol. 199, W767-74 (2012). 10.2214/AJR.11.8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan D., Madsen K. S., Kalinyak J. E., and Berg W. A., “Interpretation of positron emission mammography and MRI by experienced breast imaging radiologists: Performance and observer reproducibility,” AJR, Am. J. Roentgenol. 196, 971–981 (2011). 10.2214/AJR.10.5081 [DOI] [PMC free article] [PubMed] [Google Scholar]